Abstract
Morphogens play an essential role in cell fate specification and patterning including in laying out the mammalian body plan during gastrulation. In vivo studies have shed light on the signaling pathways involved in this process and the phenotypes involved in their disruption, however, several important open questions remain regarding how morphogens function in space and time. Self-organized patterning systems based on embryonic stem cells have emerged as a powerful platform for beginning to address these questions that is complimentary to in vivo approaches. Here we review recent progress in understanding morphogen signaling dynamics and patterning in early mammalian development by taking advantage of cutting-edge embryonic stem cell technology.
Introduction
During development, extracellular signaling molecules are essential for creating spatial patterns of cell fates. In 1952, Alan Turing introduced the term morphogen to define “a chemical signal that could produce a form” (as the name suggests) [1]. It was decades before such molecules were actually discovered and, since then, much has been learned about the mechanisms by which morphogens pattern tissues. However, much remains obscure, particularly in mammals where development is difficult to observe and to manipulate.
In Turing’s time, a broad definition of a morphogen was appropriate as nothing was known about the molecular underpinnings of development. In this review, we will use more recent definitions which add the requirement of a morphogen producing more than two fates, to exclude simpler cases in which the molecule acts as a simple switch, where cells adopt one fate in its absence and a different one in its presence [2,3]. Here we focus on mammalian development and only briefly summarize general concepts in morphogen gradient formation and interpretation. For more details about these processes we refer the reader to several recent reviews [4–8]
The simplest models for morphogen gradient generation rely on localized production, and diffusion and degradation throughout the tissue [2,9–11]. This creates a gradient that provides positional information to cells which differentiate to different fates according to its concentration [2]. This synthesis-diffusion-degradation (SDD) model predicts an exponential decay in the morphogen concentration [12–14], and has been supported by measurements of Bicoid in Drosophila and Sonic-Hedgehog in the murine neural tube, which produce a gradient with this property [14–16].
The SDD model assumes localized production, but many developmental processes are self-organized without a preexisting source. Turing showed mathematically that, under particular conditions, diffusion causes the state in which the morphogen is homogenous in space to become unstable, and results in the development of a pattern of morphogen concentration [1]. This is known as a diffusion-driven or Turing instability [1,8,17]. This pattern of morphogen could then serve as the gradient which conveys positional information to the cells [7]. Turing patterns can be generated through the coupling of two molecules: a short-range activator that activates itself as well as its own long-range inhibitor [18], and several biological examples have been demonstrated [7,19,20].
As the morphogen diffuses through the tissue, it interacts with the field of cells by binding to cell-surface receptors and initiating signaling cascades within the cells. Let us use the TGF-β pathway as an example, since we will focus on this pathway below. TGF-β ligand binding leads to the assembly of heteromeric complexes of type I and type II receptors, which causes the type II receptor to activate the type I by phosphorylation. It has been suggested that the absolute number of occupied receptors is the signal that is conveyed to the nucleus [3], although, as we discuss below, this simple formulation ignores the time-dependence of signaling. The type I receptor activates a class of signal transducers known as receptor-associated Smads (R-Smads). R-Smads then form a complex with another signal transducer, Smad4, and this complex moves into the nucleus where it regulates transcription. There are two branches of this pathway, the BMP branch and the Activin/Nodal branch, which share some components, including Smad4, but utilize different type I receptors and R-Smads. Thus, the signaling pathway relays information from outside the cell to its nucleus.
In the following sections, we summarize our current understanding of morphogens in early mammalian development, and opportunities to make progress in ESC systems. We first review what is known from in vivo experiments in the mouse, and discuss remaining open questions. We then focus on progress to date, and opportunities for the future, in addressing these questions using ESCs. The ESC systems allow for experiments that are currently impossible in the embryo, but the findings must be tested to ensure that that they reflect what occurs in vivo. They also allow for investigation of development directly in human cells, providing a powerful compliment to ongoing research using model organisms.
The role of morphogens in mammalian gastrulation
Gastrulation is the first differentiation event of the embryo proper, when the pluripotent epiblast differentiates into the three germ layers of the embryo: ectoderm, mesoderm and endoderm. Its initiation relies on three signaling pathways: the two branches of the TGF-β pathway, BMP and Activin/Nodal, and the Wnt pathway. As discussed above, BMP and Activin/Nodal signal through complexes of R-Smads (Smad1/5/8 and Smad2/3, respectively), while Wnt signals are transduced by β-catenin. In the mouse, Nodal signaling from the epiblast promotes BMP4 expression in the extra-embryonic ectoderm which activates WNT in both the visceral endoderm and the epiblast [21–23]. Wnt, in turn, increases Nodal signaling in the epiblast. Expression of Wnt and Nodal inhibitors in the anterior visceral endoderm (AVE) restricts these signals to the posterior of the embryo, where the primitive steak, the site of gastrulation, forms [24,25]. Here, we review what has been learned in vivo regarding the identity and function of these morphogen ligands, focusing on Nodal signaling as an example, and highlight open questions that have remained difficult to address.
The most direct evidence for the function of TGF-beta ligands as morphogens in vertebrates comes from experiments with dissected Xenopus animal caps. These cells will adopt dorsal ectodermal fates when cultured in isolation, however, when exposed to low doses of Activin, they adopt a ventral mesodermal fate, while high doses lead to increasing dorsalization [26]. Activin is also capable of spatially patterning a tissue in vitro, as an Activin bead placed within an animal cap will induce localized expression of Goosecoid, which requires high Activin, and a ring of Brachyury (BRA), which requires lower signaling, at a distance from the bead [27]. Similarly, exposure of dissociated animal caps to BMP4 induces increasingly ventral fates within the ectoderm with the highest doses giving rise to epidermis [28]. While these experiments demonstrate the ability of these ligands to induce multiple fates at different concentrations, they do not demonstrate that they function in this manner in vivo. Notably, Activin plays a limited role in embryonic patterning, while Nodal, which activates the same pathway, is central to patterning, but signals over a shorter range [29]. Moreover, while these experiments in which ligand is suddenly added to culture media are consistent with ligand concentration as the central factor in determining cell fate, they are also consistent with other factors, such as ligand rate of change [30]. Finally, as discussed below, similar experiments with mammalian pluripotent cells yield different results [31,32], calling into question whether these ligands can function as classical morphogens in these systems.
It is instructive to consider the case of Nodal signaling in mammalian embryos. While there are six Nodal ligands in Xenopus and two in Zebrafish, mammals have only a single ligand, and deletion of this ligand leads to severe defects in embryogenesis shortly after implantation [33,34], consistent with the roles of Nodal signaling both in maintaining epiblast pluripotency [35,36], and primitive streak induction [34]. Understanding of the role of Nodal in the mammalian embryo has come primarily from two types of experiments, measurements of expression patterns of Nodal, its inhibitors, and its targets, and assessments of the phenotypes of modified alleles of the genes that code for these extracellular molecules or for components of the intracellular signaling pathway [24,37]. Nodal is initially expressed throughout the epiblast shortly after implantation, while during gastrulation its expression is highest in the proximal posterior epiblast. At late gastrulation, it becomes restricted to the node, which forms at the anterior end of the primitive streak.
Intracellularly, Nodal signals are transduced from the receptor to the cell nucleus by two very similar proteins known as Smad2 and Smad3. Surprisingly, while Nodal knockout mice rarely form mesoderm [33], in Smad2 knockout mice, the entire embryo proper is converted to mesodermal cells [34,38]. This results from loss of anterior identity due to a failure to form the anterior visceral endoderm signaling center within the extraembryonic endoderm. Epiblast specific Smad2 knockouts do form this signaling center, however, they have severe defects in forming anterior portions of the embryo which originate in a failure to specify the anterior most mesoderm and endoderm in the primitive streak during gastrulation [39]. This phenotype is very similar to that observed in embryos which are heterozygous at the Nodal locus with one allele representing a null mutation and the other lacking an enhancer which drives Nodal expression in the primitive streak, which led the authors to conclude that the defects in epiblast specific Smad2 knockouts result from a general reduction in Nodal signaling levels [39]. In these embryos, the node and midline structures still form, however, removal of one copy of Smad3 in this context causes a loss of both structures, while embryos lacking both Smad2 and 3 fail to form mesoderm altogether [37,39]. Taken together, these results have been interpreted to indicate a dose-dependent patterning of the mesendoderm by Nodal signals [40].
There remain, however, several points of confusion. First, there is not a clear correspondence between the expression patterns of Nodal and the phenotypes induced. During gastrulation, Nodal is highest in the posterior proximal primitive streak, while reduction in Nodal levels results in a loss of the anterior most derivatives. Similarly, Nodal expression is widespread at earlier stages so it is unclear what restricts the position of the DVE/AVE signaling center to the distal and anterior side, although it has been suggested to be due to a balance between Nodal and BMP signaling [41]. Second, we largely lack measurements of signaling activity, such as the amount of phosphorylated, nuclear-localized Smad proteins, and so interpretations of phenotypes must assume what the effect on signaling has been, and these can often be counterintuitive. The case of global knockout of Smad2 discussed above provides a good example. Unexpectedly, mesoderm is drastically expanded due to the loss of the AVE and its inhibitors. As the pathway contains multiple levels of feedback, effects such as this may be common. Further, some perturbations may change the spatial or temporal patterns of signaling in a manner which is difficult to predict. Well-studied examples include changes in the expression of molecules which affect the range of signaling gradients such as inhibitors or receptors [42]. Changing expression of molecules to reduce diffusion can have the effect of sharpening the gradient – increasing levels close to the source while reducing those at a distance. Existing measurements of ligand and inhibitor expression, while informative, are largely non-quantitative and interpreting these requires determining how signaling activity is determined as a function of both activating and inhibiting influences. In the next section, we will return to these questions and discuss how in vitro experiments may provide an inroad into these issues.
ESC studies: insights and opportunities
The difficulty in addressing questions about how signaling pathways pattern the early mammalian embryo in space and time has led to the development of ESC cultures that are complimentary to in vivo models. These are particularly critical for studying early human development as ethical and practical considerations limit availability of embryos between approximately two weeks, when the 14-day rule takes effect, and 5 weeks when the earliest tissue from elective terminations is available. In this section, we briefly review ESC-based systems for studying early embryonic patterning. The basic features of most of these systems have been covered in more detail in previous reviews [30,43,44]. In this review, we will not discuss organoids, which recapitulate some of the patterning of particular organs at later developmental stages. The reader is directed to recent reviews that focus on these [45,46].
ESC systems for studying gastrulation
Organized two-dimensional ESC patterns were first reported in ref. [47]. That study took advantage of micropatterning technology to control the geometry of hESC cultures and showed that this was sufficient to overcome the hurdles of inconsistency and lack of reproducibility in regular culture. Remarkably, simple confinement and stimulation with BMP4 led to the emergence of patterns which contained all three germ layers and extra embryonic cells. In what follows, we refer to these micropatterned colonies treated with BMP4 as “2D gastruloids”. Treatment of micropatterned colonies with Wnt ligands also produces cell fate patterns, but without the extra-embryonic population at the edge [48].
While it is impossible to directly compare these micropatterns with human embryos, a recent study developed an analogous platform with mESCs, and compared the resulting patterns to the mouse embryo [49]. They showed that the patterning process of Epiblast like cells (EpiLCs) grown in two-dimensional micropatterns mimics that observed in vivo (E5.0 to E8.0), with cell differentiation following similar developmental paths. Induction of EpiLCs by FGF, BMP, Wnt and Nodal (signals observed at the posterior end of the embryo) resulted in a pattern comprised of posterior cell types including posterior epiblast, primitive streak, mesoderm, and extra-embryonic mesoderm. In agreement with in vivo observations, if BMP is not present in the induction media, the pattern is composed of anterior and distal cell types, such as anterior epiblast, anterior primitive streak, and definitive endoderm. These findings support the idea that that organized two-dimensional ESC systems can mimic some of the pattern formation that occurs in vivo.
Two-dimensional systems are inherently limited in their ability to reproduce the morphogenesis and symmetry breaking that is observed in vivo. Several three-dimensional ESC systems to mimic early development have been devised. The closest analogue of the 2D systems are three-dimensional gastruloids which are created from mESCs. These systems are built by pulsing aggregates of mESCs of a defined size with a WNT activator two days into their differentiation [50,51]. They subsequently develop an anterior-posterior axis that elongates, and even shows sequential and regionalized activation of Hox genes [52]. Other systems are generated by combining multiple cell types of the early embryo together. This is most easily done with mouse cells, because there are defined cell lines for all three lineages of the early embryo: epiblast, visceral endoderm (known as XEN cells), and trophoblast. Combining mESCs with trophoblast stem cells (TSCs) can produce configurations which look remarkably similar to the mammalian blastocyst (mouse E3.5) [53], while in a slightly different configuration it can also lead to the development of structures resembling the egg cylinder staged mouse embryo (E5) [54], known as ETS embryos. These ETS embryos go on to show some early signs of gastrulation including regionalized induction of BRA close to the boundary between the ESCs and TSCs. Combining XEN cells with trophoblasts and ESCs forms structures known as an ETX embryos, and further improves this process [55], with more robust symmetry breaking and primitive streak differentiation.
For human cells, it has been shown that culturing hESCs in Matrigel on top of a soft gel leads to differentiation to a hollow sphere of amniotic ectodermal cells [56]. Interestingly, in some fraction of cases, the initial spherical symmetry is broken and only half of the aggregate becomes amnion while the rest remains pluripotent epiblast, a configuration which resembles the amniotic sac in vivo [57]. By varying the gel composition, colonies of hESCs can also be grown as three-dimensional spheres of pluripotent cells. When treated with low doses of BMP, these also break symmetry, but instead divide into SOX2 expressing ectoderm, and BRA expressing mesoderm, which could be indicative of an anterior-posterior axis [58].
Do ligands function as morphogens in mammalian gastrulation?
BMP signal is essential for mammalian gastrulation [24,59] and has been shown to act as a morphogen in other organisms. In particular, Dpp, the BMP4 homologue in Drosophila, was shown to function as a morphogen to control dorsal-ventral patterning of the early embryo and anterior-posterior patterning and growth of the imaginal disk [60–63]. Treating dissociated cells from the Xenopus animal cap with increasing doses of BMP4 causes them to adopt progressively more ventral fates [28]. Simple treatment of mammalian ESCs by BMP ligands leads to mixtures of trophectodermal [64–66], extra-embryonic [49], and mesodermal fates [47,67], while as noted above, in the 2D gastruloid system, BMP4 treatment causes ordered differentiation to all three germ layers.
While some studies have suggested that the patterns in 2D gastruloids reflect cells reading levels of BMP4 to give rise to multiple different fates [68], there is evidence to suggest that this is not the case. First, measuring the time-dependent response to BMP treatment shows that BMP initially activates SMAD1/5/8 throughout the colony and this later becomes restricted to the edge [67,69]. This response is binary rather than graded along the radial axis: cells at the edge show a sustained response and differentiate to extra-embryonic fates, while the remainder of the cells show a transient response. Termination of BMP signaling at the colony center depends on both secreted inhibitors and polarization of BMP receptors to the basal side of the cells [67,70], and is highly synchronous between cells [69]. Thus, there is not sufficient diversity in the direct response to BMP to explain the cell fates that arise. Further, a separate study used isolated colonies containing fewer than 10 cells to show that there is a sharp transition between pluripotency and extra-embryonic fates as the BMP concentration is raised, and BMP alone never gives rise to intermediate fates [32]. Finally, in larger colonies, application of intermediate BMP concentrations does yield mesoderm differentiation, however this effect is lost upon either WNT or NODAL inhibition [32]. Similarly, in micropatterned colonies, inhibiting endogenous WNT or NODAL signals results in a loss of the rings of mesoderm and endoderm [47,71–73]. Thus, evidence from stem cells suggests that in this context, BMP does not function as a morphogen, but rather as a switch which both activates extra-embryonic differentiation and WNT/NODAL activity, and it is the combinatorial effect of these pathways that gives the appearance of a morphogen effect and patterns the 2D gastruloids.
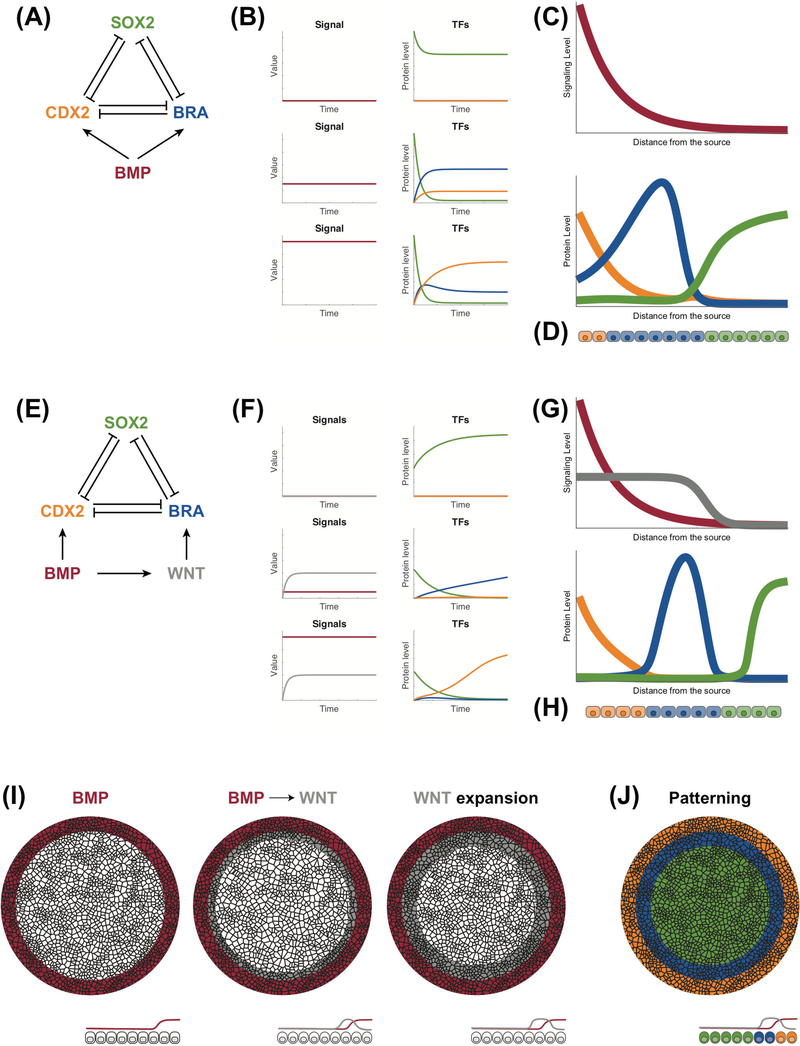
We illustrate how two signals could lead to an apparent morphogen effect as well as pattern the radial axis of 2D gastruloids with a mathematical model. We will compare this two-signal model with the classic model in which the concentration of a single signal is utilized for patterning (Figure 1A). Details of both models can be found in Appendix 1. In both cases, the concentration of the signal is interpreted by a network of three mutually repressive transcription factors, which for simplicity we label CDX2, BRA, SOX2, well established markers of the trophoectodermal, mesodermal, and pluripotent states. In the classic model in which BMP acts as a morphogen, the cell remains in a default SOX2-positive pluripotent state in the absence of signal (Figure 1A–C). When the concentration of the morphogen is sufficiently high, CDX2 is strongly induced and represses both SOX2 and BRA (Figure 1A–C). However, at intermediate morphogen concentrations, CDX2 expression is not sufficient to repress BRA, and once BRA is activated, it suppresses both CDX2 and SOX2 (Figure 1A–C). Thus, in this model, the concentration of single morphogen yields three different fates as in the French Flag model (Figure 1C).
Figure 1: Mathematical models for signal induction.
(A) Classic model for morphogen induction: three mutually repressive transcription factors (CDX2, BRA, SOX2) are activated at different rates by the morphogen ligand, in this case BMP. (B) Dynamics of the transcriptional network under different morphogen concentrations. SOX2 (green) remains active under low morphogen concentration (top). At intermediate concentrations, BRA (blue) expression quickly increases inhibiting the other two TFs (middle). High morphogen concentrations promote CDX2 (yellow) expression (bottom). (C) Morphogen signaling gradient (top) and the corresponding TF expression profile (bottom) as functions of the distance from the source. For example, a cell that is at a distance from the source will be exposed to an intermediate BMP level and will express a high level of BRA (blue). (D) Pattern created in a row of cells exposed to the gradient signal showed in (C). (E) Transcriptional network mimicking morphogen induction by induction of a secondary signal: three mutually repressive transcription factors (CDX2, BRA, SOX2) are controlled by a balance of an upstream signal (BMP) and a secondary signal (WNT). (F) Dynamics of the transcriptional network under different initial concentrations of BMP. SOX2 (green) remains active under low BMP concentration (top). At intermediate BMP concentrations, the secondary signal, WNT, is quickly induced and BRA (blue) becomes active (middle). High BMP signal promotes high CDX2 (yellow) expression which overcomes BRA (blue) inhibition (bottom). (G) Signaling levels (top) and the corresponding TF expression profile (bottom) as functions of the distance from the source of BMP. (H) Pattern created in a row of cells exposed to the signaling levels showed in (G). The blue cells are only present due to the activation of the secondary signal and not to the BMP gradient directly. (I) Sketch of the signaling dynamics of a micropattern exposed to BMP signal. First, BMP signal is restricted to the edge of the colony. Secondly, BMP signal activates the secondary signal WNT at a distance from the edge, which expands inwards in the colony. (J) The signaling dynamics result into a pattern with three regions: extra-embryonic (yellow), mesendodermal (blue) and ectodermal (green).
We asked now if two signals, neither of which acts as a morphogen on its own, could produce the same phenomena. In this model, the gene regulatory network remains the same but, the upstream signaling molecule, BMP, would activate both the expression of the transcription factor CDX2, and the production of a secondary signaling molecule, WNT, which activates the second transcription factor BRA (Figure 1D–F). At low concentrations of BMP, WNT is also not expressed, and cells remain in the default (pluripotent) state expressing SOX2. At intermediate concentrations, BMP activates the WNT signal, but does not override it, and cells differentiate to mesoderm. When BMP is sufficiently high, WNT is expressed but CDX2 is expressed too strongly and the cells differentiate to trophectoderm. A similar effect can occur in space when BMP activates a diffusible WNT signal (Figure 1I–J). Thus, an identical transcriptional network could interpret either a single signal via a classic morphogen effect or two signals, in which cells do not sense the absolute concentration of either one. These interactions could also explain the pattern observed 2D gastruloids (Figure 1I), where the BMP signal is restricted to the edge but induces WNT signaling that propagates towards the center of the colony [71], resulting in a ring of mesendoderm differentiation.
While BMP does not function directly as a morphogen in human pluripotent cells, whether WNT or NODAL can remains an open question. ACTIVIN alone is not sufficient to induce primitive streak differentiation, however, hESCs pretreated with WNT3A for 24h before ACTIVIN differentiate into mesendodermal fates [31]. Thus, whether or not these signals function as morphogens, combinatorial effects will also be essential in understanding their function during germ layer specification.
How are changing morphogen concentrations interpreted in time?
While there is a wealth of information on the phenotypes of mouse embryos when signaling components have been removed, very few direct measurements of signaling activity have been performed due to the challenge of following subcellular events in mammalian embryos. Fluorescently tagging endogenous signal transducers in ESCs and imaging them during differentiation and patterning provides a window into how cells interpret dynamic signals [31,32,69,74]. These studies have led to the conclusion that each pathway performs its own unique signal processing.
hESCs respond stably to stimulation by BMP, and the sustained response is necessary for differentiation to extra-embryonic fates [32]. In contrast, hESCs exposed to WNT or ACTIVIN signals show an adaptive response which decays even under constant stimulation [31,69,74]. ACTIVIN treated cells show a decay on the time scale of about 4 hours to level slightly above the baseline independently of ligand concentration. In contrast, in response to WNT, adaptation is slower (taking around 12 hours to reach baseline) and dose-dependent, such that it is nearly complete at lower doses of WNT, and partial at higher doses [74]. Adaptation allows the cells to read the rate of change of the signal in time instead of the absolute concentration [69,75], and changing the ligand concentration slowly abrogates the response to ACTIVIN but not to BMP. Stem cell differentiation protocols can be optimized based on this dynamic information. In the case of ACTIVIN, pulsing ligand is more efficient that constant exposure even though the integrated exposure is lower [69]. Thus, ESCs have allowed for direct measurement of signaling dynamics in pluripotent cells and in response to controlled concentrations and dynamic presentation of ligand. Whether these dynamics are relevant to the embryo remains an open question, however, insights are beginning to emerge from the patterning systems described in the next section.
How do morphogen systems result in spatial patterns of cell fates?
During development, the signaling dynamics described above lead to the formation of spatial patterns. The 2D gastruloid system has allowed for the direct observation of signaling dynamics during self-organized patterning. These results show that initially the entire colony responds to BMP before its restriction to the edge approximately 12 hours later [69]. This pattern of BMP signaling, that is high only at the edge, is then stable for the remainder of patterning. BMP signaling at the edge triggers a wave of WNT signaling which initiates around 24h after BMP treatment near the edge and moves inward at a constant rate (Figure 2A) [71]. Slightly later, WNT initiates NODAL signaling near the colony edge, and NODAL also expands inwards at a constant rate (Figure 2B) [69]. The wave of WNT signaling is initiated earlier than the wave of NODAL, but the NODAL wave travels faster allowing it to overtake the WNT wave [71].
Figure 2: Nodal and WNT signaling dynamics in BMP treated micropatterned hESC colonies.
(A) Pie sections of a live-imaged micropatterned GFP-beta-catenin hESC colony stimulated with BMP4 showing a clockwise time evolution (17h to 47h) of non-membrane beta-catenin expression. Each section is a snapshot from time-lapse imaging at clockwise increasing time points post BMP treatment. The outer orange circumference highlights the pinned back end of the WNT wave while the white spiral approximates the inward movement of the front end of the WNT wave. Data from ref [71]. (B) Pie sections of micropatterned hESC colonies stimulated with BMP4 showing a clockwise time evolution (24h to 48h) of SMAD1 (red) and SMAD2 (cyan) expression. Each section corresponds to a micropatterned colony fixed at the indicated time point post BMP treatment. The red circumference highlights that SMAD1 stays restricted to the edge at all time points. The white spiral approximates the front end of the SMAD2 wave that moves inward in time. Data from ref. [69].
Importantly, mesodermal differentiation requires both WNT and NODAL, however, the spatial extent of differentiation does not map to any particular level of these pathways [71]. As the signaling travels in a wave, the timing of signaling onset is much more variable than the level of signaling, and it is possible that it is this timing what cells sense when determining their fate. With the exception of the edge cells, each cell inside the colony experiences three events: the attenuation of the initial BMP signal, the WNT signal, and the NODAL signal. We speculate that the relative timing of these events is what determines the final pattern. As noted above, differentiation in response to NODAL signal requires previous WNT signaling [31], so one hypothesis is that the point in space where NODAL signaling overtakes WNT marks the edge of the territory of mesodermal differentiation.
A WNT wave also forms when hESCs colonies are treated with WNT3A, this time patterning the colonies into two regions: an outer ring of mesendodermal cells and an inner region of ectodermal cells [48,73]. The initial response to WNT is limited to the colony edge by cell-cell contacts through E-cadherin (E-CAD). The wave is formed by a domino effect where response to WNT ligands causes the cell to undergo EMT and downregulate E-CAD at the junctions with its neighbors, thereby freeing more cells to respond to the WNT signal. This process continues until a sufficient amount of the secreted feedback inhibitor DKK1 builds up and halts the EMT wave.
The WNT waves created by BMP or WNT3A, although similar in appearance, are mechanistically distinct. In the case of WNT3A induction, the wave created is due to a wave of competence to respond to exogenous WNT signaling, and it is not inhibited even if IWP2, an inhibitor of WNT ligand secretion, is present in the media the entire time [48]. In the case of BMP induction, the wave results from endogenous signaling, and does not start at the edge of the colony, where E-CAD expression is low. It also does not require EMT as the wave propagates farther into the colony than the territories where differentiation and EMT are observed [71].
An interesting open question is what separates the mesoderm from the endoderm. Knockout of CER1 during micropatterned differentiation, causes upregulated NODAL signaling and promotes endoderm at the expense of mesoderm differentiation [31]. However, how the NODAL signal is different upon CER1 knockout is unclear. Are its dynamics or spatial extent perturbed or only its level? Recent results with a reporter system in mouse embryos and stem cells suggest that the endoderm arises in randomly distributed cells within the anterior primitive streak and then segregates from the mesoderm [76] but, how does this observation relate to the Nodal levels or distribution? One hypothesis is that the fate choice is stochastic with a bias towards endoderm that depends on the level of Nodal signaling that a cell receives.
How are symmetries broken in the early embryo?
Two-dimensional models are very useful for studying signaling dynamics and patterning, however, they do not recapitulate the symmetry breaking or morphogenesis which is observed in vivo. This issue has driven the development of the 3D models described above. Most studies to date have concentrated on determining to what degree these models resemble in vivo embryos, however, they have provided some insights about the signals that drive patterning during early mammalian development and have set a course for future investigations.
Several of the three-dimensional models described above break symmetry from an initial spherical configuration. In the case of the human models of amnion [56,57], it is BMP signaling which separates the amnion from the epiblast. The ETS [54] and ETX [55] embryos, as well as 3D hESCs treated with low doses of BMP4 [58], localize expression of BRA to one side of the epiblast compartment. This is particularly surprising in the case of ETS embryos as they lack the visceral endoderm which places a role in positioning the primitive streak to the posterior side of the embryo. In all of these cases, symmetry breaking depends on Wnt signaling, and in the hESC model it has been shown that removing DKK1 causes the entire sphere to adopt a mesodermal fate [58]. Some caution in interpretation is warranted, however, as the markers used such as Sox2 and Bra delineate germ layers rather than the AP axis specifically. Further, in the case of the hESC model, the final object contains one axis of rotational signaling, the same as the 2D micropatterned colonies, so it is not clear that one model represents a true AP axis more than the other [30]. Nonetheless, these are useful models for how morphogen pathways are deployed to break spherical symmetry, and it will be interesting to use them to study the dynamics of the BMP and WNT pathways in these events.
The mouse gastruloid system develops a more complete AP axis, including axial elongation and the temporal and spatial emergence of Hox genes. [51,52]. A pulse of the Wnt agonist CHIR99021 (CHIR) is essential for these events, however, little is known about the response to this pulse or how downstream signals ultimately lead to patterning. In the future, this represents an exciting system to begin to explore mechanistically how signals pattern the AP axis.
Conclusions
In the previous sections we have reviewed recent insights on morphogen dynamics and patterning that have come from newly developed stem cell systems, however, the potential of these systems has only begun to be realized, and many new and exciting questions are yet to be explored. In the above sections, we highlighted several questions that are currently being addressed, including understanding precisely how the position of mesendodermal differentiation is established by the combinatorial effects of BMP, Wnt, and Nodal, how mesoderm and endoderm are separated, and what signaling dynamics lead to the symmetry breaking that underlies axis formation in the mammalian embryo. The combination of the stem cells systems and live cell reporters that have been developed should allow rapid progress on these longstanding questions. For example, DKK1 knockouts have been shown to cause defects in symmetry breaking in hESC spheroid models, and CER1 knockouts have been shown to lead to increased endoderm differentiation. Creating these knockouts directly in WNT or NODAL reporter lines, respectively, will allow for direct observation of the signaling dynamics, and how they lead to the observed phenotypes.
Here we have focused on signaling, however, much also remains to be learned about how these signals are interpreted by transcription factor networks inside of cells to implement cell fate decisions, the subject of intense study in other systems [5,77]. The simplest model is that different binding sites have different affinities for the transcription factors induced by the morphogen [77,78], however, recent studies have failed to show that there is a correlation between binding affinities and the range of morphogen induction in systems such as the vertebrate neural tube and AP patterning in fly [79–81]. Further, it is clear that understanding the interplay between morphogen dynamics and their interpretation by the individual promoters of target genes is important for understanding patterning [82]. Directly studying these issues in mammalian stem cells could shed light on the gene regulatory network that separates germ layers during gastrulation.
A major remaining challenge is comparing the results from in vitro systems with actual embryos, particularly for human. Recently, a system for culturing post-implantation human embryos has been developed, and the different cell types present at these early stages were characterized [83,84], however these are still limited by the 14-day rule, and it is also still unclear to what degree the cultured embryos mimic processes that occur in vivo. Another approach involves interspecies comparisons. Mouse ESC systems have been compared to mouse embryos [49,52,54], and comparing human and mouse gastruloids could provide a starting point for validating the human findings and highlighting species differences. Continuing to develop insights from stem cell systems and validating them in vivo is providing a window into previous inaccessible times during human development.
Acknowledgements
We thank Miguel Ángel Ortiz Salazar for a critical reading of the manuscript, Sapna Chhabra and Idse Heemskerk for providing data used to create Figure 2, and all the members of the Warmflash lab for helpful feedback. This work was funded by Rice University and grants to AW from NSF (MCB-1553228), NIH (R01GM126122), and Simons Foundation (511079).
Appendix
Mathematical model for fate patterning by a single morphogen
The dynamics of a gene regulatory network comprised of three mutually repressive transcription factors (TF1, TF2 and TF3) controlled by a morphogen signal (S), as in Figure 1A, can be described by the system of differential equations:
where the variables TF1, TF2 and TF3 represent the expression values of the corresponding transcription factors. In the main text these transcription factors are CDX2, BRA and SOX2, respectively. The parameter S corresponds to the concentration of the morphogen, which in the main text is BMP. The parameters nij,kij (with i ϵ {S,1,2,3}, j ϵ {1,2,3}) are the Hill coefficients and dissociation constants respectively, and αi,δi with i ϵ {1,2,3} are the production and degradation rates respectively.
In Figure 1B of the main text, the system of differential equations was simulated with initial condition TF1 = 0, TF2 = 0 and TF3 = 2, the parameter values given in Table 1, and the values S = 0,1,10, for the top, middle, and bottom panels, respectively. These parameters were also used to obtain Figure 1C but, this time, S decreased exponentially as a function of the distance from the source.
Table 1:
| Parameter | Value | Parameter | Value | Parameter | Value |
|---|---|---|---|---|---|
| α1 | 8 | α2 | 4 | α3 | 2 |
| δ1 | 1 | δ2 | 1 | δ3 | 1 |
| κS1 | 7 | κS2 | 0.5 | κ13 | 0.1 |
| nS1 | 1 | nS2 | 5 | n13 | 1 |
| κ21 | 0.4 | κ12 | 0.3 | κ23 | 0.1 |
| n21 | 1 | n12 | 1 | n23 | 1 |
| κ31 | 0.5 | κ32 | 1 | ||
| n31 | 1 | n32 | 1 | ||
Mathematical model for patterning multiple fates with two signals
The dynamics of a gene regulatory network comprised of three mutually repressive transcription factors (TF1, TF2 and TF3), this time controlled by a balance of an upstream signal (S1) and a secondary signal (S2), as in Figure 1E, can be described by the system of differential equations:
where the variables S2, TF1, TF2 and TF3 correspond to the expression values of the secondary signal and the corresponding transcription factors. In the main text, the secondary signal is WNT and the transcription factors are CDX2, BRA and SOX2, respectively. The parameter S1 is the concentration of the upstream signal, BMP in the main text. The parameters nij,kij (with i,j ϵ {1,2,3}) are the Hill coefficients and dissociation constants and αi,δi with i ϵ {S2,1,2,3} are the production and degradation rates, respectively.
In Figure 1F of the main text, the system of differential equations was simulated with initial condition S2 = 0, TF1 = 0, TF2 = 0 and TF3 = 3, the parameter values given in Table 2 and the main signal, and the values S1 = 0,1,10, for the top, middle, and bottom panels, respectively. These parameters were also used to obtain Figure 1G but, this time, S decreased exponentially as a function of the distance from the source.
Table 2:
| Parameter | Value | Parameter | Value | Parameter | Value |
|---|---|---|---|---|---|
| αS2 | 2 | α2 | 0.05 | α3 | 5 |
| δS2 | 0.5 | b2 | 14 | δ3 | 0.1 |
| α1 | 0.7 | δ2 | 0.5 | κ13 | 1 |
| b1 | 5 | κ12 | 0.1 | n13 | 1 |
| δ1 | 0.5 | n12 | 1 | κ23 | 0.01 |
| κ21 | 0.8 | κ32 | 0.5 | n23 | 1 |
| n21 | 1 | n32 | 1 | ||
| κ31 | 0.5 | ||||
| n31 | 1 | ||||
References
- [1].Turing AM The Chemical Basis of Morphogenesis. Phil. Trans. R. Soc. Lond. B, 1952, 237, 37–72. [Google Scholar]
- [2].Wolpert L Positional Information and the Spatial Pattern of Cellular Differentiation. J. Theor. Biol, 1969, 25, 1–47. [DOI] [PubMed] [Google Scholar]
- [3].Dyson S; Gurdon J. The Interpretation of Position in a Morphogen Gradient as Revealed by Occupancy of Activin Receptors. Cell, 1998, 93, 557–568. [DOI] [PubMed] [Google Scholar]
- [4].Rogers KW; Schier AF Morphogen Gradients: From Generation to Interpretation. Annu. Rev. Cell Dev. Biol, 2011, 27, 377–407. [DOI] [PubMed] [Google Scholar]
- [5].Briscoe J; Small S Morphogen Rules: Design Principles of Gradient-Mediated Embryo Patterning. Development, 2015, 142, 3996–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sagner A; Briscoe J Morphogen Interpretation: Concentration, Time, Competence, and Signaling Dynamics. Wiley Interdiscip. Rev. Dev. Biol, 2017, e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Green JBA; Sharpe J Positional Information and Reaction-Diffusion: Two Big Ideas in Developmental Biology Combine. 2015. [DOI] [PubMed] [Google Scholar]
- [8].Kondo S; Miura T Reaction-Diffusion Model as a Framework for Understanding Biological Pattern Formation. Science, 2010, 329(5999), 1616–1620. [DOI] [PubMed] [Google Scholar]
- [9].Crick F Diffusion in Embryogenesis. Nature, 1970, 225, 420–422. [DOI] [PubMed] [Google Scholar]
- [10].Stumpf HF Mechanism by Which Cells Estimate Their Location within the Body. Nature, 1966, 212, 430–431. [DOI] [PubMed] [Google Scholar]
- [11].Lawrence PA Gradients in the Insect Segment: The Orientation of Hairs in the Milkweed Bug Oncopeltus Fasciatus. J. Exp. Biol, 1966, 44, 607–620. [DOI] [PubMed] [Google Scholar]
- [12].Driever W; Nusslein-Volhard C A Gradient of Bicoid Protein in Drosophila Embryos. Cell, 1988, 54, 83–93. [DOI] [PubMed] [Google Scholar]
- [13].Drocco JA; Wieschaus EF; Tank DW The Synthesis–Diffusion–Degradation Model Explains Bicoid Gradient Formation in Unfertilized Eggs. Phys. Biol, 2012, 9, 055004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gregor T; Wieschaus EF; Mcgregor AP; Bialek W; Tank DW Stability and Nuclear Dynamics of the Bicoid Morphogen Gradient. Cell, 2007, 130, 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chamberlain CE; Jeong J; Guo C; Allen BL; McMahon AP Notochord-Derived Shh Concentrates in Close Association with the Apically Positioned Basal Body in Neural Target Cells and Forms a Dynamic Gradient during Neural Patterning. Development, 2008, 135, 1097–1106. [DOI] [PubMed] [Google Scholar]
- [16].Gregor T; Tank DW; Wieschaus EF; Bialek W Probing the Limits to Positional Information. Cell, 2007, 130, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Meinhardt H Space-Dependent Cell Determination under the Control of a Morphogen Gradient. J. Theor. Biol, 1978, 74, 307–327. [DOI] [PubMed] [Google Scholar]
- [18].Gierer A; Meinhardt H A Theory of Biological Pattern Formation. Kybernetik, 1972, 12, 30–39. [DOI] [PubMed] [Google Scholar]
- [19].Economou AD; Ohazama A; Porntaveetus T; Sharpe PT; Kondo S; Basson MA; Gritli-Linde A; Cobourne MT; Green JBA Periodic Stripe Formation by a Turing Mechanism Operating at Growth Zones in the Mammalian Palate. Nat. Genet, 2012, 44, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Raspopovic J; Marcon L; Russo L; Sharpe J Digit Patterning Is Controlled by a Bmp-Sox9-Wnt Turing Network Modulated by Morphogen Gradients. Science, 2014, 345(6196), 566–570. [DOI] [PubMed] [Google Scholar]
- [21].Yoon Y; Huang T; Tortelote GG; Wakamiya M; Hadjantonakis A-K; Behringer RR; Rivera-Pérez JA Extra-Embryonic Wnt3 Regulates the Establishment of the Primitive Streak in Mice. Dev. Biol, 2015, 403, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tortelote GG; Hernández-Hernández JM; Quaresma AJC; Nickerson JA; Imbalzano AN; Rivera-Pérez JA Wnt3 Function in the Epiblast Is Required for the Maintenance but Not the Initiation of Gastrulation in Mice. Dev. Biol, 2013, 374, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ben-Haim N; Lu C; Guzman-Ayala M; Pescatore L; Mesnard D; Bischofberger M; Naef F; Robertson EJ; Constam DB The Nodal Precursor Acting via Activin Receptors Induces Mesoderm by Maintaining a Source of Its Convertases and BMP4. Dev. Cell, 2006, 11, 313–323. [DOI] [PubMed] [Google Scholar]
- [24].Arnold SJ; Robertson EJ Making a Commitment: Cell Lineage Allocation and Axis Patterning in the Early Mouse Embryo. Nat. Rev. Mol. Cell Biol, 2009, 10, 91–103. [DOI] [PubMed] [Google Scholar]
- [25].Shahbazi MN; Zernicka-Goetz M Deconstructing and Reconstructing the Mouse and Human Early Embryo. Nat. Cell Biol, 2018, 20, 818–887. [DOI] [PubMed] [Google Scholar]
- [26].Green JBA; New HV; Smith JC Responses of Embryonic Xenopus Cells to Activin and FGF Are Separated by Multiple Dose Thresholds and Correspond to Distinct Axes of the Mesoderm. Cell, 1992, 71, 731–739. [DOI] [PubMed] [Google Scholar]
- [27].Gurdon JB; Harger P; Mitchell A; Lemaire P Activin Signalling and Response to a Morphogen Gradient. Nature, 1994, 371, 487–492. [DOI] [PubMed] [Google Scholar]
- [28].Wilson PA; Lagna G; Suzuki A; Hemmati-Brivanlou A Concentration-Dependent Patterning of the Xenopus Ectoderm by BMP4 and Its Signal Transducer Smad1. Development, 1997, 124, 3177–3184. [DOI] [PubMed] [Google Scholar]
- [29].Jones CM; Armes N; Smith JC Signalling by TGF-Beta Family Members: Short-Range Effects of Xnr-2 and BMP-4 Contrast with the Long-Range Effects of Activin. Curr. Biol, 1996, 6, 1468–1475. [DOI] [PubMed] [Google Scholar]
- [30].Heemskerk I; Warmflash A Pluripotent Stem Cells as a Model for Embryonic Patterning: From Signaling Dynamics to Spatial Organization in a Dish. Dev. Dyn, 2016, 245, 976–990. [DOI] [PubMed] [Google Scholar]
- [31].Yoney A; Etoc F; Ruzo A; Carroll T; Metzger JJ; Martyn I; Li S; Kirst C; Siggia ED; Brivanlou AH WNT Signaling Memory Is Required for ACTIVIN to Function as a Morphogen in Human Gastruloids. Elife, 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nemashkalo A; Ruzo A; Heemskerk I; Warmflash A Morphogen and Community Effects Determine Cell Fates in Response to BMP4 Signaling in Human Embryonic Stem Cells. Dev, 2017, 144. [DOI] [PubMed] [Google Scholar]
- [33].Conlon FL; Lyons KM; Takaesu N; Barth KS; Kispert A; Herrmann B; Robertson EJ A Primary Requirement for Nodal in the Formation and Maintenance of the Primitive Streak in the Mouse. Development, 1994, 120, 1919–1928. [DOI] [PubMed] [Google Scholar]
- [34].Brennan J; Lu CC; Norris DP; Rodriguez TA; Beddington RSP; Robertson EJ Nodal Signalling in the Epiblast Patterns the Early Mouse Embryo. Nature, 2001, 411, 965–969. [DOI] [PubMed] [Google Scholar]
- [35].James D; Levine AJ; Besser D; Hemmati-Brivanlou A TGF-Beta/Activin/Nodal Signaling Is Necessary for the Maintenance of Pluripotency in Human Embryonic Stem Cells. Development, 2005, 132, 1273–1282. [DOI] [PubMed] [Google Scholar]
- [36].Vallier L; Alexander M; Pedersen RA Activin/Nodal and FGF Pathways Cooperate to Maintain Pluripotency of Human Embryonic Stem Cells. J. Cell Sci, 2005, 118, 4495–4509. [DOI] [PubMed] [Google Scholar]
- [37].Dunn NR; Vincent SD; Oxburgh L; Robertson EJ; Bikoff EK Combinatorial Activities of Smad2 and Smad3 Regulate Mesoderm Formation and Patterning in the Mouse Embryo. Development, 2004, 131, 1717–1728. [DOI] [PubMed] [Google Scholar]
- [38].Waldrip WR; Bikoff EK; Hoodless PA; Wrana JL; Robertson EJ Smad2 Signaling in Extraembryonic Tissues Determines Anterior-Posterior Polarity of the Early Mouse Embryo. Cell, 1998, 92, 797–808. [DOI] [PubMed] [Google Scholar]
- [39].Vincent SD; Dunn NR; Hayashi S; Norris DP; Robertson EJ Cell Fate Decisions within the Mouse Organizer Are Governed by Graded Nodal Signals. Genes Dev, 2003, 17, 1646–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Robertson EJ Dose-Dependent Nodal/Smad Signals Pattern the Early Mouse Embryo. Semin. Cell Dev. Biol, 2014, 32, 73–79. [DOI] [PubMed] [Google Scholar]
- [41].Yamamoto M; Beppu H; Takaoka K; Meno C; Li E; Miyazono K; Hamada H Antagonism between Smad1 and Smad2 Signaling Determines the Site of Distal Visceral Endoderm Formation in the Mouse Embryo. J. Cell Biol, 2009, 184, 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang Y-C; Ferguson EL Spatial Bistability of Dpp-Receptor Interactions during Drosophila Dorsal-Ventral Patterning. Nature, 2005, 434, 229–234. [DOI] [PubMed] [Google Scholar]
- [43].Siggia ED; Warmflash A Modeling Mammalian Gastrulation with Embryonic Stem Cells. Curr. Top. Dev. Biol, 2018, 129, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shahbazi MN; Siggia ED; Zernicka-Goetz M Self-Organization of Stem Cells into Embryos: A Window on Early Mammalian Development. Science, 2019, 364(6444), 948–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Brassard JA; Lutolf MP Engineering Stem Cell Self-Organization to Build Better Organoids. Cell Stem Cell, 2019, 24, 860–876. [DOI] [PubMed] [Google Scholar]
- [46].Simunovic M; Brivanlou AH Embryoids, Organoids and Gastruloids: New Approaches to Understanding Embryogenesis. Development, 2017, 144, 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Warmflash A; Sorre B; Etoc F; Siggia ED; Brivanlou AH A Method to Recapitulate Early Embryonic Spatial Patterning in Human Embryonic Stem Cells. Nat. Methods, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Martyn I; Brivanlou AH; Siggia ED A Wave of WNT Signaling Balanced by Secreted Inhibitors Controls Primitive Streak Formation in Micropattern Colonies of Human Embryonic Stem Cells. Development, 2019, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Morgani SM; Metzger JJ; Nichols J; Siggia ED; Hadjantonakis A-K Micropattern Differentiation of Mouse Pluripotent Stem Cells Recapitulates Embryo Regionalized Cell Fate Patterning. Elife, 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Van Den Brink SC; Baillie-Johnson P; Balayo T; Hadjantonakis A-K; Nowotschin S; Turner DA; Arias AM Symmetry Breaking, Germ Layer Specification and Axial Organisation in Aggregates of Mouse Embryonic Stem Cells. Development, 2014, 141, 4231–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Turner DA; Girgin M; Alonso-Crisostomo L; Trivedi V; Baillie-Johnson P; Glodowski CR; Hayward PC; Jérô Me Collignon J; Gustavsen C; Serup P; Steventon B; Lutolf MP; Arias AM Anteroposterior Polarity and Elongation in the Absence of Extra-Embryonic Tissues and of Spatially Localised Signalling in Gastruloids: Mammalian Embryonic Organoids. Development, 2017, 144, 3984–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Beccari L; Moris N; Girgin M; Turner DA; Baillie-Johnson P; Cossy A-C; Lutolf MP; Duboule D; Martinez Arias A Multi-Axial Self-Organization Properties of Mouse Embryonic Stem Cells into Gastruloids. Nature, 2018, 562, 272. [DOI] [PubMed] [Google Scholar]
- [53].Rivron NC; Frias-Aldeguer J; Vrij EJ; Boisset J-C; Korving J; Vivié J; Truckenmüller RK; van Oudenaarden A; van Blitterswijk CA; Geijsen N Blastocyst-like Structures Generated Solely from Stem Cells. Nature, 2018, 557, 106–111. [DOI] [PubMed] [Google Scholar]
- [54].Harrison SE; Sozen B; Christodoulou N; Kyprianou C; Zernicka-Goetz M Assembly of Embryonic and Extraembryonic Stem Cells to Mimic Embryogenesis in Vitro. Science, 2017, 356(6334). [DOI] [PubMed] [Google Scholar]
- [55].Sozen B; Amadei G; Cox A; Wang R; Na E; Czukiewska S; Chappell L; Voet T; Michel G; Jing N; Glover DM; Zernicka-Goetz M Self-Assembly of Embryonic and Two Extra-Embryonic Stem Cell Types into Gastrulating Embryo-like Structures. Nat. Cell Biol, 2018, 20. [DOI] [PubMed] [Google Scholar]
- [56].Shao Y; Taniguchi K; Gurdziel K; Townshend RF; Xue X; Yong KMA; Sang J; Spence JR; Gumucio DL; Fu J Self-Organized Amniogenesis by Human Pluripotent Stem Cells in a Biomimetic Implantation-like Niche. Nat. Mater, 2016, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shao Y; Taniguchi K; Townshend RF; Miki T; Gumucio DL; Fu J A Pluripotent Stem Cell-Based Model for Post-Implantation Human Amniotic Sac Development. Nat. Commun, 2017, 8, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Simunovic M; Metzger JJ; Etoc F; Yoney A; Ruzo A; Martyn I; Croft G; You DS; Brivanlou AH; Siggia EDA 3D Model of a Human Epiblast Reveals BMP4-Driven Symmetry Breaking. Nat. Cell Biol, 2019, 21, 900–910. [DOI] [PubMed] [Google Scholar]
- [59].Winnier G; Blessing M; Labosky PA; Hogan BLM Bone Morphogenetic Protein-4 Is Required for Mesoderm Formation and Patterning in the Mouse. Genes Dev, 1995, 9, 2105–2116. [DOI] [PubMed] [Google Scholar]
- [60].Ferguson EL; Anderson KV Decapentaplegic Acts as a Morphogen to Organize Dorsal-Ventral Pattern in the Drosophila Embryo. Cell, 1992, 71, 451–461. [DOI] [PubMed] [Google Scholar]
- [61].Lecuit T; Brook WJ; Ng M; Calleja M; Sun H; Cohen SM Two Distinct Mechanisms for Long-Range Patterning by Decapentaplegic in the Drosophila Wing. Nature, 1996, 381, 387–393. [DOI] [PubMed] [Google Scholar]
- [62].Nellen D; Burke R; Struhl G; Basler K Direct and Long-Range Action of a DPP Morphogen Gradient. Cell, 1996, 85, 357–368. [DOI] [PubMed] [Google Scholar]
- [63].Matsuda S; Harmansa S; Affolter M BMP Morphogen Gradients in Flies. Cytokine Growth Factor Rev, 2016, 27, 119–127. [DOI] [PubMed] [Google Scholar]
- [64].Horii M; Li Y; Wakeland AK; Pizzo DP; Nelson KK; Sabatini K; Laurent LC; Liu Y; Parast MM Human Pluripotent Stem Cells as a Model of Trophoblast Differentiation in Both Normal Development and Disease. Proc. Natl. Acad. Sci. U. S. A, 2016, 113, E3882–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Xu R-H; Chen X; Li DS; Li R; Addicks GC; Glennon C; Zwaka TP; Thomson JA BMP4 Initiates Human Embryonic Stem Cell Differentiation to Trophoblast. Nat. Biotechnol, 2002, 20, 1261–1264. [DOI] [PubMed] [Google Scholar]
- [66].Li Y; Moretto-Zita M; Soncin F; Wakeland A; Wolfe L; Leon-Garcia S; Pandian R; Pizzo D; Cui L; Nazor K; Loring JF; Crum CP; Laurent LC; Parast MM BMP4-Directed Trophoblast Differentiation of Human Embryonic Stem Cells Is Mediated through a ΔNp63+ Cytotrophoblast Stem Cell State. Development, 2013, 140, 3965–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Etoc F; Metzger J; Ruzo A; Kirst C; Yoney A; Ozair MZ; Brivanlou AH; Siggia ED A Balance between Secreted Inhibitors and Edge Sensing Controls Gastruloid Self-Organization. Dev. Cell, 2016, 39, 302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tewary M; Ostblom J; Prochazka L; Zulueta-Coarasa T; Shakiba N; Fernandez-Gonzalez R; Zandstra PW A Stepwise Model of Reaction-Diffusion and Positional Information Governs Self-Organized Human Peri-Gastrulation-like Patterning. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Heemskerk I; Burt K; Miller M; Chhabra S; Guerra MC; Liu L; Warmflash A Rapid Changes in Morphogen Concentration Control Self-Organized Patterning in Human Embryonic Stem Cells. Elife, 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang Z; Zwick S; Loew E; Grimley JS; Ramanathan S Embryo Geometry Drives Formation of Robust Signaling Gradients through Receptor Localization. BioRxiv, 2018, 491290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chhabra S; Liu L; Goh R; Kong X; Warmflash A Dissecting the Dynamics of Signaling Events in the BMP, WNT, and NODAL Cascade during Self-Organized Fate Patterning in Human Gastruloids. BioRxiv, 2018, 440164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tewary M; Stblom J; Shakiba N; Zandstra PW A Defined Platform of Human Peri-Gastrulation-like Biological Fate Patterning Reveals Coordination between Reaction-Diffusion and Positional-Information. BioRxiv, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Martyn I; Kanno TY; Ruzo A; Siggia ED; Brivanlou AH Self-Organization of a Human Organizer by Combined Wnt and Nodal Signalling. Nature, 2018, 11, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Massey J; Liu Y; Alvarenga O; Saez T; Schmerer M; Warmflash A Synergy with TGFβ Ligands Switches WNT Pathway Dynamics from Transient to Sustained during Human Pluripotent Cell Differentiation. Proc. Natl. Acad. Sci, 2019, 116, 4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sorre B; Warmflash A; Brivanlou AH; Siggia ED Encoding of Temporal Signals by the TGF-β Pathway and Implications for Embryonic Patterning. Dev. Cell, 2014, 30, 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pour M; Sampath Kumar A; Walther M; Wittler L; Meissner A; Nachman I Emergence and Patterning Dynamics of Mouse Definitive Endoderm. BioRxiv, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Driever W; Thoma G; Nusslein-Volhard C Determination of Spatial Domains of Zygotic Gene Expression in the Drosophila Embryo by the Affinity of Binding Sites for the Bicoid Morphogen The Hb Regulatory Region. Nature, 1989, 340, 363–367. [DOI] [PubMed] [Google Scholar]
- [78].Struhl G; Struhl K; Macdonald PM The Gradient Morphogen Bicoid Is a Concentration-Dependent Transcriptional Activator. Cell, 1989, 57, 1259–1273. [DOI] [PubMed] [Google Scholar]
- [79].Oosterveen T; Kurdija S; Alekseenko Z; Uhde CW; Bergsland M; Sandberg M; Andersson E; Dias JM; Muhr J; Ericson J Mechanistic Differences in the Transcriptional Interpretation of Local and Long-Range Shh Morphogen Signaling. Dev. Cell, 2012, 23, 1006–1019. [DOI] [PubMed] [Google Scholar]
- [80].Peterson KA; Nishi Y; Ma W; Vedenko A; Shokri L; Zhang X; McFarlane M; Baizabal JM; Junker JP; van Oudenaarden A; Mikkelsen T; Bernstein BE; Bailey TL; Bulyk ML; Wong WH; McMahon AP Neural-Specific Sox2 Input and Differential Gli-Binding Affinity Provide Context and Positional Information in Shh-Directed Neural Patterning. Genes Dev, 2012, 26, 2802–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hannon CE; Blythe SA; Wieschaus EF Concentration Dependent Chromatin States Induced by the Bicoid Morphogen Gradient. Elife, 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dubrulle J; Jordan BM; Akhmetova L; Farrell JA; Kim S-H; Solnica-Krezel L; Schier AF Response to Nodal Morphogen Gradient Is Determined by the Kinetics of Target Gene Induction. Elife, 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Deglincerti A; Croft GF; Pietila LN; Zernicka-Goetz M; Siggia ED; Brivanlou AH Self-Organization of the in Vitro Attached Human Embryo. Nature, 2016, 533, 251–254. [DOI] [PubMed] [Google Scholar]
- [84].Shahbazi MN; Jedrusik A; Vuoristo S; Recher G; Hupalowska A; Bolton V; Fogarty NME; Campbell A; Devito LG; Ilic D; Khalaf Y; Niakan KK; Fishel S; Zernicka-Goetz M Self-Organization of the Human Embryo in the Absence of Maternal Tissues. Nat. Cell Biol, 2016, 18, 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]