Abstract
Objectives
To determine the optimal duration of continuous EEG monitoring (CEEG) for electrographic seizure (ES) identification in critically ill children.
Methods
We performed a prospective observational cohort study of 719 consecutive critically ill children with encephalopathy. We evaluated baseline clinical risk factors (age and prior clinically evident seizures) and emergent CEEG risk factors (epileptiform discharges and ictal-interictal continuum patterns) using a multistate survival model. For each subgroup, we determined the CEEG duration for which the risk of ES was <5% and <2%.
Results
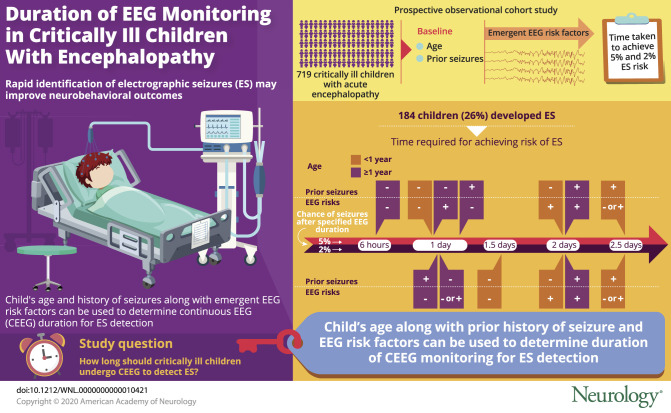
ES occurred in 184 children (26%). Patients achieved <5% risk of ES after (1) 6 hours if ≥1 year without prior seizures or EEG risk factors; (2) 1 day if <1 year without prior seizures or EEG risks; (3) 1 day if ≥1 year with either prior seizures or EEG risks; (4) 2 days if ≥1 year with prior seizures and EEG risks; (5) 2 days if <1 year without prior seizures but with EEG risks; and (6) 2.5 days if <1 year with prior seizures regardless of the presence of EEG risks. Patients achieved <2% risk of ES at the same durations except patients without prior seizures or EEG risk factors would require longer CEEG (1.5 days if <1 year of age, 1 day if ≥1 year of age).
Conclusions
A model derived from 2 baseline clinical risk factors and emergent EEG risk factors would allow clinicians to implement personalized strategies that optimally target limited CEEG resources. This would enable more widespread use of CEEG-guided management as a potential neuroprotective strategy.
ClinicalTrials.gov identifier
Electrographic seizures (ES) occur in 10% to 40% of critically ill children with acute encephalopathy who undergo continuous EEG monitoring (CEEG) in the pediatric intensive care unit (PICU),1–28 and there is increasing evidence that high ES exposure is associated with unfavorable neurobehavioral outcomes.7,10,14,21,22 Because most ES require CEEG for identification, guidelines and consensus statements recommend that children with acute encephalopathy undergo 24 to 48 hours of CEEG,29–31 and survey data indicate that an increasing number of critically ill children undergo CEEG.32 However, substantial equipment and personnel requirements make CEEG resource intense33,34 and limit widespread implementation of ES identification and treatment as a potential neuroprotective strategy. Prediction models that stratify patients by ES risk could address this problem by allowing clinicians to implement targeted strategies in which limited CEEG resources are directed to specific patients for appropriate durations. Prior studies have identified clinical and EEG risk factors for ES1,4–6,8–11,14,15,17,18,20 and combined these risk factors into prediction models that identify which critically ill children should undergo CEEG.35,36 However, these studies have not established the optimal CEEG durations for patients with varying ES risk. Optimizing CEEG duration for individual patients may be important because seemingly small differences in duration have a substantial impact on resource requirements.33,34 In critically ill adults, models are available to determine the optimal CEEG duration for individual patients on the basis of clinical risk factors and emergent CEEG features.37 Given that critically ill children are generally at higher risk for ES than adults,15,38 we aimed to extend this work to the pediatric population. In the context of a large, prospective, and contemporary observational cohort study of consecutive critically ill children undergoing CEEG, we used multistate survival analysis to determine the time-dependent risk of ES based on readily available baseline clinical risk factors and emergent EEG risk factors.
Methods
Standard protocol approvals, registrations, and patient consents
The Institutional Review Board approved the study with a waiver of consent because the study was low risk, required no follow-up data, and required data from consecutive children to avoid bias. We registered the study with ClincialTrials.gov (NCT03419260) and applied Strengthening the Reporting of Observational Studies in Epidemiology reporting standards.39
PICU CEEG prospective observational study
We performed a prospective observational cohort study of consecutive critically ill children with encephalopathy treated in the PICU of a quaternary care hospital between April 2017 and February 2019 who underwent clinically indicated CEEG to screen for ES on the basis of a guideline-adherent29–31 institutional pathway.40 We excluded neonates (<30 days old), patients who received brief postoperative epilepsy surgery care in the PICU, and patients admitted after >2 days of care for refractory status epilepticus at a different institution because the available initial clinical and EEG data were often insufficient.
Video-EEG monitoring was performed with Natus Neuroworks (Middleton, WI) with electrodes placed according to the international 10-20 system. EEG interpretation was performed by the inpatient Electroencephalography Service, and patients were managed clinically by Critical Care Medicine and the Neurology Consultation Services. Prophylactic antiseizure medications were not administered, but patients with epilepsy and some patients with clinically evident seizures before CEEG initiation were receiving antiseizure medications at CEEG onset.
Data acquisition
We prospectively collected clinical and EEG data using a Research Electronic Data Capture database. Clinical data included age, sex, prior neurodevelopmental disorders, medications, CEEG indication, and hospital and PICU admission and discharge dates. We categorized clinically evident seizures before CEEG as being present if they occurred during or just before the acute presentation. We categorized acute neurologic disorders as epilepsy related, acute structural (stroke, CNS inflammation or autoimmune disorder, traumatic brain injury, CNS infection, brain malformation, tumor/oncologic, and hypoxic-ischemic encephalopathy), or acute nonstructural (sepsis, metabolic, pharmacologic sedation, toxin, paralytic administration). The acute neurologic disorder categories were assigned on the basis of the primary presenting problems/diagnoses available at the time of PICU admission. Examples include the following: a patient with epilepsy and a ventriculoperitoneal shunt would be classified as acute symptomatic structural if a shunt malfunction were identified vs epilepsy-related if no shunt problems were identified, and a patient undergoing extracorporeal membrane oxygenation with hypoxic-ischemic brain injury after cardiac arrest would be classified as acute structural, while a patient on extracorporeal membrane oxygenation for sepsis without any known brain injury would be classified as acute nonstructural. We categorized mental status as comatose or not and worse than baseline or not.
A pediatric electroencephalographer (F.W.F.) scored the EEG tracings and clinical reports using standardized critical care EEG terminology41 for which most variables have good interrater reliability.42 EEG data included initiation and discontinuation date and time, background features, and ES and epileptiform discharges timing of onset. As in prior studies of CEEG in critically ill children,10,21,22,35,36,43,44 we categorized the background as normal/sleep, slow-disorganized, discontinuous, burst-suppression, or attenuated-featureless. We scored the presence of sporadic epileptiform discharges, brief rhythmic discharges, and periodic or rhythmic patterns along the ictal-interictal continuum.41 Consistent with prior studies15,21,36 and proposed definitions,45 we defined ES as abnormal paroxysmal events that were different from the background, lasted >10 seconds, had a plausible electrographic field, and had a temporal-spatial evolution in morphology, frequency, and amplitude. If seizures, interictal epileptiform discharges, or ictal-interictal continuum patterns were present, then we recorded the initial date and time at which the finding occurred.
Statistical analyses
We used Stata 15.1 (StataCorp, College Station, TX) for most statistical analyses and the mstate package in R46 (R Foundation for Statistical Computing, Vienna, Austria) for the multistate survival analyses. We reported summary statistics as medians and interquartile ranges (IQRs) for continuous variables and counts and percentages for categorical variables.
We performed multistate survival analysis to predict the overall risk of ES, the risk of ES after the emergence of EEG risk factors, and the risk of ES as a function of CEEG duration. The analyses estimated hazard rates, which represented the instantaneous probability that a participant would transition between 3 states (entry, EEG risk, and ES) (figure 1). These rates were parameterized as the transition probability in the multistate survival model. All participants began in the entry state. Over time, each participant could remain in the entry state (never develop EEG risk factors or ES), transition from the entry state directly to the ES state (develop ES), or transition from the entry state to the EEG risk state (develop EEG risk factors). Subjects in the EEG risk state could either remain in the that state or transition to the ES state. Subjects could not transition back to prior states. The EEG risk state was defined by the occurrence of specific EEG features that predispose to seizures, including sporadic epileptiform discharges, brief rhythmic discharges, periodic discharges (generalized, lateralized, or bilateral independent), lateralized rhythmic delta activity, and lateralized rhythmic spike-wave discharges, as defined by standardized terminology.41 Like adult ES prediction models,37,47 this inclusive approach was taken because each of the individual EEG features was too rare for individual analysis or broad clinical generalizability. Thus, the model estimated 3 rates (entry to EEG risk, entry to ES, and EEG risk to ES) that allowed us to determine the overall risk of ES, the risk of ES after development of EEG risk factors, and the risk of ES given CEEG duration. The analysis made the Markov assumption in which the transition rate does not depend on the prior state(s) or on the time spent within any given state. We used a 72-hour window from the start of CEEG because most participants underwent shorter durations of CEEG. We calculated the 95% confidence bands for the multistate survival analysis using bootstrap with 1,000 resampling.
Figure 1. Summary of the model states (entry state, EEG risk state, and ES state) and transitions.
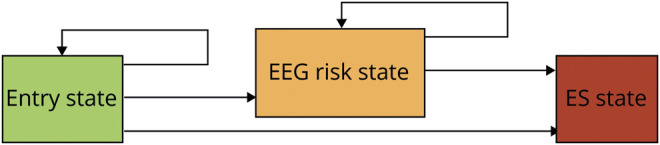
Each participant could remain in the entry state, transition from the entry state to the electrographic seizures (ES) state, or transition from the entry state to the EEG risk state. Subjects in the EEG risk state could remain in the EEG risk state or transition to the ES state.
In addition to performing the multistate survival analysis for the full cohort, we performed multistate survival analyses in which participants were stratified into 4 clinical risk categories made up of 2 dichotomous clinical variables: age (<1 year, ≥1 year) and clinically evident seizures before CEEG initiation (present, absent). We had previously performed multivariate logistic regression on this cohort to identify clinical and EEG factors associated with higher ES risk. Our analysis showed that age (<1 year) and the presence of clinically evident seizures before CEEG initiation were associated with ES.36 Consistent with our findings, these variables have been associated with ES in other cohort studies of critically ill children.1,4–6,8–11,14,15,17,18,20,35 Using these data, we calculated the CEEG duration at which participants were at <5% risk and <2% risk of experiencing ES for the full cohort and each of the 4 clinical risk categories.
We performed several additional analyses. First, we performed 2 alternative multistate survival analyses using different clinical variables to generate the 4 clinical risk categories. The first model included age (dichotomized at 1 year) and epilepsy-seizure (a combined variable scored as positive if there were clinically evident seizures before CEEG initiation or a prior diagnosis of epilepsy). The second model included age (dichotomized at 1 year) and coma (present or absent), intended to recapitulate an adult study in which a comatose state was associated with ES occurrence.37 Second, we explored whether the 2 clinical predictors (age and clinically evident seizures before CEEG initiation) were associated with CEEG duration. In addition, we evaluated the association of CEEG duration with age or prior seizures using the Wilcoxon rank-sum test and linear regression. The outcome variable was log-transformed as appropriate to meet the normality assumption for linear regression.
Data availability
We will make the underlying data available to investigators with appropriate data transfer and institutional review board approval.
Results
We enrolled 719 consecutive critically ill children who underwent CEEG. The median age was 5.6 (IQR 1.4–12.9) years. ES occurred in 184 children (26%). Table 1 provides a summary of the cohort's clinical and EEG data. Figure 2 (top row) provides swimmer plot displays of the CEEG timelines for the full cohort and stratified by each of the 4 clinical risk categories. Among the 719 patients, 16 patients (2.2%) had ES identified at the onset of CEEG (started in the ES state), 21 patients (2.9%) had EEG risk factors identified at the onset of CEEG (started in the EEG risk state), and 682 patients (94.8%) began in the entry state. Eighteen patients were excluded from further analysis, including 16 patients with ES at the onset of CEEG and 2 patients who remained in the entry state throughout but for whom data on CEEG duration were unavailable. The probability of transitioning from the entry state directly into the ES state was 0.094 (95% confidence interval [CI] 0.072–0.115, 64 of 680 patients) at a median time of 0.98 (IQR 0.26–5.23) hours. The probability of transitioning from the entry state to the EEG risk state was 0.338 (95% CI 0.303–0.374, 230 of 680 patients) at a median time of 0.38 (IQR 0.07–3.51) hours. The EEG risk state included 251 participants, including 21 participants in that state at the start of CEEG plus 230 participants who transitioned from the entry state to the EEG risk state. The probability of transitioning from the EEG risk state to the ES state was 0.414 (95% CI 0.356–0.475, 104 of 251 patients) at a median time of 1.95 (IQR 0.26–7.09) hours.
Table 1.
Summary of clinical and EEG data presented as counts (percentage) or median (IQR)
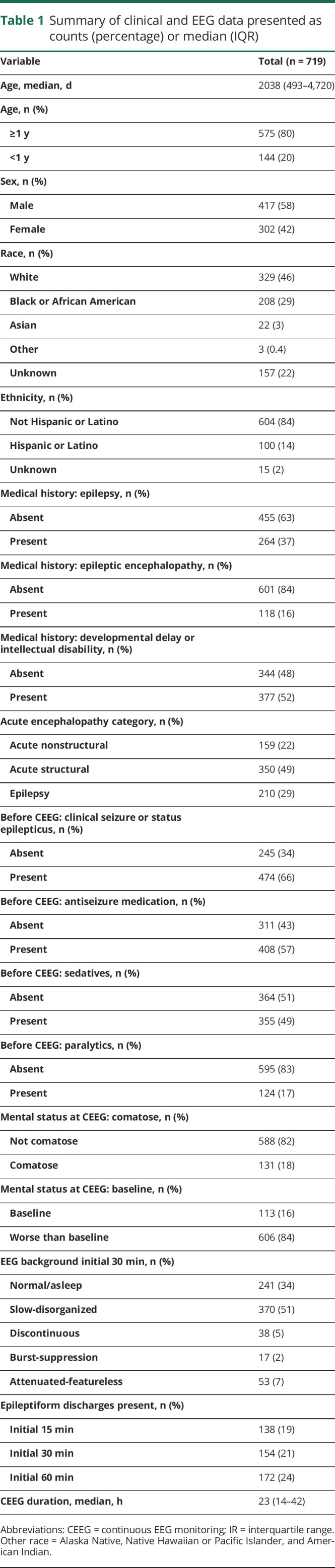
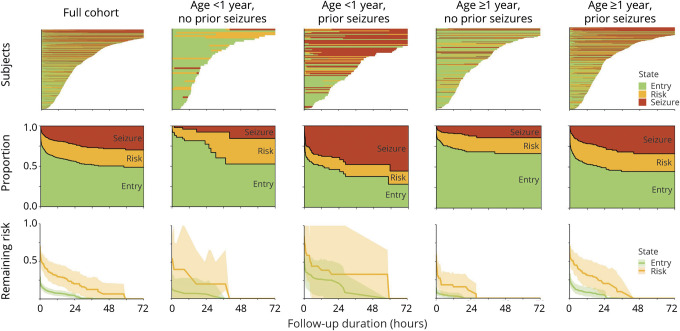
Figure 2. Swimmer plots and multistate survival analysis results for the full cohort and each clinical risk state.
Top row: swimmer plot showing the duration of continuous EEG monitoring (line length along x-axis) and state (entry = green; EEG risk = yellow; electrographic seizures [ES] = red) for each participant (y-axis). Middle row: proportion of participants in each state (entry, ES risk, ES) over time. Some participants remain in the same state over time (same color); some participants develop EEG risk factors but never experience ES (green to yellow transition); some participants experience ES without having experienced EEG risk factors (green to red transition); and some participants experience EEG risk factors and then ES (green to yellow to red transitions). Bottom row: remaining risk of transitioning to the ES state for patients in the entry (green) and EEG risk (yellow) states. Shaded areas represent the 95% confidence intervals. For the full cohort and each of the 4 clinical risk states, the risk of transitioning to the ES state remains higher over time for participants with EEG risk factors than those in the entry state.
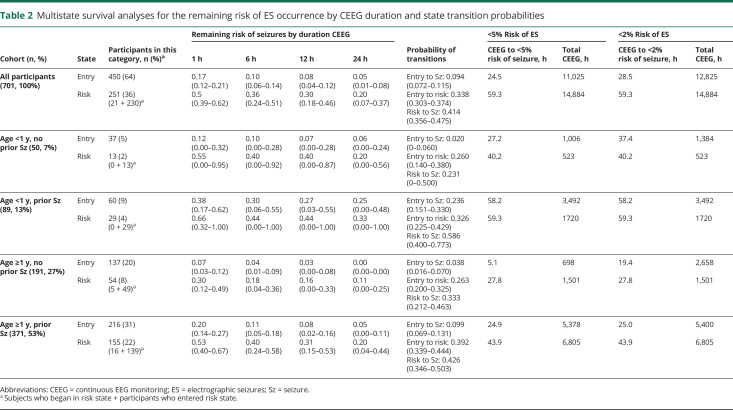
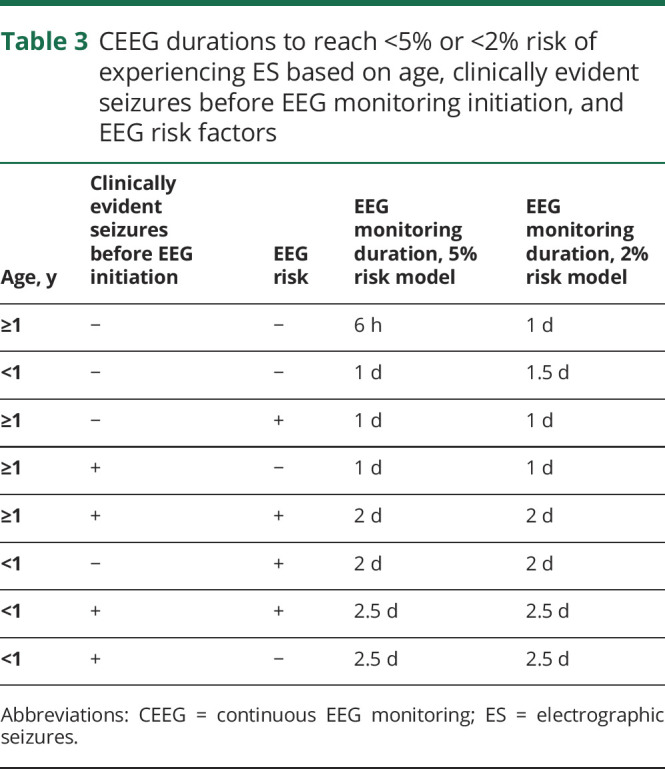
Table 2 provides the risk of developing ES in patients who were in the entry state or EEG risk state after 1, 6, 12, or 24 hours of CEEG for the full cohort and stratified by each of the 4 clinical risk categories. Figure 2 (bottom row) displays the calculated remaining risk of transitioning to the ES state as a function of CEEG duration and EEG risk state for the full cohort and each of the 4 clinical risk categories. For the full cohort, 24.5 hours or 28.5 hours of CEEG was required for participants to reach <5% or <2% risk of transitioning from the entry state to the ES state. For the full cohort, 59.3 hours of CEEG was required for participants to reach both <5% and <2% risk of transitioning from the EEG risk state to the ES state. As shown in figure 2 (middle and bottom rows) and table 2, stratification by clinical risk factor category substantially affected the CEEG duration at which participants would have <5% or <2% of transitioning to the ES state. Patients ≥1 year of age without prior seizures or EEG risk factors reached <5% risk of ES after ≈6 hours of CEEG. Patients <1 year of age without prior seizures or EEG risk factors and patients ≥1 year of age with either prior seizures or EEG risk factors achieved <5% risk of ES after ≈24 hours of CEEG. Patients ≥1 year of age with prior seizures and EEG risk factors and patients <1 year of age without prior seizures but with EEG risk factors achieved <5% risk of ES after ≈2 days of CEEG. Patients <1 year of age with prior seizures achieved <5% risk of ES after ≈2.5 days of CEEG regardless of the presence or absence of EEG risk factors (table 3). The analyses were repeated for the CEEG duration at which participants had <2% risk of ES, and the durations were comparable to the <5% risk model. However, if the 2% risk model were applied, then participants of all ages without prior seizures or EEG risk factors would undergo longer CEEG (37.4 hours if <1 year of age and 19.4 hours if ≥1 year of age) (table 3).
Table 2.
Multistate survival analyses for the remaining risk of ES occurrence by CEEG duration and state transition probabilities
Table 3.
CEEG durations to reach <5% or <2% risk of experiencing ES based on age, clinically evident seizures before EEG monitoring initiation, and EEG risk factors
If the model were applied to the 701 participants, then participants would undergo about 21,123 hours of CEEG using the <5% risk of ES model and 23,483 hours of CEEG using the <2% risk of ES model. Guideline and consensus statement recommendations29–31 would yield 16,824 and 33,648 hours of CEEG for 24 or 48 hours of CEEG, respectively. Thus, if the models were applied, there would be a 26% (5% risk model) or 39% (2% risk model) increase in CEEG hours performed compared to all participants undergoing 24 hours of CEEG and a 37% (5% risk model) or 30% (2% risk model) reduction in CEEG hours performed compared to all participants undergoing 48 hours of CEEG.
As secondary analyses, we performed 2 additional multistate survival analyses using different clinical variables to define the 4 clinical risk categories. The first analysis used age and epilepsy-seizure to define the 4 clinical risk categories, and model performance was comparable to the main model given that many patients with prior diagnosis of epilepsy presented with clinically evident seizures before CEEG initiation. The second analysis used age and coma to define the 4 clinical risk categories. Patients with and without coma had similar predicted probabilities of being in each of the 3 states.
Finally, we evaluated whether CEEG duration was associated with age or prior seizures. We applied the Wilcoxon rank-sum test due to the skewness of CEEG duration and found that prior seizures were not associated with CEEG duration (p = 0.98; median 22 [IQR 14–22] vs 24 [IQR 14–22] hours in patients with and without prior seizures, respectively), while age was significantly associated with CEEG duration (p = 0.007; median 30 [IQR 17–50] vs 22 [IQR 14–41] hours in younger and older patients, respectively). However, after controlling for ES occurrence in a multiple linear regression model on log-transformed CEEG duration, age was not significant (p = 0.07), while the ES occurrence was significant (p < 0.001). This probably occurred because younger patients were more likely to experience ES than older patients (p = 0.03; 33% vs 24%) and patients with ES underwent longer CEEG (p < 0.01; median 42 [IQR 24–74] vs 20 [IQR 13–34] hours in patients with and without ES, respectively). We also applied multiple linear regression including age, prior seizures, and their interaction. In this model, neither age (p = 0.45) nor prior seizures (p = 0.67) were significant, whereas their interaction was significantly associated with CEEG duration (p = 0.03, coefficient 25.7, 95% CI 3.1–48.3), suggesting that for younger patients (<1 year of age) the difference in CEEG duration between those with and without prior seizures is higher compared to older patients (≥1 year of age).
Discussion
The incidence of ES in this cohort was 26%, in accordance with the 10% to 40% range of incidences reported by studies of critically ill children undergoing CEEG.1–28 Given emerging evidence that high ES exposure is associated with unfavorable neurobehavioral outcomes7,10,14,21,22 and that management with existing antiseizure medications is often feasible and safe,48,49 rapid ES identification and aggressive treatment have been proposed as clinical goals. Guideline and consensus statements recommend that children with acute encephalopathy undergo 24 to 48 hours of CEEG to identify ES,29–31 and survey data document increasing CEEG use among critically ill children.32 Given that CEEG is resource intense,33,34 evidence-based strategies that allocate CEEG resources to patients at greatest risk for experiencing ES for the appropriate durations would be valuable. Prior studies have identified risk factors for ES1,4–6,8–11,14,15,17,18,20 and combined them to generate prediction models to help guide decisions on CEEG initiation.35,36 However, unlike in critically ill adult studies,37 pediatric studies have not addressed the optimal duration for CEEG. Our prior work showed that the cost-effectiveness of CEEG is substantially affected by both ES risk and CEEG duration. At every level of ES risk, strategies with longer CEEG duration had incrementally lower cost-effectiveness than strategies with shorter CEEG, but the differences became much more disparate as the risk of ES decreased.34 Therefore, strategies for refining CEEG duration are crucial for maintaining cost-effectiveness.
An effective ES prediction model would enable clinicians to implement evidence-based and tailored CEEG strategies that optimally target limited CEEG resources and minimize excessive monitoring. We performed a large and prospective study of consecutive critically ill encephalopathic children undergoing CEEG and used multistate survival analysis to stratify the risk of ES based on 2 readily available clinical variables (age and clinically evident seizures before CEEG) and emergent EEG risk factors. While we intended to follow a guideline-adherent29–31 institutional CEEG pathway,40 it is noteworthy that the median duration of CEEG was only 23 hours. This suggests that clinicians are already making practical decisions about CEEG duration that are clinically driven, reinforcing the need to ensure that these tailored approaches are evidence based. We found that when critically ill children were stratified on the basis of this combination of clinical and emergent EEG risk factors, the risk of ES decreased to <5% at various CEEG durations ranging from 6 hours to 2.5 days. If this model had been implemented for this cohort, then CEEG use would have increased by 26% compared with the 24-hour guideline recommendation or decreased by 37% compared to the 48-hour guideline recommendation.29–31 Thus, despite being more personalized and precise, the total CEEG requirement would be similar or even reduced compared to current guideline-adherent practice. If clinicians aimed to perform CEEG until a patient were at <2% risk of ES, then longer CEEG durations would be required for patients of all ages without prior seizures or emergent EEG risk factors, yielding a 39% increase in CEEG compared to the 24-hour guideline recommendation or 30% decrease compared to the 48-hour guideline recommendation.
The multistate survival analysis approach permitted adjustment for varying CEEG durations and inclusion of emergent EEG risk factors. The approach was modeled after a study of 665 consecutive critically ill adults from 2 institutions in which the incidence of ES was 23%.37 The study used a similar survival analysis approach to evaluate 2 clinical factors (coma and history of seizures) and the same emergent EEG risk factors. CEEG durations at which the risk for ES was <5% ranged from 0.4 to 44.2 hours.37 The EEG findings that defined the EEG risk state were similar in both studies and were based on EEG risk factors elucidated in prior studies of both critically ill children1,4,5,11,16 and critically ill adults.47,50 However, the studies used different clinical variables to stratify participants: age in the current pediatric study vs coma in adult study. The clinical variables in this current study were derived from a multivariable model that identified ES risk factors among critically ill children.36 These variables are consistent with other studies in which both younger age5,10,12,13,15,35 and the occurrence of clinically evident seizures before CEEG3,5–7,10–12,15–18 were associated with an increased risk for ES. While coma was associated with an increased risk for ES in adults,37 it has not been identified as an ES predictor in children. The prevalence of preexisting neurologic disorders, including baseline encephalopathy, is higher in children than in adults undergoing CEEG, potentially making the degree of encephalopathy less meaningful for stratification. In addition, whereas in the adult study patients with prior clinical seizures and patients with prior epilepsy were combined, in the current study, prior clinically evident seizures referred only to patients with clinically evident seizures during the acute presentation. We have made a distinction between patients with clinical seizures before CEEG and prior epilepsy for the following reasons: (1) in children, there may be more uncertainty about a definitive diagnosis of epilepsy (given the more common occurrence of provoked seizures, febrile seizures, and previous neurologic disorders that may not predispose to future epilepsy); and (2) we aimed to derive the model from variables apparent at the time of acute presentation with little need for information regarding medical history. However, this difference may not be substantial because a secondary analysis indicated that a model that combined clinically evident seizures before CEEG initiation with prior diagnosis of epilepsy performed similarly.
This study has several strengths. First, this was a prospective cohort study and therefore provided the optimal design for estimating incidence and identifying risk factors. Second, this was a pragmatic observational study and was therefore representative of current clinical practice with variable risks and manifestations. Third, the use of a well-established clinical pathway40 ensured consistent CEEG indications and performance. Fourth, the waiver of consent allowed inclusion of all consecutive patients. Fifth, all data were collected during the acute period, thereby avoiding loss to follow-up, withdrawal, or missing data. Sixth, the EEG variables were derived using standardized terminology41 with previously documented good interrater reliability.42 Seventh, the EEG data were derived from research-based review of the raw EEG tracing by a pediatric electroencephalographer in addition to the EEG reports rather than review of only the EEG reports. Eighth, the current cohort was similar to prior cohorts regarding the overall ES incidence1–28 and ES risk factors,1,4–6,8–11,14,15,17,18,20 suggesting validity and generalizability. Ninth, we used an advanced statistical approach (multistate survival analysis) to account for both stable baseline clinical predictors and emergent EEG predictors. Finally, while prior studies have identified ES risk factors and combined them to guide decisions regarding CEEG initiation,35,36 this study extends that work to create an evidence-based approach to determining CEEG duration.
There are several limitations to this study. First, the model requires validation in a separate cohort. Second, the study was conducted at a single center, and while the overall ES incidence and risk factors were consistent with prior studies, the variety of patients and disorders at this quaternary care hospital may differ from that in other hospitals. Thus, generalizability may be enhanced by replication at other centers or multicenter studies. Third, the median duration of CEEG was 23 hours, and although survival analysis accounted for participant dropout over time, bias could still arise if those participants are systemically different than remaining participants. Future studies, including validation studies, might benefit from performing CEEG for several additional days longer than clinically indicated in all participants to measure the decay of ES risk over time. Fourth, EEG scoring was performed by a single pediatric electroencephalographer. While EEG scoring used standardized terminology41 documented to have good interrater reliability,42 validation studies might benefit from multirater review of EEG data. Fifth, like a similar adult study,37 the EEG risk state combined numerous EEG patterns, which may each confer varying ES risk. A larger study could analyze the various patterns contained in the current EEG risk state individually, as well as other EEG features such as background components. Finally, critically ill patients can experience disease progression during their PICU course that might change their ES risk over time. Thus, further study is needed to determine how to use ES prediction models over an evolving PICU course.
Prediction models may be particularly beneficial in complex clinical situations with opportunities to reduce healthcare costs without compromising care, as is the case for CEEG in critically ill patients. Centers with limited CEEG resources unable to implement CEEG for large numbers of patients may be able to use more targeted CEEG strategies. Conversely, centers with substantial CEEG resources could improve the value of their services by refining CEEG duration. If it is validated in subsequent prospective studies, clinicians could use this approach to progress from broad, all-purpose guidelines for CEEG indications and duration29–31 to an evidence-based and targeted strategy in which patients undergo personalized durations of CEEG based on a limited number of readily available clinical and EEG variables. This advancement is a critical step in making widespread implementation of CEEG-guided management a viable neuroprotective strategy.
Glossary
- CEEG
continuous EEG monitoring
- CI
confidence interval
- ES
electrographic seizures
- IQR
interquartile range
- PICU
pediatric intensive care unit
Appendix. Authors
Study funding
N.S. Abend is funded by NIH K02NS096058.
Disclosure
F.W. Fung, J. Fan, and L. Vala report no disclosures. M. Jacobwitz, D.S. Parikh, M. Donnelly, A.A. Topjian, and R. Xiao report no disclosures. N.S. Abend reports funding from NIH (National Institute of Neurological Disorders and Stroke) K02NS096058 for this study and other funding from the Patient-Centered Outcomes Research Institute and royalties from Demos Publishing. Go to Neurology.org/N for full disclosures.
References
- 1.Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol 2006;63:1750–1755. [DOI] [PubMed] [Google Scholar]
- 2.Tay SK, Hirsch LJ, Leary L, Jette N, Wittman J, Akman CI. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia 2006;47:1504–1509. [DOI] [PubMed] [Google Scholar]
- 3.Shahwan A, Bailey C, Shekerdemian L, Harvey AS. The prevalence of seizures in comatose children in the pediatric intensive care unit: a prospective video-EEG study. Epilepsia 2010;51:1198–1204. [DOI] [PubMed] [Google Scholar]
- 4.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology 2009;72:1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia 2011;52:1130–1136. [DOI] [PubMed] [Google Scholar]
- 6.Greiner HM, Holland K, Leach JL, Horn PS, Hershey AD, Rose DF. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics 2012;129:e748–e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med 2012;38:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arango JI, Deibert CP, Brown D, Bell M, Dvorchik I, Adelson PD. Posttraumatic seizures in children with severe traumatic brain injury. Childs Nerv Syst 2012;28:1925–1929. [DOI] [PubMed] [Google Scholar]
- 9.Piantino JA, Wainwright MS, Grimason M, et al. Nonconvulsive seizures are common in children treated with extracorporeal cardiac life support. Pediatr Crit Care Med 2013;14:601–609. [DOI] [PubMed] [Google Scholar]
- 10.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology 2013;81:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCoy B, Sharma R, Ochi A, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia 2011;52:1973–1978. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber JM, Zelleke T, Gaillard WD, Kaulas H, Dean N, Carpenter JL. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care 2012;17:31–38. [DOI] [PubMed] [Google Scholar]
- 13.Arndt DH, Lerner JT, Matsumoto JH, et al. Subclinical early posttraumatic seizures detected by continuous EEG monitoring in a consecutive pediatric cohort. Epilepsia 2013;54:1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014;137:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology 2011;76:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold JJ, Crawford JR, Glaser C, Sheriff H, Wang S, Nespeca M. The role of continuous electroencephalography in childhood encephalitis. Pediatr Neurol 2014;50:318–323. [DOI] [PubMed] [Google Scholar]
- 17.Vlachy J, Jo M, Li Q, et al. Risk factors for seizures among young children monitored with continuous electroencephalography in intensive care unit: a retrospective study. Front Pediatr 2018;6:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sansevere AJ, Duncan ED, Libenson MH, Loddenkemper T, Pearl PL, Tasker RC. Continuous EEG in pediatric critical care: yield and efficiency of seizure detection. J Clin Neurophysiol 2017;34:421–426. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez Fernandez I, Sansevere AJ, Gainza-Lein M, Buraniqi E, Tasker RC, Loddenkemper T. Time to continuous electroencephalogram in repeated admissions to the pediatric intensive care unit. Seizure 2018;54:19–26. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez SM, Arndt DH, Carpenter JL, et al. Electroencephalography monitoring in critically ill children: current practice and implications for future study design. Epilepsia 2013;54:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med 2013;41:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology 2014;82:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasbani DM, Topjian AA, Friess SH, et al. Nonconvulsive electrographic seizures are common in children with abusive head trauma. Pediatr Crit Care Med 2013;14:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Neill BR, Handler MH, Tong S, Chapman KE. Incidence of seizures on continuous EEG monitoring following traumatic brain injury in children. J Neurosurg Pediatr 2015;16:167–176. [DOI] [PubMed] [Google Scholar]
- 25.Vaewpanich J, Reuter-Rice K. Continuous electroencephalography in pediatric traumatic brain injury: seizure characteristics and outcomes. Epilepsy Behav 2016;62:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gwer S, Idro R, Fegan G, et al. Continuous EEG monitoring in Kenyan children with non-traumatic coma. Arch Dis Child 2012;97:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostendorf AP, Hartman ME, Friess SH. Early electroencephalographic findings correlate with neurologic outcome in children following cardiac arrest. Pediatr Crit Care Med 2016;17:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez Fernandez I, Sansevere AJ, Guerriero RM, et al. Time to electroencephalography is independently associated with outcome in critically ill neonates and children. Epilepsia 2017;58:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 30.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol 2015;32:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol 2015;32:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG monitoring: current resources and practice in the United States and Canada. J Clin Neurophysiol 2013;30:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez-Colina AM, Topjian AA, Dlugos DJ, Abend NS. EEG monitoring in critically ill children: indications and strategies. Pediatr Neurol 2012;46:158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abend NS, Topjian AA, Williams S. How much does it cost to identify a critically ill child experiencing electrographic seizures? J Clin Neurophysiol 2015;32:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang A, Arndt DH, Berg RA, et al. Development and validation of a seizure prediction model in critically ill children. Seizure 2015;25:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fung FW, Jacobwitz M, Parikh DS, et al. Development of a model to predict electroencephalographic seizures in critically ill children. Epilepsia 2020;61:498–508. [DOI] [PubMed] [Google Scholar]
- 37.Struck AF, Osman G, Rampal N, et al. Time-dependent risk of seizures in critically ill patients on continuous electroencephalogram. Ann Neurol 2017;82:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004;62:1743–1748. [DOI] [PubMed] [Google Scholar]
- 39.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 40.Children's Hospital of Philadelphia. Critical Care Pathway for EEG Monitoring [online]. Available at: chop.edu/clinical-pathway/critical-care-pathway-eeg-monitoring-clinical-pathways. Accessed July 20, 2019. [Google Scholar]
- 41.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 42.Abend NS, Gutierrez-Colina A, Zhao H, et al. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. J Clin Neurophysiol 2011;28:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abend NS, Wiebe DJ, Xiao R, et al. EEG factors after pediatric cardiac arrest. J Clin Neurophysiol 2018;35:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abend NS, Xiao R, Kessler SK, Topjian AA. Stability of early EEG background patterns after pediatric cardiac arrest. J Clin Neurophysiol 2018;35:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia 2013;54(suppl 6):28–29. [DOI] [PubMed] [Google Scholar]
- 46.de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed 2010;99:261–274. [DOI] [PubMed] [Google Scholar]
- 47.Struck AF, Ustun B, Ruiz AR, et al. Association of an electroencephalography-based risk score with seizure probability in hospitalized patients. JAMA Neurol 2017;74:1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fung FW, Jacobwitz M, Vala L, et al. Electroencephalographic seizures in critically ill children: management and adverse events. Epilepsia 2019;60:2095–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abend NS, Sanchez SM, Berg RA, Dlugos DJ, Topjian AA. Treatment of electrographic seizures and status epilepticus in critically ill children: a single center experience. Seizure 2013;22:467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westover MB, Shafi MM, Bianchi MT, et al. The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol 2015;126:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We will make the underlying data available to investigators with appropriate data transfer and institutional review board approval.