Abstract
BACKGROUND
Clinical trials change practice in cardiology, and leading them requires research training, mentorship, sponsorship, and networking. Women report challenges in obtaining these opportunities.
OBJECTIVES
The purpose of this review was to evaluate temporal trends in representation of women as authors in heart failure (HF) randomized controlled trials (RCTs) published in high-impact medical journals and explore RCT characteristics associated with women as lead authors.
METHODS
We searched MEDLINE, EMBASE, and CINAHL for HF RCTs published in journals with an impact factor ≥10 between January 1, 2000, and May 7, 2019. We assessed temporal trends in the gender distribution of authors, and used multivariable logistic regression to determine characteristics associated with women as lead authors.
RESULTS
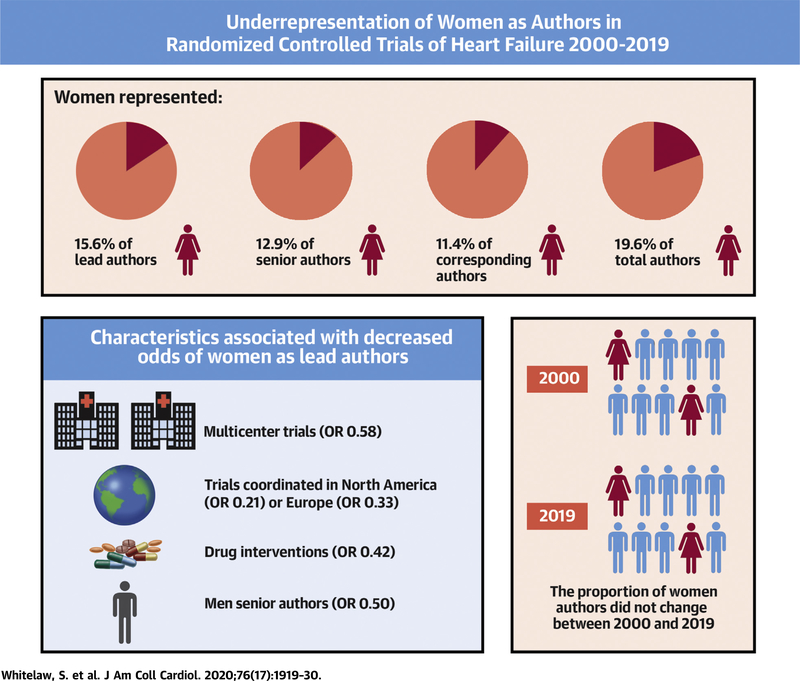
We identified 10,596 unique articles, of which 403 RCTs met inclusion criteria. Women represented 15.6% (95% confidence interval [CI]: 12.2% to 19.6%), 12.9% (95% CI: 9.8% to 16.6%), and 11.4% (95% CI: 8.5% to 14.9%) of lead, senior, and corresponding authors, respectively. The proportion of women authors has not changed over time. Women had lower odds of lead authorship in RCTs that were multicenter (odds ratio [OR]: 0.58; 95% CI: 0.18 to 0.96; p = 0.037), were coordinated in North America (OR: 0.21; 95% CI: 0.08 to 0.70; p = 0.011) or Europe (OR: 0.33; 95% CI: 0.09 to 0.91; p = 0.039), tested drug interventions (OR: 0.42; 95% CI: 0.16 to 0.97; p = 0.043), or had men as the senior author (OR: 0.50; 95% CI: 0.21 to 0.93; p = 0.043).
CONCLUSIONS
Women are under-represented as authors of HF RCTs, with no change in temporal trends. Women had lower odds of lead authorship in RCTs that were multicenter, were coordinated in North America or Europe, tested drug interventions, or had men as senior authors.
Keywords: authors, gender, heart failure, randomized controlled trials
Women are under-represented in most fields of academic medicine, and particularly in cardiology (1). A study by Blumenthal et al. (2) demonstrated that men dominate academic cardiology faculty (84% men, 17% women), and are significantly more likely to be full professors. In most academic institutions, research output is a key metric of success, and leading research studies is a path to career advancement and global reach. In the United States, women represent 25.5% of heart failure (HF) specialists, and it is unclear whether this distribution is reflected among those who lead HF research (3).
Randomized controlled trials (RCTs) generate the best-quality evidence among primary research methodologies, are often practice-changing, and receive the greatest spotlight at global meetings (4,5). Among research methodologies, RCTs pose unique challenges, require infrastructure and large amounts of funding, and can take years from planning to completion. Leading them typically requires advanced research training, mentorship, sponsorship, networking, and typically, academic appointments at research institutes. Women report obtaining these opportunities less frequently than men (6,7).
HF has experienced a revolution of practice-changing RCTs, with major advances in treatment (8–10). In this systematic review, we sought to determine the gender distribution among authors in impactful trials in HF and explore clinical trial characteristics independently associated with women as lead authors. We hypothesized that women would be under-represented as lead, senior, and corresponding authors overall, with stable temporal trends.
METHODS
STUDY OVERVIEW.
This study is registered in the International Prospective Register of Systematic Reviews (PROSPERO). Our study and the reporting followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (11).
DATA SOURCES AND SEARCHES.
With the aid of a professional information specialist, we conducted a systematic search of the published data, restricted to the English language, for manuscripts published in MEDLINE, EMBASE and CINAHL. Search terms included heart failure and randomized controlled trials. The search strategy for MEDLINE is available in the Supplemental Appendix.
STUDY SELECTION.
The authors independently screened all titles and abstracts from the search against pre-defined eligibility criteria. We performed screening and decision-making in duplicate. We included RCTs published in English between January 1, 2000, and May 7, 2019, that recruited adults (age ≥18 years) with HF. To include studies more likely to inform clinical practice, we limited the RCTs to those published in medical journals with an impact factor ≥10 in 2019 (12). The impact factor threshold of 10 was empirically chosen. We included full-text manuscripts reporting primary outcomes. We excluded protocols as well as publications subsequent to the first manuscript that described the primary outcomes of an RCT. Thus, we excluded publications describing post hoc, intermediate, or secondary analyses. We classified gender as uncertain if we were unable to ascertain the gender of authors.
DATA EXTRACTION AND ANALYSIS.
We independently extracted the following information in duplicate: year of publication, journal impact factor, region, location of recruitment, type of consent, type of intervention, level of randomization, type of follow-up, scope of trial, number of centers, funding type, journal of publication, total number of authors, and gender of authors in lead (first), middle, senior (last), and corresponding position. We only included individual authors who were listed in the author section of the paper. If applicable, we documented shared authorship roles in the marquee positions. We did not include individuals in trial investigator committees or consortia in the analysis. We determined gender via manual online searches of author names in conjunction with institution names. Sources for this information included photographs and pronoun descriptors on professional and institutional websites as well as social media accounts.
We performed a descriptive analysis, presenting continuous variables as median and interquartile range (IQR) and categorical variables as numbers and percentages. We used multivariable logistic regression to determine RCT characteristics associated with women as lead authors. The characteristics under consideration included region of RCT coordination, type of intervention, number of centers, type of funding, and gender of senior authors. We did not include journal of publication as a predictor variable because authorship is decided prior to submission for publication. We reported results as odds ratio (OR) with corresponding 95% confidence interval (CI) and associated p values. We analyzed temporal trends using the Jonckheere-Terpstra proportion trend test. All p values were 2-tailed, and the level of significance was set at alpha = 0.05. Data were analyzed using SPSS version 23 (IBM Corporation, Armonk, New York).
RESULTS
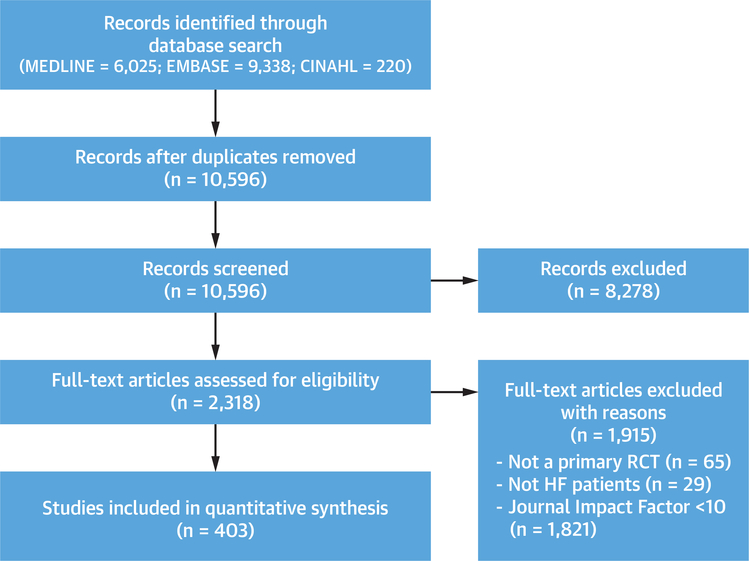
Our systematic search produced 10,596 unique manuscripts, of which 8,278 were excluded on the basis of title and/or abstract review. We assessed 2,318 full-text articles, of which 403 met eligibility criteria (Figure 1).
FIGURE 1. PRISMA Diagram of Included RCTs.
A systematic search of MEDLINE, EMBASE, and CINAHL was conducted to identify randomized controlled trials (RCTs) that recruited adults with heart failure (HF) and were published in medical journals with an impact factor ≥10.
CHARACTERISTICS OF INCLUDED RCTs.
The 403 RCTs were authored by a total of 4,346 authors (median 10 authors; IQR: 6 to 13 authors per trial). There were no RCTs with shared lead or senior authors. Most RCTs were conducted in Europe (54.3%), limited to single countries (74.9%), involved multiple centers (57.3%), and tested drug interventions (67.2%). All RCTs obtained informed consent. Most randomized individual patients (98.5%). Men comprised a majority of lead (84.4%), senior (87.1%), and corresponding authors (88.6%) (Table 1).
TABLE 1.
Characteristics of Randomized Controlled Trials (n = 403) Included in the Study
| Unit of randomization | |
| Individual | 397 (98.5) |
| Cluster | 6 (1.5) |
| Type of consent | |
| Informed | 403 (100.0) |
| Region of coordinating center | |
| North America | 147 (36.5) |
| Central and South America | 15 (3.7) |
| Australia | 10 (2.5) |
| Asia | 12 (3.0) |
| Europe | 219 (54.3) |
| Eligibility criteria | |
| Reported | 403 (100.0) |
| Recruitment | |
| Inpatient | 93 (23.1) |
| Ambulatory | 310 (76.9) |
| Type of intervention | |
| Health service | 49 (12.2) |
| Drug | 271 (67.2) |
| Device | 46 (11.4) |
| Surgery | 8 (2.0) |
| Exercise/rehabilitation | 29 (7.2) |
| Number of centers | |
| Single center | 172 (42.7) |
| Multicenter | 231 (57.3) |
| Type of follow-up | |
| Face-to-face | 392 (97.3) |
| Database | 11 (2.7) |
| Scope of trial | |
| National | 302 (74.9) |
| International | 101 (25.1) |
| Type of funding | |
| Public | 185 (45.9) |
| Industry | 163 (40.4) |
| Public and industry | 55 (13.6) |
| Gender of lead author | |
| Men | 340 (84.4) |
| Women | 63 (15.6) |
| Gender of senior author | |
| Men | 351 (87.1) |
| Women | 52 (12.9) |
| Gender of corresponding author | |
| Men | 357 (88.6) |
| Women | 46 (11.4) |
| Year of publication | |
| 2000–2003 | 127 (31.5) |
| 2004–2007 | 109 (27.0) |
| 2008–2011 | 47 (11.7) |
| 2012–2015 | 51 (12.7) |
| 2016–2019 | 69 (17.1) |
Values are n (%).
TEMPORAL TRENDS IN GENDER OF AUTHORS.
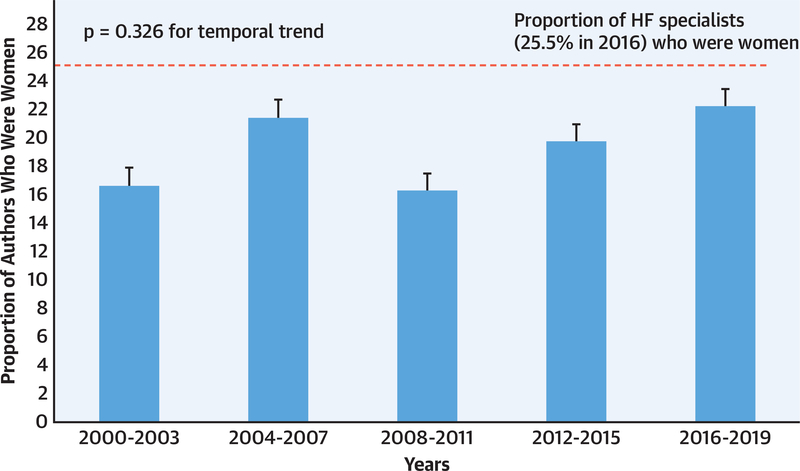
We were able to ascertain the gender of all 4,346 authors. The median number of authors per RCT increased from 8 authors (IQR: 5 to 11 authors) in 2000 to 2003 to 15 authors (IQR: 12 to 19 authors) in 2016 to 2019. Of a total of 4,346 authors, 852 (19.6%; 95% CI: 18.5% to 20.8%) were women. The proportion of women among authors in any position has not changed significantly from 2000 to present (p = 0.326) (Figure 2).
FIGURE 2. Temporal Trends in Gender of Lead, Senior, and Corresponding Authors in HF RCTs (n = 403) Published in High Impact Journals Between 2000 and 2019.
Temporal trends in the gender of authors in any position were analyzed using the Jonckheere-Terpstra proportion trend test (2-tailed testing, α = 0.05). The sample included 403 randomized controlled trials (RCTs) and 4,346 authors, 19.6% of whom were women. The proportion of women in any authorship position did not change significantly over time (p = 0.326). HF = heart failure.
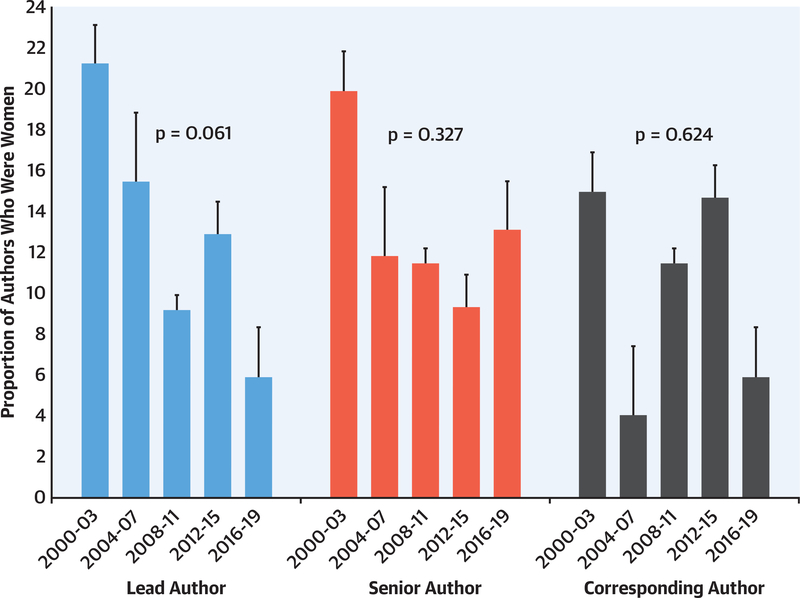
Among 403 authors in each of the lead, senior, and corresponding positions, 63 (15.6%; 95% CI: 12.2% to 19.6%), 52 (12.9%; 95% CI: 9.8% to 16.6%), and 46 (11.4%; 95% CI: 8.5% to 14.9%), respectively, were women. The proportion of women in these authorship positions decreased numerically over time, but the trends were not significant (lead author, p = 0.061; senior author, p = 0.327; corresponding author; p = 0.624) (Figure 3). Women comprised only 28 (12.1%) and 33 (14.3%) of lead and senior authors, respectively, of multicenter trials; 5 (1.2%) and 2 (0.5%) of lead and senior authors, respectively, of device trials; and 35 (8.7%) and 32 (7.9%) of lead and senior authors, respectively, of drug trials.
FIGURE 3. Temporal Trends in Gender of Lead, Senior, or Corresponding Authors in HF RCTs (n = 403) Published in High Impact Journals Between 2000 and 2019.
Temporal trends in the gender distribution of lead, senior, and corresponding authors were analyzed using the Jonckheere-Terpstra proportion trend test (2-tailed testing, α = 0.05). The sample included 403 RCTs, with 403 authors in each position. Women represented 15.6%, 12.9%, and 11.4% of the lead, senior, and corresponding authors, respectively, with no change in temporal trends (lead author, p = 0.061; senior author, p = 0.327; corresponding author; p = 0.624). Abbreviations as in Figure 2.
GENDER OF LEAD AND SENIOR AUTHORS ACCORDING TO JOURNAL OF RCT PUBLICATION.
The 403 RCTs were published in 14 major medical journals. Most RCTs were published in the European Journal of Heart Failure (n = 104), Journal of the American College of Cardiology (n = 88), and Circulation (n =60). Among journals with at least 20 RCTs published during the study period, the proportion of women as lead authors was greatest in European Journal of Heart Failure (23.1%), Journal of the American Medical Association (22.2%), and Journal of the American College of Cardiology (14.7%). Among journals with at least 20 RCTs published during the study period, the proportion of women as senior authors was greatest in Journal of the American Medical Association (22.2%), New England Journal of Medicine (15.8%), and Circulation (15.0%) (Table 2).
TABLE 2.
Gender Breakdown of Lead and Senior Authors of RCTs Published in Major Medical Journals (n = 403)
| Journal | RCTs | RCTs With Women Lead Authors | RCTs With Women Senior Authors |
|---|---|---|---|
| American Journal of Respiratory and Critical Care Medicine | 2 (0.5) | 0 (0.0) | 0 (0.0) |
| Annals of Internal Medicine | 1 (0.2) | 1 (100.0) | 0 (0.0) |
| British Medical Journal | 4 (1.0) | 1 (25.0) | 2 (50.0) |
| Circulation | 60 (14.9) | 8 (13.3) | 9 (15.0) |
| Circulation Research | 6 (1.5) | 1 (16.7) | 1 (16.7) |
| European Heart Journal | 42 (10.4) | 4 (9.5) | 6 (14.3) |
| European Journal of Heart Failure | 104 (25.8) | 24 (23.1) | 13 (12.5) |
| European Respiratory Journal | 1 (0.2) | 0 (0.0) | 0 (0.0) |
| Journal of the American Medical Association | 27 (6.7) | 6 (22.2) | 6 (22.2) |
| Journal of the American Medical Association Cardiology | 3 (0.7) | 0 (0.0) | 1 (33.3) |
| Journal of the American Medical Association Internal Medicine | 6 (1.5) | 1 (16.7) | 1 (16.7) |
| Journal of the American College of Cardiology | 88 (21.8) | 13 (14.7) | 4 (4.5) |
| Lancet | 21 (5.2) | 1 (4.8) | 3 (14.3) |
| New England Journal of Medicine | 38 (9.4) | 3 (7.9) | 6 (15.8) |
| Total | 403 | 63 | 52 |
Values are n (%) or n.
RCT = randomized controlled trial.
MULTIVARIABLE ANALYSIS OF RCT CHARACTERISTICS ASSOCIATED WITH WOMEN AS LEAD AUTHORS.
Women had lower odds of lead authorship in RCTs that were multicenter rather than single-center (OR: 0.58; 95% CI: 0.18 to 0.96; p = 0.037); coordinated in North America (OR: 0.21; 95% CI: 0.08 to 0.70; p = 0.011) or Europe (OR: 0.33; 95% CI: 0.09 to 0.91; p = 0.039) relative to Central and South America; tested drug interventions (OR: 0.42; 95% CI: 0.16 to 0.97; p = 0.043) relative to other interventions; or had men in the senior authorship position (OR: 0.50; 95% CI: 0.21 to 0.93; p = 0.043).
There was no significant association between women in lead authorship position and trials coordinated in Asia and Australia (OR: 0.24; 95% CI: 0.04 to 1.88; p = 0.162) relative to trials coordinated in Central and South America; device/surgery trials (OR: 0.37; 95% CI: 0.09 to 1.45; p = 0.213), relative to other interventions; and industry funding (OR: 0.62; 95% CI: 0.32 to 1.40; p = 0.901) relative to public funding (Table 3).
TABLE 3.
Multivariable Analysis of Clinical Trial Characteristics Associated With Women Lead Authors in RCTs of HF (n = 403)
| OR (95% CI) | p Value | |
|---|---|---|
| Region | ||
| Central and South America | 1.00 (Reference) | — |
| Europe | 0.33 (0.09–0.91) | 0.039 |
| North America | 0.21 (0.08–0.71) | 0.011 |
| Asia and Australia | 0.24 (0.04–1.88) | 0.162 |
| Type of intervention | ||
| Other | 1.00 (Reference) | — |
| Drug | 0.42 (0.16–0.97) | 0.043 |
| Device / Surgery | 0.37 (0.09–1.45) | 0.213 |
| Number of centers | ||
| Single center | 1.00 (Reference) | — |
| Multicenter | 0.58 (0.18–0.96) | 0.037 |
| Type of funding | ||
| Public | 1.00 (Reference) | — |
| Industry | 0.62 (0.32–1.40) | 0.901 |
| Gender of senior author | ||
| Women | 1.00 (Reference) | — |
| Men | 0.50 (0.21–0.93) | 0.043 |
CI = confidence interval; HF = heart failure; OR = odds ratio; RCT = randomized controlled trial.
DISCUSSION
This systematic review demonstrated that among 403 HF RCTs published in high-impact medical journals between 2000 and 2019, women comprised only 15.6%, 12.9%, and 11.4% of lead, senior, and corresponding authors, respectively. There was no significant temporal change in the proportion of women in these authorship positions. Among a total of 4,346 authors in any authorship position in these RCTs, 19.6% were women. The proportion of women authors in any authorship position did not change over time. Women had lower odds of lead authorship in RCTs that were multicenter, coordinated in North America or Europe, tested drug interventions, or had men as senior author (Central Illustration).
CENTRAL ILLUSTRATION. Under-Representation of Women as Authors in Randomized Controlled Trials of Heart Failure Published in High-Impact Journals.
Of 403 randomized controlled trials (RCTs) published in high-impact journals, women were under-represented as authors of heart failure (HF) RCTs, with no change in temporal trends. Women had lower odds of lead authorship in RCTs that were multicenter, coordinated in North America or Europe, tested drug interventions, or had men as senior authors. OR = odds ratio.
Our findings suggest that women are under-represented in leadership and collaborative roles and that there has been no change in temporal trends over the past 2 decades. This parallels the gender gap among physicians in cardiovascular subspecialties such as HF in the United States (74.5% men, 25.5% women) (3,13,14). This gap has persisted, with no change in the proportion of women HF subspecialty trainees (26%) in the United States since 2011 (15). The gender gap seen in clinical settings appears to be amplified in clinical trial leadership.
Among research methodologies, RCTs pose unique challenges—prolonged duration before academic output is generated; expense that requires external funding; and complexity that requires extended training, mentorship, research infrastructure, and networking (4,5). However, there are several gender-based inequities that make a research career challenging for women (6,7,16,17). In a survey of 507 physicians, women perceived institutes to be less supportive toward women than men, less likely to nominate them for promotion, and less likely to include them in research networks (18,19). Women face barriers in research funding and publication that may affect metrics required for promotion and retention in research careers. In a study of peer-reviewed research grants, women were assigned lower grant scores than men even after controlling for more than 20 potential confounders, including publications and history of funding success (20). Manuscripts and conference abstracts led by women were accepted more often when reviewers were blinded to the gender of the authors (21,22). Women are under-represented in editorial boards, potentially amplifying the gender bias in publication acceptances (23). These barriers may be reasons why women with an interest in cardiovascular research instead pursue full-time clinical careers, which offer greater job stability relative to funding-dependent research positions (24).
We found that women are less likely to be lead authors when men are senior authors, suggesting a gender association—either intended or unintended—between mentees and mentors. A prior analysis of publications (including primary research, viewpoints, editorials) in 6 general cardiology journals in 1996, 2006, and 2016 found that 16.5% of lead authors were women, and that there was an association between the gender of lead and senior authors (25). Another bibliometric analysis of primary research papers published in 3 high-impact general cardiology journals found that 26.7% of lead authors were women, and that there was an association between gender of lead and senior author; these papers were not restricted to RCTs (26). The estimates of women in lead positions in these 2 studies are slightly different from our study, possibly due to different date ranges (25,26), a broader focus than HF alone, inclusion of papers other than primary research (25), and inclusion of research methodologies other than clinical trials (26). A recent review of 118 HF clinical trials published between 2001 and 2016 reported a lower proportion of women as first (10%) and senior authors (8%) than our study, possibly due to the smaller number of included trials, shorter date range, and exclusion of trials with ≤400 participants (27). This study did not provide descriptive statistics or temporal trends in gender composition of each type of author (lead, corresponding, middle, or senior) due to the limited sample size, but it did report no change in the proportion of women who were either lead or senior authors (16%) over time. Importantly, this study and the ones prior to it neither assessed the role of women as collaborators nor assessed trial characteristics independently associated with women as lead authors (25–27).
Women are more likely to lead single-center rather than multicenter trials, which are logistically more complex to coordinate but have the advantage of increased generalizability and potential to change practice compared with single-center trials (28). Multicenter trials require a larger collaborative network, but a gender gap exists in large research collaborations that have a greater reach (29). For example, a recent bibliometric analysis of publications from 12 geographies and 27 subject areas found that relative to men, women had fewer collaborations both inside and outside of their institutions, as measured by the number of coauthorships of research papers (30). Collaborations broaden networks, are associated with greater number of grants and publications, and have implications on clinical trial involvement (30,31). The gender gaps in research collaboration and the types of trials women lead are likely multifactorial, may include gender bias, less prominent profiles and international recognition, less sponsorship by mentors, and exclusion from informal networks.
Women had lower odds of RCT leadership in North America and Europe, where many higher-profile RCTs are coordinated. Odds of RCT leadership were greatest in Central and South America, where there may be a slightly higher proportion of women cardiologists; for example, women represent approximately 29% of cardiologists in Brazil, 12.6% of cardiologists in the United States, and 6% to 20% of cardiologists in European countries (3,32,33). Thus, regions with the greatest proportion of women leading RCTs may be those with a greater proportion of women cardiologists. There may also be regional differences in the proportion of women in academic settings, although data is lacking in this regard (34). Finally, there may be differences in culture, networking opportunities, and research-clinical work integration that account for some differences.
Women had lower odds of leading RCTs that tested the effect of drug interventions. Most drug trials are funded by pharmaceutical companies, which are known to offer funding to women less commonly than men (35). Although not statistically significant, our results show that industry funding of a trial tended to be associated with lower odds of women in lead authorship position; the wide CIs around the estimated odds are suggestive of limited statistical power (36). An analysis of 220,908 physicians who received industry funding found that 75.1% were men, and that men received significantly greater funding than women (37). Women may be viewed less favorably as researchers by industry funding sources due to bias (38). In observational studies, reviewers have been found to assess equal productivity less positively for women than men applicants (39). Success begets success, and structural biases that favor men via collaborations, speaking engagements, grants, publications, and salary awards make them favorable candidates for downstream opportunities, including leadership of drug and device trials (38,39).
The importance of women as leaders in clinical trials is multifold. In a survey of 1,123 internal medicine trainees, most women perceived the field of cardiology to lack the mentors they desired (40). A vast majority of women researchers (77%) have men, rather than women, as their mentors according to a survey of young researchers at the National Institute of Health (41). The gender association between senior and lead authors and the under-representation of women as mentors in clinical trials—assessed using the surrogate status of senior author—may deprive women from leading clinical trials themselves, creating a cycle of under-representation of women as leaders in clinical trials. In addition, other associated benefits of having women as lead authors in clinical trials—increased enrollment of women as trial participants and increased citations per publication relative to men—may be lost (26,42).
Efforts to enhance the recruitment, retention, and career advancement of women as clinical trialists in cardiology should be a priority (24,43). Organizations such as the American Heart Association and American College of Cardiology have directed efforts to recruit women and encourage success in the field of cardiology (44,45). Both organizations have developed ‘Women in Cardiology’ committees dedicated to the advancement of women (44,45). The American Heart Association has implemented a scholarship program for trainees and a mentorship award recognizing those who have been exceptional mentors to women in cardiology (44). The American College of Cardiology has implemented mentorship programs, leadership workshops, networking opportunities, and visiting women professor programs, and most recently created a Clinical Trials Research Boot Camp program to increase the number of women and under-represented cardiologists leading clinical trials (46). Organizations such as Women As One provide platforms to mentor and promote women in cardiology (46). Most of these initiatives are not specific to research, however, and increasing women in cardiology is a first step toward closing the gender gap in cardiovascular research. To increase the proportion of women who lead research, a zero-tolerance policy for workplace bullying and harassment—reported in many research institutes as a factor in attrition of women researchers—should be enforced (24,43). Leaders of research institutes should be educated about gender disparities in research career advancement (43), eliminate inappropriate questions during interviews for recruitment and promotion, and mitigate implicit bias in selection processes (24). Programs that support career flexibility and work-life integration should be developed (24,43). Institutions should provide equal renumeration to promote the retention of women in academic settings (47).
To increase the proportion of women who lead impactful clinical trials, societies could initiate national and international collaborative research networks for women to advance their careers, broaden their reach, and increase the likelihood of multisite clinical trial involvement. Formal research networks or registries led by women for women could offer research collaboration, mentorship, and sponsorship opportunities tailored to the needs of professional women. Industry and grant funding agencies should receive antibias training, conduct blind reviews of applications, and use more objective review criteria (48,49). They should be transparent and include gender breakdowns of principal investigators who applied for and received funding (Table 4) (24,48,49). Women scientists should be included as board and executive committee members of research institutes, reviewers and chairs on grant panels, members of scientific advisory boards, key opinion leaders, and journal editorial board members. Inclusion in these positions should be proportional to their representation in the field to close some of the gender gaps (48,49). Speaking engagements as well as online and social media engagement could help increase the profile of women researchers who are not recognized or included in research networks in their home institutions.
TABLE 4.
Recommendations to Increase the Representation of Women as Authors in Randomized Controlled Trials
| Recommendations for early- and mid-career women cardiologists | Engage in online and social media networks, limiting content to science Participate in national and international research networks or registries that offer women research collaboration, mentorship, and sponsorship opportunities Invest in clinical research training (certificate programs offered by societies, advanced degrees and fellowships offered by universities) |
| Recommendations for senior men and women cardiologists | Mentor and sponsor the next generation of women trialists Create a supportive culture to ensure equal opportunity and recognition Learn to recognize and intervene during harassment |
| Recommendations for academic and departmental leadership | Receive education about gender disparities in research career advancement Eliminate inappropriate questions during interviews for recruitment and promotion, and mitigate implicit bias in selection processes Develop mentoring and sponsoring programs for career growth of researchers Include women as board or executive committee members at research institutes Ensure equal opportunity (in recruitment and retention, compensation, access to resources) and recognition for researchers based on objective criteria Encourage self-nominations and eliminate reliance on department chairs or committees to nominate researchers for awards or advancement opportunities Implement a zero-tolerance policy for workplace harassment Implement flexible promotion policies that recognize the familial and child rearing demands of early-career investigators Encourage women to apply for funding opportunities Participate in anti-bias training |
| Recommendations for industry and grant funding agencies | Conduct blind reviews of applications and use more equitable review criteria Provide gender breakdown of applicants and awards Include women scientists as reviewers and chairs on funding committees Include women in luminary networks (key opinion leaders, scientific advisory boards) |
| Recommendations for journals | Provide equitable peer review Set objective criteria and avoid informal networks for the selection of editors and editorial boards |
To our knowledge, this is the first systematic review to assess the gender breakdown of clinical trial leadership and to examine clinical trial factors associated with women as lead authors in any medical field. The strengths of our study included the comprehensive search strategy and the inclusion of RCTs published in high-impact factor journals over a 2-decade time span. The review process and data extraction were conducted independently by 2 authors and discrepancies were resolved by consultation with a third author, which reduced the likelihood that the results of our study were due to single reviewer bias or chance. The large sample size of RCTs minimized the potential for bias caused by chance.
STUDY LIMITATIONS.
This review was restricted to English language studies published in high-impact medical journals. The gender distribution of authors and associations described in this study may not apply to RCTs that were excluded from this review. It is possible that the representation of women authors in lower-impact journals do not follow the trends identified within this study. Data regarding author gender were obtained from online sources, and we cannot account for error in the primary sources. We were not able to account for gender nonbinary authors based on our search of online sources. We did not account for clustering of authorship teams or trial coordinating centers across clinical trials. We used lead and senior authorship status as surrogates for mentees and mentors as well as for leadership of RCTs, although we recognize that some trials are led by industry partners. We did not account for the degrees of authors or distinguish between clinician and nonclinician researchers, although we acknowledge that all researchers play an important role in clinical trial involvement. We could not assess race or ethnicity of authors, and we recognize that gender disparities in research are amplified among racial/ethnic groups (50). The multivariable analysis is exploratory in nature, and the results should be interpreted with caution. There is a risk of overfitting due to the low ratio of events to the degrees of freedom for the characteristic variables (51).
CONCLUSIONS
Among 403 HF RCTs published between 2000 and 2019, women were under-represented as lead, senior, and corresponding authors. The proportion of women in these authorship positions has not changed. Women had lower odds of lead authorship in RCTs that were multicenter, were coordinated in North America or Europe, tested drug interventions, or had men as the senior author. Given the independent gender association between lead and senior author, recruiting, training, and advancing women as leaders of RCTs may be a strategic way—among others—to rapidly increase the proportion of women leading RCTs.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN SYSTEMS-BASED PRACTICE:
Women are under-represented in HF clinical trials, both as participants and as authors. The odds that a woman is lead author is lower among multicenter trials, those coordinated in North America or Europe, those testing pharmacological interventions, and those with men as senior authors.
TRANSLATIONAL OUTLOOK:
Addressing the factors associated with under-representation of women may improve gender balance and advance women as leaders of clinical trials in heart failure.
ACKNOWLEDGMENTS
The authors thank Mohammad Alruwayeh, Yousif Eliya, and Kristen Sullivan for their assistance with data extraction.
Dr. Van Spall has received research salary support from McMaster Department of Medicine and the Women As One Escalator Award; and has received funding support from the Canadian Institutes of Health Research. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CI
confidence interval
- HF
heart failure
- IQR
interquartile range
- OR
odds ratio
- RCT
randomized controlled trial
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC author instructions page.
Listen to this manuscript’s audio summary by Editor-in-Chief Dr. Valentin Fuster on JACC.org.
APPENDIX For the preliminary search strategy, please see the online version of this paper.
REFERENCES
- 1.Association of American Medical Colleges. 2018–2019 The state of women in academic medicine: exploring pathways to equity. Available at: https://www.aamc.org/data-reports/data/2018-2019-state-women-academic-medicine-exploring-pathways-equity. Accessed June 3, 2020.
- 2.Blumenthal DM, Olenski AR, Yeh RW, et al. Sex differences in faculty rank among U.S. academic cardiologists. Circulation 2017;135:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta LS, Fisher K, Rzeszut AK, et al. Current demographic status of cardiologists in the United States. JAMA Cardiol 2019;4:1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaumont D, Arribas M, Frimley L, Balogen E, Roberts I, Shakur-Still H. Trial management: we need a cadre of high-class triallists to deliver the answers that patients need. Trials 2019;20:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyatt GH, Haynes RB, Jaeschke RZ, et al. , for the Evidence-Based Medicine Working Group. Users’ guides to the medical literature, XXV: evidence-based medicine: principles for applying the users’ guides to patient care. JAMA 2000;284: 1290–6. [DOI] [PubMed] [Google Scholar]
- 6.Buddeberg-Fischer B, Stamm M, Buddeberg C, et al. The impact of gender and parenthood on physicians’ careers—professional and personal situation seven years after graduation. BMC Health Serv Res 2010;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patton EW, Griffith KA, Jones RD, Stewart A, Ubel PA, Jagsi R. Differences in mentor-mentee sponsorship in male vs female recipients of National Institutes of Health grants. JAMA Intern Med 2017;177:580–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor CM. The globalization of heart failure research. J Am Coll Cardiol 2015;3:657–8. [DOI] [PubMed] [Google Scholar]
- 9.Vanduganathan M, Tahhan AS, Greene SJ, Okafor M, Kumar S, Butler J. Globalization of heart failure clinical trials; a systematic review of 305 trials conducted over 16 years. Eur J Heart Fail 2018;20:1068–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bothwell LE, Greene JA, Podolsky SH, Jones DS. Assessing the gold standard-lessons from the history of RCTS. N Engl J Med 2016; 364:2175–81. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberti A, Tetlaff J, Altman G, for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLos Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collier C Announcing the 2019 journal citation reports. Available at: https://clarivate.com/webofsciencegroup/article/announcing-the-2019-journal-citation-reports/. Accessed April 20, 2020.
- 13.Lewis SJ, Mehta LS, Douglas PS, et al. Changes in the professional lives of cardiologists over 2 decades. J Am Coll Cardiol 2017;69:452–62. [DOI] [PubMed] [Google Scholar]
- 14.Stone AT, Carlson KM, Douglas PS, Morris KL, Walsh MN. Assessment of subspecialty choices of men and women in internal medicine from 1991 to 2016. JAMA Intern Med 2019;180:140–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Board of Internal Medicine. Percentage of first-year fellows by gender and type of medical school attended. Available at: https://www.abim.org/about/statistics-data/resident-fellow-workforce-data/first-year-fellows-by-gender-type-of-medical-school-attended.aspx. Accessed August 1, 2020.
- 16.Ortega RF, Mehran R, Douglas PS. The conundrum and opportunity of gender equity for evidence generators. JAMA Cardiol 2020. April 8 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Chisholm-Burns MA, Spivey CA, Hagermann T, Josephson MA. Women in leadership and the bewildering glass ceiling. Am J Health Syst Pharm 2017;74:312–24. [DOI] [PubMed] [Google Scholar]
- 18.Foster SW, McMurray JE, Linzer M, Leavitt JW, Rosenberg M, Carnes M. Results of a gender-climate and work-environment survey at a midwestern academic health center. Acad Med 2000; 75:653–60. [DOI] [PubMed] [Google Scholar]
- 19.Mehta L, Sharma G, Douglas P, Rzeszut A. Discrimination and harassment in cardiology: insights from the 2019 American College of Cardiology global professional survey (abstr). J Am Coll Cardol 2020;Suppl 1;75:3631. [Google Scholar]
- 20.Tamblyn R, Girard N, Qian CJ, Hanley J. Assessment of potential bias in research grant peer review in Canada. CMAJ 2018;190:E489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budden AE, Tregenza T, Aarssen LW, Koricheva J, Leimu R, Lortie CJ. Double-blind review favours increased representation of female authors. Trends Ecol Evol 2008;23:4–6. [DOI] [PubMed] [Google Scholar]
- 22.Roberts SG, Verhoef T. Double blind reviewing at EvoLang 11 reveals gender bias. J Lang Evol 2016;1:163–7. [Google Scholar]
- 23.Balasubramanian S, Saberi S, Yu S, Duvernoy CS, Day SM, Agarwal PP. Women representation among cardiology journal editorial boards. Circulation 2020;141:603–5. [DOI] [PubMed] [Google Scholar]
- 24.Sharma G, Sarma AA, Walsh MN, et al. 10 recommendations to enhance recruitment, retention, and career advancement of women cardiologists. J Am Coll Cardiol 2019;74:1839–42. [DOI] [PubMed] [Google Scholar]
- 25.Ashgar M, Usman MS, Aibani R, et al. Sex differences in authorship of academic cardiology literature over the past 2 decades. J Am Coll Cardiol 2018;72:681–5. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang D, Sing D, Shah S, et al. Sex disparities in authorship order of cardiology publications. Circulation 2018;11:e005040. [DOI] [PubMed] [Google Scholar]
- 27.Reza N, Tahhan AS, Mahmud N, et al. Representation of women authors in international heart failure guidelines and contemporary clinical trials. Circ Heart Fail 2020;13:e006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhandari M, Schemitsch EH. Beyond the basics: the organization and coordination of multicenter trials. Tech Orthop 2004;19:83–7. [Google Scholar]
- 29.Elsiever. Gender in the global research landscape. Available at: https://www.elsevier.com/__data/assets/pdf_file/0008/265661/ElsevierGenderReport_final_for-web.pdf. Accessed June 10, 2020.
- 30.Tong CW, Madhur MS, Rzeszut AK, et al. Status of early-career academic cardiology: a global perspective. J Am Coll Cardiol 2017;70:2290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Block P, Weber H, Kearney P. Manpower in cardiology II in western and central Europe (1999–2000). Eur Heart J 2003;24:299–310. [DOI] [PubMed] [Google Scholar]
- 32.Mainardi GM, Cassenote AJF, Guilloux AGA, Miotto BA, Scheffer MC. What explains wage differences between male and female Brazilian physicians? A cross-sectional nationwide study. BMJ Open 2019;9:e023811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagsi R, Biga C, Poppas A, et al. Work activities and compensation of male and female cardiologists. J Am Coll Cardol 2016;67:529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Gender equity in the health workforce: Analysis of 104 countries. Available at: https://apps.who.int/iris/bitstream/handle/10665/311314/WHO-HIS-HWF-Gender-WP1-2019.1-eng.pdf?sequence=1&isAllowed=y&te=1&nl=in-her%20words&emc=edit_gn_20200312. Accessed June 11, 2020.
- 35.Chopra SS. Industry funding of clinical trials: benefit or bias? JAMA 2003;290:113–4. [DOI] [PubMed] [Google Scholar]
- 36.Du Prel JB, Hommel G, Bohrig B, Blettner M. Confidence interval or p-value: part 4 of a series on evaluation of science publications. Dtsch Arztebl Int 2009;106:335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose SL, Sanghani RM, Schmidt C, Karafa MT, Kodish E, Chisolm GM. Gender differences in physician’s financial ties to industry: a study of disclosure data. PLoS One 2015;10:e0129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witteman HO, Hendricks M, Straus S, Tannenbaum C. Are gender gaps due to evaluations of the applicant or the science? A natural experiment at a national funding agency. Lancet 2019;393:531–40. [DOI] [PubMed] [Google Scholar]
- 39.Silibiger NJ, Stubler AD. Unprofessional peer reviews disproporitionately harm under-represented groups in STEM. PeerJ 2019;7:e8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douglas PS, Rzeszut AK, Merz NB, et al. Career preferences and perceptions of cardiology among US Medicine Trainees. JAMA Cardiol 2018;3: 682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ed Martinez, Botos J Dohoney KM, et al. Falling off the academic bandwagon. EMBO Rep 2007;8:977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielson MW, Andersen JP, Schiebinger L, Schneider JW. One and a half million medical papers reveal a link between author gender and attention to gender and sex analysis. Nat Hum Behav 2017;1:791–6. [DOI] [PubMed] [Google Scholar]
- 43.Lau ES, Wood MJ. How do we attract and retain women in cardiology? Clin Cardiol 2018;41: 264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Heart Association. Women and special populations. Available at: https://professional.heart.org/professional/Communities/WomenandSpecialPopulations/UCM_475317_Women-and-Special-Populations.jsp. Accessed June 22, 2020.
- 45.American College of Cardiology. Women in cardiology section. Available at: https://www.acc.org/membership/sections-and-councils/women-in-cardiology-section/about-us/section-mission-and-objectives Accessed June 22, 2020.
- 46.Women As One. Promoting equity in medicine. Available at: https://www.womenasone.org/. Accessed June 26, 2020.
- 47.Douglas PS, Biga C, Burns KM, et al. 2019 ACC Health policy statement on cardiologist compensation and opportunity equity. J Am Coll Cardiol 2019;74:1947–65. [DOI] [PubMed] [Google Scholar]
- 48.Alverez SNE, Jagsi R, Abbuhl SB, Lee CJ, Myers ER. Promoting gender equity in grant making: what can a funder do? Lancet 2019;393:e9–11. [DOI] [PubMed] [Google Scholar]
- 49.Ross JS, Gross CO, Krumholz HM. Promoting transparency in pharmaceutical industry-sponsored research. Am J Public Health 2012; 102:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.American College of Cardiology. American College of Cardiology diversity and inclusion initiative. Available at: https://www.acc.org//w/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/About%20ACC/Diversity/2018/03/Diversity-Inclusion-Strategy-Summary.pdf. Accessed June 11, 2020.
- 51.Ogundimu EO, Altman DG, Collins GS. Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol 2016;76:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.