Abstract
Objective
To test the hypothesis that periodontal disease would be associated with increased risk for dementia and mild cognitive impairment (MCI) by assessing dementia/MCI outcomes after a baseline periodontal examination.
Methods
Participants enrolled in the Atherosclerosis Risk in Communities study with a clinical periodontal examination (or edentulous participants) at visit 4 (1996–1998; mean ± SD age 63 ± 6 years, 55% female, 21% black) and adjudicated dementia outcomes through 2016 were included (n = 8,275). A subgroup of 4,559 participants had adjudicated dementia and MCI assessments at visit 5 (2011–2013). Participants received a full-mouth periodontal examination and were classified into periodontal profile classes (PPCs) based on the severity and extent of gingival inflammation and attachment loss. MCI and dementia were determined via neurocognitive testing, neurological examination and history, informant interviews, and brain MRI in a subset. Cox proportional hazards models regressed incident dementia on PPCs. Relative risk regression models were used for the composite of MCI/dementia.
Results
The cumulative incidence and incidence density of dementia during follow-up (average 18.4 years) were 19% (n = 1,569) and 11.8 cases per 1,000 person-years. Multivariable adjusted hazard ratios for incident dementia among participants with severe PPC or edentulism (vs periodontal healthy) were 1.22 (95% confidence interval [CI] 1.01–1.47) and 1.21 (95% CI 0.99–1.48), respectively. For the combined dementia/MCI outcome, adjusted risk ratios among participants with mild/intermediate PPC, severe PPC, or edentulism (vs periodontal healthy) were 1.22 (95% CI 1.00–1.48), 1.15 (95% CI 0.88–1.51), and 1.90 (95% CI 1.40–2.58). Results were stronger among younger (≤62 years) participants (p for interaction = 0.02).
Conclusion
Periodontal disease was modestly associated with incident MCI and dementia in a community-based cohort of black and white participants.
Dementia and mild cognitive impairment (MCI) are major causes of disability and dependency.1 Dementia risk factors remain poorly understood, and in the specific case of Alzheimer disease, only half of the Alzheimer disease cases are attributable to known modifiable risk factors.2 Consequently, identification of modifiable risk factors for dementia and MCI is a priority.
Systemic inflammation has been linked to cognitive decline and the development of dementia.3 Periodontal diseases, including gingivitis and periodontitis, are highly prevalent inflammatory diseases initiated by dysbiotic subgingival biofilms. Recent research posits periodontal disease as a risk factor for dementia and cognitive impairment,4–6 consistent with a literature linking both periodontal disease and adverse microbial exposures to inflammation.7–9
Prior publications from the Atherosclerosis Risk in Communities (ARIC) study10–12 and other studies13–15 have described the interrelation between tooth loss or periodontal disease and cognitive outcomes, although many reports were cross-sectional or case-control (precluding temporal assessments) and often lacked robust confounder adjustment. There is also a lack of longitudinal data on the potential for baseline periodontal status to predict incident MCI.
We explored the associations between periodontal status, incident MCI, and dementia among participants in the ARIC study, a multicenter, community-based longitudinal cohort, providing an opportunity to build on prior research by (1) presenting longitudinal results, (2) using multivariable adjustments, and (3) including both MCI and dementia outcomes. We hypothesized that individuals with periodontal disease at baseline were at increased risk for incident MCI and dementia.
Methods
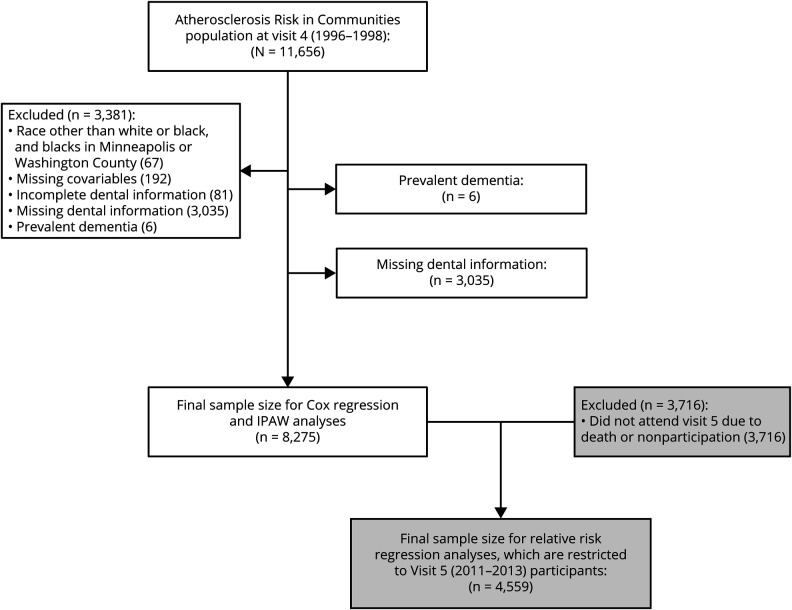
The ARIC study16 enrolled 15,792 predominantly black or white participants within the age group of 45 to 64 years in 4 different US communities (Forsyth County, North Carolina; Jackson, MS; northwestern suburbs of Minneapolis, MN; and Washington County, Maryland). In the current study, all participants who completed the fourth clinic visit (1996–1998) in ARIC (n = 11,656) were eligible for inclusion. Of the 11,656 ARIC participants seen at the fourth clinical visit, we removed 3,381, mainly due to missing dental information, for a longitudinal analytic sample of n = 8,275 (figure 1). We additionally analyzed a subgroup of 4,559 participants who attended visit 5 (2011–2013) and had an assessment for MCI and dementia.
Figure 1. Participant flowchart for incidence and IPAW analyses.
IPAW = inverse probability of attrition weighting.
Standard protocol approvals, registrations, and patient consents
All participants provided written informed consent, and the study was approved by the Institutional Review Board at each study site.
Assessment of dementia and MCI
The ARIC methods for ascertainment of MCI and dementia have been described in detail previously.17 Briefly, dementia was defined by expert review using a predetermined algorithm, incorporating data from the cognitive evaluations at visits 2, 4, and 5 and consisting of a full neuropsychological assessment (visit 5 only), interviews, informant interviews, hospital discharge codes, or diagnostic codes from death certificates.17 The algorithm was based on the National Institute on Aging–Alzheimer's Association working group formulations of dementia18 and the DSM-V. Dementia diagnoses were established by review of all information by a physician (neurologist or geriatrician) and a neuropsychologist. If the 2 disagreed on a classification, a third clinician reviewed the case to arrive at a final diagnosis.17 The clinician reviewers agreed with the algorithmic diagnosis 94% of the time.17 The panel classified participants who attended ARIC visit 5 as normal of having MCI or dementia following the criteria proposed by the National Institute of Aging–Alzheimer's Association work groups.18,19 Etiologic subtypes were additionally defined as follows. The diagnosis of Alzheimer disease–related MCI/dementia followed the National Institute of Aging–Alzheimer's Association work group criteria and was based on the presence of a nonabrupt cognitive syndrome including memory impairment in the absence of features of other specific causes of cognitive impairment.18,19 The diagnosis of vascular MCI/dementia was based on the National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria, which use information on history of stroke, its temporal relationship with the onset of cognitive impairment, the presence of vascular disease on imaging, and neurologic signs of stroke in a physical examination.20
Periodontal assessments
A full-mouth periodontal examination was conducted by trained examiners calibrated against a standard examiner. Examiners measured probing depth and gingival recession and assessed bleeding on probing at 6 sites per tooth; attachment loss was calculated from probing depth and gingival recession. Using attachment loss, probing depth, and bleeding on probing, examiners derived the periodontal profile class (PPC), which has been previously validated.21 Briefly, the PPC uses person-level latent class analysis to identify discrete classes of individuals using 7 tooth-level clinical parameters. These parameters were as follows: ≥1 site with interproximal attachment loss ≥3 mm, ≥1 site with probing depth ≥4 mm, extent of bleeding on probing (dichotomized at 50% or ≥3 sites per tooth), gingival inflammation index (0 or ≥1), plaque index (0 or ≥1), presence/absence of full prosthetic crowns for each tooth, and tooth status (present or absent; implants were counted as absent). The 7 PPC categories closely reflect increasing extent of attachment loss ≥3 mm, probing depth ≥4 mm and bleeding on probing as previously reported22 and were given the following names based on the dominant clinical features represented in each group: (1) PPC-A (Health), (2) PPC-B (mild), (3) PPC-C (high gingival index), (4) PPC-D (tooth loss), (5) PPC-E (posterior disease), (6) PPC-F (severe tooth loss), and (7) PPC-G (severe disease). We also categorized participants (1) using the periodontitis Centers for Disease Control and Prevention/American Academy of Periodontology (CDC/AAP) classification23 as no or mild periodontitis, moderate periodontitis, or severe periodontitis; (2) using the recently published by the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Disease and Conditions (abbreviated World Workshop Classifications throughout); and (3) according to levels of the periodontal inflamed surface area (PISA) metric.
Risk factor measurements
The majority of potential confounding variables were measured at the time of the periodontal examination (visit 4) via questionnaires, clinical examination, and laboratory analysis of blood samples. However, information on birth date (used to calculate age), sex, race (black or white), and education level (high school graduate, yes/no) was collected at baseline. Physical activity was assessed with a modified Baecke questionnaire24 from visit 3 (1993–1995). A physical activity index score (1 = lowest, 5 = highest) was calculated from the intensity and time dedicated to sport and exercise. Questionnaires at visit 4 were used to assess smoking (never, former, or current), and income (<$25,000, $25,000–<$50,000, $50,000–<$75,000, ≥$75,000 per year). Oral hygiene and access-to-care variables were collected during the dental history questionnaire administered at visit 4. These included medical insurance status (private insurance, Medicare/Medicaid only, none) and dental visit frequency (regularly, only for discomfort or repair, do not regularly visit the dentist).
Participants fasted for 8 hours before the visit 4 clinical examination, and blood was collected for plasma lipids (including high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol [estimated via the Friedewald equation25], and triglycerides) and glucose. APOE genotyping was performed with the TaqMan assay (Applied Biosystems, Foster City, CA) and operationalized as participants with 0, 1, or 2 APOE ε4 alleles. Body mass index (BMI) was defined as measured weight in kilograms divided by height in meters squared. Blood pressure was measured twice after a 5-minute rest, and the average was used for analysis. Use of antihypertensive medication was self-reported. Diabetes mellitus was defined as a self-reported physician diagnosis of diabetes mellitus, fasting glucose ≥126 mg/dL, ≥200 mg/dL if nonfasting, or pharmacologic treatment for diabetes mellitus.
Prevalent heart failure, coronary heart disease, stroke, and incident stroke
ARIC defined heart failure (HF) at visit 4 as the reported use of HF medication or the presence of HF according the Gothenburg criteria26 at ARIC visit 1 or having developed incident HF before visit 4, according to the presence of ICD-9-CM code 428 in any hospitalization during follow-up.27 Prevalent coronary heart disease at visit 4 was based on prior cardiovascular revascularization, self-reported physician-diagnosed myocardial infarction before visit 1, or presence of a previous myocardial infarction by ECG at visit 1 or incident coronary heart disease after visit 1 adjudicated by the ARIC Morbidity and Mortality Classification Committee.28 ARIC defined stroke at visit 4 as a self-reported physician diagnosis of a stroke before visit 1 or adjudicated probable or definite hospitalized stroke after visit 1 using criteria adapted from the National Survey of Stroke.29
Statistical analysis
All analyses were conducted with SAS version 9.4 (SAS Institute Inc, Cary, NC). Time to ARIC diagnosis of dementia was the primary outcome. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated with multivariable Cox proportional hazards models. Follow-up time began at visit 4 (1996–1998) and accrued until date of dementia diagnosis, loss to follow-up, death, or December 31, 2016, whichever occurred first. In addition, we performed a sensitivity analysis by fitting the Fine-Gray model, a proportional hazards model for the subdistribution accounting for the competing risk of death.30 We present crude incidence rates across the aforementioned periodontal disease categories in addition to 7 multivariable models constructed as follows. Model 1 (a base demographics model) adjusted for age, sex, education, race-center, income, and insurance. Model 2 (health behaviors and adiposity model) additionally adjusted for BMI, physical activity, and cigarette smoking. Model 3 further included HDL cholesterol, LDL cholesterol, prevalent HF, and APOE genotype. Model 4 added hypertension medication, systolic blood pressure, prevalent coronary heart disease, prevalent stroke, and dental visit frequency. Model 5 included all model 4 covariables but excluded participants with prevalent stroke at baseline. Model 6 added prevalent diabetes mellitus, and model 7 added incident time-dependent stroke after visit 4 (but before dementia onset) to model 4 covariables. The additional comorbid conditions, medications, and oral health care use added to models 4 to 7 could be interpreted as potential mediators linking periodontal status to incidence dementia.
In a subset of participants, we also evaluated the association of midlife periodontal disease categories and prevalence of MCI or dementia (assessed at visit 5 [2011–2013]) as a combined endpoint using relative risk regression in generalized linear models with a Poisson distribution and a log link. For these analyses, differential survival or participation at visit 5 might have introduced selection bias. Therefore, we used inverse probability of attrition weighting to adjust for attrition due to either death or failure to attend visit 5 given that the participant was alive. Logistic models for visit 5 participation, conditional on survival to visit 5, and survival included the following variables measured at or before visit 4: age, sex, race-center, high school graduate, income, insurance, APOE, BMI, physical activity, smoking status, HDL cholesterol and LDL cholesterol, diabetes mellitus, hypertension medication use, systolic blood pressure, stroke, HF, coronary heart disease, frequency of dental visits, periodontal classification (PPC and CDC/AAP variables), and global z score for cognitive tests, along with interactions between smoking status and PPC and male sex and PPC. Variables included from annual follow-up included incident stroke. The stabilized weights included age, sex, race-center, APOE, and high school graduate.
The c statistics for visit 5 participation and survival were 0.75 and 0.83, respectively.
Multiplicative interactions with periodontal classification were explored by age (<62 vs ≥62 years), sex, and race, and additive interactions were assessed via the relative excess risk due to interaction.
Data availability
ARIC data are available through NIH National Heart, Lung, and Blood Institute–sponsored Biologic Specimen and Data Repository Information Coordinating Center at biolincc.nhlbi.nih.gov/.
Results
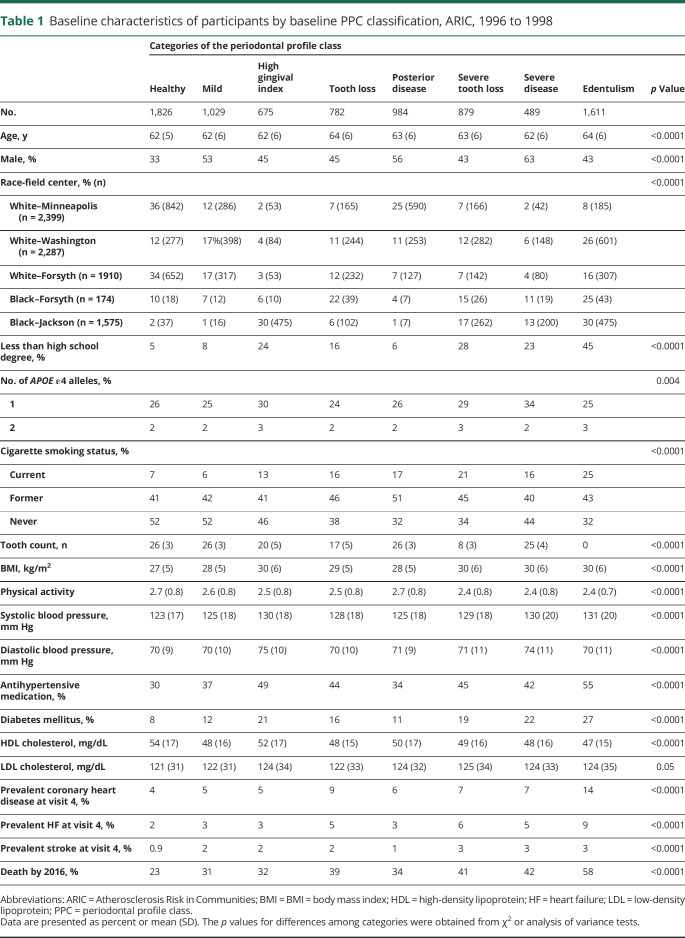
The 8,275 participants included were 55% female and 21% black and had a mean age at visit 4 of 63 (SD 6) years. The distribution of participants according to PPC was as follows: health 22%, mild disease 12%, high gingival inflammation index 8%, tooth loss 9%, posterior disease 12%, severe tooth loss 11%, severe disease 6%, and edentulous 20%. The prevalence of CDC/AAP–defined mild, moderate, and severe periodontitis was 24%, 33%, and 14%, while the remaining participants were free of periodontitis. Table 1 demonstrates that participants with worse periodontal status were more likely to have risk factors for vascular disease and dementia.
Table 1.
Baseline characteristics of participants by baseline PPC classification, ARIC, 1996 to 1998
The relationship between CDC/AAP–defined periodontitis and the latent class analysis–based PPC definition has been previously published in ARIC.21 While there is substantial agreement between most disease categories for the 2 distinct constructs, there is notable disagreement for a few PPC classes. Among participants classified as having PPC-health, 34% were classified as having moderate or mild CDC/AAP–defined periodontitis. Alternatively, among the PPC-severe tooth loss group, 48% of participants were classified as having mild or no CDC/AAP–defined periodontitis.
Periodontal disease and incident dementia
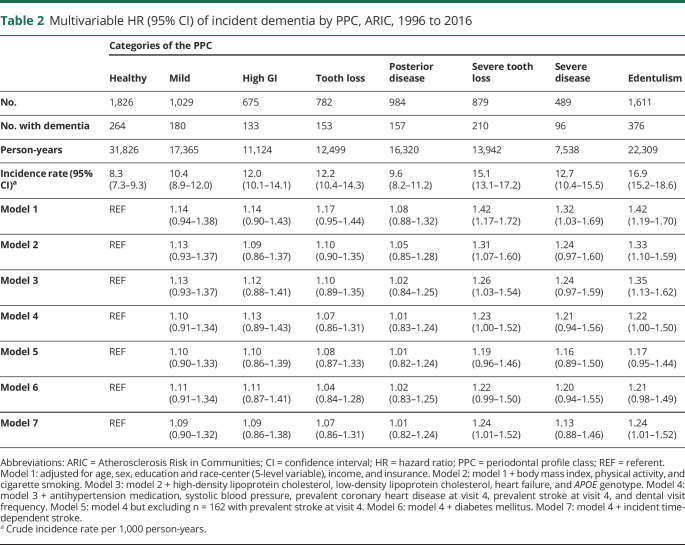
The cumulative incidence of dementia was 19.0% (n = 1,569 new cases). The median follow-up time was 18.4 (quartile 1–3, 13.8–19.6) years, and the incidence rate of dementia was 11.8 per 1,000 person-years (95% CI 11.2–12.4). PPC was associated with incident dementia. The dementia incidence rates across the 7 PPC categories and edentulism were 8.3, 10.4, 12.0, 12.2, 9.6, 15.1, 12.7, and 16.9 dementia events per 1,000 person-years, respectively (table 2). After adjustment for sociodemographic variables (age, sex, education, race-center, income, insurance), the HRs for intermediate levels of PPC (vs PPC-healthy as the reference) were near the null with CIs including 1.0, while severe tooth loss, severe disease, and edentulism HRs were 1.42 (95% CI 1.17–1.72), 1.32 (95% CI 1.03–1.69), and 1.42 (95% CI 1.19–1.70), respectively (table 2). Adjustment for BMI, smoking, physical activity, vascular risk factors, and APOE genotype modestly attenuated results for PPC-severe tooth loss, PPC-severe disease, and edentulism, but risk remained elevated in these groups with HRs as follows: 1.26 (95% CI 1.03–1.54), 1.24 (95% CI 0.97–1.59), and 1.35 (95% CI 1.13–1.62) (table 2, model 3). Additional multivariable models adjusting for evidence of prevalent vascular disease or diabetes mellitus (possible mediators of the periodontal disease–dementia association, table 2, models 4–7) resulted in additional modest attenuations of HRs, although results were consistent with the pattern of elevated risk for incident dementia in the most diseased PPC categories and the edentulous.
Table 2.
Multivariable HR (95% CI) of incident dementia by PPC, ARIC, 1996 to 2016
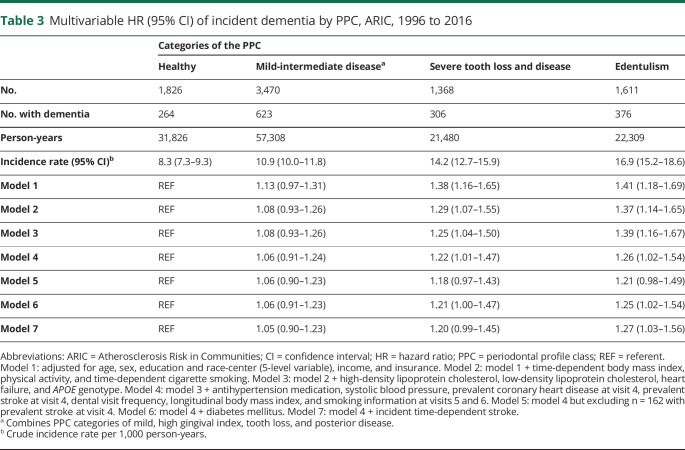
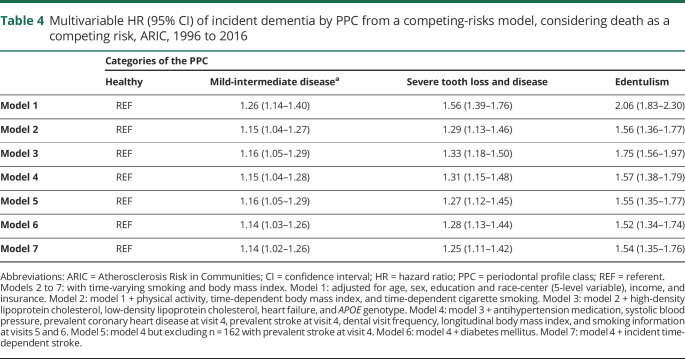
Results were consistent when the PPC categories were collapsed into 3 levels (table 3). In addition, the observed patterns were consistent when analyses were restricted to the first 15 years of follow-up: HRs among PPC-severe tooth loss and disease or edentulism (vs healthy) were 1.17 (95% CI 0.86–1.60) and 1.16 (95% CI 0.83–1.62) after model 4 multivariable adjustments. Similarly, when only incident events occurring >10 years from baseline were included, results were similar: HRs among PPC-severe tooth loss and disease or edentulism (vs healthy) were 1.12 (95% CI 0.92–1.36) and 1.11 (95% CI 0.89–1.37) after model 4 multivariable adjustments. In competing-risks analysis of the full sample, the findings were notably stronger (table 4).
Table 3.
Multivariable HR (95% CI) of incident dementia by PPC, ARIC, 1996 to 2016
Table 4.
Multivariable HR (95% CI) of incident dementia by PPC from a competing-risks model, considering death as a competing risk, ARIC, 1996 to 2016
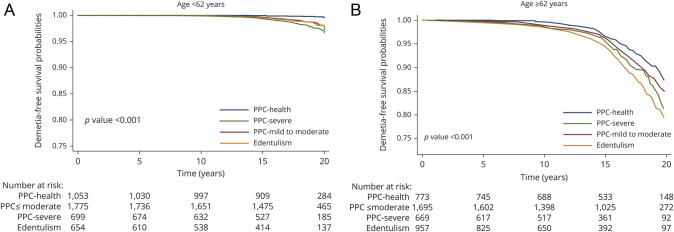
There was evidence that associations were stronger among participants <62 years (median age at the dental examination) vs ≥62 years of age (figure 2). In a multivariable model (model 4), the HRs for PPC-mild/moderate disease, severe disease, and edentulism among the younger participants were 1.16 (95% CI 0.86–1.57), 1.54 (95% CI 1.08–2.20), and 1.33 (95% CI 0.91–1.95), respectively; HRs among the older participants were 1.03 (95% CI 0.86–1.22), 1.10 (95% CI 0.88–1.38), and 1.16 (0.91–1.47), respectively (p for interaction = 0.02). The relative excess risk due to interaction was derived using results from the Cox models and was −0.33, demonstrating that the association is weaker for older participants on both the multiplicative and additive scales. No race or sex interactions were present (data not shown).
Figure 2. Kaplan-Meier dementia-free survival curves by PPC category, stratified by age, ARIC, 1996 to 2016.
Kaplan-Meier dementia-free survival curves by PPC category, stratified by age <62 years (A) vs. age >=62 years (B). Models are adjusted for adjusted for age, sex, education, and race-center (5-level variable), income, insurance, body mass index, physical activity, cigarette smoking, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, heart failure, APOE genotype, hypertension medication, systolic blood pressure, prevalent coronary heart disease, prevalent stroke, and dental visit frequency. The p value for age interaction = 0.02. ARIC = Atherosclerosis Risk in Communities; PPC = periodontal profile class.
In contrast, CDC/AAP–defined periodontitis was not associated with incident dementia. The crude event rates among those with healthy, mild, moderate, and severe CDC/AAP periodontitis were 11.1 (95% CI 9.4–13.1), 9.6 (95% CI 8.6–10.6), 10.9 (95% CI 10.0–11.9), and 12.5 (95% CI 11.0–14.2), respectively. HRs compared to the healthy reference in multivariable-adjusted models were consistently null, ranging from 0.88 to 1.12 with CIs that all included 1. Similarly, results for associations between PISA and incident dementia were null. After multivariable adjustment, a 1-SD increase in PISA values (2.9) was corresponded to an HR of 1.02 (95% CI 0.96–1.08). After multivariable adjustment, HRs for incident dementia among participants with stage III or IV periodontitis (vs those with less than stage III periodontitis) according to the world workshop classification were 1.04 (95% CI 0.72–1.50) and 1.05 (95% CI 0.72–1.52), respectively.
Periodontal disease and incident MCI or dementia
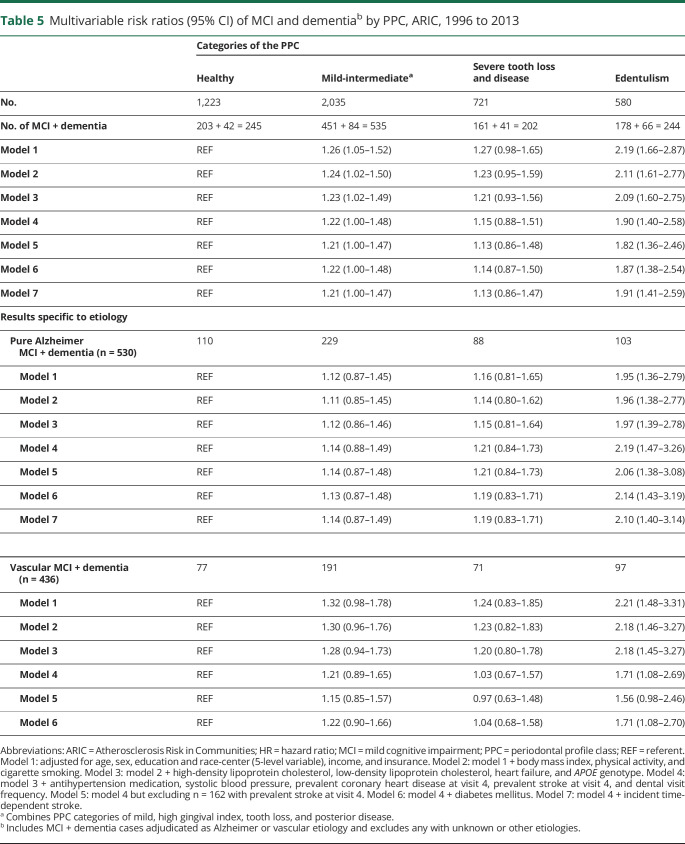
In the subset of 4,559 participants with adjudicated MCI and dementia, there were 1,226 incident MCI + dementia cases (cumulative incidence 27%), 80% of which were MCI (n = 993). PPC-defined disease and edentulism were consistently associated with increased incident MCI/dementia rates. In a multivariable model (table 5, model 3) the HRs for incident MCI/dementia among PPC-intermediate, PPC-severe, or the edentulous (vs PPC-healthy) were 1.23 (95% CI 1.02–1.49), 1.21 (95% CI 0.93–1.56), and 2.09 (95% CI 1.60–2.75), respectively.
Table 5.
Multivariable risk ratios (95% CI) of MCI and dementiab by PPC, ARIC, 1996 to 2013
Findings were generally consistent in secondary analyses for incident MCI/dementia of both Alzheimer and vascular etiologies. Adjustment for vascular risk factors (table 5, models 4–7) did not influence findings for cases with Alzheimer etiology, while adjustment for these factors resulted in greater attenuation for vascular MCI/dementia findings.
Discussion
We found periodontal disease, as defined by the PPC, to be associated with modestly increased risk for incident dementia and MCI among a sample of black and white community-dwelling adults in the ARIC study. The PPC categories characterized by severe tooth loss or extent of attachment loss were associated with an ≈20% greater dementia incidence during up to 20 years of longitudinal follow-up compared with periodontally healthy dentate participants. Edentulism was similarly associated with an ≈20% increase in the incidence of dementia. Findings were generally consistent when we considered the combined outcome of MCI and dementia, although the association between edentulism and MCI was markedly stronger; an ≈2-fold increase in MCI (or MCI + dementia) risk was observed among the edentulous relative to periodontally healthy dentate participants. In contrast, results were consistently null among groups with less severe PPC classifications, which were more reflective of gingivitis and minimal tooth loss. Similarly, the CDC/AAP definition was not associated with MCI or dementia. This might reflect a chance finding for the PPC or the high sensitivity of the CDC/AAP definition, which requires minimal extent and severity to be classified as having disease. In addition, the CDC/AAP definition does not incorporate tooth loss, which raises the possibility that participants with a history of severe periodontitis and subsequent tooth loss might be misclassified as healthy with this definition, while the PPC definition does not have this disadvantage. These results remained after extensive adjustment for a large panel of plausible sociodemographic, behavioral, and genetic risk factors and vascular comorbid conditions. The findings were also consistently positive for both Alzheimer disease–related dementia and vascular dementia.
Multiple unique biologically plausible pathways connecting periodontal disease to dementia are possible. In the case of Alzheimer disease–related dementia, multiple infectious hypotheses of Alzheimer pathology have been proposed. It is well established that spirochetes are neurotropic pathogens, and syphilitic dementia is an example of the potentially neurodegenerative effects of a classic spirochete infection. In the context of periodontal disease, Treponema denticola is a notable spirochete and an established periodontal pathogen known to be highly abundant in periodontal disease.31 Previous research has demonstrated that the gingival mucosa is a portal of entry for invasive spirochetes and that spirochetes can transmit along the trigeminal nerve and trigeminal ganglia,6,32 thus providing a plausible mechanism enabling oral microbiota to colonize in the brain. Porphyromonas gingivalis is another organism that has been studied in regard to neurodegeneration. P gingivalis is a likely keystone pathogen in periodontitis, and its translocation to extraoral sites33 is known to occur during transient bacteremias induced by dental procedures and even as the result of common activities such as toothbrushing and chewing.34 Recent evidence has directly linked toxic proteases produced by P gingivalis, called gingipains, to increased production of β-amyloid and detrimental effects on the tau protein.35
The possible link between periodontal disease and vascular dementia is potentially more indirect, with cardiometabolic diseases serving as causal intermediates. A robust body of literature suggests that chronic periodontal infections might contribute to insulin resistance, prediabetes,36,37 incident diabetes mellitus,38–40 and incident stroke,41–43 including in the ARIC study.43,44 Accordingly, insulin resistance, diabetes mellitus, and stroke are strong predictors of future cognitive decline.45,46 Currently, adjustment for vascular risk factors did not influence findings for cases with Alzheimer disease–related dementia, while adjustment for these factors resulted in greater attenuation of periodontitis HRs for vascular dementia cases, which is consistent with the concept of mediation via vascular pathways.
The observation that edentulism was more strongly associated with incident dementia than poor periodontal status among dentate individuals may appear to be counterintuitive because the removal of teeth (and periodontal disease) presumably reduces or resolves the adverse microbial challenge. However, these findings are consistent with several previous studies that have linked edentulism to incident mortality and cardiovascular disease after multivariable adjustment.47–49 Because complete tooth loss among adults is often the end consequence of chronic periodontal infections during the life course (although the reasons for tooth loss are unknown in ARIC), it is possible that the key risk period occurs during young to middle adulthood, and subsequent tooth extraction is unable to reverse risk. It is also plausible that adverse microbial profiles remain, living on oral mucosal surfaces and dentures after complete tooth extraction. Alternatively, translocation of oral bacteria to systemic sites during the life course could enable oral pathogens to remain viable and immunomodulatory after their portal of entry is blocked, at least partially, with tooth extraction and resolution of periodontal lesions. Data from the Taiwan national Health Insurance Research Database found that among individuals with periodontitis, those with tooth extraction had a higher annual incidence of dementia (0.57%) than those who receiving intensive periodontal treatment (0.35%).13 Alternatively, edentulous individuals might simply have an inflammatory phenotype with edentulism serving as a surrogate of lifelong accumulation of inflammation-induced morbidity as reflected by the increased prevalence of cardiovascular disease among edentulous individuals. Our adjustments for comorbid conditions should minimize but not eliminate this form of confounding.
There was evidence for an interaction between age and periodontal disease such that periodontal associations with dementia were strongest among younger members of the ARIC cohort. This was consistent on both the additive and multiplicative scales. The observation that the relative risk is great among younger individuals accords with prior research relating periodontal disease to cardiovascular disease.49,50 If true, these findings suggest that future intervention studies, should they be justified, may need to consider including younger adults during vulnerable periods and before the onset of MCI. Nevertheless, even smaller relative risks observed in the older participants are of population importance given the high prevalence of periodontitis and the high lifetime risk of dementia.
Some important limitations of our current report should be noted. The baseline periodontal assessments in ARIC were made among participants with a mean age of 63 years, and it is possible that cognitive decline might have been begun before the development of periodontitis and tooth loss. The main hypotheses linking periodontal disease to dementia centrally involve adverse oral microbial exposures, but we had no direct assessments of the oral microbiota to test more refined hypotheses. We are also unable to account for longitudinal change in periodontal status, resulting in potential misclassification of periodontal disease status during the follow-up period, although this limitation is likely to have biased results toward the null. Attrition might also have attenuated our findings because those with periodontal disease or dementia are less likely to remain in the study longitudinally. The use of inverse probability of attrition weighting minimizes these concerns, as previously discussed.17
We have found clinical measures of severe periodontitis and tooth loss to be associated with a modestly enhanced dementia risk during up to 20 years of longitudinal follow-up among a racially diverse cohort of adult men and women. Future studies are justified to further characterize the potential role of oral microbiota in explaining this relationship and the potential for anti-infective periodontal interventions to prevent cognitive decline.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important contributions. The PPC stages and computational algorithms, as well as the application to stages and grades, are protected under copyright (2017–2018) with the University of North Carolina.
Glossary
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CDC-AAP
Centers for Disease Control and Prevention/American Academy of Periodontology
- CI
confidence interval
- DSM-V
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- HDL
high-density lipoprotein
- HF
heart failure
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, 9th revision, clinical modification
- LDL
low-density lipoprotein
- MCI
mild cognitive impairment
- PISA
periodontal inflamed surface area
- PPC
periodontal profile class
Appendix. Authors
Study funding
The ARIC study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). Neurocognitive data were collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, and 2U01HL096917 from the NIH (National Heart, Lung, and Blood Institute, National Institute for Neurological Disorders and Stroke, National Institute on Aging, and National Institute on Deafness and Other Communication Disorders) and with previous brain MRI examinations funded by R01-HL70825 from the National Heart, Lung, and Blood Institute.
Disclosure
R.T. Demmer, F.L. Norby, K. Lakshminarayan, K.A. Walker, J.S. Pankow, A.R. Folsom, T. Mosley, J. Beck, and P.L. Lutsey report no disclosures relevant to the manuscript. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000010312 for full disclosures.
References
- 1.World Health Organization. Dementia fact sheet. 2016. World Health Organization Available at: who.int/mediacentre/factsheets/fs362/en/. Accessed January 27, 2017. [Google Scholar]
- 2.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol 2002;52:168–174. [DOI] [PubMed] [Google Scholar]
- 4.Noble JM, Scarmeas N, Papapanou PN. Poor oral health as a chronic, potentially modifiable dementia risk factor: review of the literature. Curr Neurol Neurosci Rep 2013;13:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly B, Thompsell A, Sharpling J, et al. Evidence summary: the relationship between oral health and dementia. Br Dent J 2018;223:846–853. [DOI] [PubMed] [Google Scholar]
- 6.Sochocka M, Zwolinska K, Leszek J. The infectious etiology of Alzheimer's disease. Curr Neuropharmacol 2017;15:996–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behle JH, Sedaghatfar MH, Demmer RT, et al. Heterogeneity of systemic inflammatory responses to periodontal therapy. J Clin Periodontol 2009;36:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demmer RT, Breskin A, Rosenbaum M, et al. The subgingival microbiome, systemic inflammation and insulin resistance: the Oral Infections, Glucose Intolerance and Insulin Resistance study. J Clin Periodontol 2017;44:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demmer RT, Trinquart L, Zuk A, et al. The influence of anti-infective periodontal treatment on C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2013;8:e77441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naorungroj S, Schoenbach VJ, Beck J, et al. Cross-sectional associations of oral health measures with cognitive function in late middle-aged adults: a community-based study. J Am Dent Assoc 2013;144:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naorungroj S, Schoenbach VJ, Wruck L, et al. Tooth loss, periodontal disease, and cognitive decline in the Atherosclerosis Risk in Communities (ARIC) study. Community Dent Oral Epidemiol 2015;43:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naorungroj S, Slade GD, Beck JD, et al. Cognitive decline and oral health in middle-aged adults in the ARIC study. J Dent Res 2013;92:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YL, Hu HY, Huang LY, Chou P, Chu D. Periodontal disease associated with higher risk of dementia: population-based cohort study in Taiwan. J Am Geriatr Soc 2017;65:1975–1980. [DOI] [PubMed] [Google Scholar]
- 14.Fang WL, Jiang MJ, Gu BB, et al. Tooth loss as a risk factor for dementia: systematic review and meta-analysis of 21 observational studies. BMC Psychiatry 2018;18:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley NC, Affoo RH, Siqueira WL, Martin RE. A systematic review examining the oral health status of persons with dementia. JDR Clin Trans Res 2017;2:330–342. [DOI] [PubMed] [Google Scholar]
- 16.The Atherosclerosis Risk in Communities (ARIC) study: design and objectives: the ARIC Investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 17.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst) 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 21.Beck JD, Moss KL, Morelli T, Offenbacher S. In search of appropriate measures of periodontal status: the periodontal profile phenotype (P(3) ) system. J Periodontol 2018;89:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morelli T, Moss KL, Beck J, et al. Derivation and validation of the periodontal and tooth profile classification system for patient stratification. J Periodontol 2017;88:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol 2007;78(suppl):1387–1399. [DOI] [PubMed] [Google Scholar]
- 24.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 26.Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea: validation of a scoring test for clinical-epidemiological use: the study of men born in 1913. Eur Heart J 1987;8:1007–1014. [DOI] [PubMed] [Google Scholar]
- 27.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 28.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years' experience. J Clin Epidemiol 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 29.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 30.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 31.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000 2005;38:135–187. [DOI] [PubMed] [Google Scholar]
- 32.Miklossy J. Alzheimer's disease: a neurospirochetosis: analysis of the evidence following Koch's and Hill's criteria. J Neuroinflammation 2011;8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kebschull M, Demmer RT, Papapanou PN. “Gum bug, leave my heart alone!” Epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res 2010;89:879–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol 2006;33:401–407. [DOI] [PubMed] [Google Scholar]
- 35.Dominy SS, Lynch C, Ermini F, et al. Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 2019;5:eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arora N, Papapanou PN, Rosenbaum M, Jacobs DR Jr, Desvarieux M, Demmer RT. Periodontal infection, impaired fasting glucose and impaired glucose tolerance: results from the Continuous National Health and Nutrition Examination Survey 2009-2010. J Clin Periodontol 2014;41:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demmer RT, Jacobs DR Jr, Singh R, et al. Periodontal bacteria and prediabetes prevalence in ORIGINS: the Oral Infections, Glucose Intolerance, and Insulin Resistance study. J Dent Res 2015;94(suppl):201S–211S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demmer RT, Jacobs DR Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care 2008;31:1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winning L, Patterson CC, Neville CE, Kee F, Linden GJ. Periodontitis and incident type 2 diabetes: a prospective cohort study. J Clin Periodontol 2017;44:266–274. [DOI] [PubMed] [Google Scholar]
- 40.Ide R, Hoshuyama T, Wilson D, Takahashi K, Higashi T. Periodontal disease and incident diabetes: a seven-year study. J Dent Res 2011;90:41–46. [DOI] [PubMed] [Google Scholar]
- 41.Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease: the first National Health and Nutrition Examination Survey and its follow-up study. Arch Intern Med 2000;160:2749–2755. [DOI] [PubMed] [Google Scholar]
- 42.Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke 2003;34:47–52. [DOI] [PubMed] [Google Scholar]
- 43.Sen S, Giamberardino LD, Moss K, et al. Periodontal disease, regular dental care use, and incident ischemic stroke. Stroke 2018;49:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beck JD, Moss KL, Morelli T, Offenbacher S. Periodontal profile class is associated with prevalent diabetes, coronary heart disease, stroke, and systemic markers of C-reactive protein and interleukin-6. J Periodontol 2018;89:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young SE, Mainous AG III, Carnemolla M. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes Care 2006;29:2688–2693. [DOI] [PubMed] [Google Scholar]
- 46.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol 2017;74:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tu YK, Galobardes B, Smith GD, McCarron P, Jeffreys M, Gilthorpe MS. Associations between tooth loss and mortality patterns in the Glasgow Alumni Cohort. Heart 2007;93:1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desvarieux M, Demmer RT, Rundek T, et al. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Stroke 2003;34:2120–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desvarieux M, Schwahn C, Volzke H, et al. Gender differences in the relationship between periodontal disease, tooth loss, and atherosclerosis. Stroke 2004;35:2029–2035. [DOI] [PubMed] [Google Scholar]
- 50.Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS, Garcia RI. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation 2008;117:1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
ARIC data are available through NIH National Heart, Lung, and Blood Institute–sponsored Biologic Specimen and Data Repository Information Coordinating Center at biolincc.nhlbi.nih.gov/.