Abstract
Exposures to poly- and perfluoroalkyl substances (PFASs) have been linked to metabolic disruption, immunotoxicity and cancer in humans. PFASs are known to be present in diverse consumer products including textiles and food packaging. Here we present a new method for quantifying the atomic percent fluorine (% F) in the surficial 0.01 μm of consumer products using X-ray photoelectron spectroscopy (XPS). The surface of food contact materials and textiles measured in this study contained up to 28% F and 45% F, respectively. PTFE tape was measured to demonstrate XPS accuracy and precision. Depth profiles of fluorine content in consumer products measured using XPS showed highest levels at the upper-most surface in contact with the surrounding environment and a decrease below the surface. PFASs released in methanol extracts and quantified using traditional liquid chromatography-tandem mass spectrometry typically accounted for <1% of the fluorine measured with XPS in consumer products. We conclude that XPS is a useful technique for characterizing PFASs in consumer products because it can precisely quantify the surficial fluorine content of materials. XPS also allows identification of CF2 and CF3 groups in materials and can elucidate the depth dependent distribution of fluorine in products.
Graphical Abstract
Introduction
Elevated exposures to poly- and perfluoroalkyl substances (PFASs) have been linked to many adverse health effects, including diabetes, obesity risk, and immune suppression.1–3 More than 4000 PFASs have been produced since the late 1940s and hundreds have been detected in environmental samples.4–6 PFASs are defined by the presence of the CnF2n+1- moiety. They are often applied as “non-stick” surface coatings on products,7 can migrate from treated food contact papers into food or food-simulants such as butter, water, vinegar, and water/ethanol mixtures,4, 5, 7–10 and can leach from textiles into simulated saliva and sweat.11, 12 While PFASs are often applied as surface coatings on products,7 quantifying this surficial fluorine remains a challenge.
Traditional methods for measuring PFASs (liquid chromatography-tandem mass spectrometry: LC-MS/MS, or gas chromatography-mass spectrometry: GC-MS) only capture a small fraction of the fluorinated substances present in consumer products.13 Recently, particle-induced gamma ray emission (PIGE) has been proposed as a rapid screening technique for measuring the cumulative fluorine in consumer products.13–15 PIGE has been validated as a reliable method for detecting the presence of fluorine in the upper 100–250 μm of consumer products but does not distinguish between inorganic and organic fluorine.13–15 In addition, measurement techniques that can distinguish between surficial and subsurface fluorine in a product are needed.
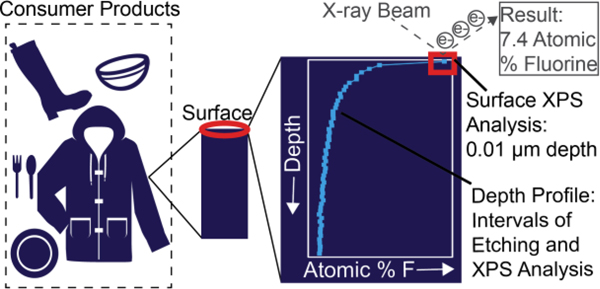
Here we investigate the utility of using X-ray photoelectron spectroscopy (XPS) to quantify surficial (0.01 μm) fluorine content in consumer materials. XPS represents a true surface measurement (surface defined as ≤ 0.1 μm)16, with a penetration depth four orders of magnitude smaller than PIGE (100–250 μm). Penetration depth is important because contact with consumer products (food, skin, children’s saliva) typically occurs at the surface of the material. We selected 45 products from a college campus for XPS analysis after determining concentrations of the suite of standardly measured PFASs using LC-MS/MS. XPS measurements were performed before and after a methanol extraction to understand fluorine mobility potential. Finally, we examined heterogeneity in concentrations with depth to determine whether fluorine is concentrated at the surface or evenly distributed with product depth (several micrometers thickness).
Materials and Methods
Consumer Product Sample Collection.
With the assistance of the Harvard University Office for Sustainability, we collected 94 consumer products that represent frequently used items on a college campus. These included: 45 food contact materials, 37 textiles, and 12 domestic products such as lens wipes, bandages, masks, and a shower curtain (Table S1 provides a complete sample inventory). All samples were sealed in low density polyethylene bags for storage at room temperature and cut with methanol-cleaned scissors as needed.
LC-MS/MS and Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (LC-QTOF-MS) Analysis.
We performed a methanol extraction on products following a method modified from prior work.10, 13 Methanol is commonly used for consumer product extractions10, 13, 17, 18 and is optimal for extracting the anionic compounds quantified with LC-MS/MS (see analyte list below). PFAS analysis was performed using an Agilent (Santa Clara, CA, U.S.A.) 6460 triple quadrupole LC-MS/MS with electrospray ionization in negative ion mode, as described elsewhere,19 with modifications detailed in the Supporting Information (SI). Native and isotopically labeled PFAS standards were purchased from Wellington Laboratories (Guelph, ON, Canada) (Table S2 and Table S3). A total of 16 PFASs were quantified: perfluorobutanoate (PFBA), perfluoropentanoate (PFPeA), perfluorohexanoate (PFHxA), perfluoroheptanoate (PFHpA), perfluorooctanoate (PFOA), perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUnDA), perfluorododecanoate (PFDoDA), perfluorobutane sulfonate (PFBS), perfluorohexane sulfonate (PFHxS), perfluorooctane sulfonate (PFOS), perfluorooctane sulfonamide (FOSA), 6:2 fluorotelomer sulfonate (6:2 FtS), N-Methyl perfluorooctane sulfonamidoacetic acid (N-MeFOSAA), and N-Ethyl perfluorooctane sulfonamidoacetic acid (N-EtFOSAA). LC-QTOF-MS analysis was completed for selected samples using a Shimadzu high performance reverse-phase liquid chromatography system coupled with a Sciex 5600 triple QTOF-MS. Samples were scanned in both positive and negative mode. Additional information on analytical methods and quality assurance/quality control measures are provided in the SI.
XPS Analysis.
A subset of 45 samples (19 food contact materials, 14 textiles, and 12 domestic products) with a wide range of PFAS concentrations (determined by LC-MS/MS analysis) were selected for analysis of surficial percent atomic fluorine (hereon % F will refer to atomic percent fluorine) using a Thermo Scientific Al K-Alpha XPS (Waltham, MA, U.S.A), 1.4866 keV. The X-rays had a 400 μm spot size. Samples were mounted on carbon tape, and at least two points on each sample were analyzed (we report the average). The instrumental error for atomic composition is ±1%. A survey scan was used to quantify the atomic composition of all samples. A high-resolution scan for carbon (C1s) and fluorine (F1s) was completed for samples with detectable fluorine. The “1s” refers to the atomic orbital. The high resolution scan provides information on specific bonds (for example CF2 and CF3 groups) present in the sample.20 PTFE tape was included as a positive control. Volatile PFASs account for a small fraction (<3%) of total fluorine in consumer products,13 and we therefore neglect such potential losses during XPS vacuum pump-down.
We used an argon ion beam (ion gun energy = 250 eV) to etch selected samples in 10 second intervals. We performed a survey scan of % F after each etching interval to construct a fluorine depth profile. Details on XPS analysis, and sample preparation for XPS measurements pre- and post- methanol extraction are provided in the SI.
RESULTS AND DISCUSSION
LC-MS/MS PFAS Analysis of Methanol Extracts.
LC-MS/MS is the most commonly used and widely available instrument for aqueous PFAS analysis. Based on this method, at least one PFAS was detected in 43% of the 94 samples examined in this study. PFOA and PFHxA were detected most frequently, with individual detection frequencies of 29%. All LC-MS/MS results are reported in Tables S4 and S5.
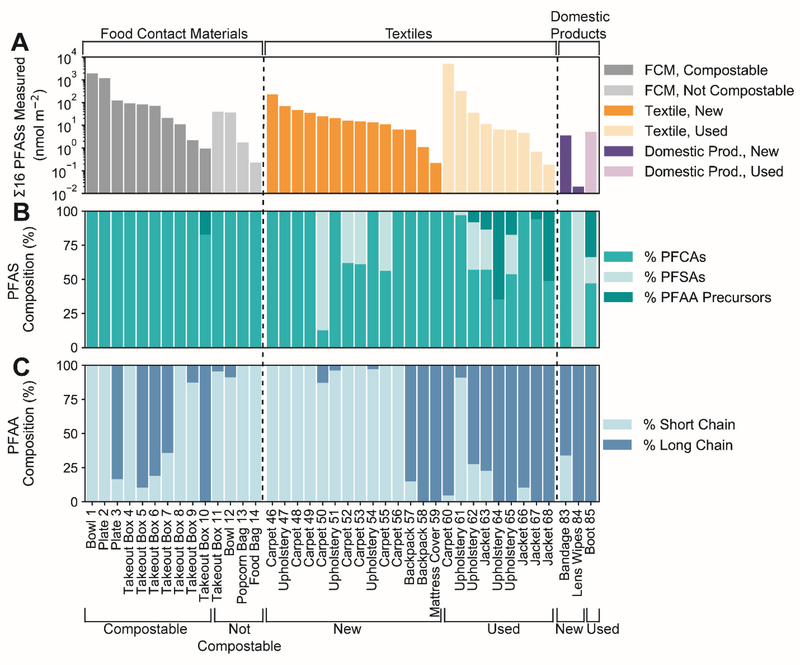
The maximum concentration of any one PFAS was PFOA measured in a carpet sample (sample 60) collected from an actively used faculty office (3200 nmol m−2 or 0.38 mg kg−1). Risk-based concentration limits have not been established for most PFASs in consumer products. For comparison, the maximum concentration observed is very close to the New Hampshire Department of Environmental Services Direct Contact Risk Based PFOA soil concentration of 0.5 mg kg−1 for young children.21 A compostable disposable bowl (sample 1) contained the highest single PFAS concentration (PFBA) measured in a food contact material at 960 nmol m−2 (0.60 mg kg−1). High concentrations of PFASs detected in numerous compostable food contact materials (Figure 1A) suggest potential environmental impacts and requires further evaluation.
Figure 1.
Concentrations and composition of 16 PFASs measured in consumer products. Samples include compostable food contact materials, non-compostable (or unknown) food contact materials, new textiles, used textiles, new domestic consumer products, and used domestic consumer products. Panel (A) shows concentrations of the sum of 16 measured PFASs for all samples above detection. FCM denotes food contact material. Panel (B) shows PFAS composition. The PFAA precursors are N-EtFOSAA, N-MeFOSAA, FOSA, and 6:2 FtS. Panel (C) shows the fraction of short- and long-chain PFAAs.
Perfluorinated carboxylates (PFCAs) dominated the PFAS composition for both compostable and other food contact materials (Figure 1B). Used textiles contained a more diverse composition of PFCAs, perfluorinated sulfonates (PFSAs), and perfluoroalkyl acid (PFAA) precursors (hereon referred to as precursors) than other products. Precursors quantified using LC-MS/MS were N-EtFOSAA, N-MeFOSAA, FOSA, and 6:2 FtS.
Of the compostable food contact materials measured, 50% contained over 64% long-chain PFAAs (Figure 1C) (n ≥ 7 for PFCAs, and n ≥ 6 for PFSAs, were n is the number of perfluorinated carbons)5. Long-chain PFASs have higher bioaccumulation potential than other PFASs and thus are of particular concern.22 Used textiles were primarily composed of long-chain PFAAs, while new textiles were predominantly composed of short-chain PFAAs (Figure 1C). This is consistent with reported shifts in chemical production, where longer-chain PFASs have been replaced by shorter-chain alternatives.22
Atomic percent fluorine (% F).
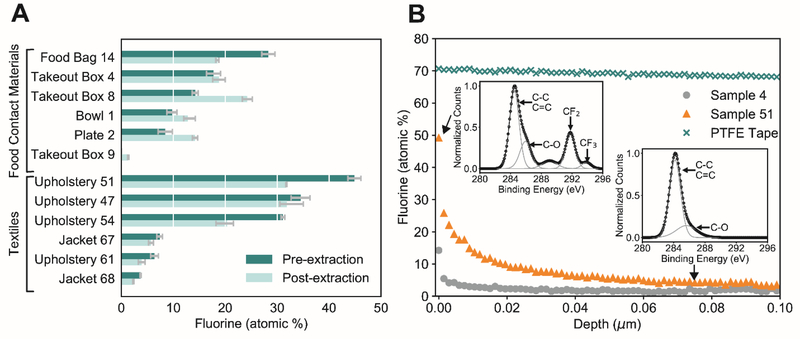
The highest interfacial fluorine measured in a consumer product was 45% F from a new upholstery sample (sample 51) (Figure 2A). The maximum atomic percent fluorine of any food contact material analyzed was in a disposable food bag (sample 14) that contained 28% F (Figure 2A). We did not detect fluorine in any of the domestic consumer products using XPS (detection limit of 1%).
Figure 2.
(A) XPS results showing % F in food contact materials (n=6) and textiles (n=6) that contained detectable fluorine. Sample 9 was included as a blank control but contained fluorine post-extraction (see Methanol Extraction for XPS section of SI). Whiskers are the minimum and maximum measured, the bar is the average % F. (B) Depth profiles of a food contact material (sample 4), a textile (sample 51), and PTFE tape. Each point represents a XPS measurement of atomic percent fluorine, followed by etching with an argon ion beam for 10 seconds. The depth of etching for a tantalum pentoxide reference is plotted: we estimate the actual maximum total depth etched to be 10 μm. The insets show the high-resolution C1s scan for two selected data points in sample 51: the surface measurement and one measurement taken at estimated 7.5 μm depth. The inset C1s scans show the disappearance of the 292 eV peak with depth. The 292 eV peak corresponds with the CF2 group binding energy.
XPS allows identification of CF2 and CF3 groups in a sample and can thus confirm the presence of perfluorinated carbons. An example of CF2 and CF3 groups identified using a high resolution C1s scan is shown in Figure 2B. The CF2 group is usually located at ~292 eV (verified with PTFE tape) and CF3 at ~293 eV.20 Out of the 11 samples with detectable fluorine pre-extraction, nine samples displayed peaks at ~292 eV (CF2 group) and/or at ~293 eV (CF3 group). The two samples that did not display peaks at ~292 eV or ~293 eV were samples 61, and 68, which had the lowest % F (Figure 2A). Further confirming the presence of organofluorine, all 11 samples that had detectable fluorine pre-extraction displayed a peak at ~689 eV in the F1s scan, which corresponds to the binding energy of highly fluorinated carbon groups (such as CF2 and CF3).23 These results illustrate how high resolution C1s and F1s scans can be used to distinguish inorganic from organic fluorine.
The reproducibility of XPS measurements was verified with sample 67 (textile, rough surface). Variability was greatest for spatially independent samples randomly taken from around the textile (relative standard deviation: RSD=27%, n=9). Within a sample (<1cm2), RSD decreased to 11.4% (n=10). PTFE tape was measured as a positive control and contained 70% F, which agrees well with the expected value of 67% F for pure CF2 groups (this value would increase if there were CF3 groups present). PTFE tape (smooth surface) had a low RSD (0.53%, n=10), indicating that XPS measurements are reproducible and material coating method/roughness are the main sources of variability.
XPS of Samples Pre- and Post-Extraction.
Results shown in Figure 2A indicate methanol extraction does not remove most fluorine from consumer materials. The % F measured before and after extraction (n=12) was not statistically different (p > 0.05) based on a two-tailed Wilcoxon signed-rank test. The location of the C1s peaks associated with perfluorinated carbons did not shift after the methanol extraction, further confirming the persistence of organic fluorine in these materials. Robel et al.13 also reported that a large fraction of fluorine remains in products post-extraction. These findings suggest substantial quantities of fluorine (perhaps fluorinated polymers and associated monomers) could be released to landfills or composting facilities at the end of the product’s life.13
XPS Depth Profiles.
Figure 2B shows fluorine depth profiles for one food contact material (a takeout box, sample 4), one textile sample (upholstery, sample 51), and PTFE tape as a control. Figure 2B indicates the total etching depth reached a maximum of ~0.1 μm based on the etching rate from a tantalum pentoxide reference material. We estimated the consumer products in this study were etched to a maximum of 10 μm (see SI Sputtering Yield for Consumer Products section for details24). A single XPS measurement represents a depth of 0.01 μm, but the depth profiles shown (Figure 2B) are composed of >60 individual XPS measurements taken in between etching intervals.
For both products, the % F declined rapidly below the surface before stabilizing (Figure 2B). A relatively constant fluorine profile with depth was observed in PTFE tape (Figure 2B), reflecting the difference between a coating and a dense polymer. After five rounds of etching (maximum 0.75 μm estimated depth), the % F decreased by 64% in the textile (sample 51) and 77% in the food contact material (sample 4). After etching to a maximum estimated depth of 10 μm, the % F decreased by 93% in the textile and 88% in the food contact material. Depth profiles of additional samples also show decreasing % F with depth (Figure S1). The C1s spectra displayed a peak at ~292 eV (CF2 group) at the beginning of the depth profile for sample 51 but not at ~7.5 μm estimated depth where fluorine is detected in small amounts (<5% F) (Figure 2B). Three different samples from the same food contact material (sample 4) were subjected to XPS depth profiling to demonstrate reproducibility (Figure S2). XPS depth-profiling indicates that fluorine is not homogenously distributed throughout the material and can be heavily concentrated in the near surface (e.g., fluorocarbon coatings).
XPS LC-MS/MS Comparison.
XPS and LC-MS/MS results were converted to weight percent for comparison (See SI for calculation). The LC-MS/MS results were scaled from the original sample thickness to 0.01 μm for direct comparison to XPS. Scaling the thickness to 0.01 μm provides an upper bound weight percent value for LC-MS/MS, since the extraction process likely extracts fluorine from deeper than 0.01 μm (Figure 2B).
A maximum of 53% of the fluorine was accounted for using the targeted LC-MS/MS approach based on samples that had detectable fluorine using both LC-MS/MS and XPS (Figure S3). In 7 out of the 11 samples, LC-MS/MS measurements accounted for <1% of the fluorine, indicating the vast majority remains unmeasured. We did not find a correlation between LC-MS/MS and XPS fluorine (Figure S4), similar to the results reported by PIGE analysis.13 LC-MS/MS only measures the selected methanol-extractable ionic PFAS fraction, whereas XPS measures the total fluorine content of the material interface, which includes any fluoropolymers or fluoromonomers.
The XPS detection limit is ~1 atomic % (~1.6 wt.% F assuming the rest of the material is carbon). The LC-MS/MS can detect much lower concentrations, ~4 × 10−5 wt.% F for PFOA (assuming a representative detection limit of 1 ng g−1, a 1 g sample, and the average thickness of 0.055 cm from samples in Figure S3). Therefore, while LC-MS/MS does not capture a large fraction of the fluorine, it does have a significantly lower detection limit. XPS and LC-MS/MS are thus complementary techniques that together provide a more holistic approach to fluorine analysis in consumer products.
We screened for a total of 109 negative and 57 positive PFAS compounds by using LC-QTOF-MS analysis to identify PFASs that contribute to the % F measured using XPS that were not detected using LC-MS/MS. Across all samples, 42 PFASs were detected with LC-QTOF-MS and 50% were PFAS compounds measured with LC-MS/MS (Table S9). Therefore, it is unlikely that quantitative LC-QTOF-MS analysis of these compounds will close the mass balance between XPS and measured PFAS compounds. Volatile and precursors compounds measured with the total oxidizable precursor assay25, 26 have also been shown to represent a small fraction of the total fluorine in consumer products.13 Most research institutions lack the specialized equipment to conduct such investigations. Thus, we propose that widely available analytical techniques such as XPS are useful for screening for total fluorine in the surface of samples.
Discussion.
PFASs are often applied as surface coatings to products,7 which is the component in contact with the surrounding environment. However, the surface-specific (≤ 0.1 μm)16 fluorine content of consumer products has not previously been characterized. We characterized the total fluorine content in the true surface (0.01 μm) of 45 consumer products using XPS. We find that the surface of the consumer products measured here contain up to 45% F, and that fluorine is persistent in the consumer products based on methanol extractions, indicating the potential for long-term environmental impacts.
To the best of our knowledge, PIGE is the only other analytical technique that has been used to determine the presence of total fluorine in consumer products. Both XPS and PIGE could be used to screen products for fluorine content. However, there are important differences between the two techniques. XPS is a surface-specific (0.01 μm) technique that can discern the presence and depth distribution of organic fluorine. PIGE measures the bulk material (100–250 μm) and cannot distinguish between inorganic and organic fluorine, and cannot provide depth resolution. XPS systems are commonly available at research universities and could thus be widely leveraged for fluorine analyses. Future work is needed to investigate whether there is a link between surficial fluorine (XPS measurements) and human exposure. We conclude that XPS provides a powerful new method for quantification of % F in the surface of consumer products.
Supplementary Material
Acknowledgements.
This work was supported by the National Institute for Environmental Health Sciences Superfund Research Program (P42ES027706) and the Campus Sustainability Innovation Fund administered by the Harvard Office for Sustainability. We acknowledge the Harvard University Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation under NSF award no. 1541959. We thank Hao-Yu Greg Lin for his valuable assistance in the XPS measurements and Linda Lee for LC-QTOF-MS support.
Footnotes
Supporting Information
Details on materials, LC-MS/MS extraction, LC-MS/MS analysis, quality assurance/quality control, XPS analysis, methanol extraction for XPS, and LC-QTOF-MS analysis. Calculations on sputtering yield for consumer products, and XPS and LC-MS/MS weight percent. Tables containing sample inventory, compound names, abbreviations, internal standards, molecular formulas, and molecular weights, LC-MS/MS parameters, LC-MS/MS results (nmol m−2), LC-MS/MS results (μg kg−1), LC-MS/MS method quantification limits, LC-MS/MS recovery and precision results, XPS results, and LC-QTOF-MS results. Figures showing additional consumer product depth profiles, repetitive depth profile analysis of sample 4, XPS wt.% F and LC-MS/MS wt.% F comparison, and comparison of LC-MS/MS and XPS data.
References
- (1).Grandjean P; Heilmann C; Weihe P; Nielsen F; Mogensen UB; Timmermann A; Budtz-Jørgensen E Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J. Immunotoxicol. 2017, 14 (1), 188–195, DOI: 10.1080/1547691X.2017.1360968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Liu G; Dhana K; Furtado JD; Rood J; Zong G; Liang L; Qi L; Bray GA; DeJonge L; Coull B; et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Med. 2018, 15 (2), e1002502, DOI: 10.1371/journal.pmed.1002502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Sun Q; Zong G; Valvi D; Nielsen F; Coull B; Grandjean P Plasma concentrations of perfluoroalkyl substances and risk of Type 2 diabetes: A prospective investigation among U.S. women. Environ. Health Perspect. 2018, 126 (3), DOI: 10.1289/EHP2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; de Voogt P; Jensen AA; Kannan K; Mabury SA; van Leeuwen SPJ Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manage. 2011, 7 (4), 513–541, DOI: 10.1002/ieam.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wang Z; DeWitt JC; Higgins CP; Cousins IT A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ. Sci. Technol. 2017, 51 (5), 2508–2518, DOI: 10.1021/acs.est.6b04806 [DOI] [PubMed] [Google Scholar]
- (6).OECD. Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per - and Polyfluoroalkyl Substances (PFASs); OECD Environment Directorate, Environment, Health and Safety Division: Paris, France, 2018. [Google Scholar]
- (7).Kotthoff M; Müller J; Jürling H; Schlummer M; Fiedler D Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ. Sci. Pollut. Res. 2015, 22 (19), 14546–14559, DOI: 10.1007/s11356-015-4202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Begley TH; White K; Honigfort P; Twaroski ML; Neches R; Walker RA Perfluorochemicals: Potential sources of and migration from food packaging. Food Addit. Contam. 2005, 22 (10), 1023–1031, DOI: 10.1080/02652030500183474 [DOI] [PubMed] [Google Scholar]
- (9).Begley TH; Hsu W; Noonan G; Diachenko G Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit. Contam., Part A 2008, 25 (3), 384–390, DOI: 10.1080/02652030701513784 [DOI] [PubMed] [Google Scholar]
- (10).Yuan G; Peng H; Huang C; Hu J Ubiquitous occurrence of fluorotelomer alcohols in eco-friendly paper-made food-contact materials and their implication for human exposure. Environ. Sci. Technol. 2016, 50 (2), 942–950, DOI: 10.1021/acs.est.5b03806 [DOI] [PubMed] [Google Scholar]
- (11).CEC. Furthering the Understanding of the Migration of Chemicals from Consumer Products – A Study of Per- and Polyfluoroalkyl substances (PFASs) in Clothing, Apparel, and Children’s Items; Montreal, Canada, 2017; p 201. [Google Scholar]
- (12).Polyfluoroalkyl Substances (PFASs) in Textiles for Children; Survey of chemical substances in consumer products No. 136, 2015; The Danish Environmental Protection Agency: Copenhagen, Denmark, 2015. [Google Scholar]
- (13).Robel AE; Marshall K; Dickinson M; Lunderberg D; Butt C; Peaslee G; Stapleton HM; Field JA Closing the mass balance on fluorine on papers and textiles. Environ. Sci. Technol. 2017, 51 (16), 9022–9032, DOI: 10.1021/acs.est.7b02080 [DOI] [PubMed] [Google Scholar]
- (14).Ritter EE; Dickinson ME; Harron JP; Lunderberg DM; DeYoung PA; Robel AE; Field JA; Peaslee GF PIGE as a screening tool for per- and polyfluorinated substances in papers and textiles. Nucl. Instrum. Methods Phys. Res., Sect. B 2017, 407, 47–54, DOI: 10.1016/j.nimb.2017.05.052 [DOI] [Google Scholar]
- (15).Schaider LA; Balan SA; Blum A; Andrews DQ; Strynar MJ; Dickinson ME; Lunderberg DM; Lang JR; Peaslee GF Fluorinated compounds in U.S. fast food packaging. Environ. Sci. Technol. Lett. 2017, 4 (3), 105–111, DOI: 10.1021/acs.estlett.6b00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Vickerman JC; Gilmore IS, Surface Analysis, The Principal Techniques. 2nd ed.; John Wiley & Sons Ltd: West Sussex, U.K, 2009. [Google Scholar]
- (17).Ye F; Zushi Y; Masunaga S Survey of perfluoroalkyl acids (PFAAs) and their precursors present in Japanese consumer products. Chemosphere 2015, 127, 262–268, DOI: 10.1016/j.chemosphere.2015.02.026 [DOI] [PubMed] [Google Scholar]
- (18).van der Veen I; Weiss JM; Hanning A-C; de Boer J; Leonards PEG Development and validation of a method for the quantification of extractable perfluoroalkyl acids (PFAAs) and perfluorooctane sulfonamide (FOSA) in textiles. Talanta 2016, 147, 8–15, DOI: 10.1016/j.talanta.2015.09.021 [DOI] [PubMed] [Google Scholar]
- (19).Weber AK; Barber LB; LeBlanc DR; Sunderland EM; Vecitis CD Geochemical and hydrologic factors controlling subsurface transport of poly- and perfluoroalkyl substances, Cape Cod, Massachusetts. Environ. Sci. Technol. 2017, 51 (8), 4269–4279, DOI: 10.1021/acs.est.6b05573 [DOI] [PubMed] [Google Scholar]
- (20).Ferraria AM; Lopes da Silva JD; Botelho do Rego AM XPS studies of directly fluorinated HDPE: problems and solutions. Polymer 2003, 44 (23), 7241–7249, DOI: 10.1016/j.polymer.2003.08.038 [DOI] [Google Scholar]
- (21).Direct Contact Risk-Based Soil Concentration: Perfluorooctanoic Acid: CAS #335–67-1 New Hampshire Department of Environmental Services, Environmental Health Program: Concord, NH, 2016. [Google Scholar]
- (22).Wang Z; Cousins IT; Scheringer M; Hungerbuehler K Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions. Environ. Int. 2015, 75, 172–179, DOI: 10.1016/j.envint.2014.11.013 [DOI] [PubMed] [Google Scholar]
- (23).Nansé G; Papirer E; Fioux P; Moguet F; Tressaud A Fluorination of carbon blacks: An X-ray photoelectron spectroscopy study: I. A literature review of XPS studies of fluorinated carbons. XPS investigation of some reference compounds. Carbon 1997, 35 (2), 175–194, DOI: 10.1016/S0008-6223(96)00095-4 [DOI] [Google Scholar]
- (24).Seah MP Universal equation for argon gas cluster sputtering yields. J. Phys. Chem. C 2013, 117 (24), 12622–12632, DOI: 10.1021/jp402684c [DOI] [Google Scholar]
- (25).Houtz EF; Higgins CP; Field JA; Sedlak DL Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ. Sci. Technol. 2013, 47 (15), 8187–8195, DOI: 10.1021/es4018877 [DOI] [PubMed] [Google Scholar]
- (26).Houtz EF; Sedlak DL Oxidative conversion as a means of detecting precursors to perfluoroalkyl acids in urban runoff. Environ. Sci. Technol. 2012, 46 (17), 9342–9349, DOI: 10.1021/es302274g [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.