Abstract
Objective
Since the strongest risk factor for sudden unexpected death in epilepsy (SUDEP) is frequent bilateral tonic-clonic seizures (BTCS), our aim was to determine whether postictal hypoperfusion in brainstem respiratory centers (BRCs) is more common following tonic-clonic seizures.
Methods
We studied 21 patients with focal epilepsies who underwent perfusion imaging with arterial spin labeling MRI. Subtraction maps of cerebral blood flow were obtained from the postictal and baseline scans. We identified 6 regions of interest in the brainstem that contain key BRCs. Patients were considered to have postictal BRC hypoperfusion if any of the 6 regions of interest were significantly hypoperfused.
Results
All 6 patients who experienced BTCS during the study had significant clusters of postictal hypoperfusion in BRCs compared to 7 who had focal impaired awareness seizures (7/15). The association between seizure type studied and the presence of BRC hypoperfusion was significant. Duration of epilepsy and frequency of BTCS were not associated with postictal brainstem hypoperfusion despite also being associated with risk for SUDEP.
Conclusion
Postictal hypoperfusion in brainstem respiratory centers occurs more often following BTCS than other seizure types, providing a possible explanation for the increased risk of SUDEP in patients who regularly experience BTCS.
Sudden unexpected death in epilepsy (SUDEP) is a considerable cause of epilepsy-related direct deaths and premature death in people with refractory epilepsy who attend specialized epilepsy centres.1,2 The strongest risk factor for SUDEP is frequent bilateral tonic-clonic seizures (BTCSs), where SUDEP has been observed within minutes of seizure termination.3,4 In MORTEMUS (the Mortality in Epilepsy Monitoring Unit Study), all 11 SUDEP deaths occurred 2.5–16 minutes after a BTCS. Terminal apnea, occurring 2.8–8 minutes before terminal cardiac arrest, was identified as the possible cause of death.4 This led to the hypothesis that SUDEP, a postictal phenomenon, may result from disruption of brainstem function, in particular, brainstem respiratory centers (BRCs).
A number of mechanisms have been proposed for SUDEP, including neurogenic pulmonary edema, central apnea, cardiac arrhythmias, and postictal asphyxiation.5 Increasing evidence suggests respiratory dysfunction as the major cause.6–9 Respiratory control in the human brainstem is mediated by a pontomedullary network of neurons. Physiologically, they are divided into 3 respiratory groups: the dorsal respiratory group (DRG), located in the dorsal medulla, is composed of cells in the nucleus solitarius; the ventral respiratory group (VRG), located in the ventral medulla, includes the nucleus ambiguus, the nucleus retroambiguus, and the pre-Bötzinger complex; and the pontine respiratory group (PRG), located in the dorsal pons, includes the pneumotaxic and apneustic centers, which are related to the parabrachial nuclei.
Although the dysfunction of respiratory mechanisms is hypothesized to be involved in SUDEP, there is limited evidence for BRC hypoxia.10–12 In humans, we have shown significant postictal hypoperfusion in the seizure onset zone (within 90 minutes of seizure termination) using postictal arterial spin labeling (ASL) MRI13 and CT perfusion.14 These 2 studies validate the usage of perfusion studies in detecting postictal hypoperfusion in humans. We therefore hypothesized that postictal hypoperfusion in BRCs can be detected and would more likely be seen following a BTCS.
Methods
Standard protocol approvals, registrations, and patient consents
This study was approved by the Conjoint Health Research Ethics Board, University of Calgary. Written informed consent was obtained from all participants.
Participants
Twenty-five adult patients with refractory focal epilepsy provided informed consent and were prospectively recruited for the study between January 2014 and March 2016. These were the same patients used for our previous ASL study that investigated postictal hypoperfusion at the presumed seizure onset zone.13 All patients underwent scalp video-EEG monitoring at the Foothills Medical Centre Seizure Monitoring Unit as part of their presurgical workup. Patients were weaned off anticonvulsant medications in order to capture habitual seizures. All patients underwent our center's standard epilepsy protocol structural 3T MRI scan. Most patients also underwent ictal SPECT, PET, neuropsychological testing, and language functional MRI. Demographic information was collected including age, sex, age at seizure onset, habitual seizure types, seizure frequency, and duration of epilepsy.
Image acquisition
Postictal perfusion scans were obtained within 90 minutes of a habitual seizure captured by video-EEG monitoring. EEG electrodes were removed, and the patient was transferred to the magnetic resonance (MR) scanner, accompanied by a physician. Following a minimum of 24 hours of seizure freedom, a subsequent MR perfusion scan was performed to obtain a baseline, interictal cerebral blood flow (CBF) measurement.
ASL MR images were acquired using a 3T GE Discovery MRI 750 system (GE, Waukesha, WI) with a 12-channel phase array head receive coil. A 3D fast-spin-echo pseudo-continuous arterial spin labeling (pCASL) with background suppression sequence was used. The pCASL sequence had the following parameters: scan duration = 5:50 minutes, repetition time = 5,513 ms, echo time = 4.7 ms, in-plane resolution = 1.875 × 1.875 mm, slice thickness = 5 mm, 28 slices, postlabel delay = 2,525 ms, number of excitations = 4, and spiral acquisition with 1,024 points and 8 arms. Quantitative CBF maps were generated in CBF units (mL/100 g/min) using the formula:
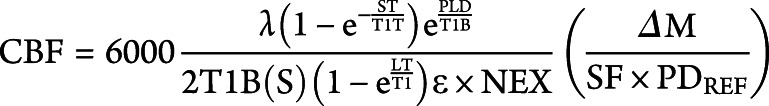 |
where T1B and T1T represent T1 values (1.6 seconds at 3T) for blood and tissue respectively, ε (efficiency) is set to 0.80 × 0.75, λ (partial coefficient) is set to 0.9, ΔM is the difference between tag and no tag images, PDREF is the reference proton density images, NEX is the number of excitations, SF is the scaling factor of 45, and PLD is the postlabeling delay.13 A high-resolution T1-weighted anatomical scan was also collected in the same scanning session (postictal or baseline) for each patient. The parameters used were repetition time 9.4 ms, echo time min full, in-plane resolution 0.5 × 0.5 mm, and slice thickness 1.3 mm. Perfusion maps were overlaid onto these images in order to localize blood flow changes within the brainstem.
Data analysis
Brain extraction was performed on baseline and postictal quantitative CBF maps and high-resolution T1-weighted images using the brain extraction tool (BET) from FMRIB Software Library (FSL-BET v2.1).15 Both CBF maps were subsequently registered onto each patient's high-resolution baseline anatomical scan using an affine transformation (12 degrees of freedom) from the FSL linear image registration tool (FLIRT v5.5).15 The data were spatially smoothed using a Gaussian Kernel filter (6-mm full-width half-maximum). Next, a subtraction CBF map (baseline − postictal) was generated for each patient and overlaid onto their own baseline anatomical T1-weighted image to identify brainstem areas where, following a seizure, perfusion was decreased relative to their own baseline.
Evaluation of brainstem CBF changes and SUDEP risk factors
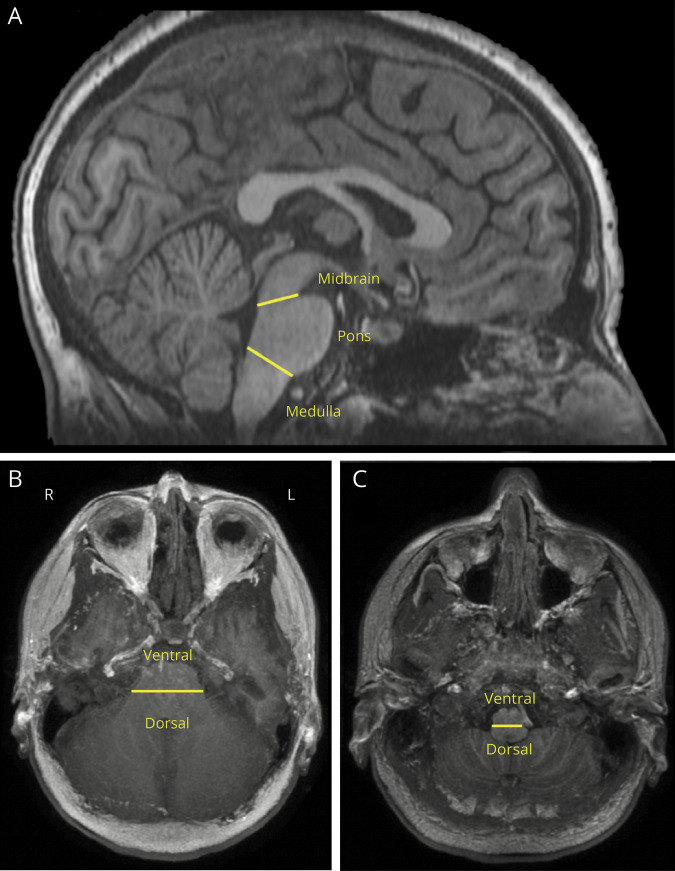
Six brainstem regions were identified as regions of interest (ROIs) for visual analysis of clusters of hypoperfusion: left and right dorsal pons, left and right dorsal medulla, and left and right ventral medulla (figure 1). These ROIs contain the key brainstem respiratory groups: the PRG, the DRG, and the VRG. The seizure immediately preceding the postictal perfusion study is referred to as the index seizure. Patients were separated into 2 groups based on the type of their index seizure: BTCS or focal impaired awareness seizure (FIAS).
Figure 1. T1-weighted image showing brainstem divisions used to define brainstem respiratory center regions of interest (dorsal pons, dorsal medulla, ventral medulla).
(A) Sagittal view of the brainstem with divisions between the midbrain, pons, and medulla denoted by the yellow lines. (B) Axial view of the pons with the division between the ventral and dorsal portions denoted by the yellow line. (C) Axial view of the medulla with the division between the ventral and dorsal portions denoted by the yellow line.
Brainstem perfusion changes were visualized on the subtraction ASL images using FSLView (3.2.0) and 3D ROIs were identified by a single individual (J.S.P.) in a blinded fashion. A minimum decrease of 15 CBF units was used as a threshold to identify hypoperfused regions. This threshold is supported by studies in rodents showing that hippocampal seizures are followed by a minimum of 25%–30% reduction of CBF16 and that normal gray matter CBF in humans, as measured using ASL MRI, is 45–50 CBF units.17 Additional cluster filtering (minimum of 400 voxels) was applied, as this was found to retain physiologic clusters of hypoperfusion while reducing the number of artefactual clusters caused by vessel artifacts and small errors in registration.14 Patients were determined to have BRC hypoperfusion if there was at least one cluster of hypoperfusion in any of the 6 ROIs after application of the CBF decrease threshold and cluster filtering.
Statistical analysis
Relative risk and the corresponding 95% confidence interval (CI) was calculated in order to examine whether there was an association between the type of index seizure (BTCS or FIAS) and the distribution of patients who did and did not have hypoperfusion in brainstem ROIs. Associations between BRC hypoperfusion and other factors associated with SUDEP were also tested. Relative risk and the corresponding 95% CI was calculated to investigate the association of hypoperfusion with categorical predictor variables, while the odds ratio (OR) and corresponding 95% CI was calculated for continuous predictor variables. Seizure duration and MRI scan latency were compared between FIAS and FBTCS groups as well as between the hypoperfused and nonhypoperfused patients with FIAS in order to preclude these 2 factors as influences on the presence or absence of hypoperfusion. Ninety-five percent CIs were calculated for each group and differences were considered to be statistically significant if the CIs did not overlap. Statistical analyses were performed using IBM SPSS Statistics 26.
Data availability
Raw and processed ASL MRI data are available on request.
Results
Of the 25 participants who underwent ASL MRI, 1 patient did not receive a baseline ASL scan, 1 did not receive a postictal scan, and the data for 2 patients had significant motion artifact, leaving 21 patients to be included in the study. Clinical and demographic features are summarized in table 1. There were 10 men (48%), mean age was 33.95 ± 10.64 years (range 20–58), mean age at onset of epilepsy was 16.19 ± 11.16 years (range 1–43), and mean duration of epilepsy was 17.76 ± 13.08 years (range 1–57). The mean time to postictal ASL image acquisition was 65.76 ± 17.22 minutes (range 45–116). The mean index seizure duration was 82.14 ± 40.34 seconds (range 26–166).
Table 1.
Patient demographics, clinical features, and structural abnormalities
Of the 21 patients studied, 13 (61%) exhibited hypoperfusion in at least one of the brainstem ROIs (table 2). MRI scan latency was similar between patients who exhibited hypoperfusion (66.92 ± 18.05 minutes) and those who did not (63.88 ± 16.79 minutes). Hypoperfusion was observed in all 6 brainstem ROIs and was observed in both BTCS and FIAS patient groups (figures 2 and 3). The number of BRC ROIs exhibiting hypoperfusion ranged from 1 to 5 (table 2). Three of the patients in the BTCS group experienced postictal EEG suppression (44, 16, and 36 seconds for patients 15, 17, and 21, respectively). The other 3 patients in the BTCS group experienced postictal EEG slowing but not suppression. There was no difference in duration of epilepsy (χ2[1] = 0.097, OR 0.99, 95% CI 0.92–1.06). Six patients had BTCS and 15 patients had FIAS as their index seizure. All 6 patients who had an index BTCS and 7/15 (47%) with an index FIAS exhibited postictal hypoperfusion in at least 1 BRC and this difference was statistically significant (RR 2.14, 95% CI 1.25–3.68). Eighteen out of 21 patients (86%) had history of BTCS, of whom 11 (61%) had postictal hypoperfusion in BRC. Six of the patients (6/7; 86%) with at least annual BTCS had BRC postictal hypoperfusion, whereas 5 (5/11; 45%) of those with less than annual BTCS had BRC postictal hypoperfusion. Although the proportion is almost twice as high in those with at least annual BTCS, this difference was not statistically significant (RR 1.89, 95% CI 0.92–3.85).
Table 2.
Index seizure characteristics and subtraction arterial spin labeling results showing the number of hypoperfused brainstem respiratory centers
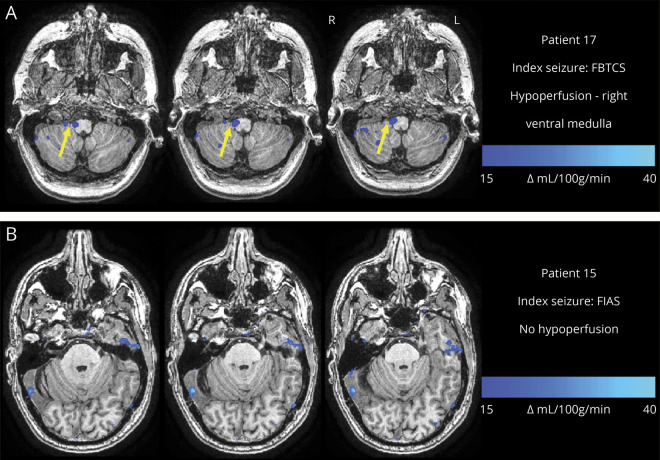
Figure 2. Examples of different patterns of postictal hypoperfusion in brainstem respiratory centers.
(A) Image from a 25-year-old woman with monthly focal to bilateral tonic-clonic seizures (FBTCS) who had a FBTCS as the index seizure. Subtraction cerebral blood flow (CBF) map (baseline − postictal) superimposed onto the patient's T1-weighted anatomical image shows hypoperfusion > Δ15 CBF units in the right ventral medulla (yellow arrow) across 3 axial slices. (B) Image from a 21-year-old woman who has infrequent FBTCS (<1 per year) and had a FIAS as the index seizure. Subtraction CBF map (baseline − postictal) superimposed onto the patient's T1-weighted anatomical image showed no significant hypoperfusion in any of the brainstem respiratory center regions of interest. FIAS = focal impaired awareness seizure.
Figure 3. Example of postictal hypoperfusion in a pontine region of interest.
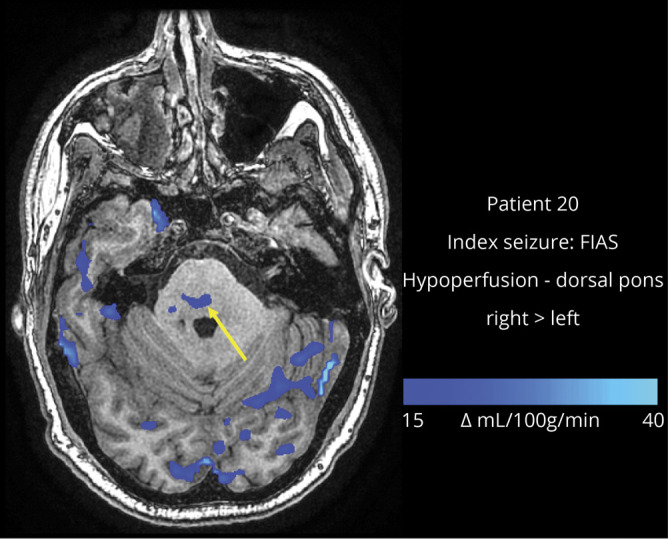
Image from a 26-year-old man who has fewer than 1 focal to bilateral tonic-clonic seizure per year and had a focal impaired awareness seizure (FIAS) as the index seizure. Subtraction cerebral blood flow (CBF) map (baseline − postictal) superimposed onto the patient's T1-weighted anatomical image shows hypoperfusion > Δ15 CBF units in the dorsal pons (arrow).
There was no significant difference in MRI scan latency between the BTCS (66.67 ± 12.56 minutes) and FIAS (65.40 ± 19.15 minutes) groups. There was no significant difference in index seizure duration between the BTCS (112.00 ± 47.29 seconds) and FIAS (70.20 ± 31.48 seconds) groups. MRI scan latency and index seizure duration also did not significantly differ between the hypoperfused and nonhypoperfused patients who had experienced a FIAS. For the group that experienced BRC hypoperfusion, the mean scan latency was 67.14 ± 22.80 minutes and the mean seizure duration was 72.86 ± 23.32 seconds. For the group that did not experience hypoperfusion, the mean scan latency was 63.88 ± 16.79 minutes and the mean seizure duration was 67.88 ± 38.77 seconds.
Discussion
Our study demonstrated the existence of postictal BRC hypoperfusion and showed that BTCS is an important risk factor for this hypoperfusion. One of the earliest theories linking the postictal state to cerebral hypoperfusion comes from Todd's paresis, a phenomenon that mimics ischemic stroke. Case reports have subsequently shown that regional hypoperfusion does occur in Todd's paresis.18,19 However, only recently was a prolonged period (minimum of 60 minutes) of severe postictal hypoxia (pO2 < 10 mm Hg) demonstrated in the rodent hippocampus following seizure termination.16 This was found to be the result of local arteriolar vasoconstriction mediated by cyclooxygenase-2 (COX-2) and L-type calcium channels leading to postictal hypoperfusion.16 In rodents, it was observed that pretreatment with L-type calcium channel blockers or COX-2 antagonists, but not COX-1 antagonists, could mitigate postictal forelimb weakness and memory deficits. These findings suggest that postictal behavioral deficits are independent of the preceding seizure and are remediable. This has led some to theorize that hypoperfusion in the postictal state could have both acute and chronic effects, causing network dysfunction in brain regions that participate in seizures.12 Thus multiple potential pathophysiologic and behavioral consequences of the postictal state have been postulated including amnesia, Todd's paresis, and SUDEP.
In rodents, the medullary respiratory center is mediated by 2 rhythm generators: the parafacial respiratory group and the pre-Bötzinger complex, which are modulated by pontine structures and the Bötzinger complex. In rat seizure models, population firing of serotonergic neurons in medullary raphe nuclei—responsible for modulation of respiratory and cardiac functions—was found to be substantially reduced during the ictal and postictal periods.20 This led to exploration of the use of selective serotonin reuptake inhibitors for reducing SUDEP risk.21 In a recent study with mouse models, during and following fatal seizures, pre-Bötzinger complex tissue became severely hypoxic, causing widespread brainstem hypoxia followed by breathing cessation and subsequent cardiac arrest.10 Studies of mice with fatal audiogenic seizures showed that mechanical ventilation could prevent sudden death.6 Autopsy of 14 SUDEP cases showed alterations in neuropeptidergic and monoaminergic neuronal populations in the pre-Bötzinger complex when compared to epilepsy and nonepilepsy controls.11 Studies of rodents have demonstrated the presence of postictal hypoperfusion-induced hypoxia in regions of seizure onset and propagation, including BRCs, which may provide a possible explanation for a mechanism underlying SUDEP.10,12 Mouse SUDEP models have confirmed the presence of spreading depression in the brainstem that causes cardiorespiratory arrest and may be the critical event in SUDEP.22 Further studies in mice linked dorsal medullary spreading depression with associated cardiorespiratory collapse to leaky ryanodine receptor-2 mutations, which have been implicated in cases of sudden cardiac death and SUDEP in humans.23
Despite a paucity of human data, the MORTEMUS and some case reports are supportive. A case report of 2 patients who died of SUDEP during EEG monitoring showed seizure-associated hypoxemia as the inciting event.7 Another single case report found postictal central apnea to be the cause of a near-SUDEP incident.8 Furthermore, continuous cardiorespiratory monitoring of 17 patients in a seizure monitoring unit showed ictal central apnea in 59% of patients and in 20 of 47 recorded seizures.9 Another study demonstrated ictal central apnea exclusively in focal epilepsy and questioned the existence of isolated postictal central apnea, considering postictal apnea to be persistence of ictal central apnea into the postictal period.
The same study postulated ictal central apnea as a potential biomarker for SUDEP and found higher association with temporal lobe epilepsy, focal impaired awareness seizures with motor onset, and focal aware seizures with nonmotor onset.24 Another study reported 2 cases of near SUDEP with ictal central apnea and postconvulsive central apnea as cause for peri-ictal apnea independently. The same study suggested that postconvulsive central apnea may be associated with brainstem dysfunction rather than ictal central apnea and that ictal central apnea was more common and seen exclusively with focal epilepsies.25
Brainstem and SUDEP mechanisms
It remains unclear why, in SUDEP, a particular seizure becomes fatal in a patient who may have previously had multiple similar seizures. It is possible that repeated seizures may increase susceptibility to SUDEP. This is supported by the fact that SUDEP is more common in patients with refractory epilepsy, higher seizure frequency, younger age at onset, and longer duration of epilepsy.3 It has been suggested that SUDEP could be a subset of sudden unexpected death due to medullary brain lesions, an autopsy entity that affects patients of all ages who harbor medullary lesions but were otherwise healthy and neurologically normal. Patients with SUDEP may be acquiring de novo structural or dysfunctional medullary lesions as a result of repeated seizures until a terminal event occurs.26 A “two-hit” pathomechanism has been put forth, whereby the “first hit” results from the excitotoxic effects of the propagation of seizure activity to sites beyond the seizure onset zone that are important in seizure control, arousal, and cardiopulmonary function. These include dorsomesencephalic structures like the periaqueductal gray, the colliculi, the cuneiform nucleus, and the raphe. After repeated seizures, these areas sustain repeated insults leading to the accumulation of structural abnormalities, which may increase the duration and severity of seizures such that they destabilize crucial autonomic system functioning.27 Whether repeated episodes of postictal hypoperfusion and hypoxia could cause structural abnormalities in the brainstem severe enough to precipitate SUDEP needs to be determined.
Other postulated mechanisms for SUDEP include channelopathies causing cardioautonomic dysfunction, airway obstruction, and pulmonary edema. Dravet syndrome (DS), which confers a high risk for SUDEP, was thought to result from cardiac dysfunction due to mutations in the SCN1A gene. One study showed peri-ictal breathing dysfunction in 7 patients with DS lasting as long as 4 hours postictally with 1 patient dying of SUDEP.28 The same authors demonstrated using muscarinic receptor antagonists in SCN1A loss-of-function mouse models that apnea, subsequent bradycardia, and death could be prevented by low-dose intracerebroventricular infusion and not by peripheral parasympathetic blockade, and thus concluded that central apnea could be the cause of cardiac dysfunction, and ultimately SUDEP.
Brainstem hypoperfusion and SUDEP risk factors
Our study demonstrated a variable degree of BRC hypoperfusion in a heterogenous patient group with focal epilepsies with variable duration of epilepsy, age at onset, and seizure types, suggesting that BRC hypoperfusion is likely insufficient to cause SUDEP alone. Rather, a combination of different risk factors is needed, as is described by the proposed two-hit pathomechanism. An association between BRC hypoperfusion and BTCS is not surprising as postictal generalized EEG suppression, commonly seen following BTCS, is often associated with prolonged hypoxemia.29,30 However, we do not have sufficient power to determine whether a relationship exists between the duration of suppression and the number of hypoperfused brainstem respiratory nuclei.
The observation that BTCSs are the risk factor most strongly associated with BRC hypoperfusion is notable as having BTCSs is the single greatest risk factor for SUDEP. Other risk factors including higher frequency of BTCS and longer duration of epilepsy were not found to be significant risk factors for BRC hypoperfusion. These findings suggest that multiple pathophysiologic mechanisms may interact to increase the risk for SUDEP, with BRC hypoperfusion being just one among them.
SUDEP and brainstem imaging
Numerous efforts have been made to identify neuroimaging biomarkers for SUDEP. One study used deformation-based morphometry to compare the brainstems of 2 patients with temporal lobe epilepsy (TLE) who died of SUDEP to healthy controls, patients with unilateral TLE–mesial temporal sclerosis (MTS), and patients with nonlesional unilateral TLE. Patients with TLE-MTS had volume loss in the dorsal mesencephalon, and this was more severe and extensive in patients who died SUDEP.31 Recent unpublished research by the same authors using deformation-based morphometry of the brainstems of 27 patients, 1–10 years prior to SUDEP, demonstrated extensive brainstem damage in the mesencephalon and medulla.32 This provides evidence that brainstem changes can occur well before the terminal event in SUDEP.
The extent of brainstem atrophy is also associated with impaired autonomic control, and SUDEP risk could be increased if atrophy extends to the lower brainstem.27 One study found an association between the extent and severity of brainstem volume loss and survival time before SUDEP, implying that it may be possible to predict progression to SUDEP using imaging.27 This study also showed that volume loss in the mesencephalon, raphe nuclei, and upper medulla were most strongly correlated with progression to SUDEP. Functional MRI studies have demonstrated differences in brainstem functional connectivity in patients with TLE and in patients at a high risk for SUDEP.33,34
Our study presents a novel imaging strategy to observe postictal perfusion changes in the brainstem. Whether this imaging procedure can be used to stratify patients with epilepsy for risk of developing SUDEP depending on the site and number of brainstem regions exhibiting postictal hypoperfusion needs to be verified with further studies.
The main limitation of this study is the relatively small sample size. For this reason, we could not assess the relationship between all known risk factors for SUDEP with postictal brainstem hypoperfusion, which could have strengthened the association between SUDEP and postictal brainstem hypoperfusion. In addition, our cohort only included patients with focal epilepsies due to the design of the parent study. However, it is possible that our findings could be reproduced in generalized epilepsies as postictal cerebral hypoperfusion has been demonstrated using ASL MRI in patients with generalized absence epilepsy.35 The time elapsed from the termination of the index seizure to scanning in this study was also longer than the longest time to SUDEP observed in the MORTEMUS study.4 The longer time to scanning is a considerable limitation; however, it should be noted that the most severe hypoxia in rodent models is observed between 20 and 60 minutes postictally and arteriole constriction is sustained for up to 90 minutes.12 In addition, our study could not provide insight into the cause for postictal brainstem hypoperfusion. It is not known if the observed hypoperfusion is the result of primary reduction in blood flow or a consequence of reduced neuronal activity and resultant decreased perfusion due to neurovascular coupling. However, work in animal models has shown seizure-induced vasoconstriction to be primarily responsible for hypoperfusion.16
Postictal hypoperfusion can occur in brainstem regions distant from the seizure onset zone. Patients who experience BTCSs are more likely to have postictal hypoperfusion in BRCs. With BTCSs being an established risk factor for SUDEP and evidence pointing to central respiratory dysfunction in human and rodent studies of SUDEP, postictal BRC hypoperfusion could be a risk factor or a mechanism for SUDEP. Applying the “two-hit” pathomechanism, it is possible that repeated seizures with postictal hypoperfusion could lead to the accumulation of structural abnormalities in BRCs until the terminal event in SUDEP. Thus BRC hypoperfusion may be a potential neuroimaging marker for risk for SUDEP and a target for therapeutic intervention.
Glossary
- ASL
arterial spin labeling
- BET
brain extraction tool
- BRC
brainstem respiratory center
- BTCS
bilateral tonic-clonic seizure
- CBF
cerebral blood flow
- CI
confidence interval
- COX-2
cyclooxygenase-2
- DRG
dorsal respiratory group
- DS
Dravet syndrome
- FIAS
focal impaired awareness seizure
- MORTEMUS
the Mortality in Epilepsy Monitoring Unit Study
- MR
magnetic resonance
- MTS
mesial temporal sclerosis
- OR
odds ratio
- pCASL
pseudo-continuous arterial spin labeling
- PRG
pontine respiratory group
- ROI
region of interest
- SUDEP
sudden unexpected death in epilepsy
- TLE
temporal lobe epilepsy
- VRG
ventral respiratory group
Appendix 1. Authors
Appendix 2. Calgary Comprehensive Epilepsy Program Collaborators
Contributor Information
on behalf of the Calgary Comprehensive Epilepsy Program Collaborators:
Yahya Agha-Khani, Nathalie Jetté, Colin Josephson, William Murphy, Neelan Pillay, and Samuel Wiebe
Study funding
Study funded by the Canadian Institutes of Health Research (MOP-136839).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet 2011;378:2028–2038. [DOI] [PubMed] [Google Scholar]
- 2.Tomson T, Walczak T, Sillanpaa M, Sander JW. Sudden unexpected death in epilepsy: a review of incidence and risk factors. Epilepsia 2005;46(suppl 11):54–61. [DOI] [PubMed] [Google Scholar]
- 3.Hesdorffer DC, Tomson T, Benn E, et al. Combined analysis of risk factors for SUDEP. Epilepsia 2011;52:1150–1159. [DOI] [PubMed] [Google Scholar]
- 4.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 5.Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol 2014;10:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venit EL, Shepard BD, Seyfried TN. Oxygenation prevents sudden death in seizure-prone mice. Epilepsia 2004;45:993–996. [DOI] [PubMed] [Google Scholar]
- 7.Bateman LM, Spitz M, Seyal M. Ictal hypoventilation contributes to cardiac arrhythmia and SUDEP: report on two deaths in video-EEG-monitored patients. Epilepsia 2010;51:916–920. [DOI] [PubMed] [Google Scholar]
- 8.So EL, Sam MC, Lagerlund TL. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia 2000;41:1494–1497. [DOI] [PubMed] [Google Scholar]
- 9.Nashef L, Walker F, Allen P, Sander JW, Shorvon SD, Fish DR. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry 1996;60:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George AG, Wall A, Teskey GC. Postictal hypoperfusion/hypoxia as a mechanism of sudden unexpected death in epilepsy: acute pharmacological interventions. AES 2018 Annual Meeting Abstract Database. Chicago: American Epilepsy Society; 2018. [Google Scholar]
- 11.Patodia S, Somani A, O'Hare M, et al. The ventrolateral medulla and medullary raphe in sudden unexpected death in epilepsy. Brain 2018;141:1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrell JS, Colangeli R, Wolff MD, et al. Postictal hypoperfusion/hypoxia provides the foundation for a unified theory of seizure-induced brain abnormalities and behavioral dysfunction. Epilepsia 2017;58:1493–1501. [DOI] [PubMed] [Google Scholar]
- 13.Gaxiola-Valdez I, Singh S, Perera T, Sandy S, Li E, Federico P. Seizure onset zone localization using postictal hypoperfusion detected by arterial spin labelling MRI. Brain 2017;140:2895–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li E, d'Esterre CD, Gaxiola-Valdez I, et al. CT perfusion measurement of postictal hypoperfusion: localization of the seizure onset zone and patterns of spread. Neuroradiology 2019;61:991–1010. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 16.Farrell JS, Gaxiola-Valdez I, Wolff MD, et al. Postictal behavioural impairments are due to a severe prolonged hypoperfusion/hypoxia event that is COX-2 dependent. elife 2016;5:e19352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong EC, Buxton RB, Frank LR. Quantitative perfusion imaging using arterial spin labeling. Neuroimaging Clin N Am 1999;9:333–342. [PubMed] [Google Scholar]
- 18.Yarnell PR. Todd's paralysis: a cerebrovascular phenomenon? Stroke 1975;6:301–303. [DOI] [PubMed] [Google Scholar]
- 19.Mathews MS, Smith WS, Wintermark M, Dillon WP, Binder DK. Local cortical hypoperfusion imaged with CT perfusion during postictal Todd's paresis. Neuroradiology 2008;50:397–401. [DOI] [PubMed] [Google Scholar]
- 20.Zhan Q, Buchanan GF, Motelow JE, et al. Impaired serotonergic brainstem function during and after seizures. J Neurosci 2016;36:2711–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faingold CL, Tupal S, Randall M. Prevention of seizure-induced sudden death in a chronic SUDEP model by semichronic administration of a selective serotonin reuptake inhibitor. Epilepsy Behav 2011;22:186–190. [DOI] [PubMed] [Google Scholar]
- 22.Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med 2015;7:282ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aiba I, Wehrens XH, Noebels JL. Leaky RyR2 channels unleash a brainstem spreading depolarization mechanism of sudden cardiac death. Proc Natl Acad Sci USA 2016;113:E4895–E4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacuey N, Zonjy B, Hampson JP, et al. The incidence and significance of periictal apnea in epileptic seizures. Epilepsia 2018;59:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilella L, Lacuey N, Hampson JP, et al. Incidence, recurrence, and risk factors for peri-ictal central apnea and sudden unexpected death in epilepsy. Front Neurol 2019;10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaster JH, Ottaviani G, Matturri L, Lavezzi AM, Zamecnik J, Smith TW. Sudden unexpected death related to medullary brain lesions. Am J Forensic Med Pathol 2008;29:371–374. [DOI] [PubMed] [Google Scholar]
- 27.Mueller SG, Nei M, Bateman LM, et al. Brainstem network disruption: a pathway to sudden unexplained death in epilepsy? Hum Brain Mapp 2018;39:4820–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y, Bravo E, Thirnbeck CK, et al. Severe peri-ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest 2018;128:1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freitas J, Kaur G, Fernandez GB, et al. Age-specific periictal electroclinical features of generalized tonic-clonic seizures and potential risk of sudden unexpected death in epilepsy (SUDEP). Epilepsy Behav 2013;29:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seyal M, Hardin KA, Bateman LM. Postictal generalized EEG suppression is linked to seizure-associated respiratory dysfunction but not postictal apnea. Epilepsia 2012;53:825–831. [DOI] [PubMed] [Google Scholar]
- 31.Mueller SG, Bateman LM, Laxer KD. Evidence for brainstem network disruption in temporal lobe epilepsy and sudden unexplained death in epilepsy. Neuroimage Clin 2014;5:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller SG, Nei M, Bateman L, et al. Evidence for brainstem network disruption in focal epilepsy and sudden unexplained death in epilepsy: a first validation study. American Epilepsy Society Annual Meeting; December 1–5, 2017; Washington, DC.
- 33.Allen LA, Harper RM, Kumar R, et al. Dysfunctional brain networking among autonomic regulatory structures in temporal lobe epilepsy patients at high risk of sudden unexpected death in epilepsy. Front Neurol 2017;8:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y, Chen Q, Yu X, et al. A resting-state functional connectivity study in patients at high risk for sudden unexpected death in epilepsy. Epilepsy Behav 2014;41:33–38. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Lei D, Ren J, et al. Patterns of postictal cerebral perfusion in idiopathic generalized epilepsy: a multi-delay multi-parametric arterial spin labelling perfusion MRI study. Sci Rep 2016;6:28867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw and processed ASL MRI data are available on request.