Abstract
Objective
To examine whether neuropathologic burden is associated with hearing impairment.
Methods
We studied 2,755 autopsied participants ≥55 years of age from the National Alzheimer's Coordinating Center database. Participants had at least 1 clinical evaluation at US National Institute on Aging–funded Alzheimer's Disease Center no more than 2 years before death. Patients were classified as hearing impaired by clinician report at baseline. Common dementia neuropathologies included Alzheimer disease pathologic change (Consortium to Establish a Registry for Alzheimer's Disease neuritic plaque density, neurofibrillary degeneration Braak stage), Lewy body disease, gross infarcts, and microinfarcts. Logistic regression models predicted impaired hearing with adjustment for age at death, sex, race, education, center, and follow-up time. Relative risks were calculated with the use of marginal standardization.
Results
Impaired hearing was common (32%). In participants who were cognitively normal at baseline (n = 580), impaired hearing was associated with higher Braak stage (relative risk [RR] 1.33 per 2-stage increase, 95% confidence interval [CI] 1.06–1.66) but not other pathologies. In participants with dementia (n = 2,175), impaired hearing was positively associated with microinfarcts (RR 1.18, 95% CI 1.00–1.39) and inversely associated with neuritic plaque density (RR 0.91 per score increase, 95% CI 0.85–0.99). Development of impaired hearing in those with cognitive impairment was associated with neocortical Lewy bodies (1.26, 95% CI 1.02–1.55).
Conclusions
Impaired hearing, reported before the onset of cognitive impairment, was associated with increased neurofibrillary tangle burden. Impaired hearing in those with cognitive impairment was associated with microinfarcts and neocortical Lewy bodies but not typical Alzheimer disease pathologic change. Functional hearing problems may be a preclinical marker of neurofibrillary neurodegeneration, although replication is needed.
Emerging evidence suggests that age-related hearing impairment is associated with increased risk of dementia.1–6 Multiple biological mechanisms have been posited to explain this association, but it is unclear whether hearing impairment is associated with a specific pattern of neurodegenerative disease neuropathology. Hearing impairments can be caused by degradation in peripheral structures in the inner ear or central auditory processing dysfunction in brain.7,8 Impaired hearing could indirectly accelerate cognitive decline through effects on social isolation or reduced cognitive reserve.7,9,10 Alternatively, underlying neurodegeneration associated with dementia may affect central auditory processing and cause difficulty hearing.8,11,12 However, hearing impairment may increase misclassification of cognitive impairment,13–15 in which case dementia-causing pathologies would be less common in individuals with compared to those without hearing impairment. Hearing loss is associated with structural volumes in some studies,16–18 but it is not established whether specific neuropathologic changes are linked to hearing impairment. Such research may help disentangle mechanisms and is important to understanding whether hearing loss is a relevant target for dementia prevention or preclinical detection.
The objective of this study was to use data from one of the largest autopsy samples, the National Alzheimer's Coordinating Center (NACC) database, to examine whether neuropathologic profiles differ between those with and those without clinician-reported hearing impairment. We focus on common neuropathologies of aging related to Alzheimer disease (AD), Lewy body disease, vascular brain injury (VBI), and primary age-related tauopathy (PART). We compare findings between those with and those without cognitive impairment at the time of reported hearing function.
Methods
Standard protocol approvals, registrations, and patient consents
Individual Alzheimer’s Disease Centers (ADCs) received institutional review board approval, and written informed consent was obtained from all participants and their study coparticipants. NACC received institutional review board approval from the University of Washington for release of deidentified data.
Data sources and study populations
NACC participants were prospectively evaluated at 1 of ≈30 US ADCs between September 2005 and September 2018. Each ADC recruits and enrolls participants according to its own protocol. Participants were evaluated by trained clinicians or interviewers approximately annually at an ADC using a standardized clinical protocol, the Uniform Data Set (UDS). Participant medical and health history is assessed, and participants receive physical and neurologic examinations, plus a battery of neuropsychological assessments. Additional details are available online (alz.washington.edu/WEB/forms_uds.html) and are published elsewhere.19,20 Neuropathologic data are collected from neuropathologists on the basis of autopsy results among participants who die and had consented to autopsy evaluation at an ADC.21,22
The current analyses focused on UDS participants who had been autopsied as of September 2018. To focus on the most common types of dementia that occur in community-dwelling older adults,23 we excluded participants from the primary analysis sample if they had (1) a rare disease (n = 1,441 excluded) such as Down syndrome, prion disease, autosomal dominant genetic diseases (i.e., early-onset AD), or frontotemporal lobar degeneration (FTLD) or (2) age at death <55 years (n = 128 excluded). We also excluded participants missing a clinical visit proximal to death (within 2 years; n = 875 excluded) so that we would have near-death information on cognitive status and covariates. Participants missing information on hearing abilities, demographics (age, sex, race, education), or common neuropathologies were also excluded (n = 234). In total, 2,755 autopsied participants met inclusion criteria for primary analyses. In a secondary analysis, we added participants with FTLDs (n = 703) to the analytic sample to examine associations with impaired hearing and FTLD.
Hearing impairment
History of impaired hearing was assessed at each annual UDS visit on the basis of clinician interview (UDS Form B1; type of clinician is not specified for this form and could vary by ADC). Clinicians were asked 3 related hearing questions: (1) Without a hearing aid, is the individual’s hearing functionally normal?(yes, no, unknown)? (2) Does the individual usually wear a hearing aid (yes, no, unknown)? (3) If yes, is the individual’s hearing functionally normal with a hearing aid? Clinicians filling out the form were instructed to select “no” to questions 1 and 3 if any functional impairment exists (reduced ability to do everyday activities such as listening to the radio or television, talking with family or friends). Answers could be based on report of participant or coparticipant, clinician judgement, basic clinical examination, or medical history with audiology results.
In the current analysis, we defined impaired hearing according to whether the participant was noted to have impaired hearing at baseline (e.g., not functional hearing in question 1) regardless of hearing aid status. We conducted a subanalysis to evaluate associations with development of hearing impairment over follow-up (e.g., those who had normal hearing reported at baseline but who had hearing impairment reported in a follow-up visit). We also examined hearing aid use defined on the basis of whether the participant was noted to use hearing aids at baseline.
Covariates
NACC UDS forms collect a variety of participant characteristics, medical history, neurologic evaluation, neuropsychological battery, and clinician ratings. We selected a limited set of covariates for the current analyses. Demographic characteristics included age, sex, education, and race/ethnicity. History of health conditions, including vascular risk factors (hypertension and hypercholesterolemia), diabetes mellitus, any cardiovascular disease, and stroke, was recorded at each clinical visit as recent/active, remote/inactive, or absent. Participants were asked whether they had depression in the 2 years before the visit or presence of depressive episodes >2 years before the clinical visit; we defined a history of depression as any recent or remote depressive episodes. We defined a history of each comorbidity (recent or remote) according to the last clinical visit before death. APOE genotyping was performed on consenting participants. APOE ε4 allele status was classified as at least 1 or none. The Clinical Dementia Rating Dementia Staging Instrument Sum of Boxes (CDR-SB) score,24 a composite measure of the overall level of cognitive impairment and functional disability that is based on clinical judgment and study coparticipant report, was collected at each study visit. Participants also completed a neuropsychological battery of 11 tests.25,26 but those with severe dementia often are unable to complete tests, and exact tests changed in 2015, so we focused on the CDR-SB as a measure of overall cognition and dementia staging in the current study because it is available for all participants. Cognitive status was evaluated at each visit, and a diagnosis was made by either a single clinician or a consensus group of clinicians after a review of all evaluation information available, including neuropsychological testing and neurologic examination. Normal cognition (UDS Form D1) was defined as (1) no diagnosis of mild cognitive impairment27 or dementia on the basis of consensus criteria28 and (2) either a CDR score of 0 or neuropsychological testing within normal range (or both). We dichotomized participants on the basis of normal cognition vs cognitively impaired (range from mild impairments to severe dementia).
Neuropathologic features
ADCs follow consensus-based guidelines but conduct neuropathologic assessments according to center-specific protocols.21,22 Neuropathologists used a standardized form, and results were uploaded to the NACC database. AD neuropathologic change (ADNC) included Consortium to Establish a Registry for Alzheimer's Disease (CERAD) scores of neuritic plaque densities (none, sparse, moderate, frequent)29 and Braak stages for neurofibrillary tangle pathology (categorized as none, I/II, III/IV, V/VI).30 ADNC was also categorized semiquantitatively (no/low, intermediate, and high). No/low ADNC was defined as no/sparse neuritic plaques and any Braak stage or any neuritic plaques density and Braak stage 0 to II. Intermediate ADNC was defined as moderate or frequent CERAD plaques and Braak stage III to IV, and high ADNC was defined as moderate or frequent plaques and Braak stage V to VI. This assessment does not include Thal phasing31 for amyloid plaques, so this operationalization overlaps with but does not correspond exactly to the levels of ADNC as defined by the National Institute on Aging–Alzheimer's Association criteria.32 PART was classified as present in participants with definite PART as defined by Braak stage I to IV and no neuritic plaques in the absence of consistent Thal phase assessments historically.33 Cerebrovascular pathology encompassed VBI and indicators of vessel disease. In all samples, VBI was defined as any gross infarcts or cortical microinfarcts. In NACC, gross infarcts (present, absent) were defined as large artery or lacunar infarcts identified macroscopically regardless of age. Cortical microinfarcts (present, absent) were defined as infarcts in the cortex that were detected only microscopically. Overall severity of cerebral amyloid angiopathy (identified with stains for amyloid) and atherosclerosis (identified grossly) was recorded as none, mild, moderate, or severe. Presence of Lewy bodies was assessed according to established guidelines and classified as none, brainstem predominant, limbic (transitional), neocortical (diffuse), or region not specified/other.34 Hippocampal sclerosis was classified as present or absent. Presence of FTLD subtypes was documented. For this analysis, we categorized FTLD into tau-positive subtypes (FTLD-tau; e.g., Pick disease, corticobasal degeneration, progressive supranuclear palsy, and other tauopathies) and nontauopathy FTLD subtypes (e.g., FTLD with TAR DNA-binding protein 43 [TDP-43], ubiquitin-positive/tau-negative inclusions, no distinctive histology, or not specified but not a tauopathy). Assessment of TDP-43 was not questioned on the NACC neuropathology forms until 2014.
Statistical analyses
Participant characteristics were described for those with and without hearing impairment. As indirect evidence of the validity of our measure of impaired hearing, we evaluated whether the measure corresponded with well-established epidemiologic patterns in age-related hearing impairment,35 i.e., that the prevalence of impaired hearing increased with age and was higher for men. We plotted sex-stratified predicted age curves of the prevalence of impaired hearing on the basis of logistic regression with natural cubic splines for age. Multivariable logistic regression models assessed associations between impaired hearing and neuropathologic burden, focusing on the most common neuropathologic features in NACC: neuritic plaques, Braak stage, neocortical Lewy bodies, gross infarcts, and microinfarcts. We fitted models stratified on baseline cognitive status (normal and cognitively impaired) to compare associations by disease stage. Primary models included adjustment for demographics (age at death, sex, race, education) and months between last visit and death, follow-up time, and ADC. Because late-life health conditions may represent shared risk factors or potential mediators of the relationship between hearing impairment and dementia, we ran a secondary model with additional adjustment for APOE ε4 allele and history of the following comorbid conditions: hypertension, hypercholesterolemia, diabetes mellitus, smoking, atrial fibrillation, heart attack, congestive heart failure, depression, and CDR-SB score at the last visit. Odds ratios are commonly interpreted as relative risks (RRs), but odds ratios overestimate the RR for common outcomes such as impaired hearing; we therefore estimated RRs using marginal standardization based on predicted probabilities from logistic regressions in the total sample.36
We ran several sensitivity analyses of the primary models to identify and account for potential biases in the sample to further generalize results. We reran models with hearing aid use as the outcome. Next, we used stabilized inverse probability weights37,38 to account for potential sample selection bias and to refer study findings to the overall NACC sample. Weights for autopsy selection and missing hearing measurement were calculated as predicted probabilities according to separate logistic regressions with age, birth year, education, sex, race, APOE genotyping conducted, CDR-SB score at the last visit, ADC, and interactions between age and education, sex, race, and CDR-SB score at the last visit as primary predictors; weights for each model were multiplied together. The 95% confidence intervals (CIs) for weighted models were derived from 1,000 bootstrap replications.39 Finally, because criteria for pathologies became more standardized in NACC forms after 2014, we reran analyses in those who had died after 2014.
We also ran several secondary analyses. First, we further examined the association between impaired hearing and diagnostic categories of AD neuropathologic features and PART because both are defined by neuritic plaque and Braak staging. We evaluated the association between impaired hearing and ADNC (low ADNC not PART, intermediate ADNC, high ADNC), PART, and the APOE ε4 allele as primary predictors. This analysis focused on those who were cognitively normal at baseline. Next, we evaluated whether the development of impaired hearing over follow-up was associated with any particular neuropathologies as a clue as to whether impaired hearing could be an emerging symptom of dementia-related pathology. This secondary analysis was limited to people with cognitive impairment at baseline who did not have impaired hearing at their first ADC visit. Finally, we evaluated the association between impaired hearing and FTLD subtypes (tauopathy and nontauopathy) by adding 704 participants with FTLD to the study sample (n = 2,755). Because of the small numbers of participants with FTLD without dementia, we did not stratify by baseline cognitive status. All secondary models were adjusted for age at death, sex, non-White race, education, months between last visit and death, follow-up time, and ADC.
We report 95% CIs, and all tests were 2 sided with α = 0.05. Analyses were conducted with R (version 3.2.1, R Foundation for Statistical Computing, Vienna, Austria).
Data availability
Data maintained by NACC are publicly available to researchers by request: alz.washington.edu/WEB/researcher_home.html.
Results
Participant characteristics
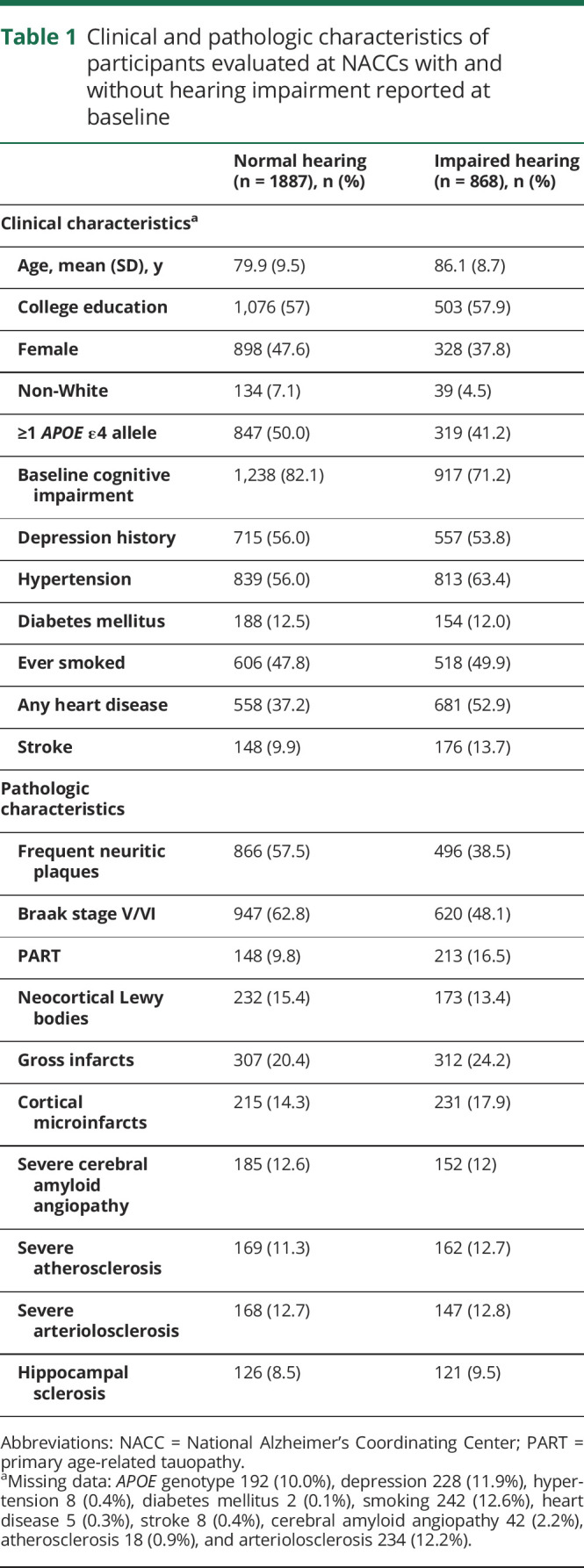
Participants (n = 2,755) were followed up for an average of 4.4 years (SD 2.8 years, range 0–12 years) before death. Impaired hearing was common at baseline (32%), and 22% reported hearing aid use (68% of those with impaired hearing). Impaired hearing increased exponentially with age at death and was higher in men (figure 1). Twenty two percent of participants without impaired hearing at baseline developed impaired hearing at a follow-up visit. Table 1 shows participant characteristics by baseline hearing impairment status. Participants with impaired hearing at baseline were on average less likely to be female, to have cognitive impairment, and to carry an APOE ε4 allele. They were on average older and more likely to have heart disease, stroke, and hypertension. ADNC and Lewy body disease were less common in those with impaired hearing, but PART and gross or microscopic infarcts were more common in those with impaired hearing (table 1).
Figure 1. Prevalence of impaired hearing by age at death.

Table 1.
Clinical and pathologic characteristics of participants evaluated at NACCs with and without hearing impairment reported at baseline
Hearing impairment and neuropathologies in participants cognitively normal at baseline
There were 580 participants with normal cognition at baseline (the time of hearing reporting), among whom 37.6% had impaired hearing. One hundred ninety-eight participants were seen multiple times; those with impaired hearing at baseline were slightly more likely to become cognitively impaired by the last visit compared to those without impaired hearing (55% vs 45%).
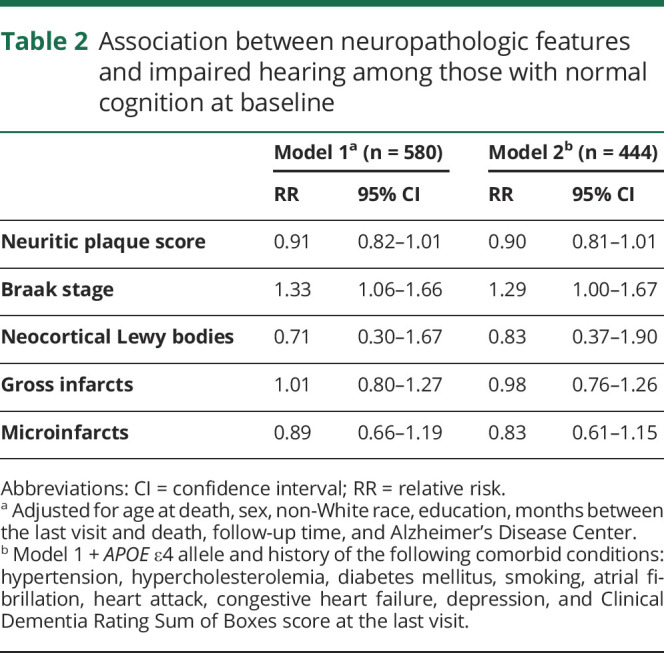
Table 2 shows the association between impaired hearing and common neuropathologies. In models adjusted for demographics, impaired hearing at baseline was associated with higher Braak stage: participants were 1.33 times more likely to have impaired hearing per increase in Braak stage (operationalized as 0, I/II, III/IV, V/VI) (95% CI 1.06–1.66; table 2). There was a trend toward an inverse association with neuritic plaque density (RR 0.91, 95% CI 0.82–1.01). Neocortical Lewy bodies, gross infarcts, and microinfarcts were not associated with impaired hearing (all p > 0.05; table 2). Additional adjustment for APOE ε4 allele and health conditions did not substantially change estimates but reduced the sample sizes (table 2).
Table 2.
Association between neuropathologic features and impaired hearing among those with normal cognition at baseline
Estimates were also similar under sensitivity analyses when we included weighting to account for potential selection bias, in models with hearing aid use as the outcome, and in those restricted to autopsies since 2014, but estimates were less precise, and all CIs were consistent with the null (data not shown).
Hearing impairment, ADNC, and PART in participants cognitively normal at baseline
In a separate model, we further examined the link between hearing impairment and the continuum of AD neuropathologic features and PART diagnostic categories (table 3). Impaired hearing was more common in those with high ADNC (RR 1.49, 95% CI 1.15–1.94) and PART (RR 1.32, 95% CI 1.06–1.66) compared to those with low ADNC and not PART. There was a trend toward an inverse relationship with APOE ε4 allele (RR 0.85, 95% CI 0.65–1.10).
Table 3.
Associations between impaired hearing and ADNC and PART in those with normal cognition at baseline (n = 580)

Because trends seemed to be driven by association with Braak stage, we also plotted the prevalence of impaired hearing by Braak stage (figure 2, which was higher for each increased stage from Braak 0 to V; figure 2). This trend did not differ between those with and those without any neuritic plaques, so results were not stratified.
Figure 2. Prevalence of impaired hearing increases by Braak stage in participants with normal baseline cognition (n = 545).
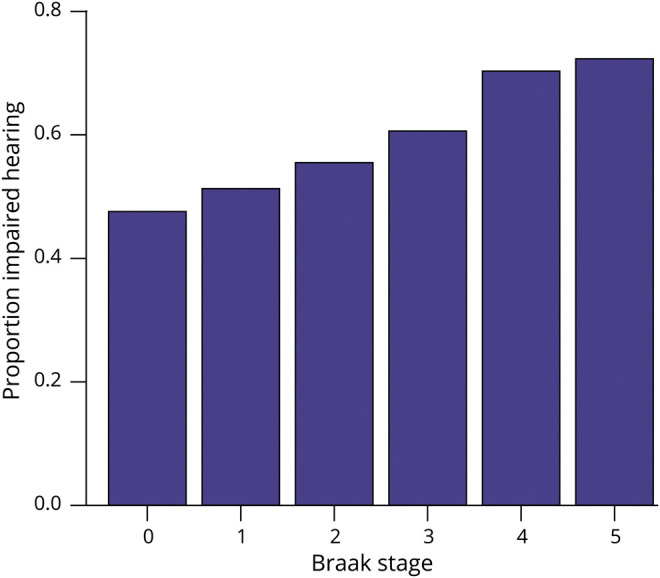
Only Braak stages 0 to V are shown because there were few participants with Braak stage VI (n = 35).
Hearing impairment and neuropathologies in participants cognitively impaired at baseline
There were 2,175 participants with cognitive impairment at baseline. Table 4 shows the association between impaired hearing and common neuropathologies in participants with cognitive impairment at baseline. In adjusted models, microinfarcts were associated with 1.18-higher likelihood of impaired hearing (95% CI 1.00–1.39; table 4). Neuritic plaques were inversely associated with impaired hearing (RR 0.91 per increase in score, 95% CI 0.85–0.99). Impaired hearing was not associated with Braak stage, neocortical Lewy bodies, or gross infarcts (all p > 0.05; table 4). Additional adjustment for APOE ε4 allele and health conditions did not substantially change estimates but reduced the sample sizes (table 4). We next examined associations with development of impaired hearing; among people who did not have impaired hearing at their first ADC visit, neocortical Lewy bodies (but not other pathologies) were associated with impaired hearing developed after baseline (RR 1.26, 95% CI 1.02–1.55).
Table 4.
Association between neuropathologic features and impaired hearing among those with cognitive impairment at baseline

Estimates and overall findings were similar in models with hearing aid use as the outcome. Estimates were also similar under sensitivity analyses when we included weighting to account for potential selection bias and in those restricted to autopsies since 2014, but they were less precise, and all CIs were consistent with the null (data not shown).
Hearing impairment and FTLDs
There were 704 participants with FTLDs; 415 had FTLD-tau, 247 had nontauopathy FTLD, and 41 had both tau and nontau inclusions. In a secondary analysis, we added these 704 participants to the 2,755 participants included in the above analyses and examined the association of impaired hearing at baseline with FLTD subtype with adjustment for age at death, sex, non-White race, education, months between the last visit and death, follow-up time, and ADC. Impaired hearing at baseline was ≈20% less likely in FTLD-tau (RR 0.81, 95% CI 0.68–0.96). There was no association between nontauopathy FTLD and impaired hearing (RR 1.04, 95% CI 0.85–1.27).
Discussion
We evaluated the association between clinician-reported impaired hearing and common neuropathologies of aging among older adults evaluated at ADCs. We also examined neuropathologic profiles associated with hearing impairment across stages of cognitive impairment. We found a positive association with higher Braak stage (tau neurofibrillary degeneration) in those with normal cognition at baseline. When considering the ADNC continuum and diagnostic categories, we found that impaired hearing was associated with both high ADNC and PART. In those with cognitive impairment at baseline, there was a positive association with microinfarcts but an inverse association with neuritic plaques (amyloid). Neocortical Lewy bodies were associated with development of hearing impairment over follow-up. We did not find an association between impaired hearing and gross infarcts. In a secondary analysis, FTLD-tau was inversely associated with impaired hearing; however, there was no association between nontauopathy FTLD and impaired hearing. Estimates in sensitivity analyses were generally similar to primary findings, but CIs were wider. Together, these results suggest that reported hearing impairment before dementia onset may be associated with neurofibrillary degeneration related to aging and AD. Hearing impairment later in the clinical disease course may be more strongly associated with other pathologies such as neocortical Lewy bodies and microinfarcts; however, future replication will be needed in studies using biomarkers, objective measures of hearing loss, and population-based samples.
Our finding of an association between impaired hearing (measured before cognitive impairment) and higher Braak stage adds to the literature supporting a biological link of functional hearing impairment before the onset of cognitive impairment with underlying dementia neuropathologies. Prior studies have found associations between hearing loss and future risk of dementia.1–6 However, imaging studies focusing on hearing loss (with audiometric tests for peripheral hearing) have produced mixed findings. Some find decreased whole-brain volumes, reduced temporal lobe or auditory cortex volumes,16,17 or reduced hippocampal volume.18 Other studies have found no association between hearing loss and brain volumes.40 Animal studies have found that noise-induced (peripheral) hearing loss is associated with increased neurodegeneration in the hippocampus, decreased neurogenesis, and poor memory function.41,42 Our finding of an association between clinician-rated hearing impairment before dementia onset and higher neurofibrillary tangles does not establish the direction or causality of the relationship but is consistent with a link between hearing impairment and hippocampal and temporal lobe neurodegeneration.
Clinical judgment of hearing impairment in this study focuses on functional hearing abilities (e.g., hearing radio or conversation) and could encompass deficits in peripheral or central auditory processing,7,8 which further adds to the difficulty of inferring causality. Peripheral hearing loss, in particular, may increase cognitive demands or lead to social isolation, which in turn may lead to neurodegeneration.9,10 Dementia-related neurodegeneration can affect the central auditory pathways, and thus, we may be capturing effects due to AD or PART that are independent of peripheral hearing loss.10 Central auditory processing dysfunction, in particular, affects functional hearing such as speech in noise and is thought to be an indicator of preclinical or early AD.8,11 However, central auditory dysfunction is difficult to tease apart from cognitive function,8,11 and studies in those without cognitive impairment are limited and have focused on specific tests for auditory processing.43 Some studies question a biological link between hearing and cognition; many cognitive tests rely on hearing, and poor hearing may lead to more errors in hearing-based cognitive tests.13,14 However, if measurement error were the only explanation for an association, we would expect to find consistent inverse associations between hearing impairment and neuropathologies. Regardless of the mechanism, our findings suggest that clinician-reported impaired hearing may be a preclinical indicator for underlying neurofibrillary pathology. Future studies with biomarkers and objective measures of peripheral hearing and central auditory processing will be needed to establish temporal order and causal mechanisms. Our findings of an inverse association with FTLD-tauopathy suggest that the association between impaired hearing and Braak stage is not related to tauopathy in general but rather neurofibrillary degeneration seen in AD and aging (PART). Studies to examine the distribution of neurofibrillary degeneration within brain regions and other tauopathies could also help better tease apart these relationships.
This study provides a comparison of pathologies associated with hearing impairment before the onset of cognitive impairment and in those with existing cognitive impairment. Difficulty hearing is often reported in patients with AD,44 and neurodegeneration in AD affects anatomic structures, including the auditory pathways: neuritic plaques and tangles have been found in auditory association cortex and subcortical auditory pathways, which include the medial temporal lobe.45,46 However, these prior studies were small and had no comparison groups.47 Unexpectedly, we found a trend toward an inverse association with amyloid plaques in this sample, particularly among those with cognitive impairment at baseline. This inverse association in our study may have resulted because the APOE ε4 allele tended to be less common in those with hearing impairment, as has been found in population-based studies.48 It is unclear whether this is due to a biological mechanism or survival bias. Participants with cognitive and hearing impairment tended to live to older ages than those with the APOE ε4 allele. Alternatively, dementia may affect accuracy or missingness of our hearing measure, or those with hearing impairment and dementia may be more likely to drop out of ADCs; if this was the case, we may have underestimated the associations between pathologies and hearing impairment in those with dementia. The inverse association with amyloid plaques was weaker (and not significant) in those with normal cognition at baseline; those with high ADNC and PART were more likely to have impaired hearing. The presence of neocortical Lewy bodies was associated with the development of impaired hearing in many of our analyses, particularly in those analyses focused on participants with cognitive impairment and the development of hearing impairment after baseline. Patients with dementia with Lewy bodies often have auditory hallucinations suggesting involvement of the auditory cortex, which may also affect functional hearing abilities.34
VBI (gross or microscopic infarcts) was also more prevalent in older ages, and cortical microinfarcts were more common in those with impaired hearing and dementia. We found an association between cortical microinfarcts and hearing impairment in those with cognitive impairment. Microinfarcts are microscopic infarcts, are often distributed widely49 throughout brain regions, and are strongly associated with dementia and cognitive decline in multiple domains.50,51 Thus, it is possible that auditory regions are also affected. Vascular disease may contribute to both hearing loss52,53 and microinfarct development49; however, there was no association between VBI and impaired hearing in those without dementia at baseline. We may have had limited power to detect an association because the severity or burden of infarcts was not collected in a standard way until 2014. Alternatively, this finding may be due to chance. Future work is needed to confirm these associations in other settings.
Our study has a number of limitations. US ADCs, which contribute to NACC, follow up samples that differ from a broader population, mostly comprising White older adults with relatively high socioeconomic status and high risk for clinical AD. In addition, those in the autopsy sample tended to have severe dementia by the last visit. Neuropathologic assessments may not reflect burden of pathology when hearing impairment was reported, although neurodegenerative pathologies begin accumulating decades before symptom onset.54,55 We did not have objective audiometric data or separate peripheral and central hearing measurements. Some participants’ hearing abilities may have been misclassified. We also did not have information on type of hearing loss, so some participants with genetic or congenital hearing loss may have been included; however, we saw a strong association of the impaired hearing measure with older age. Those with severe cognitive impairment were more likely to have missing data and were not rated as having impaired hearing at baseline as would otherwise be expected. This suggests that our findings may underestimate the magnitude of the association between hearing impairment and neuropathologies. Despite these concerns, the NACC database represents one of the world's largest and highest-quality multicenter databases, with both detailed clinical and pathologic information. The database has been extensively audited. In addition, we conducted additional secondary and sensitivity analyses to inform the generalizability of our results and potential for selection bias.
Even with the important limitations of our measurements and study sample, this study provides intriguing preliminary evidence that brain pathologies are associated with hearing impairment in a large autopsy sample. We found that impaired hearing before the onset of cognitive impairment was associated with increased neurofibrillary tangles both in AD and in PART. This association was independent of neuritic plaques, which tended to be less frequent in those with hearing impairment, suggesting an association of pathologic neuronal tau and impaired hearing that is independent of β-amyloid. We saw an inverse association, however, with FTLD-tau. Future studies evaluating other tauopathies such chronic traumatic encephalopathy and aging-related tau astrogliopathy may be informative. Microinfarcts and neocortical Lewy bodies were associated with hearing impairment after the onset of cognitive impairment. Together, these findings are consistent with the hypotheses that hearing impairment may affect brain atrophy and neuropathologic burden or that underlying pathologies may impair functional hearing abilities even before dementia onset. Future studies with biomarker and audiometric information are needed to establish the causal direction of these associations and to verify these findings with objective measures of hearing impairment and in more diverse study populations.
Acknowledgment
Acknowledgments
They authors are deeply grateful to all of the study participants, clinicians, and other workers at the ADCs and NACC who made this research possible. They also thank the NACC staff for help obtaining data. Preliminary versions of this work were presented at the Alzheimer's Disease and Parkinson's Disease Conference in March 2019 and the Alzheimer's Association International Conference in July 2019.
Glossary
- AD
Alzheimer disease
- ADC
Alzheimer's Disease Center
- ADNC
AD neuropathologic change
- CDR-SB
Clinical Dementia Rating Dementia Staging Instrument Sum of Boxes
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- CI
confidence interval
- FTLD
frontotemporal lobar degeneration
- NACC
National Alzheimer's Coordinating Center
- PART
primary age-related tauopathy
- RR
relative risk
- TDP-43
TAR DNA-binding protein 43
- UDS
Uniform Data Set
- VBI
vascular brain injury
Appendix. Authors
Footnotes
Editorial, page 511
Study funding
This research was also supported by National Institute on Aging grants T32AG049663, K01AG062722, K01AG063895, and K24AG031155, the Alzheimer's Association AARF-18-565846, the Nancy and Buster Alvord Endowment, and the University of California, San Francisco Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging, P30 AG044281. The NACC database is funded by National Institute on Aging/NIH grant U01 AG016976. NACC data are contributed by the National Institute on Aging–funded ADCs: P30 AG019610 (principal investigator [PI] Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG062428-01 (PI James Leverenz, MD) P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P30 AG062421-01 (PI Bradley Hyman, MD, PhD), P30 AG062422-01 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P30 AG062429-01 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P30 AG062715-01 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc 2004;52:1996–2002. [DOI] [PubMed] [Google Scholar]
- 2.Moore DR, Edmondson-Jones M, Dawes P, et al. Relation between speech-in-noise threshold, hearing loss and cognition from 40–69 years of age. PLoS One 2014;9:e107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer ME, Cruickshanks KJ, Schubert CR, et al. Age-related sensory impairments and risk of cognitive impairment. J Am Geriatr Soc 2016;64:1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies HR, Cadar D, Herbert A, Orrell M, Steptoe A. Hearing impairment and incident dementia: findings from the English Longitudinal Study of Ageing. J Am Geriatr Soc 2017;65:2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deal JA, Betz J, Yaffe K, et al. Hearing impairment and incident dementia and cognitive decline in older adults: the Health ABC Study. J Gerontol A Biol Sci Med Sci 2017;72:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2018;144:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panza F, Solfrizzi V, Logroscino G. Age-related hearing impairment-a risk factor and frailty marker for dementia and AD. Nat Rev Neurol 2015;11:166–175. [DOI] [PubMed] [Google Scholar]
- 8.Sardone R, Battista P, Panza F, et al. The age-related central auditory processing disorder: silent impairment of the cognitive ear. Front Neurosci 2019;13:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis A, McMahon CM, Pichora-Fuller KM, et al. Aging and hearing health: the life-course approach. Gerontologist 2016;56(suppl 2):S256–S267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin FR, Albert M. Hearing loss and dementia: who's listening? Aging Ment Health 2014;18:671–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swords GM, Nguyen LT, Mudar RA, Llano DA. Auditory system dysfunction in Alzheimer disease and its prodromal states: a review. Ageing Res Rev 2018;44:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panza F, Solfrizzi V, Seripa D, et al. Age-related hearing impairment and frailty in Alzheimer's disease: interconnected associations and mechanisms. Front Aging Neurosci 2015;7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorgensen LE, Palmer CV, Pratt S, Erickson KI, Moncrieff D. The effect of decreased audibility on MMSE performance: a measure commonly used for diagnosing dementia. J Am Acad Audiol 2016;27:311–323. [DOI] [PubMed] [Google Scholar]
- 14.Lim MYL, Loo JHY. Screening an elderly hearing impaired population for mild cognitive impairment using Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). Int J Geriatr Psychiatry 2018;33:972–979. [DOI] [PubMed] [Google Scholar]
- 15.van Boxtel MP, van Beijsterveldt CE, Houx PJ, Anteunis LJ, Metsemakers JF, Jolles J. Mild hearing impairment can reduce verbal memory performance in a healthy adult population. J Clin Exp Neuropsychol 2000;22:147–154. [DOI] [PubMed] [Google Scholar]
- 16.Lin FR, Ferrucci L, An Y, et al. Association of hearing impairment with brain volume changes in older adults. NeuroImage 2014;90:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rigters SC, Bos D, Metselaar M, et al. Hearing impairment is associated with smaller brain volume in aging. Front Aging Neurosci 2017;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchida Y, Nishita Y, Kato T, et al. Smaller hippocampal volume and degraded peripheral hearing among Japanese community dwellers. Front Aging Neurosci 2018;10:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord 2007;21:249–258. [DOI] [PubMed] [Google Scholar]
- 20.Besser L, Kukull W, Knopman DS, et al. Version 3 of the National Alzheimer's Coordinating Center's Uniform Data Set. Alzheimer Dis Assoc Disord 2018;32:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer's Coordinating Center (NACC) database: an Alzheimer disease database. Alzheimer Dis Assoc Disord 2004;18:270–277. [PubMed] [Google Scholar]
- 22.Besser LM, Kukull WA, Teylan MA, et al. The revised National Alzheimer's Coordinating Center's neuropathology form: available data and new analyses. J Neuropathol Exp Neurol 2018;77:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonnen JA, Santa Cruz K, Hemmy LS, et al. Ecology of the aging human brain. Arch Neurol 2011;68:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 25.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009;23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weintraub S, Besser L, Dodge HH, et al. Version 3 of the Alzheimer Disease Centers' neuropsychological test battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord 2018;32:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 28.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol (Berl) 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thal DR, Rüb U, Orantes M, Braak H. Phases of a beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–1800. [DOI] [PubMed] [Google Scholar]
- 32.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol (Berl) 2014;128:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 35.Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med 2011;171:1851–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol 2014;43:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiol 2004;15:615–625. [DOI] [PubMed] [Google Scholar]
- 38.Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York: Wiley; 1987. [Google Scholar]
- 39.Bradley E. An Introduction to the Bootstrap. New York: Chapman & Hall; 1994. [Google Scholar]
- 40.Profant O, Škoch A, Balogová Z, Tintěra J, Hlinka J, Syka J. Diffusion tensor imaging and MR morphometry of the central auditory pathway and auditory cortex in aging. Neuroscience 2014;260:87–97. [DOI] [PubMed] [Google Scholar]
- 41.Park SY, Kim MJ, Sikandaner H, Kim DK, Yeo SW, Park SN. A causal relationship between hearing loss and cognitive impairment. Acta Otolaryngol (Stockh) 2016;136:480–483. [DOI] [PubMed] [Google Scholar]
- 42.Liu L, Shen P, He T, et al. Noise induced hearing loss impairs spatial learning/memory and hippocampal neurogenesis in mice. Sci Rep 2016;6:20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gates GA, Anderson ML, McCurry SM, Feeney MP, Larson EB. Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol Head Neck Surg 2011;137:390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gold M, Lightfoot LA, Hnath-Chisolm T. Hearing loss in a memory disorders clinic: a specially vulnerable population. Arch Neurol 1996;53:922–928. [DOI] [PubMed] [Google Scholar]
- 45.Lewis DA, Campbell MJ, Terry RD, Morrison JH. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer's disease: a quantitative study of visual and auditory cortices. J Neurosci Off J Soc Neurosci 1987;7:1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer's disease. Neurology 1993;43:779–785. [DOI] [PubMed] [Google Scholar]
- 47.Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc 2015;11:70–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mener DJ, Betz J, Yaffe K, et al. Apolipoprotein E allele and hearing thresholds in older adults. Am J Alzheimers Dis Other Demen 2016;31:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol 2012;11:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study autopsy study. Ann Neurol 2011;70:774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenowitz WD, Hubbard RA, Keene CD, et al. Mixed neuropathologies and associations with domain-specific cognitive decline. Neurology 2017;89:1773–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckert MA, Kuchinsky SE, Vaden KI, Cute SL, Spampinato MV, Dubno JR. White matter hyperintensities predict low frequency hearing in older adults. J Assoc Res Otolaryngol 2013;14:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer ME, Schubert CR, Nondahl DM, et al. Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis 2015;238:344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data maintained by NACC are publicly available to researchers by request: alz.washington.edu/WEB/researcher_home.html.


