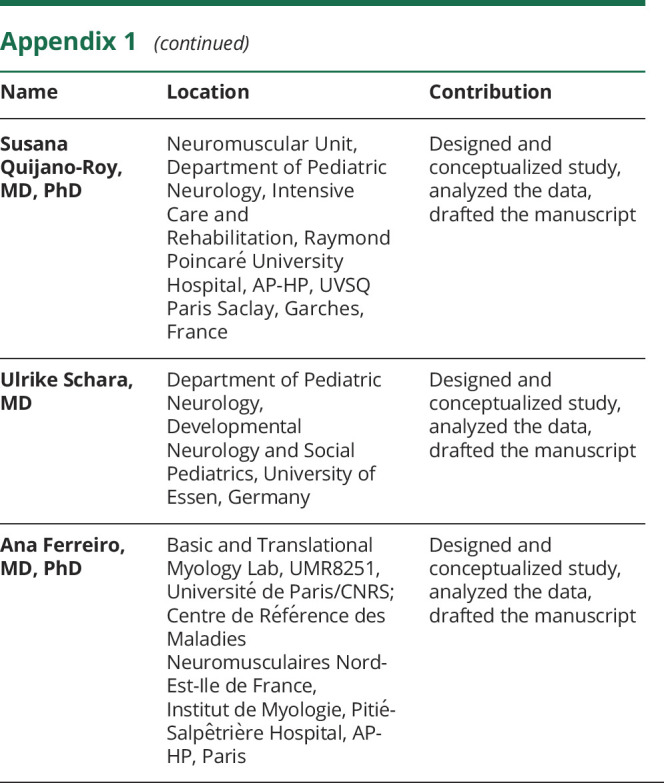
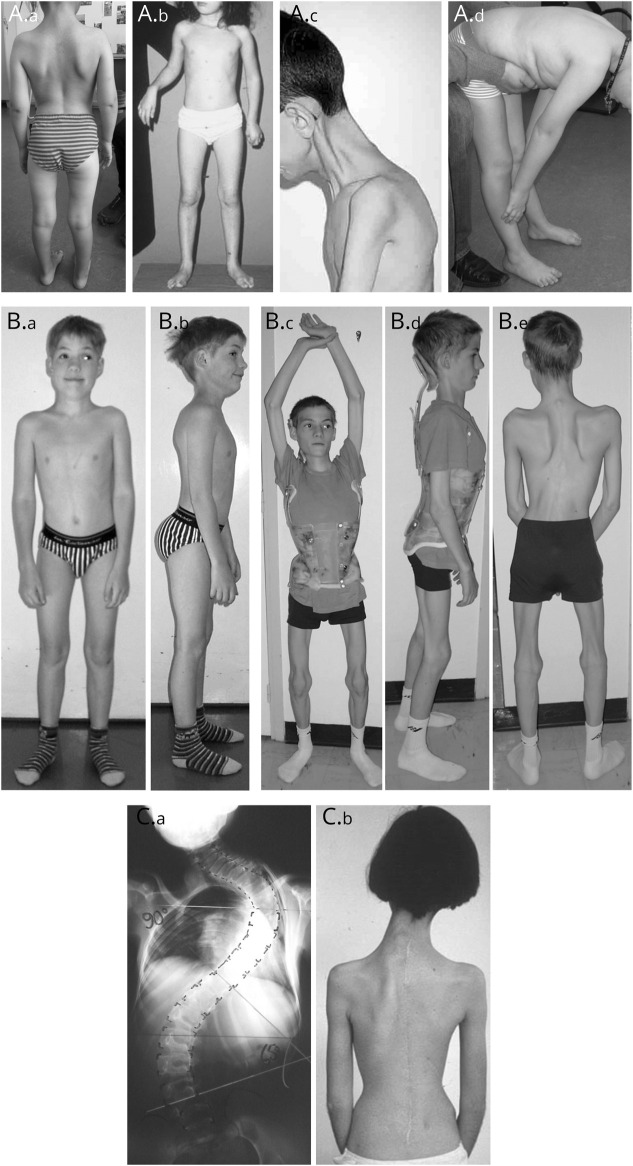
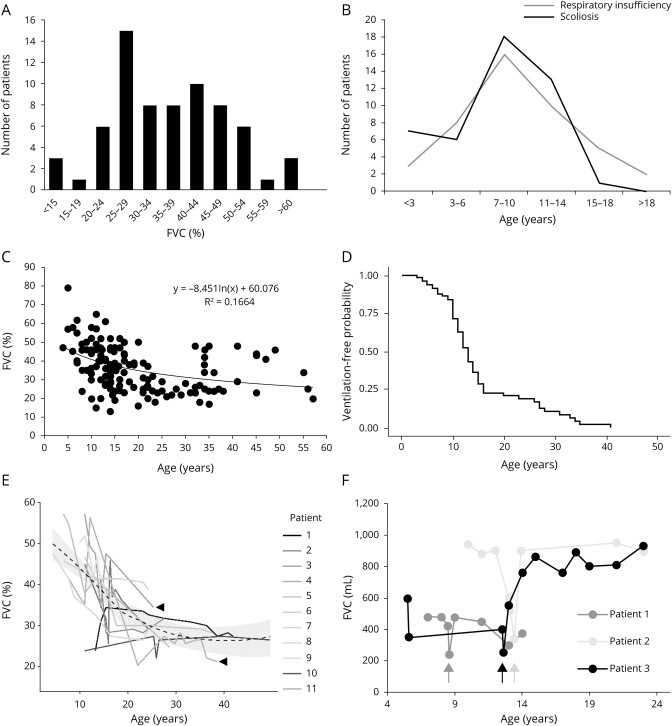
Rocio N Villar-Quiles
Rocio N Villar-Quiles, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Maja von der Hagen
Maja von der Hagen, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Corinne Métay
Corinne Métay, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Victoria Gonzalez
Victoria Gonzalez, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Sandra Donkervoort
Sandra Donkervoort, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Enrico Bertini
Enrico Bertini, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Claudia Castiglioni
Claudia Castiglioni, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Denys Chaigne
Denys Chaigne, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Jaume Colomer
Jaume Colomer, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Maria Luz Cuadrado
Maria Luz Cuadrado, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Marianne de Visser
Marianne de Visser, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Isabelle Desguerre
Isabelle Desguerre, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Bruno Eymard
Bruno Eymard, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Nathalie Goemans
Nathalie Goemans, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Angela Kaindl
Angela Kaindl, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Emmanuelle Lagrue
Emmanuelle Lagrue, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Jürg Lütschg
Jürg Lütschg, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Edoardo Malfatti
Edoardo Malfatti, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Michèle Mayer
Michèle Mayer, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Luciano Merlini
Luciano Merlini, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
David Orlikowski
David Orlikowski, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Ulrike Reuner
Ulrike Reuner, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Mustafa A Salih
Mustafa A Salih, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Beate Schlotter-Weigel
Beate Schlotter-Weigel, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Mechthild Stoetter
Mechthild Stoetter, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Volker Straub
Volker Straub, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Haluk Topaloglu
Haluk Topaloglu, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
J Andoni Urtizberea
J Andoni Urtizberea, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Anneke van der Kooi
Anneke van der Kooi, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Ekkehard Wilichowski
Ekkehard Wilichowski, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Norma B Romero
Norma B Romero, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Michel Fardeau
Michel Fardeau, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Carsten G Bönnemann
Carsten G Bönnemann, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Brigitte Estournet
Brigitte Estournet, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Pascale Richard
Pascale Richard, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Susana Quijano-Roy
Susana Quijano-Roy, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Ulrike Schara
Ulrike Schara, MD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,
Ana Ferreiro
Ana Ferreiro, MD, PhD
1From the Basic and Translational Myology Lab (R.N.V.-Q., V.G., A.F.), UMR8251, Université de Paris/CNRS; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (R.N.V.-Q., B. Eymard, N.B.R., A.F.) and Neuromuscular Morphology Unit (N.B.R., M.F.), Institut de Myologie, Pitié-Salpêtrière Hospital, AP-HP, Paris, France; Department of Paediatric Neurology (M.v.d.H.), Medinzinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Germany; AP-HP (C.M., P.R.), Centre de Génétique Moléculaire et Chromosomique, UF Cardiogénétique et Myogénétique Moléculaire et Cellulaire, GH Pitié-Salpêtrière, Paris; Department of Neurology (V.G.), University Hospital of Montpellier, France; Neuromuscular and Neurogenetic Disorders of Childhood Section (S.D.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Unit of Neuromuscular and Neurodegenerative Disorders (E.B.), Bambino Gesu' Children's Research Hospital, Rome, Italy; Departamento de Neurología Pediátrica (C.C.), Clínica Las Condes, Santiago, Chile; Paediatrics Department (D.C.), Hôpital de Hautepierre, Strasbourg, France; Neuromuscular Unit (J.C.), Neuropaediatrics Department, Institut de Recerca Hospital Universitari Sant Joan de Deu, Barcelona; Center for the Biomedical Research on Rare Diseases (CIBERER) (J.C.), ISCIII; Department of Neurology (M.L.C.), Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos; Department of Medicine (M.L.C.), Universidad Complutense de Madrid, Spain; Department of Neurology (M.d.V.), Amsterdam University Medical Centre, Amsterdam Neuroscience, the Netherlands; Department of Pediatric Neurology (I.D.), Necker Enfants Malades Hospital, Paris Descartes University, France; Department of Child Neurology (N.G.), University Hospitals Leuven, Belgium; Department of Pediatric Neurology (A.K.), Center for Chronically Sick Children, Institute of Cell Biology and Neurobiology, Charité-Universitätsmedizin Berlin, Germany; Department of Neuropediatrics (E.L.), CHRU de Tours, Université François Rabelais de Tours, UMR INSERM U1253, Tours, France; Department of Neuropediatrics (J.L.), University Children's Hospital of Basel (UKBB), Switzerland; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (E.M.), Neurology Department, Raymond-Poincaré Hospital, AP-HP, Garches; Centre de Référence des Maladies Neuromusculaires Nord/Est/Ile de France (M.M.), Service de Neuropédiatrie, Hôpital Trousseau, Paris, France; Department of Biomedical and Neuromotor Sciences (L.M.), University of Bologna, Italy; Réanimation Médicale, Physiologie-Explorations Fonctionnelles et Centre d'Investigation Clinique, UMR 1429 (D.O.), INSERM-UMR, 1179, UVSQ (D.O.), and Neuromuscular Unit, Department of Pediatric Neurology, Intensive Care and Rehabilitation, AP-HP, UVSQ Paris Saclay (B. Estournet, S.Q.-R.), Hôpital Raymond Poincaré, Garches, France; Department of Neurology (U.R.), Medizinische Fakultät Carl Gustav Carus Technische Universität Dresden, German; Division of Pediatric Neurology, Department of Pediatrics (M.A.S.), College of Medicine, King Saud University, Riyadh, Saudi Arabia; Friedrich-Baur-Institut (B.S.-W.), Department of Neurology, Ludwig-Maximilians-University of Munich; Department of Pediatric Neurology (M.S.), University of Tübingen, Germany; The John Walton Muscular Dystrophy Research Centre (V.S.), Institute of Genetic Medicine, Newcastle University, Newcastle Hospitals NHS Foundation Trust, UK; Department of Child Neurology (H.T.), Hacettepe University, School of Medicine, Ankara, Turkey; Centre de Compétence Neuromusculaire (J.A.U.), Hôpital Marin, Hendaye, France; Department of Neurology (A.v.d.K.), Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, the Netherlands; Pediatrics and Adolescent Medicine, Division of Pediatric Neurology (E.W.), University Medical Center Göttingen, Georg-August University Göttingen, Germany; Neuromuscular and Neurogenetic Disorders of Childhood Section (C.G.B.), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; and Department of Pediatric Neurology (U.S.), Developmental Neurology and Social Pediatrics, University of Essen, Germany.
1,✉