Abstract
Objective
The association of limbic-predominant age-related transactive response DNA-binding protein 43 encephalopathy neuropathologic change (LATE-NC) with cognition and dementia was assessed in community-dwelling Black elders, and racial differences in these associations were tested.
Methods
Black (n = 76) and White (n = 152) decedents from 4 longitudinal clinical pathologic studies of aging were matched 2 to 1 by age at death, sex, years of education, dementia status, and follow-up time. LATE-NC detected by immunohistochemistry was dichotomized into none/mild and moderate/severe groups. Distribution and clinical and pathologic characteristics of LATE-NC and its association with cognitive profiles and odds of dementia were determined in Black decedents, and racial differences in these associations were assessed.
Results
The overall frequency of LATE-NC in Black and White decedents was similar (40.8% vs 45.4%). Black decedents with moderate/severe LATE-NC were older, had significantly lower global cognition scores, particularly in memory domains, and had higher frequency of Alzheimer disease, hippocampal sclerosis, and cerebral amyloid angiopathy than the LATE-NC none/mild group. LATE-NC in Black decents was independently associated with impaired global cognition, episodic and semantic memory, and visuospatial abilities. There were no racial differences in clinical features or pathologic distribution of LATE-NC except for a significant increase in the mean cytoplasmic inclusions in the entorhinal and mid temporal cortices in White compared to Black decedents. In addition, no racial differences in the cognitive profiles or the odds of dementia were observed in Black vs White decedents.
Conclusions
Consistent with findings in White decedents, LATE-NC in Black decedents is associated with impaired cognition, including memory domains.
More than a decade has elapsed since the initial reports of the localization of the transactive response DNA-binding protein 43 (TDP-43) in brains of frontotemporal lobar degeneration cases and in brain and spinal cord of amyotrophic lateral sclerosis cases.1,2 In these studies, neurons and glia showed loss of normal nuclear TDP-43 immunoreactivity associated with translocation of TDP-43 into the cytoplasm and neurites in the form of inclusions, findings referred to as TDP-43 pathology or proteinopathy. TDP-43 proteinopathy was subsequently reported in Alzheimer disease (AD),3 Lewy body diseases,4,5 age-related hippocampal sclerosis (HS),6,7 and chronic traumatic encephalopathy.8 TDP-43 pathology was also reported in the aging brain in the absence of a pathologic diagnosis of AD.4,9,10 Recently, localization of TDP-43 pathology predominantly in the limbic areas with or without coexisting HS occurring in older adults (>80 years of age) has been the focus of attention, especially because these patients have an amnestic dementia syndrome that mimics AD. This syndrome is now called limbic-predominant age-related TDP-43 encephalopathy (LATE),11 and the neuropathologic findings are referred to as LATE neuropathologic change (LATE-NC). This terminology is used in this article.
Most reported studies of LATE-NC have been performed in White decedents. Therefore, little is known about LATE-NC in Black decedents. LATE-NC is known to be independently related to lower function in global cognition and episodic and semantic memory in White decedents.9 It is well documented that members of racial minority populations, including older Black patients, have increased risk for cognitive impairment and an almost 2-fold increased risk for AD,12–15 but reasons for the racial disparities remain unknown. One possibility to account for this disparity is an increased frequency or severity of LATE-NC in Black decedents with greater impairment in cognition from this pathology than observed in White decedents. Although no current treatment exists for LATE-NC, understanding racial differences in this pathology could provide important clues for future therapeutic targets and ultimately could have implications for health care in a vulnerable, at-risk population. This study was undertaken to document the distribution and severity of LATE-NC in Black decedents, to compare the clinical and pathologic characteristics of those with and without LATE-NC, and to determine the association of LATE-NC with cognition and odds of dementia. In addition, the distribution and severity of LATE-NC in Black and White decedents were compared, as were the associations of LATE-NC with memory, other cognitive domains, and odds of dementia to test for racial differences. Participants were from 1 of 4 longitudinal studies of aging and dementia: the Rush Memory and Aging Project (MAP), the Religious Orders Study (ROS), the Minority Aging Research Study (MARS), and the African American Core (AA Core).
Methods
Standard protocol approvals, registrations, and patient consents
This study included Black and White decedents from clinical-pathologic cohort studies of aging and dementia, each approved by the Institutional Review Board of Rush University Medical Center. A signed, informed consent was obtained from each participant for annual clinical evaluations, as was a signed Anatomical Gift Act for brain donation. Brain donation was required in MAP and ROS but was optional in MARS and the AA Core.
Participants and clinical evaluation
Racial category (Black vs White) was determined by self-report. By June 2019, autopsy was performed on 82 community-dwelling Black decedents. Because complete clinical and pathologic data were available from only 76, only these cases were included in the present study (Rush MAP n = 15, ROS n = 24, MARS n = 27, and AA Core n = 10). In all these studies, antemortem and postmortem data collections were similar, allowing combined analyses of the cohorts. Black participants had a mean age at death of 82.8 years; their education was a mean of 14.7 years; and their mean follow-up time in the studies was 6.1 years. Because there were many more autopsies from White than Black decedents, the Black decedents were matched 2 to 1 to 152 White decedents by age at death, sex, years of education, dementia status, and their follow-up time in the studies using Mahalanobis distance matching as described previously.16 Matching could not be done by socioeconomic status because data on income were not available for many of the participants. The matched White decedents from Rush MAP (n = 100) and ROS (n = 52) had a mean age at death of 84.7 years; their education was a mean of 15.2 years; and their mean follow-up time in the study was 6.1 years.
Participants underwent uniform clinical evaluation at baseline and annually thereafter. Their assessment included a standardized battery of 21 neuropsychological tests as described previously.17–19 The Mini-Mental State Examination (MMSE) and Complex Ideational Material were used for descriptive and diagnostic purposes only. The remaining 19 tests assessed the level of and change in episodic, semantic, and working memory; perceptual speed; and visuospatial abilities. To reduce ceiling and floor artifacts and random variability, composite measures were obtained as described previously.19,20 The global cognitive function score was computed by averaging the z scores of all 19 tests. Criteria of the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke and the AD and Related Disorders Association21 were used to diagnose dementia and clinical AD. Proximate to death, dementia status was assigned by a board-certified neurologist after review of all clinical information.
Neuropathologic analyses
The mean postmortem interval was 11.2 hours (SD 12.6 hours). Autopsies, brain fixation, and dissection of 11 brain regions for microscopy were performed following a standard protocol as described previously.20 Blocks processed with standard techniques were embedded in paraffin, and sections (6 µm) stained with hematoxylin-eosin were used to detect microinfarcts and to assess arteriolosclerosis, as described below, and HS. HS was evaluated unilaterally in a coronal section of the mid hippocampus and graded as absent or present according to severe neuronal loss and gliosis in CA1 and/or subiculum or other sectors.6
LATE-NC assessment
TDP-43 protein was localized by immunohistochemistry in 4 brain regions (amygdala, entorhinal cortex, hippocampus CA1 and subiculum, and dentate neurons) and 4 neocortical areas (anterior temporal pole, mid temporal, orbital frontal, and mid frontal cortices) as described previously.20 Immunohistochemistry was performed with phosphorylated monoclonal TAR5P-1D3, pS409/410 antibodies from 2 sources. Before 2015, the antibody source was Ascenion (Munich, Germany; 1:10022); after 2015, the antibody source was Millipore Sigma (Burlington, MA; 1:400). Phosphorylated TDP-43 was visualized as a brown reaction product produced by reagents in the Bond Polymer Refine Detection Kit (Leica Microsystems, New Buffalo, IL). A semiquantitative estimate of TDP-43 cytoplasmic inclusions in neurons and glia in the 8 regions mentioned above was obtained as described previously.20 As recommended by the Consensus Committee,11 LATE-NC progression was divided into 3 stages. In stage 1, LATE-NC was localized to the amygdala; in stage 2, there was extension to the hippocampus or entorhinal cortex; and in stage 3, there was extension to neocortical areas.9 All 228 cases had LATE-NC data available from the amygdala because previous studies6,23 reported that in aging and AD the amygdala is the first site to be involved by LATE-NC. Because our previous study20 showed that stage 1 is not related to cognitive impairment and because there were very few Black decedents with stage 2 in the present study (table 1), LATE-NC distribution was dichotomized into none/mild (none and stage 1) and moderate/severe (stages 2–3) for descriptive and analytic purposes.
Table 1.
Frequency of LATE-NC in brain regions and mean TDP-43 cytoplasmic inclusions by stage in 31 Black decedents
AD pathology
A pathologic diagnosis of AD included intermediate- and high-likelihood cases diagnosed with the National Institute on Aging–Reagan criteria.24 Neuritic and diffuse plaques and neurofibrillary tangles in 5 brain regions (mid frontal, mid temporal, inferior parietal, and entorhinal cortices and hippocampal CA1), having the highest density of these structures, were quantified from sections stained by modified Bielschowsky silver stain as described previously.25
Infarcts
Macroscopic and microscopic infarcts were assessed as described previously,20 and only chronic macroinfarcts and microinfarcts were included in the analyses as dichotomous variables.
Vascular diseases
Atherosclerosis was assessed in basal cerebral arteries, and arteriolosclerosis was assessed in the basal ganglia. Both vessel pathologies were graded with a 6-tiered semiquantitative scale from 0 (none) to 6 (severe) as described previously.20 Cerebral amyloid angiopathy (CAA) was assessed in meningeal and intracortical vessels in 4 cortical sections (mid frontal, mid temporal, inferior parietal and occipital) immunostained for β-amyloid and graded as described previously.26
Lewy bodies
Six regions (mid frontal, mid temporal, entorhinal and cingulate cortices, amygdala, and substantia nigra) were assessed for the presence of Lewy bodies as described previously,18 and they were recorded and analyzed as a dichotomous variable.
Statistical analyses
Demographics, clinical characteristics, and age-related pathologies, including macroinfarcts and microinfarcts, HS, Lewy bodies, and AD and vascular pathologies (arteriolosclerosis, atherosclerosis, and CAA), were compared between Black decedents with none/mild and those with moderate/severe LATE-NC and between Black and White decedents with moderate/severe LATE-NC with the use of χ2 test or t statistics. The mean cytoplasmic inclusions in brain regions of Black and White decedents were compared with the Wilcoxon rank-sum test.
Multivariable linear regression analyses were used to determine the association of moderate/severe LATE-NC in Black decedents with the outcome measures of global cognition and separately episodic, semantic, and working memory; perceptual speed; and visuospatial skills. Multivariable logistic regression analyses evaluated the association of LATE-NC and age-related pathologies in Black decedents with dementia as a binary outcome. Additional linear and logistic regression models were run that included both Black and White decedents, and terms for race and LATE-NC were included as the primary predictors to test for racial differences in the association of LATE-NC with cognitive and dementia outcomes. All models controlled for age, sex, education, and the other age-related pathologies listed above. Results were presented as point estimates with 95% confidence intervals (CIs). All analyses were carried out with Statistical Analysis Software (SAS/STAT 14.1 User's Guide, SAS Institute Inc, Cary, NC). Model assumptions were examined graphically and analytically and were adequately met. A nominal threshold of p < 0.05 was used for statistical significance, while for the memory domain–specific analyses, a Bonferroni correction for 5 domains (α = 0.05/5) was applied to adjust for multiple testing.
Data availability
The data used in this study can be requested from the Rush Alzheimer Disease Center Research Resource Sharing Hub (radc.rush.edu).
Results
LATE-NC in Black decedents
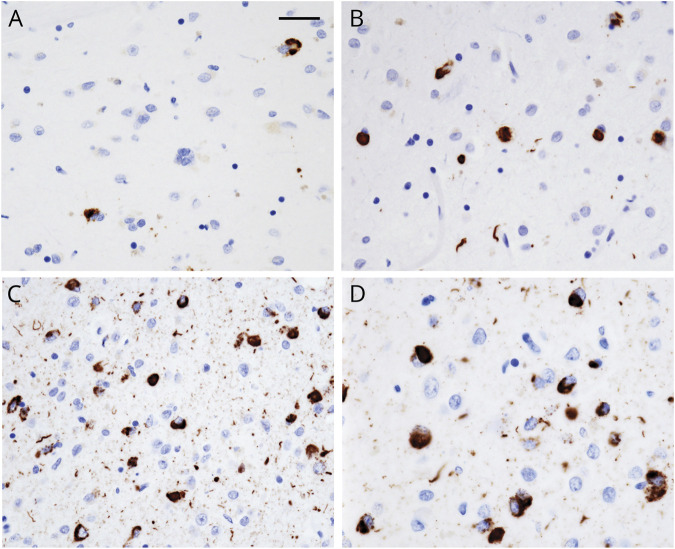
Overall, LATE-NC changes in neurons and glia were present in 31 of 76 (40.8%) Black decedents. Most inclusions were homogeneous (figure, A–D), while granular inclusions were less frequent. Neurite immunostaining was associated with moderate numbers of cytoplasmic inclusions (figure, B) and was maximal in association with frequent inclusions (figure, C). The frequency of TDP-43 inclusions was highest in the amygdala and least in the orbital frontal and mid frontal cortices (table 1). The distribution of TDP-43 inclusions in brain regions was similar to our previous observations.6,9 Inclusions were confined to the amygdala (stage 1) in 45.2% of decedents; 12.9% showed additional involvement of the hippocampus and entorhinal cortex (stage 2); and 41.9% of decedents showed additional LATE-NC in the temporal and frontal cortices (stage 3). The mean density of TDP-43 inclusions was also highest in the amygdala in all stages, and mean inclusions in all regions were highest in the stage 3 decedents.
Figure. TDP-43 inclusions in the amygdala.
Representative areas of the amygdala show (A) sparse, (B) moderate, and (C) frequent TDP-43 inclusions in neuronal cytoplasm and neurites. Areas depicted (A–C) are smaller than the 0.25-mm2 counting frame used to quantify the inclusions. (D) Transactive response DNA-binding protein 43 (TDP-43) cytoplasmic inclusions and neurite immunostaining are shown at high magnification from an area of frequent inclusions. Scale bar = 50 µm (A–C) and 25 µm (D).
Black decedents with moderate/severe LATE-NC were significantly older at the time of death compared to those in the none/mild LATE-NC group (table 2). There was no difference in sex or years of education between the 2 groups. Frequency of dementia was higher (64.7%) in the moderate/severe LATE-NC group than in the none/mild group (33.9%).
Table 2.
Clinical pathologic characteristics of 76 Black decedents by LATE-NC
LATE-NC and other age-related pathologies in Black decedents
HS was present only in Black decedents having moderate/severe LATE-NC (table 2), while the frequency of AD was higher in Black decedents having moderate/severe LATE-NC compared to the none/mild group. The frequency of Lewy body disease was similar in both groups. Among vascular pathologies, the frequency of CAA in the moderate/severe group was twice that of the none/mild LATE-NC group. The frequency of other age-related pathologies, including macroinfarcts and microinfarcts, atherosclerosis, and arteriolosclerosis, did not differ in Black decedents in the none/mild vs moderate/severe LATE-NC group. None of the Black decedents had a pathologic diagnosis of frontotemporal lobar degeneration.
Cognition and odds of dementia in Black decedents
The mean scores for global cognition and all cognitive domains were lower in Black decedents with moderate/severe LATE-NC compared to the none/mild group (table 2). In linear regression models, LATE-NC in Black decedents was independently associated with impaired global cognition, episodic and semantic memory, and visuospatial abilities (table 3), and these associations persisted on application of a Bonferroni correction for multiple testing. The mean MMSE scores were lower and dementia was more frequent in the Black decedents with moderate/severe LATE-NC compared to the group with none/mild LATE-NC (table 2). Pathologic examination of those with dementia showed that 65% had moderate/severe LATE-NC. In both the none/mild and moderate/severe groups, other pathologies were present to account for dementia. In logistic regression analyses controlling for demographics and other age-related pathologies, the odds of dementia in Black decedents with LATE-NC was elevated 2-fold. However, this value did not reach statistical significance (table 4).
Table 3.
Relation of LATE-NC and age-related pathologies to cognitive outcomes in Black decedents
Table 4.
Relation of LATE-NC and age-related pathologies to dementia in Black decedents
Moderate/severe LATE-NC in Black vs White decedents
Black and White decedents matched on key demographics, dementia status, and their follow-up time in the studies showed no differences in these characteristics or MMSE and cognition scores proximate to death (data not shown). The overall frequency of LATE-NC in White decedents was 45.4%, which was not statistically different from the frequency of 40.8% in Black decedents (p = 0.509). The frequency of the APOE ε4 allele was significantly higher in Black than White decedents (41.9% vs 26.5%, p = 0.045). In logistic regression analyses that included both Black and White decedents, the APOE ε4 allele was associated with LATE-NC (3.23, 95% CI 1.59–6.55), and this association did not differ by race because in an additional model the interaction between the APOE ε4 allele and race was not significant.
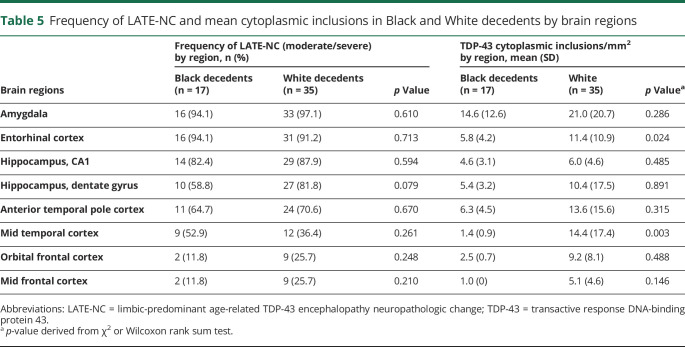
There were no racial differences in the distribution and severity of LATE-NC in the brain except that higher mean cytoplasmic inclusions in the entorhinal and mid temporal cortices were noted in White decedents (table 5). Furthermore, there were no racial differences in the frequency of AD, other age-related pathologies, or the frequency of vascular diseases in Black decedents compared to White decedents with moderate/severe LATE-NC.
Table 5.
Frequency of LATE-NC and mean cytoplasmic inclusions in Black and White decedents by brain regions
Cognition and dementia in Black vs White decedents with moderate/severe LATE-NC
No racial differences were observed in the global cognition scores and those of the 5 cognitive domains in Black decedents compared to White decedents with moderate/severe LATE-NC (data not shown). In linear regression models that included both White and Black decedents, LATE-NC was independently associated with a lower level of global cognitive function (−0.42, 95% CI −0.76 to 0.07) and episodic (−0.50, 95% CI −0.90 to 0.09) and semantic (−0.99, 95% CI −1.51 to 0.48) memory. On application of the Bonferroni correction to analyses of the cognitive domains, LATE-NC in both groups was associated only with impaired semantic memory. In additional models, a term for an interaction between LATE-NC and race was added to each of the linear regression models with global cognitive function and 5 separate cognitive domains as outcomes. In these models, the interaction for LATE-NC and race did not reach statistical significance, suggesting no racial difference in these associations.
In logistic regression analyses that included both Black and White decedents and controlling for demographics and other age-related pathologies, the odds of dementia was elevated in moderate/severe LATE-NC. However, this value did not reach statistical significance (odds ratio 1.39, 95% CI 0.58–3.31). To determine whether these associations differed by race, a term for the interaction of LATE-NC with race was added to each model, but in these models, the interaction between LATE-NC and race did not reach statistical significance.
Discussion
To the best of our knowledge, this is the largest group of community-dwelling Black decedents in the literature in whom the frequency and distribution of LATE-NC and its association with cognition and odds of dementia are documented. LATE-NC in Black decedents was independently associated with impaired global cognition, episodic and semantic memory, and visuospatial abilities. In a sample of Black and White participants matched on key demographics, dementia, and follow-up time, no racial differences in the clinical or pathologic characteristics were noted in the moderate/severe LATE-NC groups. Severity of LATE-NC in the 2 groups showed that White decedents had significantly higher inclusions in the entorhinal and mid temporal cortices. In addition, no racial differences in the cognitive profiles or the odds of dementia were observed in Black vs White decedents.
The overall frequency of LATE-NC was similar in Black vs White decedents (40.8% vs 45.4%). A similar frequency of LATE-NC was reported in previous studies of White decedents of a similar age in whom the amygdala was sampled.9,10 A single multiethnic study of cognitively normal elders reported LATE-NC in 6 (17.7%) Black decedents and Black decedents admixed with other races.27 This low frequency was attributed to the lower age of the study participants, the mean age being 74 years.
Our previous studies of mainly White decedents with LATE-NC have reported 36,9 and even 5 stages20 of LATE-NC, which could not be applied to the present study due to the small sample size because just over half the Black participants (17 of 31) had LATE-NC beyond the amygdala. The regional distribution of LATE-NC with involvement of amygdala before neocortical structures is similar to the reported distribution in White decedents with AD4,23 and elders without a pathologic diagnosis of AD.9
The current study did not show racial differences in the regional distribution of LATE-NC except that the severity of LATE-NC in the entorhinal and mid temporal cortices was greater in White decedents than Black decedents. The reason for this difference is not readily apparent because the ages of participants in the 2 groups were matched. The observed higher frequency of the APOE ε4 allele in Black vs White decedents in this study has been reported previously.28,29 It is interesting that in this study the APOE ε4 allele is related to LATE-NC in both Black and White decedents and that it is also reported to be related to faster decline in episodic memory,28 a clinical characteristic of LATE.11
The age of Black decedents and the burden of LATE-NC in the moderate/severe group were significantly higher than in those with none/mild LATE-NC (p = 0.017 and p < 0.001 respectively). The association of a higher burden of LATE-NC with age is well documented in previous studies of White decedents, including those with minimal AD pathology,10 older mentally ill adults,30 a population-based study that included AD cases,31 and our longitudinal studies of aging and dementia that included AD cases.20 Also related to age may be the higher frequency of AD in the moderate/severe group of Black decedents compared to the none/mild group. The higher frequency of AD in the moderate/severe group may explain the higher frequency of CAA in this group because >90% of AD cases have coexisting CAA.32 HS was found only in Black decedents with moderate/severe LATE-NC, and the stated frequency of HS may be an underestimate because only 1 section of the mid hippocampus was analyzed. Therefore, future studies with more extensive sampling of the hippocampus are warranted to document the frequency of HS in Black decedents.
The relationship between LATE-NC and cognition in Black decedents is unknown. In the present study, linear regression models that controlled for demographics and age-related pathologies showed that LATE-NC is associated with impaired global cognition and the specific domains of episodic and semantic memory and visuospatial abilities. This pattern of cognitive impairment is similar to previous findings in White decedents with or without AD.9,20,33 Involvement of semantic memory was reported in White decedents when LATE-NC extended to the mid frontal cortex.20 In Black decedents, impaired semantic memory was noted even though only 2 cases showed LATE-NC in the mid frontal cortex. The association of LATE-NC with cognition is not confounded by age because our previous studies have consistently shown that the association of age with cognition is mediated by neuropathology and cognitive impairment is not a normative age-related process but reflects nonnormative pathologic and mortality-related processes.34
Although dementia was more frequent in Black decedents with moderate/severe LATE-NC, logistic regression models did not show an association with higher odds of dementia when age-related pathologies were added to the model. This finding is possibly related to the small sample size because our previous study of mainly White decedents (n = 1,108) showed higher odds of dementia in those with moderate/severe LATE-NC.20
Strengths of this study include the availability of detailed clinical and neuropsychological data and detailed data on multiple neuropathologies obtained in a blinded manner. This study also has limitations. The ROS participants likely have better dietary intake, access to health care, and levels of education, which may affect cognitive risk. To alleviate this concern, Black decedents from ROS were matched only with White decedents from ROS. Although a robust matching technique was used that included important demographic characteristics, because of missing data, we did not match on income, a socioeconomic indicator that is typically lower among Black decedents compared with White decedents. We did, however, match on education, a common proxy for socioeconomic status and one that is more closely related to long-term economic position. Although this is the largest study in the literature of LATE-NC in Black decedents, the total number of cases is still relatively small. Given the frequency of LATE-NC in Black decedents and the strong association with lower cognition, further study of LATE-NC in older Black decedents is warranted.
Acknowledgment
The authors thank all the participants of MARS, AA Core, Rush MAP, and ROS and the staff of Rush Alzheimer's Disease Center, including Er-Yun Chen and Dominika Burba.
Glossary
- AA Core
African American Core
- AD
Alzheimer disease
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- HS
hippocampal sclerosis
- LATE
limbic-predominant age-related TDP-43 encephalopathy
- LATE-NC
LATE neuropathologic change
- MAP
Rush Memory and Aging Project
- MARS
Minority Aging Research Study
- MMSE
Mini-Mental State Examination
- ROS
Religious Orders Study
- TDP-43
transactive response DNA-binding protein 43
Appendix. Authors
Study funding
This work was supported by the NIH, National Institute on Aging (R01AG017917, P30AG10161, R01AG15819, RF1AG022018, R01AG042210), and Illinois Department of Public Health.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 2006;351:602–611. [DOI] [PubMed] [Google Scholar]
- 2.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006;314:130–133. [DOI] [PubMed] [Google Scholar]
- 3.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol 2014;127:811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J. TDP-43 pathology in Alzheimer's disease, dementia with Lewy bodies and ageing. Brain Pathol 2017;27:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakashima-Yasuda H, Uryu K, Robinson J, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 2007;114:221–229. [DOI] [PubMed] [Google Scholar]
- 6.Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol 2015;77:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson PT, Smith CD, Abner EL, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol 2013;126:161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee AC, Gavett BE, Stern RA, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 2010;69:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nag S, Yu L, Wilson RS, Chen EY, Bennett DA, Schneider JA. TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology 2017;88:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchino A, Takao M, Hatsuta H, et al. Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol Commun 2015;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 2019;142:1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016;12:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steenland K, Goldstein FC, Levey A, Wharton W. A meta-analysis of Alzheimer's disease incidence and prevalence comparing African-Americans and Caucasians. J Alzheimers Dis 2016;50:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 2001;56:49–56. [DOI] [PubMed] [Google Scholar]
- 15.Weuve J, Rajan KB, Barnes LL, Wilson RS, Evans DA. Secular trends in cognitive performance in older Black and White U.S. adults, 1993-2012: findings from the Chicago Health and Aging Project. J Gerontol B Psychol Sci Soc Sci 2018;73(suppl 1):S73–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han SD, Barnes LL, Leurgans S, Yu L, Bennett DA, Boyle PA. Literacy mediates racial differences in financial and healthcare decision making in older adults. J Am Geriatr Soc 2020;68:1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006;66:1837–1844. [DOI] [PubMed] [Google Scholar]
- 18.Nag S, Yu L, VanderHorst VG, et al. Neocortical Lewy bodies are associated with impaired odor identification in community-dwelling elders without clinical PD. J Neurol 2019;266:3108–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology 2002;59:1910–1914. [DOI] [PubMed] [Google Scholar]
- 20.Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer's disease. Acta Neuropathol Commun 2018;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 22.Neumann M, Kwong LK, Lee EB, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol 2009;117:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Josephs KA, Murray ME, Whitwell JL, et al. Updated TDP-43 in Alzheimer's disease staging scheme. Acta Neuropathol 2016;131:571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 1997;56:1095–1097. [DOI] [PubMed] [Google Scholar]
- 25.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 2004;62:1148–1155. [DOI] [PubMed] [Google Scholar]
- 26.Yu L, Boyle PA, Nag S, et al. APOE and cerebral amyloid angiopathy in community-dwelling older persons. Neurobiol Aging 2015;36:2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nascimento C, Suemoto CK, Rodriguez RD, et al. Higher prevalence of TDP-43 proteinopathy in cognitively normal Asians: a clinicopathological study on a multiethnic sample. Brain Pathol 2016;26:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes LL, Arvanitakis Z, Yu L, Kelly J, De Jager PL, Bennett DA. Apolipoprotein E and change in episodic memory in Blacks and Whites. Neuroepidemiology 2013;40:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajan KB, Barnes LL, Wilson RS, Weuve J, McAninch EA, Evans DA. Apolipoprotein E genotypes, age, race, and cognitive decline in a population sample. J Am Geriatr Soc 2019;67:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geser F, Robinson JL, Malunda JA, et al. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol 2010;67:1238–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keage HA, Hunter S, Matthews FE, et al. TDP-43 in the population: prevalence and associations with dementia and age. J Alzheimers Dis 2014;42:641–650. [DOI] [PubMed] [Google Scholar]
- 32.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm (Vienna) 2002;109:813–836. [DOI] [PubMed] [Google Scholar]
- 33.Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 2013;70:1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson RS, Wang T, Yu L, Bennett DA, Boyle PA. Normative cognitive decline in old age. Ann Neurol 2020;87:816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study can be requested from the Rush Alzheimer Disease Center Research Resource Sharing Hub (radc.rush.edu).