Abstract
Objective
To determine whether vascular and neurodegenerative factors influence cognition before clinically relevant Alzheimer disease pathology, we analyzed MRI measures and amyloid imaging in an ethnoracially diverse cohort of cognitively normal individuals older than 60 years.
Methods
Participants (n = 154; mean age 74.15 ± 6.94; 50% female; 54% Caucasian, 22.1% Hispanic, 14.9% African American) were recruited from the University of California, Davis Alzheimer's Disease Research Center, who were cognitively normal at baseline, time of PET, and MRI, and received yearly cognitive assessment for 6.23 ± 4.16 years. Mixed model regression with random slope and intercept was calculated for episodic memory and executive function, adjusting for age, sex, education, and ethnicity.
Results
Vascular burden score was associated with total white matter hyperintensity (WMH) volume (β, 0.171; 95% confidence interval [CI], 0.024–0.318). WMH volume was associated with low baseline executive function (−0.115; −0.226 to −0.003) and rate of change in memory (−0.029; −0.045 to −0.012). Hippocampal volume was associated with the rate of change in memory (0.040; 0.021–0.059) and executive function (0.024; 0.008–0.039). Continuous measures of amyloid status influenced change in memory (−0.026; −0.044 to −0.008) and executive function (−0.033; −0.046 to −0.021) independently of MRI measures.
Conclusion
Vascular brain injury and neurodegeneration are associated with baseline cognitive performance and the rate of longitudinal change independent of amyloid status among community-dwelling, ethnicity diverse cognitively normal individuals, supporting the role of vascular diseases as risk factors for later-life dementia.
Advancing age is associated with changes in brain structure1 and varying trajectories of cognitive performance.2 Cerebral amyloid burden accumulates with age3 and is associated with poorer memory performance,4 incident cognitive impairment, and dementia.5 Nearly 75% of cognitively normal individuals aged 70–795 and about one-third of individuals with mild cognitive impairment (MCI), however, are free of significant amyloid on PET imaging,6 suggesting that factors other than amyloidosis are associated with declining cognitive performance.
Cerebrovascular disease (CVD) is common and associated with cognitive decline,7 incident MCI, and dementia,8 independent of Alzheimer pathology,9 particularly among Blacks and Hispanics.10 While the effect of CVD is generally assumed to be associated with MRI measures of white matter hyperintensities (WMH), and infarcts on MRI,8 additional evidence shows that CVD also can cause cerebral and hippocampal atrophy.11
Previous studies show that MRI measures of vascular brain injury (WMH and infarcts) have an early and independent effect on cognition, even among those who have substantial co-occurring amyloid burden.7,12,13 However, given evidence that the population burden of age-related cognitive decline may be considerably lessened through prevention and treatment of vascular risk factors,14 it is important to identify the extent and consequences of vascular brain injury in the absence of clinically relevant amyloid pathology. This study aimed to examine the individual and combined effect of MRI measures of WMH, global and regional atrophy, and cerebral amyloid burden on the trajectory of cognitive performance comprehensively among an ethnically diverse, cognitively normal population over an extended period of observation.
Methods
Study population
A total of 154 participants were recruited for this study from the University of California, Davis Alzheimer's Disease Research Center (UCD ADRC) diversity cohort.15 All participants were cognitively normal at baseline and time of PET and MRI and received yearly cognitive assessment at least 2 or more times (figure 1). Inclusion criteria required the participant to be over 60 years of age at enrollment. Exclusion criteria included unstable major medical illness, major primary psychiatric disorder (history of schizophrenia, bipolar disorder, or recurrent major depression), or substance abuse or dependence in the last 5 years. The presence of vascular risk factors or stroke that did not render the participant incapable of cognitive testing and longitudinal assessment were not exclusionary.15
Figure 1. Timeline of study measurements.
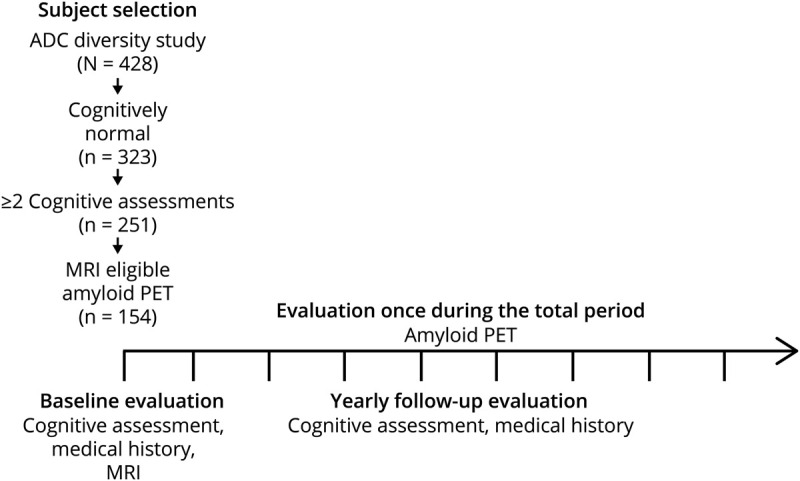
The mean follow-up duration was 6.23 ± 4.16 years, and the mean number of assessments was 6.46 ± 3.65 times. The mean time lag from baseline assessment to amyloid PET was 2.87 ± 3.68 years. ADC = Alzheimer's Disease Research Center.
Participants received a thorough multidisciplinary clinical evaluation, which included detailed medical history, neurologic examination, laboratory tests, and neuropsychological testing using the Uniform Data Set battery.16,17 Diagnosis of cognitive status (normal, MCI, or dementia) was made according to standard criteria and methods.17 Each participant was initially diagnosed as cognitively normal at a multidisciplinary consensus conference.
Neuropsychological measures
We used measures of episodic memory and executive function to assess cognition. Individuals in this sample were originally recruited and evaluated by 2 different batteries of neuropsychological tests, although the majority of participants in this study came from the UCD ADRC diversity cohort that used the Spanish and English Neuropsychological Assessment Scale (SENAS).18 The second group was enrolled in a program project grant on ischemic vascular contributions to cognitive decline and dementia (IVD) that ran from 1996 to 2012 using a different neuropsychological test battery. Individuals from the IVD cohort were transitioned to follow-up with the UCD ADRC neuropsychological battery at the close of the IVD study. A total of 95 individuals received neuropsychological test batteries for both protocols.
Conceptually similar measures of episodic memory and executive function were derived from the different tests that composed the ADRC and IVD neuropsychological test batteries. Item response theory methods were used to create equated episodic memory and executive function scores from these 2 batteries that are on the same measurement metric and can be combined in analyses using data from both projects. The general approach followed methods described in previous publications19 and a detailed description of methods and results are available for review in the supplementary files.
Vascular burden score
The presence and number of vascular risk factors for each participant was based on a thorough review of the participant's medical history, medical records, and medications brought into the study site at the time of evaluation. The vascular burden score (VBS) for each individual was the sum of 5 vascular risk factors and vascular diseases (presence, 1; absence, 0; per each) and could vary from 0 to 5: (1) hypertension, (2) diabetes, (3) hyperlipidemia, (4) cardiovascular disease (1 or more among heart attack, atrial fibrillation, angioplasty, coronary artery bypass surgery, pacemaker, and congestive heart failure), and (5) CVD (1 or more among stroke and TIA) as previously described.7
MRI
Image acquisition
Structural MRI scans for 85 participants were obtained at the UCD MRI research center on a 3T Siemens (Munich, Germany) Magnetom Trio Syngo System with an 8-channel head coil. Acquired images included a T1-weighted volumetric magnetization-prepared rapid gradient echo (repetition time [TR] 2,500, echo time [TE] 2.94 or 2.98, inversion time [TI] 1,100, with 1 mm3 isotropic resolution) and a fluid-attenuated inversion recovery (FLAIR) scan (TR 5,000, TE 403, TI 1700, with 1.00 × 1.00 mm2 in-plane resolution and 2.00 mm slice thickness). Sixty-nine participants received scans using a 1.5T GE (Cleveland, OH) Signa Genesis system at the UCD research center. Each session included a T1-weighted 3D spoiled gradient recalled echo scan (TR 9, TE 1.9, with 0.98 × 0.98 mm2 in-plane resolution and 1.5 mm slice thickness) and a FLAIR scan (TR 11,002, TE 147, TI 2,250, with 0.98 × 0.98 mm2 in-plane resolution and 3.00 mm slice thickness). The mean time lag from initial assessment to MRI was 0.13 ± 0.14 years.
MRI tissue classification
MRI measurements were made as part of our in-house processing pipeline as previously described.20 Structural MRIs were processed to remove the skull using an atlas-based method.21 Gray, white, and CSF tissues segmentation was performed using an algorithm designed to enhance accuracy at likely tissue boundaries.22 Segmentation of WMHs utilized a Bayesian approach where the likelihood of WMH was estimated from FLAIR signal characteristics, the prior probability of WMH occurrence was calculated from previous supervised segmentations of independent FLAIR images, and additional posterior probability constraints were applied at each image voxel.7 Hippocampal volume was computed by a multiatlas hippocampal segmentation algorithm.21
PET
Image acquisition
Pittsburgh compound B (PiB) PET images were acquired for 65 individuals at the Lawrence Berkeley National Laboratory on a Siemens ECAT EXACT HR PET scanner in 3D acquisition model. PiB radiotracer was synthesized at this facility using a standard protocol23 where 10–15 mCi of [11C] PiB was injected into an antecubital vein. Dynamic acquisition frames (34–35 frames total) were obtained over 90 minutes.
Florbetapir-PET scans were acquired on a Siemens Biograph mCT 40 PET machine for 89 individuals during a 50- to 70-minute interval following a 10 mCi (370 MBq) bolus injection of florbetapir (18F).
PET analysis
PET analysis was performed in 2 ways. PiB data were preprocessed with procedures described previously using a gray matter cerebellar reference region to calculate distribution volume ratio (DVR) images.13 The Global PiB Index was generated from the mean DVRs from regions of interest vulnerable to early β-amyloid (Aβ) deposition, which include the frontal cortex (anterior to the precentral gyrus), lateral parietal cortex, lateral temporal cortex, posterior cingulate, and precuneus.13 The occipital cortex was also examined because of its susceptibility to cerebral amyloid angiopathy.
Florbetapir data were analyzed using standard uptake value ratio (SUVR) measures.24 Four 5-minute frames 50–70 minutes after injection were averaged and the image data were spatially normalized to a standard anatomical atlas in our laboratory. Mean tracer retention was calculated from 6 predefined target cortical regions of interest (medial orbital frontal, temporal, parietal, anterior cingulate, posterior cingulate, and precuneus) and whole cerebellar gray matter reference region, based on T1-weighted high-resolution MRI.
Participants were determined to be amyloid-positive using published DVR13 or SUVR24 thresholds according to each radiotracer. Secondary analyses included scaled DVR or SUVR values from the PiB and florbetapir images as additional predictor variables to assess the effect of amyloid retention as a continuous effect on cognitive change. The scaled DVR or SUVR values were calculated based on a Gaussian 2 mixture model. The fitted parameters for the normal distributions of SUVR or DVR of each tracer were then used to create z score variance from the mean for each individual and each tracer.
Statistical analysis
Because of our hypothesis related to vascular brain injury and cognition, initial linear regression models evaluated the association of VBS with WMH and hippocampal volumes and the scaled DVR or SUVR values. Demographic differences between amyloid-positive and -negative groups were tested using Pearson χ2 tests for dichotomous variables and independent sample t tests for continuous variables.
Association of demographics and baseline MRI measures with baseline and change in cognitive scores
To assess cross-sectional associations, we used linear models including baseline cognitive performance (episodic memory or executive function) as the dependent variable, and baseline demographic variables (age, sex, education, ethnicity, cognitive test form) and baseline MRI variables as independent variables. Longitudinal associations were assessed using linear mixed effect models with repeated measures of cognition as the dependent variable. Time since initial assessment, demographic variables, and baseline MRI variables were included as predictors, as well as the interaction with time. Random effects for the intercept and time were also included.
Additional effect of amyloid burden on baseline and change in cognitive scores
We used an analytical approach similar to that described in the previous study7 based on serial regression analyses to investigate the additional effect of amyloid burden, as reflected by PiB and florbetapir imaging, on cognition. This goal was assessed by adding in the linear mixed models amyloid positivity (yes/no, model A) or continuous measures of scaled DVR or SUVR values (model B) as a predictor. Tracer type and a time-lag variable computed as the difference in years between the date of PET imaging and the date of the first neuropsychological testing were added as covariates. All continuous independent variables, except time, were transformed into z scores to facilitate regression coefficient comparisons.
All tests used were 2-tailed, with α = 0.05. All statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Standard protocol approvals, registrations, and patient consents
This study was approved by the institutional review board at UC Davis and all study participants provided written informed consent.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request. Supplementary file is available from Dryad (doi.org/10.25338/B8P60R).
Results
Sample characteristics
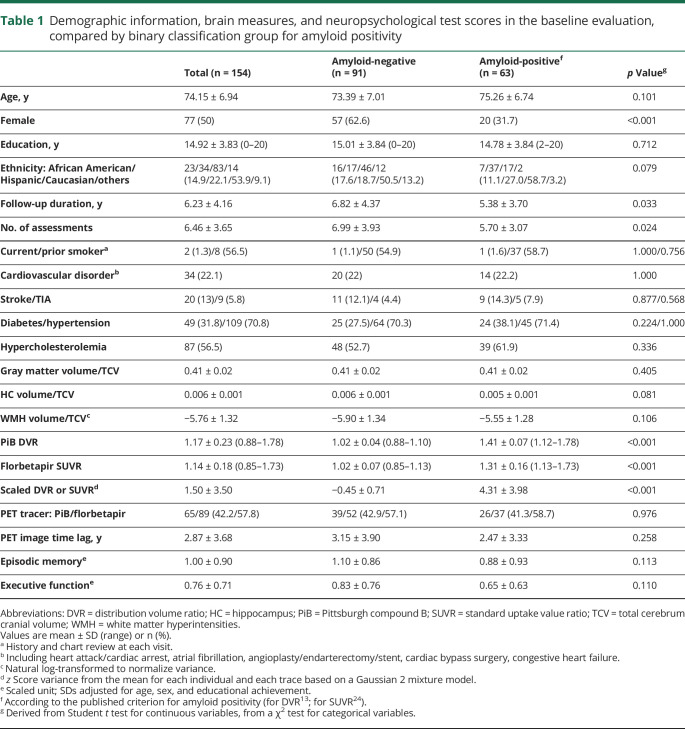
Participants were 74.15 ± 6.94 years of age on average, 50% female, and had an average of 14.92 ± 3.83 years of educational achievement (table 1) at baseline evaluation. The overall sample consisted of 23 African Americans, 34 Hispanics, 83 Caucasians, and 14 persons from other racial or ethnic groups. A total of 42 participants had the IVD cognitive testing paradigm at baseline enrollment whereas the remaining received SENAS testing throughout. The mean follow-up duration was 6.23 ± 4.16 years, and the mean number of assessments was 6.46 ± 3.65. The ranges of hippocampal and WMH volume were 4.68–8.16 and 0.10–59.94 mL. The mean time lag from initial assessment to PET was 2.87 ± 3.68 years. Secondary analysis of bias due to death or dropout did not indicate bias due to incomplete follow-up or subject representation (data not shown).
Table 1.
Demographic information, brain measures, and neuropsychological test scores in the baseline evaluation, compared by binary classification group for amyloid positivity
The frequency of cardiovascular disease history was 22.1% (heart attack 9.1%, atrial fibrillation 5.9%, angioplasty 10.5%, coronary artery bypass surgery 5.8%, congestive heart failure 2.6%), and that of CVD was 16.9% (stroke 13.0%, TIA 5.8%). Hypertension, hypercholesterolemia, and diabetes had frequencies of 70.8%, 56.5%, and 31.8% (table 1), respectively. There were no significant differences in prevalence of vascular risk factors or disease by race/ethnicity.
VBS was positively associated with WMH volume (β, 0.171; 95% confidence interval [CI], 0.024–0.318) but not with cerebral gray matter volume (−0.027; −0.179 to 0.124), hippocampal volume (0.044; −0.092 to 0.179), or the scaled DVR or SUVR values (0.034; −0.118 to 0.186), after adjusting age, sex, and ethnicity, indicating that vascular risk and disease were associated with WMH, but not atrophy or PET measures. WMH volumes were associated with scaled DVR or SUVR values (0.188; 0.025–0.351) whereas hippocampal volume was not associated with WMH (0.098; −0.049 to 0.245).
Binary classification of participants according to amyloid positivity produced no significant differences between the 2 groups except for sex, follow-up duration, and the number of assessments (table 1).
Association of demographics and baseline MRI measures with baseline and change in cognitive scores
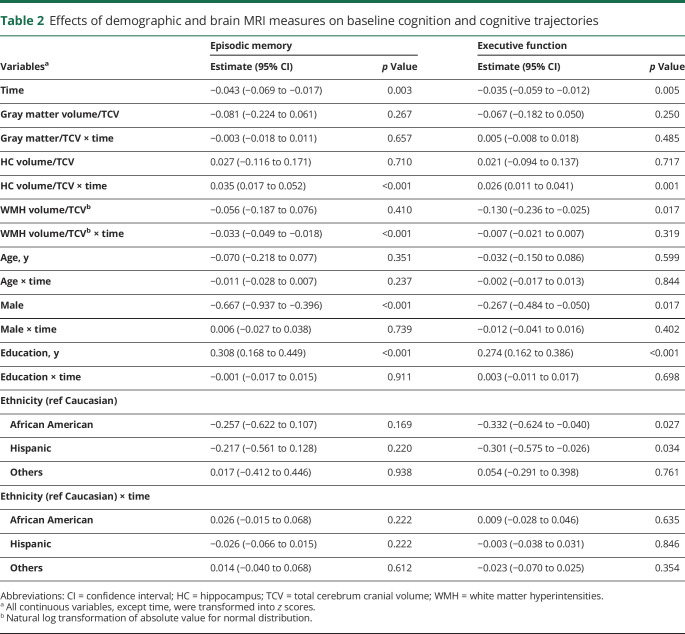
Over the course of observation, the estimated slopes of episodic memory (β, −0.043 SD/year; 95% CI, −0.069 to −0.017) and executive function (β, −0.035 SD/year; 95% CI, −0.059 to −0.012; table 2) showed small declines in both cognitive domains. None of the brain measures was associated with baseline episodic memory. Normalized WMH volume, however, had a significant negative effect on baseline executive function. For longitudinal differences in cognition, WMH and hippocampal volumes were associated with trajectories of episodic memory, but only the hippocampus was associated with executive change. Female sex and higher educational attainment were associated with higher memory and higher executive performance. None of the demographic variables, however, showed significant interactions with time.
Table 2.
Effects of demographic and brain MRI measures on baseline cognition and cognitive trajectories
Additional effect of amyloid burden on baseline and change in cognitive scores
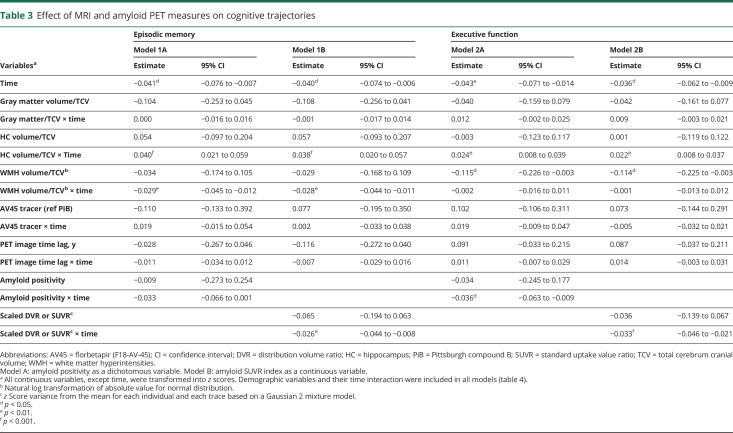
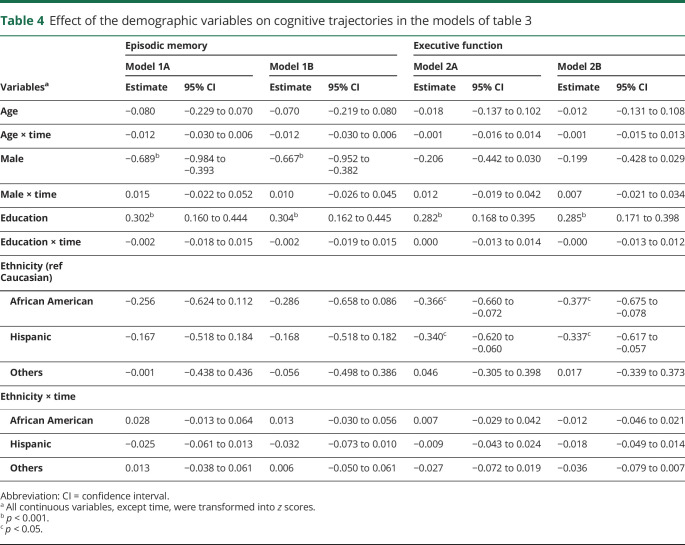
Sequential modeling that added amyloid retention is summarized in tables 3 and 4. Models 1A and 2A revealed that amyloid positivity was not independently associated with baseline performance (β, −0.009; 95% CI, −0.273 to 0.254) or the rate of change (β, −0.033; 95% CI, −0.066 to 0.001) for episodic memory, whereas the effect of hippocampal volume and WMH volume on the rate of change was maintained (table 3). The statistical significance of each demographic variable including interaction with time was not changed compared to the model that did not include PET information (table 4). For executive function, amyloid positivity was associated with the rate of change/decline (β, −0.036; 95% CI, −0.063 to −0.009). Hippocampal volume also maintained its effect on the rate of change in executive function, and WMH volume maintained a significant association with baseline executive performance (table 3). Sex was not associated with executive performance, and other demographic variables (including interactions with time) were not changed in statistical significance compared to the model that did not include PET information (table 4).
Table 3.
Effect of MRI and amyloid PET measures on cognitive trajectories
Table 4.
Effect of the demographic variables on cognitive trajectories in the models of table 3
When continuous amyloid retention was entered into the sequential model (models 1B and 2B in table 3), amyloid retention was associated with the rate of change in both cognitive functions (β, −0.026; 95% CI, −0.044 to −0.008 for episodic memory; β, −0.033; 95% CI, −0.046 to −0.021 for executive function). The significant effects of the MRI measures on both memory and executive function were not changed when compared to models 1A and 2A. The kind of PET tracer and PET image time lag were not associated with cognitive performance or the rate of changes in any model.
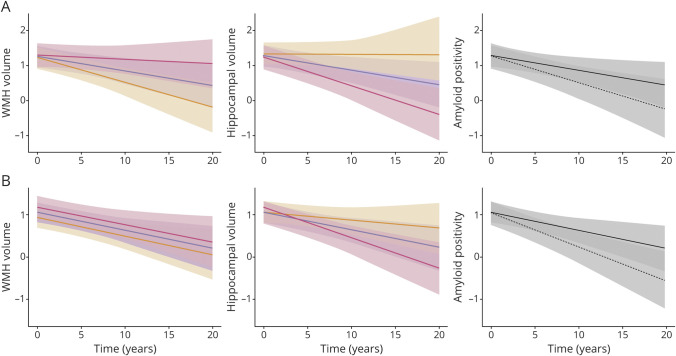
Figure 2 summarizes the marginal effects of MRI measures and amyloid positivity from model A in table 3 according to SDs of MRI measures and amyloid positivity for PET measures. Individuals with −1 SD of WMH and 1 SD of hippocampal volume both showed little change in memory performance over time. Executive function declined for all groups, but performance remained overall better for those with the −1 SD of WMH and the 1 SD of hippocampal volume. Conversely, those individuals with +1 SD of WMH or −1 SD of hippocampal volume performed significantly worse on baseline executive function and trajectories of memory and executive function. Modest but significant divergence over time as an effect of amyloid positivity is seen with executive function performance, but there are no overlapping CIs of the estimate in trajectories of memory performance.
Figure 2. Plots of marginal effects of mixed regression models.
Each graph shows cognitive trajectories for various levels of a single brain measure. (A) Predictive values of episodic memory performance. (B) Predictive values of executive function performance. Y axes represent cognitive performance. X axes are time. Red line, 1.0 SD below the mean; blue line, mean; yellow line, 1.0 SD above the mean for white matter hyperintensities (WMH) and hippocampal volumes; solid black line, amyloid-negative; dotted black line, amyloid-positive.
Discussion
This study found a consistent effect of WMH and hippocampal volumes on cognitive function independent of amyloid status in a community-based, ethnically diverse cohort of cognitively normal individuals. WMH volume, which was significantly associated with VBS in this study, had the strongest effect on level of baseline executive function among various brain measures, and showed an effect similar to hippocampal volume and continuous amyloid status on the rate of change in episodic memory. Hippocampal volume also had a consistent effect on the rate of change in both episodic memory and executive function. Amyloid burden, when treated as a dichotomous variable, did not affect the level or the rate of change in memory performance, though there was a modest effect on the rate of change in executive function. Amyloid burden, when considered as a continuous measure, however, had a significant association with change in both memory and executive function, but this influence remained independent of structural brain measures. We further show that individuals with WMH volume 1 SD below average (nonvascular path) had limited change in memory performance over the period of observation, whereas those having 1 SD above average (vascular path) had significant decline. While similar to the previous study,25 we further extend the findings by showing that hippocampal volumes 1 SD above average showed limited change in memory and executive performance over the period of observation (figure 2), whereas those with volumes 1 SD below average resulted in significant decline in cognition. In sum, cognitively normal individuals with low WMH burden or high hippocampal volume underwent modest changes in memory performance, independently of amyloid status.
These results emphasize the important role of nonamyloid processes as early risk factors for cognitive decline and future dementia in the general population and challenge current concepts regarding the role of Alzheimer disease (AD) pathologies in cognitive aging.26 The fact that WMH volume was associated with degree of amyloid retention when considered as a continuous variable is consistent with previous studies examining the effect of vascular risk and disease on cerebral amyloid retention27 and indicates an association among vascular risk, amyloid, and WMH.28
Figure 3A summarizes currently available data regarding population prevalence of hypertension (the most common vascular risk factor), extensive WMH, hippocampal atrophy, and excessive amyloid burden. Given that vascular risk factors and vascular disease become prevalent at a relatively early age29 and the findings that vascular risk factors have an early and cumulative effect on brain injury,30 we confirmed the association of vascular risk factors with WMH volume in our population and hypothesized that vascular risk factors throughout the lifespan increase susceptibility to late-life dementia.30 Figure 3B summarizes this hypotheses.
Figure 3. Proposed models of timing of amyloid and nonamyloid processes in the general population.
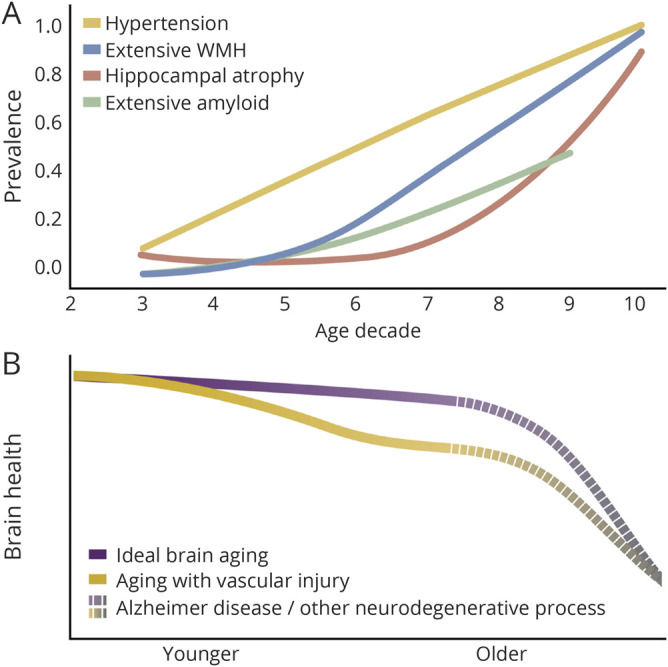
Vascular and degenerative factors influence cognition before clinically relevant Alzheimer disease pathology and likely create susceptibility to later life dementia. (A) Age-specific prevalence of hypertension, abnormal white matter hyperintensities (WMH), hippocampus, and amyloid status modified from references 1, 29, and 50. (B) Hypothetical model by which vascular risk factors cause subtle brain injury increasing risk for later-life dementia when neurodegeneration is more prevalent.
The combined effects of cerebrovascular pathology on dementia appear even more prominent for African Americans and Hispanics than non-Hispanic Whites.10 Given that vascular risk factors such as systolic blood pressure and obesity are associated with increased likelihood of dementia11 and recent evidence that lifestyle modification31 or ideal control of systolic blood pressure32 are associated with lower risk for incident cognitive impairment or decline, the increasingly recognized role of risk modification for dementia prevention supports the relevance of these findings.
Increased volumes of WMH are considered a common consequence of the aging process,1 but are exacerbated by vascular risk factors that influence brain and cognitive health, even during middle life.11 In later life, evolution of WMH is associated with declines in both memory and executive function33 and extensive WMH predict incident MCI and dementia.8 While WMH also accompany AD20,34 and mediate cognitive effect,34 previous cross-sectional studies examining individuals with normal cognition or MCI find that WMH and infarction have a negative effect on cognition independent of concurrent amyloid status.12,13
WMH, which was associated with VBS, had the greatest effect on the level of baseline executive function (nearly 3 times that of any other measures) in our study. Previous studies from our group show that WMH was associated with executive function by reducing the functional connectivity of the prefrontal cortex with other cortical or subcortical regions35 independent of location.36 We also found that WMHs affect memory trajectory, which might be also likely via frontal systems impairment.35 The lack of association between WMH volume and decline in executive function could indicate that change in executive function is more sensitive to the progression of WMH than to baseline WMH.33 We conclude from previous data and the results of this study that WMH have early and consistent effects on cognition not influenced by amyloid burden, consistent with data indicating an increased risk of WMH for future MCI and dementia8 likely through increased susceptibility to later life neurodegeneration as depicted in figure 3B.
Age-related differences in global and regional brain volumes, including hippocampal volume, are well described.1 Vascular risk factors increase these age-related differences and are associated with declines in cognitive function37 even when the diseases are mild38 or well controlled.39 Similarly, even pure vascular disease is known to cause both generalized40 and hippocampal atrophy41 and associated decline in cognition. Although neurodegeneration markers are commonly used in AD research,26 they are not specific to AD, but rather are indicators of damage that may be derived from a variety of etiologies including vascular brain injury.26 Given that CVD is associated with cerebral and hippocampal atrophy,11,37,39 we investigated the effect of hippocampal volume and gray matter volume as markers of neurodegeneration on cognition along with WMH volume. While hippocampal volume was associated with both memory and executive function trajectories, amyloid burden and WMH volume were not associated with hippocampal volume in the present study. Further studies are needed to understand potential causes of hippocampal atrophy among diverse, cognitively normal individuals.
Lack of effect of gray matter atrophy on cognition most likely reflects the intact cognitive status of this study group. Conversely, the effect of hippocampal atrophy is more specific to cognition and therefore had greater influence, suggesting that gray matter volume atrophy may be related to cognitive decline at a later pathophysiologic stage42 in our population. Longitudinal imaging of this group would further elucidate the relationship between generalized atrophy measures and cognition.
Individuals of this study were diverse, not only in relation to race or ethnicity, but also along demographic variables such as social economic status and education. In addition, we enrolled individuals irrespective of medical history, resulting in an increased prevalence of vascular risk factors more in line with the US population.29
Including a more representative older population in our study likely accounts for some of the differences found. First, as expected,43 there was a significant decline in performance across both memory and executive function among this group of cognitively normal individuals, which differs from cognitive trajectories for normal individuals from other studies such as the Alzheimer's Disease Neuroimaging Initiative (ADNI). While there were some baseline differences in cognitive performance in relation to race or ethnicity, sex, and level of education in our group (tables 2 and 4), these demographic variables did not influence longitudinal cognitive performance in this cohort, similar to findings previously reported by our group.44 This lack of longitudinal effect of demographic variables may support generalizability of our findings, despite the demographic diversity of the cohort.
The findings that VBS was associated with WMH and WMH was associated with continuous amyloid burden are also likely influenced by population characteristics. The prevalence of vascular risk factors and vascular diseases among the participants of our study was higher than in other biomarker studies such as ADNI. In our study, the prevalence of these diseases did not vary significantly by race/ethnicity and, therefore, it is more likely that it was the result of our efforts to recruit participants directly from the community without regard to risk factors or concurrent vascular disease that led to this difference.
In clinically normal study populations, the effects of amyloid deposition on cognition have been mixed. Studies that find no definite effect on cognition12,13 note that Aβ is an initiating factor in the progression toward AD that may not influence cognition early in the course of disease. In cognitively intact older adults, therefore, it may be the delayed sequelae of Aβ deposition, such as tau aggregation and subsequent tau-mediated neurodegeneration, that produce cognitive deficits.45 Given that the effect of amyloidosis on cognitive impairment is most strongly associated with the combined effects of tauopathy and neurodegeneration,26,46 the degree of amyloid burden, which was relatively mild (maximum values of DVR or SUVR were under 2.0) in our cognitively normal population, might explain the lack of effect on cognition in this study. Our lower values of amyloid burden likely reflect that individuals were determined to be cognitively normal at enrollment and time of PET imaging. Although the relative effect sizes of amyloid, hippocampal, and WMH volumes on cognitive trajectory were similar in this study as well as previous studies,25,47 the distinguishing finding of this study is the large effect size of WMH volume on the level of executive function. This effect was about 3–3.5 fold compared to the effect of amyloid status (whether categorical or continuous) on cognitive trajectory. Our finding that WMH volume was associated with increased amyloid retention may support the possibility of an interaction between vascular brain injury and amyloid deposition, but there was no interaction between WMH volume and amyloid status (or 3-way interaction with time) in the linear mixed model (data not shown); therefore, further research is needed to test potential hypotheses.
Our study has a number of limitations. First, while our study emphasized community-based recruitment, the number of participants is relatively small and likely does not represent the entire local population. Second, amyloid PET was obtained later in the course of follow-up, thereby reducing the ability of PET to estimate the level of cognitive performance at baseline. Amyloid accumulation, however, is likely to be relatively modest during the period of follow-up in this cognitively normal cohort.48 Moreover, model adjustment by the variable for the time lag of PET acquisition showed no effect on cognitive function or trajectory in every model. Third, participants in this study are a select cohort designed to examine a specific hypothesis from an ongoing longitudinal cohort limiting generalizability.
This study found that vascular brain injury measured by WMH volume and neurodegeneration measured by hippocampal volume were associated with decreased level of baseline cognitive performance and increased rate of cognitive decline independent of amyloid status among community-dwelling, ethnicity diverse (53.9% non-Hispanic White) cognitively normal individuals. The first strength of this study is the sample characteristics including diverse race/ethnicity, which better represents the representativeness of the community in the United States, and the second is the comprehensive examination of WMH, global/regional atrophy, and cerebral amyloid burden. The study that has been conducted on the effect of these 4 comprehensive brain variables in ethnically diverse, cognitive normal community populations is rare. Finally, we proposed the model indicating that vascular and degenerative factors influence cognition before clinically relevant AD pathology and likely play a role in later life dementia risk (figure 3B). Given evidence that vascular risk can be mitigated with positive effects on cognition,32 aggressive treatment of vascular risk factors may substantially reduce dementia incidence. This is particularly true for African Americans and Hispanics, where dementia prevalence is estimated to be nearly twice that of non-Hispanic Whites49 and who have a higher prevalence of vascular risk factors and vascular disease.29
Acknowledgment
The authors thank the participants of this study and the staff who support this effort.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- CI
confidence interval
- CVD
cerebrovascular disease
- DVR
distribution volume ratio
- FLAIR
fluid-attenuated inversion recovery
- IVD
ischemic vascular contributions to cognitive decline and dementia
- MCI
mild cognitive impairment
- PiB
Pittsburgh compound B
- SENAS
Spanish and English Neuropsychological Assessment Scale
- SUVR
standard uptake value ratio
- TE
echo time
- TI
inversion time
- TR
repetition time
- UCD ADRC
University of California, Davis Alzheimer's Disease Research Center
- VBS
vascular burden score
- WMH
white matter hyperintensities
Appendix. Authors
Study funding
This study was supported by grants NIH P30 AG10129, P01 AG12435, R01 AG047827, and R01 AG031563.
Disclosure
J. Han, P. Maillard, D. Harvey, E. Fletcher, O. Martinez, D. Johnson, J. Olichney, S. Farias, S. Villeneuve, W. Jagust, and D. Mungas report no disclosure relevant to the manuscript. C. DeCarli is a consultant to Novartis on a safety study of heart failure. Go to Neurology.org/N for full disclosures.
References
- 1.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 2.Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in cognitive function in older persons from a community population: relation to age and Alzheimer disease. Arch Neurol 1999;56:1274–1279. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010;67:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirth M, Oh H, Mormino EC, Markley C, Landau SM, Jagust WJ. The effect of amyloid beta on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimers Dement 2013;9:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol 2009;66:1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology 2007;68:1718–1725. [DOI] [PubMed] [Google Scholar]
- 7.DeCarli C, Villeneuve S, Maillard P, et al. Vascular burden score impacts cognition independent of amyloid PET and MRI measures of Alzheimer's disease and vascular brain injury. J Alzheimers Dis 2019;68:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke 2010;41:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 2004;62:1148–1155. [DOI] [PubMed] [Google Scholar]
- 10.Filshtein TJ, Dugger BN, Jin LW, et al. Neuropathological diagnoses of demented Hispanic, black, and non-Hispanic white decedents seen at an Alzheimer's disease center. J Alzheimers Dis 2019;68:145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011;77:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchant NL, Reed BR, DeCarli CS, et al. Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiol Aging 2012;33:1006.e25–1006.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchant NL, Reed BR, Sanossian N, et al. The aging brain and cognition: contribution of vascular injury and abeta to mild cognitive dysfunction. JAMA Neurol 2013;70:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinton L, Carter K, Reed BR, et al. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Dis Assoc Disord 2010;24:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009;23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer disease centers. Alzheimer Dis Assoc Disord 2006;20:210–216. [DOI] [PubMed] [Google Scholar]
- 18.Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess 2004;16:347–359. [DOI] [PubMed] [Google Scholar]
- 19.Mungas D, Reed BR, Kramer JH. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology 2003;17:380–392. [DOI] [PubMed] [Google Scholar]
- 20.Lee DY, Fletcher E, Martinez O, et al. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer disease. Stroke 2010;41:1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aljabar P, Heckemann RA, Hammers A, Hajnal JV, Rueckert D. Multi-atlas based segmentation of brain images: atlas selection and its effect on accuracy. Neuroimage 2009;46:726–738. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher E, Singh B, Harvey D, Carmichael O, DeCarli C. Adaptive image segmentation for robust measurement of longitudinal brain tissue change. Conf Proc IEEE Eng Med Biol Soc 2012;2012:5319–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem 2003;46:2740–2754. [DOI] [PubMed] [Google Scholar]
- 24.Landau SM, Breault C, Joshi AD, et al. Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med 2013;54:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 2015;138:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott JA, Braskie MN, Tosun D, et al. Cerebral amyloid and hypertension are independently associated with white matter lesions in elderly. Front Aging Neurosci 2015;7:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics: 2019 update: a report from the American heart association. Circulation 2019;139:e56–e66. [DOI] [PubMed] [Google Scholar]
- 30.DeCarli C. Clinically asymptomatic vascular brain injury: a potent cause of cognitive impairment among older individuals. J Alzheimers Dis 2013;33(suppl 1):S417–S426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg A, Ngandu T, Rusanen M, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimers Dement 2018;14:263–270. [DOI] [PubMed] [Google Scholar]
- 32.SPRINT MIND Investigators for the SPRINT Research Group, Williamson JD, Pajewski NM, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maillard P, Carmichael O, Fletcher E, Reed B, Mungas D, DeCarli C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology 2012;79:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Zimmerman ME, Narkhede A, et al. White matter hyperintensities and the mediating role of cerebral amyloid angiopathy in dominantly-inherited Alzheimer's disease. PLoS One 2018;13:e0195838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lockhart SN, Mayda AB, Roach AE, et al. Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Front Hum Neurosci 2012;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology 2004;63:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology 2004;63:1591–1599. [DOI] [PubMed] [Google Scholar]
- 38.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995;45:2077–2084. [DOI] [PubMed] [Google Scholar]
- 39.Salerno JA, Murphy DG, Horwitz B, et al. Brain atrophy in hypertension: a volumetric magnetic resonance imaging study. Hypertension 1992;20:340–348. [DOI] [PubMed] [Google Scholar]
- 40.Peters N, Holtmannspotter M, Opherk C, et al. Brain volume changes in CADASIL: a serial MRI study in pure subcortical ischemic vascular disease. Neurology 2006;66:1517–1522. [DOI] [PubMed] [Google Scholar]
- 41.O'Sullivan M, Ngo E, Viswanathan A, et al. Hippocampal volume is an independent predictor of cognitive performance in CADASIL. Neurobiol Aging 2009;30:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fletcher E, Gavett B, Harvey D, et al. Brain volume change and cognitive trajectories in aging. Neuropsychology 2018;32:436–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown SC, Park DC. Theoretical models of cognitive aging and implications for translational research in medicine. Gerontologist 2003;43:57–67. [DOI] [PubMed] [Google Scholar]
- 44.Early DR, Widaman KF, Harvey D, et al. Demographic predictors of cognitive change in ethnically diverse older persons. Psychol Aging 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jack CR Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 2009;132:1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fletcher E, Filshtein TJ, Harvey D, Renaud A, Mungas D, DeCarli C. Staging of amyloid beta, t-tau, regional atrophy rates, and cognitive change in a nondemented cohort: results of serial mediation analyses. Alzheimers Dement 2018;10:382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabin JS, Schultz AP, Hedden T, et al. Interactive associations of vascular risk and beta-amyloid burden with cognitive decline in clinically normal elderly individuals: findings from the Harvard Aging Brain Study. JAMA Neurol 2018;75:1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villemagne VL, Pike KE, Chetelat G, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol 2011;69:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alzheimer's Association. 2019 Alzheimer's disease facts and figures. Alzheimers Dement 2019;15:321–387. [Google Scholar]
- 50.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015;313:1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request. Supplementary file is available from Dryad (doi.org/10.25338/B8P60R).