Abstract
Objective
To determine the cognitive consequences of anticholinergic medications (aCH) in cognitively normal older adults as well as interactive effects of genetic and CSF Alzheimer disease (AD) risk factors.
Methods
A total of 688 cognitively normal participants from the Alzheimer's Disease Neuroimaging Initiative were evaluated (mean age 73.5 years, 49.6% female). Cox regression examined risk of progression to mild cognitive impairment (MCI) over a 10-year period and linear mixed effects models examined 3-year rates of decline in memory, executive function, and language as a function of aCH. Interactions with APOE ε4 genotype and CSF biomarker evidence of AD pathology were also assessed.
Results
aCH+ participants had increased risk of progression to MCI (hazard ratio [HR] 1.47, p = 0.02), and there was a significant aCH × AD risk interaction such that aCH+/ε4+ individuals showed greater than 2-fold increased risk (HR 2.69, p < 0.001) for incident MCI relative to aCH−/ε4−), while aCH+/CSF+) individuals demonstrated greater than 4-fold (HR 4.89, p < 0.001) increased risk relative to aCH−/CSF−. Linear mixed effects models revealed that aCH predicted a steeper slope of decline in memory (t = −2.35, p = 0.02) and language (t = −2.35, p = 0.02), with effects exacerbated in individuals with AD risk factors.
Conclusions
aCH increased risk of incident MCI and cognitive decline, and effects were significantly enhanced among individuals with genetic risk factors and CSF-based AD pathophysiologic markers. Findings underscore the adverse impact of aCH medications on cognition and the need for deprescribing trials, particularly among individuals with elevated risk for AD.
As public concern grows over the increasing prevalence of Alzheimer disease (AD) and the paucity of reliable treatments, studies emphasizing modifiable risk factors that may accelerate cognitive impairment in older adults are of high importance.1 Use of anticholinergic medications (aCH) may represent one such risk factor,2 yet the longitudinal cognitive changes associated with aCH are often underappreciated, especially in cognitively unimpaired older adults.3,4 Exploring the potential risks of aCH in older adults is warranted given animal studies demonstrating that cholinergic deprivation promotes pathologic plaque and tangle formation and neurodegeneration in AD-vulnerable brain regions, as well as accelerated decline in learning and memory.5,6 These findings highlight the potential for aCH to precipitate cognitive dysfunction through interactions with AD pathology, yet studies of aCH in older adults often assess its influence in isolation rather than in conjunction with other known risk factors.7,8
Given that aCH may promote damage to AD-vulnerable brain regions and incipient cognitive impairment, it is critical to enhance our understanding of the mechanisms driving negative cognitive outcomes associated with aCH and determine whether individuals with AD risk factors would benefit most from aCH reduction. Therefore, we conducted a comprehensive longitudinal analysis with sensitive neuropsychological diagnostics to investigate the effect of aCH and its interaction with genetic and CSF AD risk factors on progression to MCI and domain-specific rates of cognitive decline in a well-characterized cohort of cognitively normal participants. We expected that aCH would increase risk of incident MCI and accelerate cognitive decline, with effects exacerbated among those possessing AD risk factors.
Methods
Standard protocol approvals, registrations, and patient consents
Research was approved by the institutional review boards of participating sites within the Alzheimer's Disease Neuroimaging Initiative (ADNI), and written informed consent was obtained for study participants. Approval for this specific project was obtained from the local institutional review board prior to data analysis.
Participants
A total of 688 participants from the ADNI with normal cognition at baseline and at least 1 yearly neuropsychological follow-up were included. Participants who reverted from MCI to normal during follow-up were excluded from survival analyses that assessed conversion from cognitively normal to MCI (n = 98; 37% aCH+, 63% aCH−). Participants came from a well-educated (mean 16.4 years, SD 2.67) and primarily non-Hispanic white (94% White, 97% non-Hispanic) sample.
Anticholinergic medication use
Data from the ADNI medications log (name of medication, dosage, frequency) were examined to identify aCH at each participant's baseline visit. Any aCH (as self-reported and identified in the Anticholinergic Cognitive Burden scale) within 3 months of baseline was included if records indicated continuous use (i.e., at least once a week for more than 6 months). Notably, use of certain aCH within 4 weeks prior to baseline resulted in exclusion from ADNI, potentially restricting our sample of aCH+ individuals (data available from Dryad, table B1, doi.org/10.6075/J0BC3WZS). The total number of medications taken by each participant was tallied as an indication of overall health and to approximate comorbid medical conditions.9
aCH burden was measured using a dichotomous score as well as a novel cumulative burden metric. The dichotomous score indicates presence or absence of aCH identified in the Anticholinergic Cognitive Burden (ACB) Scale.10 aCH+ participants were also categorized as high aCH+ if they were taking one or more medications with an ACB of 2 or 3, and low aCH+ if they were taking one or more medications with an ACB of 1. The ACB scale was used because it was most reliably associated with adverse cognitive outcomes in a comprehensive meta-analytic comparison of anticholinergic scales.3,4
The cumulative aCH burden metric was derived in the following manner for each individual: for each ACB drug, we (1) applied an equation11 based on daily dosage relative to the geriatric minimally efficacious dosage12 for a given drug (see equation 1); (2) multiplied the resulting value by the rank of anticholinergic potential (i.e., 1, 2, or 3) for the drug10; and (3) summed the resulting values for each ACB drug taken. The cumulative aCH burden metric uses the following formula (equation 1): D/(D + MED), where D is the daily dosage for a given drug and MED is the minimally efficacious dosage for that drug in the geriatric population.12 Although prior studies have used the MED for the general adult population, we used thresholds specific to the geriatric population given that different metabolic processes in this group may necessitate lower doses. Table B2 (data available from Dryad, doi.org/10.6075/J0BC3WZS) provides an example of this calculation for an individual participant. As a reference, participants could receive a score of 2 if they (1) take 1 aCH with an ACB score of 2 at the MED, (2) take 2 aCH with an ACB score of 1 at the MED, or (3) take 1 aCH with an ACB score of 1 at twice the MED.
Individuals taking at least one aCH are referred to as aCH+ and individuals not taking any aCH are referred to as aCH−. For analyses assessing interactions between aCH and AD risk factors, the following nomenclature is used: (1) aCH−/ε4− or aCH−/CSF− refers to aCH− individuals who are APOE noncarriers or CSF biomarker negative; (2) aCH−/ε4+ or aCH−/CSF+ refers to aCH− individuals who are APOE carriers or CSF biomarker positive; (3) aCH+/ε4− or aCH+/CSF− refers to aCH+ individuals who are APOE noncarriers or CSF biomarker negative; and (4) aCH+/ε4+ or aCH+/CSF+ refers to aCH+ individuals who are APOE carriers or CSF biomarker positive.
Clinical variables
Depressive symptoms were measured using the Geriatric Depression Scale (GDS). Notably, ADNI excludes any participants with a score greater than 5 on the GDS, limiting the influence of depressive symptoms in the current study. Vascular risk was indexed in 2 ways: (1) a continuous variable measuring pulse pressure, defined as systolic minus diastolic blood pressure, which reflects vascular risk and arterial stiffening13,14; and (2) a dichotomous variable indicating history of cardiovascular problems (e.g., hypertension, atrial fibrillation, coronary artery disease, stroke). APOE carrier status was defined by the presence of at least 1 ε4 allele (ε4+). CSF AD pathology was assessed as the ratio of phosphorylated tau181 and β-amyloid1–42 (p-tau/Aβ), obtained using Roche ELECSYS assays and dichotomized with positivity (CSF+) defined as a ratio value >0.0251 pg/mL.15
Cognitive outcome
Incidence of MCI was determined using a well-validated actuarial neuropsychological approach.16–18 Participants were diagnosed with MCI if they met the following criteria: (1) at least 2 scores within 1 cognitive domain that fell more than 1 SD below the age-, education-, and sex-corrected normative mean; (2) at least 1 score in each of 3 cognitive domains that fell more than 1 SD below the normative mean; or (3) a total score ≥6 on the Functional Activities Questionnaire. If none of these criteria were met, the participant was considered cognitively normal.
To assess the effect of aCH on domain-specific cognitive decline, data from neuropsychological tests comprising language, attention/executive function, and memory domains were examined: Rey Auditory Verbal Learning Test Delayed Recall and Recognition (memory); Boston Naming Test and Animal Fluency (language); and Trail-Making Test Parts A and B (attention/executive function). Scores from each measure were converted to z scores based on age-, education-, and sex-adjusted regression coefficients derived from a normative control group within ADNI and averaged within domains to create domain-specific z scores. These 3 cognitive domains were assessed in order to comprehensively examine the effects of aCH on areas of cognition whose neural substrates are influenced by cholinergic innervation, as described in the discussion.
Statistical analyses
χ2 tests for independence and independent-sample t tests were conducted to compare aCH+ and aCH− group characteristics. Cox proportional hazard models examined the influence of aCH on risk of progression from cognitive normality to MCI or AD across 10 years while adjusting for age, sex, education, race, GDS, pulse pressure, cardiovascular history, and total number of medications. For all Cox models, Schoenfeld residuals were examined to test for the proportional hazards assumption. Linear mixed effects models analyzed longitudinal rate of change in domain z scores as a function of aCH over 3 years. The aforementioned variables and time were included as continuous covariates, and subject and intercept were modeled as random effects. Slope was also initially modeled as a random effect; if the model failed to converge, the maximum number of iterations was increased, and if convergence still failed, the random effect of slope was removed from the model. Time was centered to the baseline visit. Bonferroni correction was applied for the cognitive domain comparisons, resulting in an α value of p < 0.05/3 < 0.02. For significant effects of aCH, secondary analyses tested for interactions with APOE and CSF biomarkers. Models assessing 3-way interactions controlled for main effects and all 2-way interactions. Sensitivity analyses tested these models only in the high aCH+ group, relative to the aCH− group, to assess for modulation of effects based on anticholinergic strength. Analyses and figures were completed using SPSS version 25 (SPSS, Inc., Chicago, IL) and R version 3.6.1 (R-project.org/) including the following packages: survival, survminer, lme4, afex, dplyr, ggplot2, ipw. All tests were 2-tailed and significance was determined based on an α level of 0.05, unless otherwise indicated.
Data availability
Data used in this article were obtained from the ADNI database (adni.loni.usc.edu). ADNI was launched in 2003 as a public–private partnership. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD.
Results
aCH summary statistics
Of the 688 participants, 230 (33%) were aCH+, and 73 of the 230 aCH+ participants were high aCH+. An average of 4.7 aCH medications were taken per aCH+ individual. Metoprolol, atenolol, loratadine, and bupropion were the most common medications taken (see table B3 for a full list, data available from Dryad, doi.org/10.6075/J0BC3WZS). The majority of medications were being taken at levels much higher than the MED for a geriatric population (57% of aCH medications were taken at dosages at least 2× higher than the MED, and 18% were taken at least 4× higher than the MED).
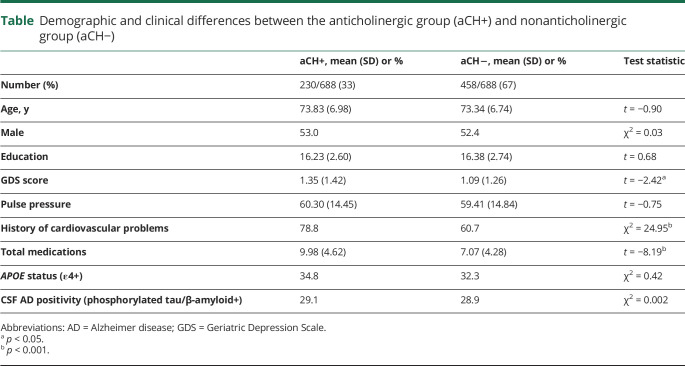
Demographic and clinical characteristics
As seen in the table, there were no differences in demographic characteristics between the aCH+ and aCH− groups. There was also no difference in proportion of ε4+ or p-tau/Aβ+ participants between groups. GDS scores, number of medications, and history of cardiac problems were higher in the aCH+ group, whereas pulse pressure did not differ. These 4 clinical variables were controlled for in all subsequent analyses to account for potential confounds of overall health and medical conditions for which aCH are commonly prescribed.
Table.
Demographic and clinical differences between the anticholinergic group (aCH+) and nonanticholinergic group (aCH−)
Associations among aCH, AD risk factors, and progression to MCI
aCH effect
Compared to aCH− participants, aCH+ participants demonstrated an increased risk of incident MCI over the 10-year period such that at least 1 aCH conferred nearly 1.5× increased risk (hazard ratio [HR] 1.47, 95% confidence interval [CI] 1.10–1.98, p = 0.01). Examination of cumulative aCH burden scores in aCH+ participants showed that higher burden was associated with increased risk of incident MCI such that each point increase in the burden metric conferred nearly 1.5× increased risk (HR 1.46, 95% CI 1.11–1.91, p = 0.01).
aCH by APOE interaction
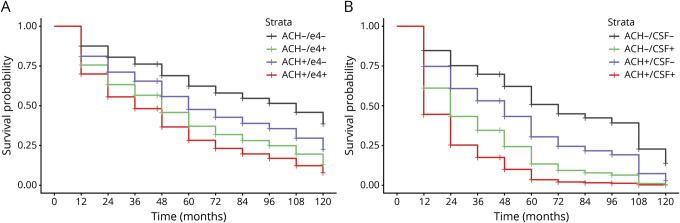
Genetic risk interaction effects revealed an aCH by APOE interaction (log-likelihood ratio [LLR] 69.31, p < 0.001), such that compared to the aCH−/ε4− group, aCH+/ε4+ participants had more than 2.5-fold risk of incident MCI (HR 2.69, 95% CI 1.78–4.07, p < 0.001; figure 1A). Further comparisons indicated that the aCH+/ε4− group (HR 1.57, 95% CI 1.07–2.30, p = 0.02) and aCH−/ε4+ group (HR 2.10, 95% CI 1.48–2.99, p < 0.001) also differed from the aCH−/ε4− group.
Figure 1. Rates of progression to mild cognitive impairment (MCI).
Progression to MCI over 10 years as a function of anticholinergic (ACH) medication use and (A) APOE ε4 status (ε4− or ε4+) or (B) CSF Alzheimer disease [AD] pathology (β-amyloid [Aβ]/phosphorylated-tau [p-tau]− or Aβ/p-tau+).
aCH by CSF interaction
CSF risk interaction effects revealed an aCH by CSF interaction (LLR 68.31, p < 0.001), such that compared to the aCH−/CSF− group, aCH+/CSF+ participants demonstrated nearly 5-fold increased risk of incident MCI (HR 4.89, 95% CI 2.86–8.36, p < 0.001; figure 1B). Further comparisons indicated that the aCH+/CSF− group (HR 1.77, 95% CI 1.09–2.87, p = 0.03) and aCH−/CSF+ group (HR 2.98, 95% CI 1.93–4.59, p < 0.001) also differed from the aCH−/CSF− group.
High aCH
When considering only the high aCH+ group relative to the aCH− group, the effect of aCH on risk of incident MCI was retained (HR 2.11, 95% CI 1.36–3.28, p < 0.001) such that high aCH+ participants demonstrated greater than 2-fold increased risk. Interaction effects of the high aCH+ group with APOE status and CSF AD risk status were retained. Compared to the aCH−/ε4− group, the high aCH+/ε4+ group had greater than 3-fold increased risk of incident MCI (HR 3.35, 95% CI 1.84–6.12, p < 0.001); compared to the aCH−/CSF− group, the high aCH+/CSF+ group had nearly 7-fold increased risk of incident MCI (HR 6.85, 95% CI 2.89–16.26, p < 0.001).
Associations among aCH, AD risk factors, and cognitive trajectories
Memory
aCH effect
Linear mixed effects models revealed no main effect of aCH (i.e., intercept at baseline visit; t = −0.82, p = 0.41). There was an interaction between aCH and visit, such that the aCH+ group exhibited a steeper slope of decline in memory performance than the aCH− group (t = −2.35, p = 0.02). There was no interaction between aCH burden and visit (t = −0.69, p = 0.49).
aCH by APOE interaction
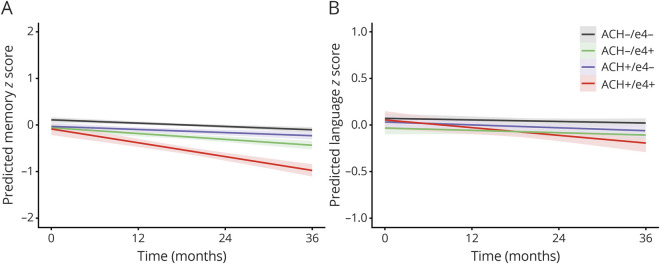
There was no 2-way interaction for the difference in baseline memory score between aCH−/ε4− and aCH+/ε4+ groups (t = −1.25, p = 0.22). There was a 3-way interaction between aCH, APOE status, and visit (F = 15.89, p < 0.001), such that the aCH+/ε4+ group exhibited a steeper decline in memory relative to the aCH−/ε4− group (t = −6.53, p < 0.001; figure 2A). Participants in the aCH+/ε4+ group also exhibited a steeper decline in memory relative to aCH+/ε4− (t = −6.08, p < 0.001) and aCH−/ε4+ groups (t = −4.38, p < 0.001).
Figure 2. Cognitive trajectories and APOE ε4 status.
Rate of change in memory (A) and language (B) performance over 36 months as a function of anticholinergic (ACH) medication use and APOE ε4 status (ε4− or ε4+).
aCH by CSF interaction
There was no 2-way interaction for the difference in baseline memory score between aCH−/CSF− and aCH+/CSF+ groups (t = −1.82, p = 0.07). There was a 3-way interaction between aCH, CSF AD positivity, and visit (F = 6.59, p < 0.001), such that the aCH+/CSF+ group exhibited a steeper decline in memory relative to the aCH−/CSF− group (t = −4.18, p < 0.001; figure 3A). Participants in the aCH+/CSF+ group also exhibited a steeper decline in memory relative to aCH+/CSF− (t = −3.26, p = 0.001) and aCH−/CSF+ groups (t = −2.86, p = 0.004).
Figure 3. Cognitive trajectories and Alzheimer disease (AD) CSF status.
Rate of change in memory (A) and language (B) performance over 36 months as a function of anticholinergic (ACH) medication use and CSF AD pathology (β-amyloid [Aβ]/phosphorylated-tau [p-tau]− or Aβ/p-tau+).
High aCH
The effect of aCH on memory decline was retained when considering only high aCH+ participants (t = −2.10, p = 0.04), although this did not survive Bonferroni correction. aCH by APOE (F = 6.55, p < 0.001) and aCH by CSF (F = 4.00, p = 0.008) interaction effects were retained when considering only high aCH+ participants.
Language
aCH effect
Linear mixed effects models revealed no main effect of aCH (t = −0.14, p = 0.89). There was an interaction between aCH and visit such that the aCH+ group exhibited a steeper slope of decline in language performance (t = −2.35, p = 0.02). There was no interaction between aCH burden and visit (t = 0.51, p = 0.61).
aCH by APOE interaction
There was no 2-way interaction for the difference in baseline language score between aCH−/ε4− and aCH+/ε4+ groups (t = −0.39, p = 0.69). There was a 3-way interaction between aCH, APOE status, and visit (F = 3.52, p = 0.02), such that the aCH+/ε4+ group exhibited a steeper decline in language relative to the aCH−/ε4− group (t = −3.22, p = 0.001; figure 2B). Participants in the aCH+/ε4+ group also exhibited a steeper decline in language relative to aCH+/ε4− (t = −2.16, p = 0.03; this did not survive Bonferroni correction) and aCH−/ε4+ groups (t = −2.54, p = 0.01).
aCH by CSF interaction
There was no 2-way interaction for the difference in baseline language score between aCH−/CSF− and aCH+/CSF+ groups (t = −0.15, p = 0.88). There was a 3-way interaction between aCH, CSF AD positivity, and visit (F = 5.98, p = 0.001), such that the aCH+/CSF+ group exhibited a steeper decline in language relative to the aCH−/CSF− group (t = −3.37, p = 0.001; figure 3B). The aCH+/CSF+ group also exhibited a steeper decline in language relative to aCH+/CSF− (t = −3.82, p < 0.001) and aCH−/CSF+ groups (t = −2.82, p = 0.005).
High aCH
The effect of aCH on language decline was not retained when considering only high aCH+ participants (t = −1.26, p = 0.21). The interaction effect for aCH by APOE was not retained when considering only high aCH+ participants (F = 1.23, p = 0.30). The interaction effect for aCH by CSF was retained when considering only high aCH+ participants (F = 3.28, p = 0.02).
Executive function
There was no main effect of aCH (t = 0.52, p = 0.61) or interaction between aCH and visit on executive function (t = −0.59, p = 0.56), even when considering only high aCH+ participants (t = −0.79, p = 0.43). Therefore, no further interactions in this domain were explored.
Additional sensitivity analyses
Several additional sensitivity analyses were conducted, the results of which can be found in appendices C–G (data available from Dryad, doi.org/10.6075/J0BC3WZS). These analyses included, for both Cox proportional hazards and linear mixed effects models, exclusion of individuals who converted to MCI within 1 year (i.e., 1-year lagging) to control for potential poor recall of baseline medication use (see appendix C, doi.org/10.6075/J0BC3WZS), exclusion of individuals taking psychiatric medications at baseline (see appendix D, doi.org/10.6075/J0BC3WZS), additional adjustment for history of psychiatric difficulties (see appendix E, doi.org/10.6075/J0BC3WZS), and inverse probability of treatment weighting for depressive symptoms and cardiovascular indications (see appendices F and G, doi.org/10.6075/J0BC3WZS).
Discussion
Our findings of an association between use of aCH and subsequent progression to MCI over 10 years, accelerated memory and language decline, and exacerbation with increased AD biomarker risk among older adults who were initially cognitively normal extend prior studies that have primarily investigated cross-sectional associations between aCH and cognition or risk of progression to dementia.2,3,19 Previous research has indicated adverse outcomes only with definite/strong or cumulative exposure to aCH, possibly due to use of insensitive cognitive diagnostic approaches18 or samples collapsed across cognitively normal and impaired individuals.2,19–21 The current study, in contrast, employed an actuarial diagnostic approach known to reduce false-positive diagnoses and produce reliable groups of cognitively normal individuals and individuals with MCI,17 improving our ability to elucidate the link between aCH and cognitive outcomes.
Despite our liberal classification of aCH (i.e., fewer than 20% of the aCH taken in this sample would be classified as definite anticholinergics), employment of our novel cumulative aCH risk metric indicated that there is an effect of aCH burden on risk of progression to MCI, suggesting that cumulative exposure to medications of greater aCH potential or higher dosages confers increased risk. This effect of aCH potential is corroborated by secondary analyses indicating that effects were generally retained when considering only high aCH+ participants. Notably, the restricted sample size for the high aCH+ group resulted in greater variability around the estimate (i.e., a larger CI) likely due to fewer participants taking stronger aCH medications, resulting in reduced power and disproportionately low cell sizes for the high aCH+ groups stratified by biomarker status. Whether cumulative use of multiple weaker aCHs is tantamount to use of one strong aCH remains unknown. However, it is clear from our results that use of aCH in general has detrimental consequences on cognitive functioning in older adults.
Our findings also demonstrate that the association between aCH and progression to MCI was moderated by both APOE ε4 genotype status and CSF AD pathology. Prior studies investigating interactions between APOE and aCH are mixed, with some studies suggesting a positive interaction22,23 and others indicating decreased risk among ε4 carriers.24 To our knowledge, no studies have investigated the interaction between aCH and CSF biomarkers of AD pathology. Our results demonstrate an interaction between aCH and AD biomarkers that increase risk of incident MCI and accelerate decline in memory and language beyond either risk factor alone. The substantially greater cognitive risk of aCH in ε4+ or p-tau/Aβ+ participants observed are in line with recent work identifying the basal forebrain, the primary region of acetylcholine production, as an early predilection site for plaque and tangle pathology25,26 that induces neuronal loss and impairs cholinergic transmission.27,28 Because ε4+ and p-tau/Aβ+ individuals are more susceptible to these pathologic changes, their use of aCH may cause a double hit on acetylcholine such that there is (1) loss of central cholinergic neurons in the basal forebrain due to AD-related pathology, compounded by (2) the acetylcholine-depleting effects of aCH. These independent risks may act synergistically to further reduce cholinergic transmission relative to either risk factor alone, consequently accelerating cognitive decline.
Assessment of domain-specific rates of cognitive decline indicates robust associations between aCH, AD pathologic risk factors, and trajectories of memory decline and language. At least one previous study corroborates our findings of associations between aCH and memory performance; this previous study, however, also found associations with executive function, which were not observed in our study.29 The observed domain-specific influence of aCH, and discrepancies with previous results, might be explained by differential susceptibility of specific basal forebrain nuclei and receptor subtypes to AD pathology.
The septal nucleus and ventral dorsal band of the basal forebrain provide cholinergic input to the hippocampus and associated medial temporal structures,30,31 regions strongly associated with memory function. Although imaging studies with MCI and AD groups have observed basal forebrain atrophy predominately within the nucleus basalis of Meynert (NBM),32,33 a recent study demonstrated enlargement of the septal nucleus in cognitively healthy older adults who later progressed to MCI and AD,34 possibly indicating an early pathologic hypertrophy of this region.35 Thus, early pathologic changes in the septal subregion of the basal forebrain may partially explain, via the proposed 2-hit model, the predominant memory decline associated with compounded aCH and AD pathologic risk in our cognitively normal sample. Furthermore, nicotinic acetylcholine receptor subtypes that mediate several functions involved in long-term potentiation are primarily expressed within the hippocampus and are affected by amyloid pathology, providing another mechanism through which memory may be the most susceptible cognitive domain to adverse consequences of aCH.30
The accelerated rate of decline observed for language, although less robust than memory, was a notable effect that has not been examined in other studies. The NBM, the primary location of acetylcholine production with 90% of its cells identified as cholinergic,30,32,33 can be subdivided into several anatomically and functionally distinct sections with differentially preferred targets for innervation. Convergent findings from several studies using high-resolution imaging have identified the posterior NBM as the subdivision that first exhibits significant atrophy in individuals with MCI.32 The posterior NBM projects primarily to the superior temporal gyrus and adjacent cortical regions31 that are associated with language function.36,37
Although AD pathology targets the septal nucleus in the preclinical period and the NBM in MCI, there is relative sparing of other basal forebrain nuclei that have prefrontal cortex projections until later stages of the disease.31 This may explain why we did not observe effects in the executive function domain. Furthermore, it has been proposed that executive function deficits do not develop until later in the canonical sequence of cognitive changes in AD.38,39
Our study has a number of strengths. First, we examined the consequences of aCH in a well-characterized sample of cognitively normal older adults using sensitive and reliable actuarial neuropsychological diagnostics that improve identification of MCI. Second, this is the first study to our knowledge to investigate the association between aCH and longitudinal decline across multiple neuropsychological domains in cognitively normal older adults. Our findings suggest that reduction of aCH prior to the onset of overt cognitive problems may represent a critical period for intervention before these medications lead to lasting alterations in important cholinergic networks. Although deprescribing among all older adults may prove beneficial, the synergistic effect observed between aCH and AD pathologic risk suggests that reducing aCH burden during the preclinical stages of AD may be especially warranted given that this group is particularly vulnerable. Third, all analyses controlled for comorbidities frequently associated with aCH (i.e., depressive symptoms, cardiovascular risk) and total medications to reduce potential confounding effects of conditions for which aCH are commonly prescribed.
Our findings are limited by the relative health of ADNI participants compared to the general population and the exclusion of particular medications from ADNI. For example, only one-third of participants were taking aCH medications, with even fewer in the high aCH+ group, whereas other studies report exposure in up to 70% of primary care older adult populations.19,40 Thus our ability to investigate the effects of various aCH properties, such as number of medications, strength of anticholinergic effects, and dosages, was somewhat limited. Nonetheless, we found that our novel aCH burden metric was associated with increased risk of incident MCI among aCH+ individuals, indicating an effect of cumulative aCH burden beyond use of any single aCH. Importantly, despite the robust use of covariates in our models and retained effects for only high aCH+ participants, confounding effects of health indications that vary with time (e.g., cardiovascular health, depressive symptoms) may contribute to cognitive change and therefore continue to present a challenge when differentiating comorbidity effects from aCH effects. We have attempted to address these health confounds, as well as the possibility of reverse causality with psychiatric difficulties preceding cognitive impairment, through several sensitivity analyses available in appendices C–G (data available from Dryad, doi.org/10.6075/J0BC3WZS), for which statistical significance was largely retained. Furthermore, although competing risk of mortality should be considered, it should be noted that there were no statistically significant group differences in study withdrawal due to death. The findings are also limited by the inability to account for complete prescribing data including participants' aCH use prior to the study period and changes in medications at follow-up time points. Moreover, the relatively small sample size, particularly when categorizing participants into aCH and biomarker groups, may have resulted in wide CIs and imprecise estimates. Furthermore, there is a potential for selection bias due to unobserved group differences. Finally, it is important to acknowledge the homogeneity of the sample in terms of educational, ethnic, and racial diversity. The current findings may not generalize to the entire population and we encourage continued research in this area within more representative samples.
Our results demonstrate that use of aCH in cognitively normal, highly educated, and healthy older adults is associated with increased risk of progression to MCI and accelerated cognitive decline, which are exacerbated in the presence of AD biomarkers. Future studies investigating brain-based structural and functional changes associated with aCH and AD risk factors are warranted to further elucidate the mechanisms driving these consequences of aCH. Findings of this study underscore the potential for negative consequences of aCH in older adults and support deprescribing trials, especially for individuals with elevated risk for AD.
Acknowledgment
The authors thank the participants of the Alzheimer's Disease Neuroimaging Initiative for providing data for this study as well as the individuals who work to make these data available for public use.
Glossary
- Aβ
β-amyloid
- ACB
Anticholinergic Cognitive Burden
- aCH
anticholinergic medications
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- CI
confidence interval
- D
daily dosage for a given drug
- GDS
Geriatric Depression Scale
- HR
hazard ratio
- LLR
log-likelihood ratio
- MED
minimally efficacious dosage
- NBM
nucleus basalis of Meynert
- p-tau
phosphorylated tau
Appendix. Authors
Study funding
This work was supported by NSF fellowship DGE-1650112 (A.J.W.), NIH grants R01 AG049810 (M.W.B.), K24 AG026431 (M.W.B.), P30 AG062429 (J.B.B, D.R.G, D.P.S.), and NIA R01AG061452 (N.L.C.). Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH grant U01 AG024904) and Department of Defense ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and the following: AbbVie, Alzheimer's Association, Alzheimer's Drug Discovery Foundation, Araclon Biotech, BioClinica, Inc., Biogen, Bristol-Myers Squibb Company, CereSpir, Inc., Cogstate, Eisai Inc., Elan Pharmaceuticals, Inc., Eli Lilly and Company, EuroImmun, F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc., Fujirebio, GE Healthcare, IXICO Ltd., Janssen Alzheimer Immunotherapy Research & Development, LLC, Johnson & Johnson Pharmaceutical Research & Development LLC, Lumosity, Lundbeck, Merck & Co., Inc., Meso Scale Diagnostics, LLC, NeuroRx Research, Neurotrack Technologies, Novartis Pharmaceuticals Corporation, Pfizer Inc., Piramal Imaging, Servier, Takeda Pharmaceutical Company, and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the NIH (fnih.org). The grantee organization is the Northern California Institute for Research and Education and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for NeuroImaging at the University of Southern California.
Disclosure
A.J. Weigand reports no disclosures relevant to the manuscript. M.W. Bondi receives royalties from Oxford University Press and serves as a consultant for Eisai, Novartis, and Roche Pharmaceutical. K.R. Thomas reports no disclosures. N.L. Campbell participated in a data safety monitoring board for NIA award R01AG067631 and serves as a consultant to Astellas Pharma, US. D.R. Galasko serves as a paid consultant for vTv Pharmaceuticals, Inc., serves on data safety monitoring boards for Cognition Therapeutics, Inc., and Proclara, Inc., and serves as paid Editor-in-Chief for Alzheimer's Research & Therapy, and receives clinical trial funding from Biogen, Eli Lilly, and Genentech. D.P. Salmon reports personal consulting fees from Takeda Pharmaceuticals, Inc., and Aptinyx, Inc. outside the submitted work. D. Sewell reports other from Medical Advisory Board and ActivCare Living–Residential Memory Care, Inc. and grants from Higi SH LLC, DHHS/HRSA Geriatric Workforce Enhancement Program Award, and DHHS/HRSA Geriatric Workforce Enhancement Program Award, outside the submitted work. J.B. Brewer reports nonfinancial support from CorTechs Laboratories, Inc., and nonfinancial support from Human Longevity, Inc., outside the submitted work. The terms of these arrangements have been reviewed by and approved by UCSD in accordance with its conflict of interest policies. H.H. Feldman reports grants from National Institutes of Aging including Alzheimer Disease Cooperative Study U19AG10483-26, DIAN Network 2UF1AG032438-07, CIHR 287674, Biohaven Pharmaceuticals, and Toyama Pharmaceuticals, and development grant funding from Probiodrug, during the conduct of the study; service agreements with Eisai Pharmaceuticals, Genentech/Roche Pharmaceuticals, Banner Health Institute, Samus Therapeutics, Axon Neurosciences, Samumed, Merck Pharmaceuticals, Tau RX, and Arkuda Therapeutics; speaker fees from World Events Forum, Medscape, Optum, and San Diego Academy of Family Physicians; and travel expenses from Axon Neurosciences, Alion Pharmaceuticals, and Probiodrug. L. Delano-Wood reports no disclosures relevant to the manuscript. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000010643 for full disclosures.
References
- 1.World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025. Available at: who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/. Accessed March 14, 2018. [Google Scholar]
- 2.Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: a case-control study. BMJ 2018;361:k1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Safe 2016;7:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos-Rodriguez JJ, Pacheco-Herrero M, Thyssen D, et al. Rapid β-Amyloid deposition and cognitive impairment after cholinergic denervation in APP/PS1 mice. J Neuropathol Exp Neurol 2013;72:272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshiyama Y, Kojima A, Itoh K, et al. Does anticholinergic activity affect neuropathology? Implication of neuroinflammation in Alzheimer's disease. Neurodegener Dis 2015;15:140–148. [DOI] [PubMed] [Google Scholar]
- 7.Cai X, Campbell N, Khan B, Callahan C, Boustani M. Chronic anticholinergic use and the aging brain. Alzheimers Dement 2013;9:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc 2008;56:2203–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins AJ, Kroenke K, Unutzer J, et al. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol 2004;57:1040–1048. [DOI] [PubMed] [Google Scholar]
- 10.Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health 2008;4:311–320. [Google Scholar]
- 11.Hilmer SN, Mager DE, Simonsick EM, et al. Drug burden index score and functional decline in older people. Am J Med 2009;122:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semla TP, Beizer JL, Higbee MD, eds. Geriatric Dosage Handbook. 18th ed. Hudson: Lexicomp; 2013. [Google Scholar]
- 13.Nation DA, Edland SD, Bondi MW, et al. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology 2013;81:2024–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strandberg TE, Pitkala K. What is the most important component of blood pressure: systolic, diastolic, or pulse pressure? Curr Opin Nephrol Hypertens 2003;12:293–297. [DOI] [PubMed] [Google Scholar]
- 15.Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 2018;S1552-5260:30029–30033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bondi MW, Edmonds EC, Jak AJ, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis 2014;42:275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edmonds EC, Delano-Wood L, Clark LR, et al. Susceptibility of the conventional criteria for MCI to false positive diagnostic errors. Alzheimers Dement 2015;11:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 2009;17:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell N, Boustani M, Limbil T, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging 2009;4:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bali V, Chatterjee S, Carnahan R, Chen H, Johnson M, Aparasu R. Risk of dementia among elderly nursing home patients using paroxetine and other selective serotonin reuptake inhibitors. Psychiatr Serv 2015;66:1333–1140. [DOI] [PubMed] [Google Scholar]
- 21.Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergic medications and incident dementia. JAMA Intern Med 2015;175:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrière I, Fourrier-Reglat A, Dartigues JF, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-City Study. Arch Intern Med 2009;169:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nebes RD, Pollock BG, Perera S, Halligan EM, Saxton JA. The greater sensitivity of elderly APOE ε4 carriers to anticholinergic medications is independent of cerebrovascular disease risk. Am J Geriatr Pharmacother 2012;10:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell NL, Boustani MA, Lane KA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology 2010;75:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz TW, Spreng RN. Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer's pathology. Nat Commun 2016;7:13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesulam M. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show? Learn Mem 2004;11:43–49. [DOI] [PubMed] [Google Scholar]
- 27.Bales KR, Tzavara ET, Wu S, et al. Cholinergic dysfunction in a mouse model of Alzheimer disease is reversed by an anti-Aβ antibody. J Clin Invest 2006;116:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellucci A, Luccarini I, Scali C, et al. Cholinergic dysfunction, neuronal damage and axonal loss in TgCRND8 mice. Neurobiol Dis 2006;23:260–272. [DOI] [PubMed] [Google Scholar]
- 29.Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016;73:721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 2016;91:1199–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 1983;214:170–197. [DOI] [PubMed] [Google Scholar]
- 32.Kilimann I, Grothe M, Heinsen H, et al. Subregional basal forebrain atrophy in Alzheimer's disease: a multicenter study. J Alzheimers Dis 2014;40:687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grothe M, Zaborszky L, Atienza M, et al. Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer's disease. Cereb Cortex 2009;20:1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butler T, Harvey P, Deshpande A, et al. Basal forebrain septal nuclei are enlarged in healthy subjects prior to the development of Alzheimer’s disease. Neurobiol Aging 2018;65:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler T, Bowen R, Atwood CS. Septal hypertrophy and cell cycle re-entry in AD. Aging 2019;11:297–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davey J, Thompson HE, Hallam G, et al. Exploring the role of the posterior middle temporal gyrus in semantic cognition: integration of anterior temporal lobe with executive processes. Neuroimage 2016;137:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malone PS, Glezer LS, Kim J, Jiang X, Riesenhuber M. Multivariate pattern analysis reveals category-related organization of semantic representations in anterior temporal cortex. J Neurosci 2016;36:10089–10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belleville S, Fouquet C, Hudon C, Zomahoun HTV, Croteau J. Consortium for the early identification of Alzheimer's disease–Quebec: neuropsychological measures that predict progression from mild cognitive impairment to Alzheimer's type dementia in older adults: a systematic review and meta-analysis. Neuropsychol Rev 2017;27:328–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duke Han S, Nguyen CP, Stricker NH, Nation DA. Detectable neuropsychological differences in early preclinical Alzheimer's disease: a meta-analysis. Neuropsychol Rev 2017;27:305–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell NL, Lane KA, Gao S, Boustani MA, Unverzagt F. Anticholinergics influence transition for normal cognition to mild cognitive impairment in older adults primary care. Pharmacotherapy 2018;38:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this article were obtained from the ADNI database (adni.loni.usc.edu). ADNI was launched in 2003 as a public–private partnership. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD.