Abstract
Objective
To test the hypothesis that incipient Alzheimer disease (AD) may adversely affect hearing and that hearing loss may adversely affect cognition, we evaluated whether genetic variants that increase AD risk also increase problem hearing and genetic variants that increase hearing impairment risk do not influence cognition.
Methods
UK Biobank participants without dementia ≥56 years of age with Caucasian genetic ancestry completed a Digit Triplets Test of speech-in-noise hearing (n = 80,074), self-reported problem hearing and hearing with background noise (n = 244,915), and completed brief cognitive assessments. A genetic risk score for AD (AD-GRS) was calculated as a weighted sum of 23 previously identified AD-related polymorphisms. A genetic risk score for hearing (hearing-GRS) was calculated using 3 previously identified polymorphisms related to hearing impairment. Using age-, sex-, and genetic ancestry–adjusted logistic and linear regression models, we evaluated whether the AD-GRS predicted poor hearing and whether the hearing-GRS predicted worse cognition.
Results
Poor speech-in-noise hearing (>-5.5-dB speech reception threshold; prevalence 14%) was associated with lower cognitive scores (ß = −1.28; 95% confidence interval [CI] −1.54 to −1.03). Higher AD-GRS was significantly associated with poor speech-in-noise hearing (odds ratio [OR] 1.06; 95% CI 1.01–1.11) and self-reported problems hearing with background noise (OR 1.03; 95% CI 1.00–1.05). Hearing-GRS was not significantly associated with cognitive scores (ß = −0.05; 95% CI −0.17 to 0.07).
Conclusions
Genetic risk for AD also influences speech-in-noise hearing. We failed to find evidence that genetic risk for hearing impairment affects cognition. AD disease processes or a that shared etiology may cause speech-in-noise difficulty before dementia onset.
Age-related hearing impairment is among the most important potentially modifiable determinants of Alzheimer disease (AD).1 Many observational studies have found that hearing impairment is associated with cognitive decline.2–7 However, given the long, insidious development of hearing loss and dementia, it is difficult to establish temporal order or to rule out shared etiologies.
Age-related hearing impairment is caused by loss of peripheral hearing (involving the ear) or loss of central hearing (brain processing abilities). Hearing loss, especially peripheral, is thought to cause dementia through effects on social isolation or cognitive capacity,8 but the evidence is not conclusive. Underlying AD-related neurodegeneration may affect central hearing loss even in prodromal stages.9–11 In addition, other diseases, including vascular or metabolic disease, may affect hearing12,13 and promote cognitive decline.14,15 Prior observational studies cannot evaluate these different hypothesized mechanisms; novel study designs are needed to test these alternatives.
To begin to evaluate these hypotheses; we used an approach paralleling a bidirectional mendelian randomization (MR) study, which uses genetic variants to evaluate the causal effect of a risk factor on a health outcome.16–18 First, we took advantage of known genetic variation in AD risk,19 which is established at birth, to test the hypothesis that shared genetics or incipient AD may influence hearing in older adults without dementia (figure 1A), focusing on a speech-in-noise test, which may be sensitive to central hearing loss. Second, we took advantage of known genetic variation in hearing-impairment risk20 to test the hypothesis that hearing impairment may influence cognition (figure 1B).
Figure 1. Conceptual models of AD genetic risk, dementia, and hearing loss motivating analysis.
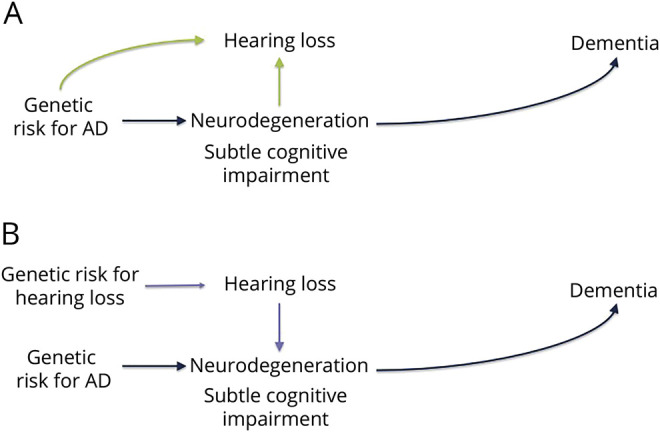
Genetic variants known to increase Alzheimer disease (AD) risk can be used to distinguish between mechanisms that may explain the previously documented association between hearing loss and dementia risk. If incipient neurodegeneration or other shared etiologies influence hearing loss, it implies that genetic variants that increase the risk of dementia will also be associated with increased risk of hearing loss (A). If hearing loss has effects on biological or social processes that increase risk of dementia, it implies that genetic risk for AD will be independent of (not associated with) hearing loss (B) but that genetic risk for hearing impairment will be associated with risk of dementia.
Methods
Study setting and participants
UK Biobank is an ongoing study of >500,000 adults. Participants 40 to 69 years of age were recruited from 2006 to 2010 from across the United Kingdom to provide detailed information about themselves via computerized questionnaires, provide biological samples, undergo clinical measurements, and have their health followed up prospectively.21 Participants are generally healthy volunteers compared to the general UK population and do not have dementia at enrollment.22
The current analysis was restricted to UK Biobank participants ≥56 years of age (n = 291,516) to focus on older adults in whom hearing loss was likely to be common. We excluded those with missing genetic information (n = 8,560) or who were flagged as recommended for genetic analysis exclusion (n = 226) and those without any hearing assessments (n = 693). We also excluded participants classified as of non-European genetic ancestry (n = 37,122) on the basis of a combination of self-report and genetic ancestry principal components (PCs) because genetic predictors of AD may differ by race/population stratification.23 This left an analytic sample of 244,915 with at least 1 hearing measure. A speech-in-noise hearing test was available for 80,074 of these participants.
Standard protocol approvals, registrations, and patient consents
Ethics approval was obtained from the National Health Service National Research Ethics Service, and all participants provided written informed consent.
Hearing
A Digit Triplet Test (DTT), a measure of speech-in-noise hearing ability, was incorporated into visits during 2009 and was completed by 80,136 eligible participants. Participants with hearing aids were asked to remove hearing aids before testing, and those with cochlear implants were asked not to attempt the test. Participants were guided by a video demonstration, performed the test via touchscreen, and wore circumaural headphones (Sennheiser HD-25, Wedemark, Germany). The English speech materials for the UK Biobank DTT were developed at the University of Southampton and are described elsewhere.24,25 Digit triplets (e.g., group of 3 monosyllabic digits such as 2-8-5) were presented in a total of 15 sets. A background noise matched to the spectrum of speech stimuli played simultaneously while digit triplets were presented. In initial triplets, both noise and speech levels were adjusted together to a comfortable level. The speech level was then fixed, and noise levels were increased or decreased adaptively after each triplet to estimate the signal-to-noise ratio at which a participant had 50% correct recognition of 3 digits. A speech reception threshold (SRT) was used as the primary measure of speech-in-noise hearing. SRT was calculated as the mean signal-to-noise ratio for triplets 8 to 15. Higher scores correspond to worse performance. In analyses, we used the SRT of the better-hearing ear, following the approach of other studies.3,26 Due to outliers and skewed distribution, we log-transformed the SRT (calculated as log [SRT + 13] to account for negative SRT values) when analyzing it as a continuous measure of speech-in-noise hearing. We also created a dichotomous indicator for poor speech-in-noise hearing, defined as an SRT >−5.5 dB, corresponding to cutoffs used in previous research.3,26 The DTT test requires both peripheral and central hearing processing.27
All participants were also asked about problems hearing (“Do you have any difficulty with your hearing?”) and problems hearing in noise (e.g., “Do you find it difficult to follow a conversation if there is background noise [such as television, radio, children playing]?”). Possible answers were “yes,” “no,” “do not know,” and “prefer not to answer.” We considered the answers “do not know” or “prefer not to answer” as missing data. Self-reports of hearing difficulty are complementary to audiometric measurements and have been validated against audiometric measures of hearing impairment for use in large epidemiologic studies,28 and prior work has found that the measures of hearing with background noise are highly correlated with the UK Biobank DTT.3
Genotyping and genetic risk scores for AD
Genotyping of UK Biobank samples was conducted with 2 closely related arrays (Affymetrix using a bespoke BiLEVE Axiom array and Affymetrix UK Biobank Axiom array; Santa Clara, CA) and is described in detail elsewhere.29,30 Briefly, all genetic data were quality controlled and imputed by UK Biobank (downloaded on December 1, 2017) to a reference panel that merged the 1,000 Genomes phase 3 and UK10K reference panels. A secondary imputation was completed with the Haplotype Reference Consortium reference panel, and results from the Haplotype Reference Consortium imputation were preferentially used at single nucleotide polymorphisms (SNPs) present in both panels. Before the release of the UK Biobank genetic data, a stringent quality control protocol was applied, which was performed at the Welcome Trust Centre for Human Genetics and is described elsewhere.31 We additionally excluded participants from the present study if they had non-European ancestry (as described above), were missing genetic information, or were recommended for genetic exclusion by UK Biobank due to a high heterozygosity rate (after correction for ancestry) or high missing rate.32
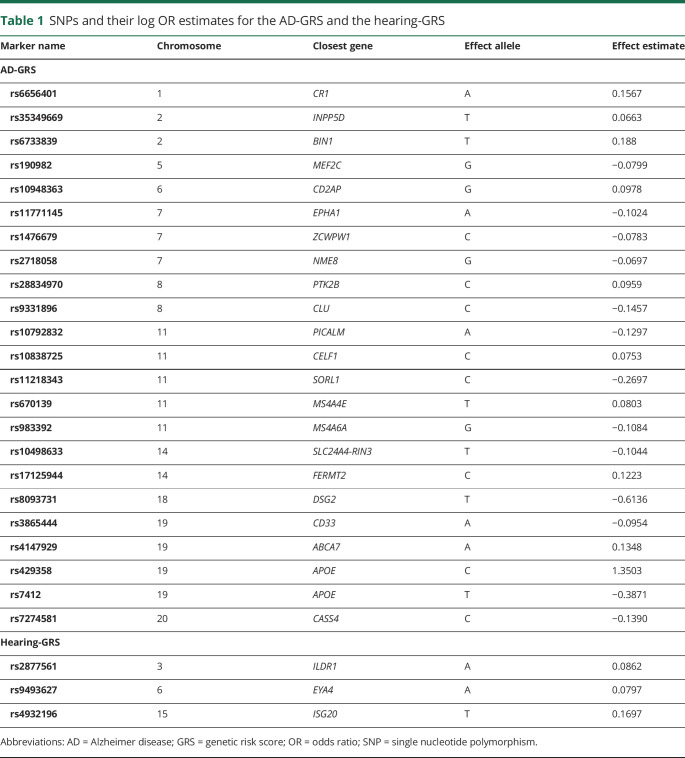
We used summary results from the 2013 International Genomics of Alzheimer's Project (IGAP) meta-analyzed genome-wide association study on late-onset AD in White populations19 to calculate an AD genetic risk score (GRS) for each participant. The IGAP study identified 23 loci associated with AD, including 2 SNPs used to characterize APOE ε4 allele status. The GRS was based on the meta-analyzed β coefficients obtained in the IGAP stage 1 study, which included genotyped and imputed data (7,055,881 SNPs, 1000 G phase 1α imputation, build 37, assembly Hg19) of 17,008 AD cases and 37,154 controls. We calculated the AD-GRS by multiplying each individual's risk allele count for each locus by the β coefficient (expressed as the log odds ratio [OR]) for that polymorphism (table 1) and summing the products for all 23 loci. This step weights each SNP in proportion to its anticipated effect (either positive or negative) on AD risk. The scores can be interpreted as the log OR for AD conferred by that individual's profile on the 23 SNPs compared to a person who had the major allele at each locus. With this construction, a 1-unit increase in the AD-GRS connotes a 2.7 times higher risk of AD. To assess the association with the AD-GRS beyond the effects of APOE ε4 (AD-GRS without APOE), we also calculated an alternative AD-GRS after removing the 2 SNPs associated with APOE. In a sensitivity analysis of the effects of APOE genotype alone, we created a score based on the 2 SNPs associated with APOE. From the APOE-only scores, we derived a dichotomous variable noting the presence of at least 1 APOE ε4 allele.
Table 1.
SNPs and their log OR estimates for the AD-GRS and the hearing-GRS
Using similar methods, we also calculated a GRS for age-related hearing impairment (hearing-GRS). This score was based on 3 SNPs that have previously been identified to be significantly associated with age-related hearing impairment in the Kaiser Permanente Northern California Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort with replication in the UK Biobank.20 Age-related hearing impairment in GERA was defined by ICD-9 codes 388.01 (presbycusis), 389.12 (bilateral neural hearing loss), and 389.19 (bilateral sensorineural hearing loss). We used effect estimates from GERA to calculate the hearing-GRS (table 1).20
Cognition
The verbal reasoning assessment (also called fluid intelligence in some UK Biobank reports) was a visual touchscreen-based test that required participants to solve problems based on logic and reasoning. A summary score was calculated from the number of correct responses to 13 questions within a 2-minute time limit. Although other cognitive measures were assessed for some UK Biobank participants, we used these 2 measure for several reasons: the test was completed on a large fraction of the eligible sample; it declines with age; and it is correlated with all other cognitive measures.33 Associations between poor hearing and cognition using other cognitive tests have been reported previously.3
Other covariates
Age and sex were reported during baseline assessment via touchscreen questionnaires. The UK Biobank provides PCs related to genetic population stratification, we used the first 5 PCs in our analyses to adjust for confounding by population stratification.
Statistical analysis
Following the general approach of a bidirectional MR study, we evaluated bidirectional relationships between hearing impairment and cognition using genetic risk as an analytic tool.16–18 First, we estimated whether genetic variants known to increase risk for AD were associated with problem hearing by DDT or self-report. Next, we evaluated whether genetic variants known to increase risk for age-related hearing impairment were also associated with poor cognition. This approach takes advantage of the fact that genetic variants have an established temporal order (i.e., determined at conception, before disease onset) before the exposure (e.g., AD or hearing loss). Furthermore, one's genotypes are not as susceptible to traditional confounders in observational studies such as an one's socioeconomic status, health behaviors, or health conditions. This established temporal order helps improve causal inference in observational data.16 If AD-related genetic variants influence hearing impairment, this provides evidence that either AD influences hearing impairment or the variants share a genetic etiology (figure 1A). In contrast, if hearing impairment–related genetic variants influence cognition, this provides evidence that hearing loss influences cognition (figure 1B).
First, we confirmed that, consistent with previous literature, (1) AD-GRS was associated with cognition (verbal reasoning), (2) hearing-GRS was associated with hearing impairment, and (3) hearing impairment was associated with cognition in our sample. We tested our primary hypotheses by evaluating the association between (1) AD-GRS and speech-in-noise hearing impairment and (2) hearing-GRS and cognition. We evaluated associations between AD-GRS (with and without APOE) and self-reported hearing measures as secondary analyses to evaluate the consistency of results because self-reported measures were available on a larger sample of participants. We estimated separate logistic regressions with each AD-GRS as the predictor and each hearing impairment measure as the outcome (poor speech-in-noise hearing, self-reported hearing problems, and self-reported problems with hearing in noise). We conducted a sensitivity analysis to attempt to isolate the effects of AD-GRS on peripheral hearing because the available hearing measures represent both central and peripheral hearing. We therefore adjusted for cognition (verbal reasoning) to evaluate the association between AD-GRS and speech-in-noise hearing loss, which may suggest an effect of the AD-GRS on peripheral hearing. SRT is a continuous measure, so we also ran a linear regression model between the AD-GRS and continuous log SRT.
We used linear regressions to test the association between hearing-GRS and cognitive test scores. We also estimated a traditional 2-stage least-squares instrumental variable model using the hearing-GRS as an instrument for hearing problems to further test whether findings were consistent with a causal effect of worse hearing on cognition.34 The coefficients can be interpreted as the cognitive effects of differences in hearing resulting from the genetic variants and can be directly compared to the observational estimate of poor hearing on cognitive scores. All regression models included adjustment for age, sex, and 5 PCs to account for ancestry differences. We ran MR-Egger regression as a sensitivity analyses to test for any horizontal pleiotropy (e.g., independent effect of genetic variants on both hearing and cognition).35 We report intercept terms and the intercept test from MR-Egger analysis for primary models; a nonzero intercept suggests horizontal pleiotropy. Analyses were conducted in R (version 3.3.2, R Foundation for Statistical Computing, Vienna, Austria). All tests were 2 sided with α = 0.05, and we report 95% confidence intervals (CIs) to represent uncertainty in our effect estimates.
Data availability
Researchers can apply to UK Biobank to access the data used in this study (ukbiobank.ac.uk/).
Results
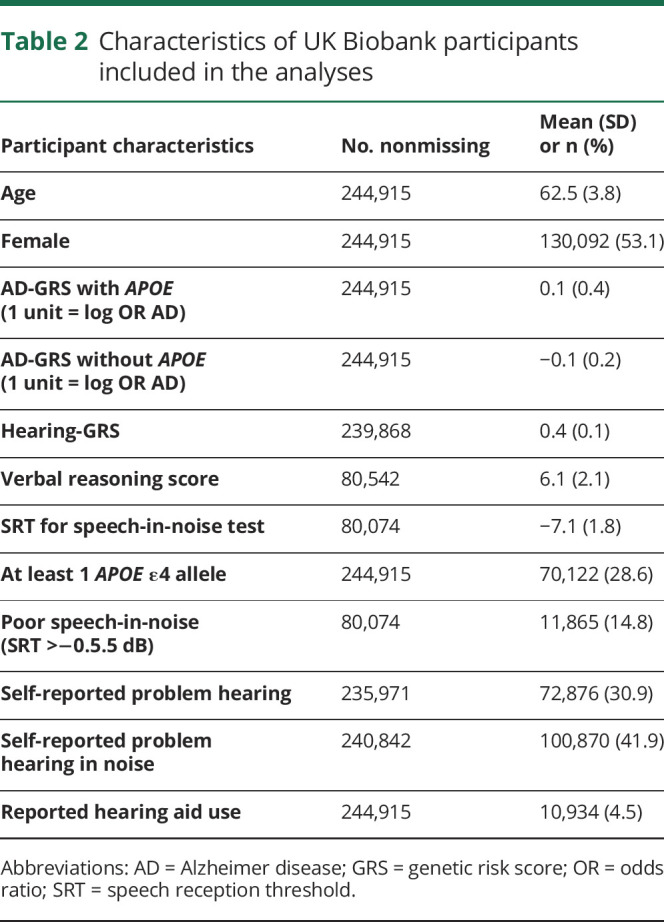
Average age of participants was 62.5 years (SD 3.8 years; table 2). The SRT was on average −7.1 dB (SD 1.8 dB); 14% had poor speech-in-noise hearing (SRT >−5.5 dB). Self-report of hearing problems was common (30%–40%). Of those who reported any hearing problems, 85% also reported problems hearing in noise; of those who reported any hearing problems in noise, 64.5% also reported problems hearing in general. Speech-in-noise hearing was significantly worse (higher value) in those with self-reported hearing problems (mean [SD] SRT −6.7 dB [2.1 dB] compared to −7.4 dB [1.5 dB]) and those with self-reported hearing problems in noise (mean [SD] SRT −6.8 dB [2.1 dB] compared to −7.4 dB [1.5 dB]) (both p < 0.001, Kruskal-Wallis test). Among those with poor speech-in-noise hearing (SRT >−5.5 dB), 52% had self-reported problems hearing, and 57% had self-reported problems hearing in noise.
Table 2.
Characteristics of UK Biobank participants included in the analyses
Confirming associations of hearing and AD-GRS with cognition
Every measure of poor hearing was associated with worse verbal reasoning (table 3). Higher AD-GRS with APOE was also associated with worse verbal reasoning (table 3), although the AD-GRS without APOE was not.
Table 3.
Linear regression coefficients predicting verbal reasoning scores as a function of self-reported and speech-in-noise measures of hearing and AD-GRS
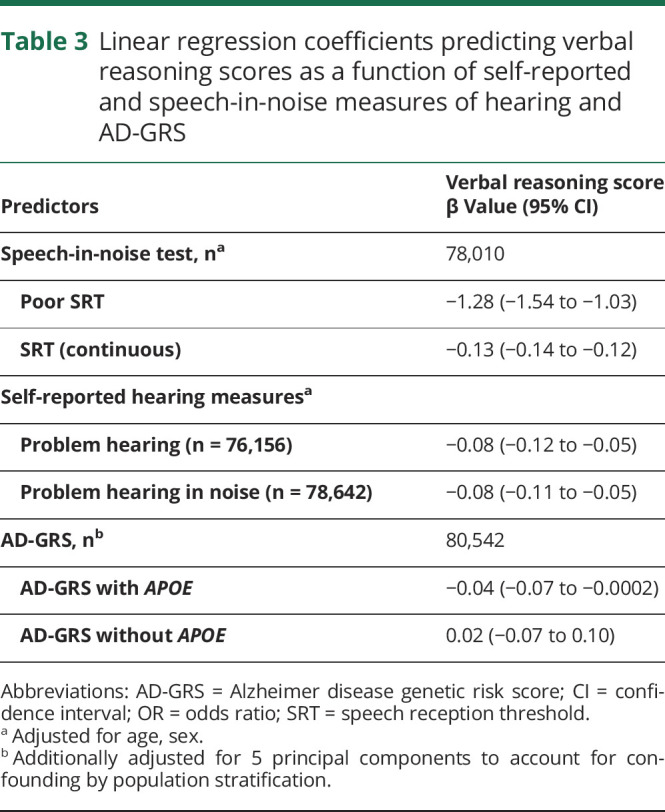
AD-GRS and hearing associations
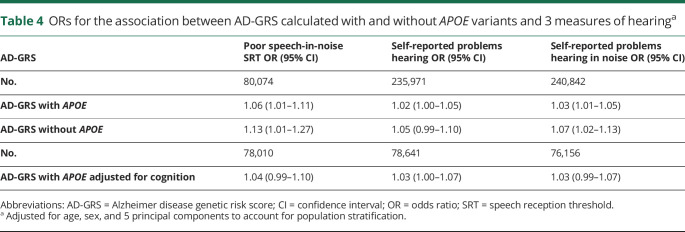
AD-GRS was associated with poor speech in-noise hearing (SRT) (p < 0.02 for AD-GRS with APOE and p < 0.02 for AD-GRS without APOE). AD-GRS also predicted self-reported problems hearing in noise (table 4). The association of the AD-GRS and self-reported problems hearing was in the same direction but not statistically significantly. Effect sizes across all hearing outcomes ranged from a 2% to 13% increased odds of poor hearing per 1-unit increase in AD-GRS; to put this into more interpretable terms, an increase in the AD-GRS that would roughly triple the odds of AD would also increase risk of hearing impairment by 2% to 13%. There was a trend toward slightly higher effect sizes in analyses using poor speech-in-noise hearing.
Table 4.
ORs for the association between AD-GRS calculated with and without APOE variants and 3 measures of hearinga
Results were relatively similar with adjustment for cognition (verbal reasoning) as a covariate (table 4). The point estimate for poor speech-in-noise hearing was slightly reduced and CIs were slightly wider; however, fewer participants were included in these analyses due to missing cognitive data. APOE alone was not significantly associated with poor SRT (OR 1.04; 95% CI 0.98–1.10). Tests for horizontal pleiotropy of AD-GRS SNPs with MR-Egger regression were not significant, and intercepts were close to 0 for each hearing outcome (self-reported problem hearing:β = 0.000, standard error [SE] 0.000, p = 0.97; self-reported problem hearing in noise: β = 0.004, SE 0.002, p = 0.11; poor SRT: β = 0.006, SE 0.004, p = 0.18).
When SRT was used as a continuous measure of hearing, the AD-GRS with APOE (but not AD-GRS without APOE) was associated with worse average speech-noise hearing (SRT) (figure 2 shows effects by sample quartiles of AD-GRS with APOE).
Figure 2. Association between speech-in-noise SRT and quartiles of AD-GRS (n = 80,074).
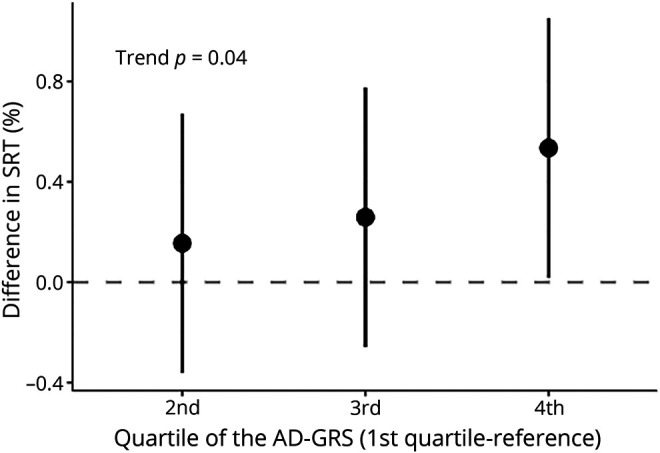
There was a significant trend (p = 0.04) for increasing quartile of Alzheimer disease genetic risk score (AD-GRS) and worse speech reception threshold(SRT) (positive change) on a continuous scale. However, only the fourth quartile had a significantly worse SRT than the first quartile of AD-GRS (reference group). Based on a linear regression of log SRT adjusted for age, sex, and 5 principal components to account for confounding by population stratification.
Hearing-GRS and associations with hearing and cognition
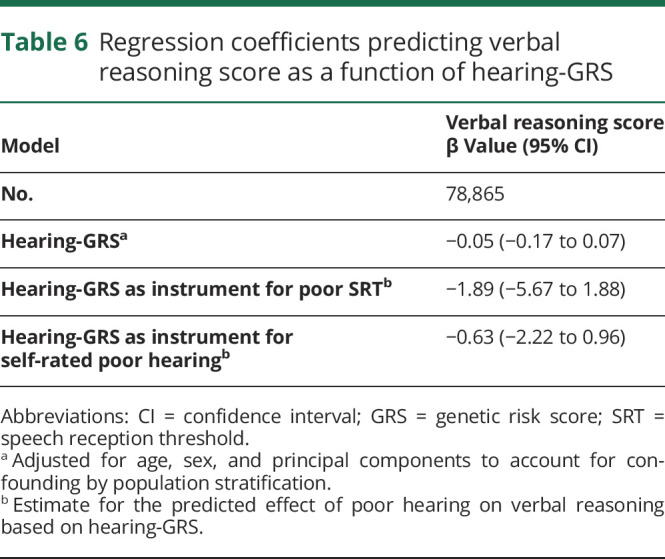
The hearing-GRS was significantly associated with worse speech-in-noise hearing (table 5) as expected: a 1-log-unit higher GRS was associated with 35% higher odds of poor speech-in-noise hearing (F statistic 9.62%) and 43% higher odds of self-rated problem hearing (F statistic 29.7). However, there was no significant association between the hearing-GRS and verbal reasoning scores (table 6). Conducted as an instrumental variable analysis in which the hearing-GRS was used as an instrument for SRT values, the point estimates for the effect of poor speech-in-noise hearing (SRT) on cognition (−1.89 points) were similar to the observational point estimate (−1.28 points); however, the instrumental variables–based effect estimate was lacking precision (table 6).
Table 5.
Regression coefficients predicting hearing as a function of hearing-GRSa
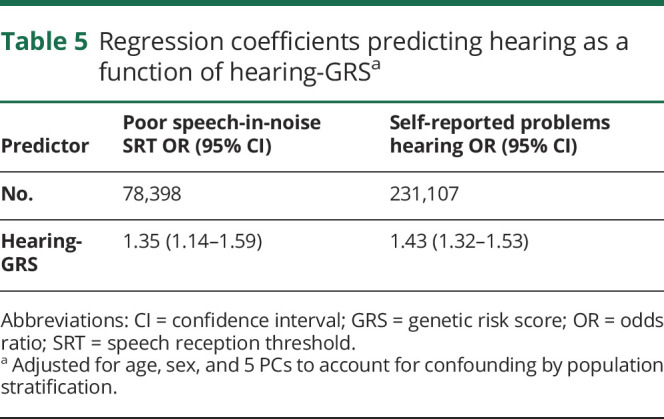
Table 6.
Regression coefficients predicting verbal reasoning score as a function of hearing-GRS
Discussion
In this study, we found that higher AD-GRS was associated with worse speech-in-noise hearing in a large sample of middle-aged and older adults without dementia. Findings were consistent across self-reported hearing problems and poor speech-in-noise hearing measurements. Effect estimates were generally small; the largest effect estimates were for the association of AD-GRS and speech-in-noise hearing assessment. Results were similar but slightly stronger for the AD-GRS without APOE compared to the AD-GRS with APOE. Participants did not have dementia at baseline, and these associations were independent of cognitive abilities at baseline; in addition, we did not find evidence for pleiotropic effects. We did not find evidence for an association between hearing-GRS and cognition, although estimates were in the expected direction. Future work will be necessary to fully tease apart the interplay of neurodegeneration, cognitive decline, and hearing loss. However, our current findings are consistent with the hypothesis that the underlying AD process influences speech-in-noise hearing ability before the onset of cognitive impairment.
Sensory impairments are emerging as a potential marker of preclinical AD.36 Amyloid plaque and neurofibrillary tangle deposition occurs in auditory pathways in patients with dementia.10,37 Several studies have found that central auditory processing is significantly worse in those with AD dementia or even mild cognitive impairment11,38–40; 1 longitudinal study suggests that this auditory dysfunction may predict dementia onset.11 Speech-in-noise hearing is correlated with cognitive tests, including attention and memory, beyond correlations with pure tone audiometry.27 In addition, children with auditory processing disorders often can present with comorbid cognitive, reading, and language deficits41 and have white matter structural abnormalities on brain MRI.42 Participants with high AD-GRS in this study are disproportionately likely to be experiencing early effects of AD but do not have severe cognitive impairments at baseline; in fact, only a small fraction have developed dementia over follow-up.43 Because neurodegeneration and even very subtle cognitive impairments may occur many years before the development of dementia symptoms,44 these changes may in turn lead to worse hearing, consistent with our finding that higher genetic AD risk is associated with worse speech-in-noise hearing.
We used GRSs to begin to evaluate alternative causal hypotheses linking poor hearing and dementia. The majority of studies on hearing and dementia focus on peripheral hearing loss as a risk factor for developing dementia.2–7 However, detecting speech in noise requires both peripheral and central hearing processing, as well as general cognitive abilities to discriminate digits.3 As an important caveat, it is unclear from our findings whether genetic risk for AD is associated with peripheral hearing loss or purely central hearing. In prior studies, the APOE ε4 allele was both inversely45 and positively46 associated with peripheral hearing loss. However, in this study, the AD-GRS without APOE tended to have slightly stronger associations with hearing impairment. Our findings suggest the AD-GRS is associated with hearing abilities independently of cognition and that further research with pure tone audiometric testing on a large sample of older adults is warranted.
Genetic variants related to AD may have pleiotropic pathways via which they influence hearing such as by affecting nonneurodegenerative factors such as vascular disease that may act as shared risk factors for hearing loss and cognitive decline. Variation in several AD genes, including APOE, affect lipid metabolism47 and cardiovascular risk factors.48 Our sensitivity analyses using MR-Egger regression, however, did not find strong evidence for pleiotropic effects. Furthermore, a genome-wide association study of neuropathologic contributors to dementia found that AD genetic loci are associated with AD neuropathology but not with vascular brain injury.49 This lends further evidence to the hypothesis that the AD disease process may contribute to impairment in hearing but does not exclude the possibility of pleiotropic effects. Our analyses cannot distinguish whether these findings are the result of neurodegeneration in auditory or other brain regions or very early symptoms of cognitive impairment, although effects were independent of overall cognition. Regardless of this ambiguity in interpretation, our results suggest that problem hearing in noise may be an early marker for AD.
We did not find an association between the GRS for hearing impairment and cognition. Given the imprecision of our estimates, however, our findings do not conclusively exclude the possibility that hearing loss causes cognitive decline. Point estimates were similar between our observational and instrumental variable analyses; thus, it is possible that this analysis was underpowered. Age-related hearing impairment is likely highly polygenic, and few SNPs have shown significant associations in genome-wide association studies.20 In future studies, it may be worth investigating the use of whole-genome information or combining samples to enhance analytic power.
The Lancet commission on dementia prevention calculated a relative risk of impaired peripheral hearing on dementia of 1.9,1 equating to an OR of 2.0 to 2.5, depending on the prevalence of dementia. This estimated association between hearing and dementia is much higher than could be attributed to the effects of known AD genetic risk on impaired hearing in this study (OR 1.05–1.13). This suggests other mechanisms link hearing and dementia but does not establish that those other mechanisms are necessarily a causal effect of hearing on dementia. If peripheral hearing loss does cause dementia, then auditory interventions maybe be an effective a way to reduce dementia burden.1 However, such an intervention would not be as effective if the association between peripheral hearing and dementia is partly explained by underlying AD or dementia processes, shared etiologies, or noncausal links. Although interventions to improve hearing and communication for older adults likely have broad benefits for social engagement and quality of life,50 future studies are still needed to show that prevention of hearing loss slows cognitive decline.
There are several important caveats and limitations to our analysis. Effect sizes are small, and it is unclear how well speech-in-noise hearing impairment predicts future development of dementia. Our results are potentially influenced by selection bias due to selective survival because the AD-GRS is associated with mortality.43 However, this effect is likely limited because the sample is relatively young and healthy with a low mortality rate, especially due to dementia. There is substantial variation in risk for AD that is not captured by our risk score. This limitation would likely reduce our ability to detect associations, and estimates may be underestimated. This analysis focused on genetic variants for AD, so we may not have captured associations specific to other dementia subtypes. However, these findings likely broadly apply to dementia because AD is the most common form of dementia and often co-occurs with other pathologies.1 There may have been misclassification in the self-reported hearing measures, and directions for answering questions were unclear for participants with hearing aids. However, this also likely would bias results toward the null. This sample did not include participants of non-European ancestry, which may limit generalizability. There are also considerable strengths to this study, particularly the size of sample, the use of several measures of hearing abilities, and an innovative analytic approach.
We used variation in genetic risk for AD as an approach to examine whether the AD disease process or a shared etiology influences hearing. In support of this hypothesis, we found that higher genetic risk for AD was positively associated with worse hearing ratings and worse speech-in-noise testing. We also used variation in genetic risk for hearing impairment to examine whether hearing loss influences cognition. Although we did not find evidence to support this effect of hearing loss on cognition, our estimates were imprecise. Our findings suggest that poor speech-in-noise hearing may be an early marker of underlying dementia processes. However, additional research will be needed to replicate findings in other samples, to investigate measures of peripheral hearing loss, and to determine whether loss of speech-in-noise testing can be useful as a tool to identify preclinical AD.
Acknowledgment
The authors thank the UK Biobank participants and staff. The UK Biobank project is funded by the Medical Research Council, The Wellcome Trust, the Department of Health for England and Wales, the North West Regional Development Agency, and the Scottish Executive.
Glossary
- AD
Alzheimer disease
- CI
confidence interval
- DTT
Digit Triplet Test
- GERA
Genetic Epidemiology Research on Adult Health and Aging
- GRS
genetic risk score
- ICD-9
International Classification of Diseases, 9th revision
- IGAP
International Genomics of Alzheimer's Project
- MR
mendelian randomization
- OR
odds ratio
- PC
principal component
- SE
standard error
- SNP
single nucleotide polymorphism
- SRT
speech reception threshold
Appendix. Authors
Study funding
This work was supported by National Institute on Aging grants T32-AG-049663 (W.D.B.), K01AG062722 (W.D.B.), K24-AG031155 (K.Y.), and RF1AG052132-01 (R.A.W. and M.M.G.), the Alzheimer's Association AARF-18-565846 (W.D.B.) and the University of California, San Francisco Claude D. Pepper Older Americans Independence Center funded by National Institute on Aging, P30 AG044281 (W.D.B.), and TALENT 2018-T1/BMD-11226 from the Community of Madrid (SW).
Disclosures
W. Brenowitz reports no disclosures relevant to the manuscript. T. Filshtein works at 23andMe but conducted the work while at the University of California, San Francisco. K. Yaffe, S. Walter, S. Ackley, T. Hoffmann, E. Jorgenson, R. Whitmer. and M. Glymour reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Publication history
This manuscript was previously published in bioRxiv: http://biorxiv.org/cgi/content/short/556506v1. Received by Neurology November 11, 2019. Accepted in final form May 12, 2020.
References
- 1.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 2.Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med 2013;173:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore DR, Edmondson-Jones M, Dawes P, et al. Relation between speech-in-noise threshold, hearing loss and cognition from 40–69 years of age. PLoS One 2014;9:e107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer ME, Cruickshanks KJ, Schubert CR, et al. Age-related sensory impairments and risk of cognitive impairment. J Am Geriatr Soc 2016;64:1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deal JA, Betz J, Yaffe K, et al. Hearing impairment and incident dementia and cognitive decline in older adults: the Health ABC Study. J Gerontol A Biol Sci Med Sci 2017;72:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies HR, Cadar D, Herbert A, Orrell M, Steptoe A. Hearing impairment and incident dementia: findings from the English Longitudinal Study of Ageing. J Am Geriatr Soc 2017;65:2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan J, Sun Y, Sang S, Pham JH, Kong WJ. The risk of cognitive impairment associated with hearing function in older adults: a pooled analysis of data from eleven studies. Sci Rep 2018;8:2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin FR, Albert M. Hearing loss and dementia: who's listening? Aging Ment Health 2014;18:671–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swords GM, Nguyen LT, Mudar RA, Llano DA. Auditory system dysfunction in Alzheimer disease and its prodromal states: a review. Ageing Res Rev 2018;44:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer's disease. Neurology 1993;43:779–785. [DOI] [PubMed] [Google Scholar]
- 11.Gates GA, Beiser A, Rees TS, D'Agostino RB, Wolf PA. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer's disease. J Am Geriatr Soc 2002;50:482–488. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell P, Gopinath B, McMahon CM, et al. Relationship of type 2 diabetes to the prevalence, incidence and progression of age-related hearing loss. Diabet Med 2009;26:483–488. [DOI] [PubMed] [Google Scholar]
- 13.Fischer ME, Schubert CR, Nondahl DM, et al. Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis 2015;238:344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esiri MM. Which vascular lesions are of importance in vascular dementia? Ann NY Acad Sci 2000;903:239–243. [DOI] [PubMed] [Google Scholar]
- 15.Abner EL, Nelson PT, Kryscio RJ, et al. Diabetes is associated with cerebrovascular but not Alzheimer's disease neuropathology. Alzheimers Dement 2016;12:882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008;27:1133–1163. [DOI] [PubMed] [Google Scholar]
- 17.Glymour MM, Tchetgen T, JE, Robins JM. Credible mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am J Epidemiol 2012;175:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess S, Butterworth AS, Thompson JR. Beyond mendelian randomization: how to interpret evidence of shared genetic predictors. J Clin Epidemiol 2016;69:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet 2013;45:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann TJ, Keats BJ, Yoshikawa N, Schaefer C, Risch N, Lustig LR. A large genome-wide association study of age-related hearing impairment using electronic health records. PLoS Genet 2016;12:e1006371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. Plos Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 2017;186:1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marden JR, Walter S, Tchetgen EJT, Kawachi I, Glymour MM. Validation of a polygenic risk score for dementia in Black and White individuals. Brain Behav 2014;4:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall SJ. The Development of a New English Sentence in Noise Test and an English Number Recognition Test [online]: Faculty of Engineering, Science, and Mathematics, Institute of Sound and Vibration Research, University of Southampton; 2006. Available at: biobank.ctsu.ox.ac.uk/crystal/docs/audiology_msc2006.pdf. Accessed August 6, 2018 [Google Scholar]
- 25.UK Biobank: Hearing “Speech-In-Noise” Test [online]. Available at: biobank.ctsu.ox.ac.uk/crystal/docs/Hearing.pdf. Accessed August 6, 2018. [Google Scholar]
- 26.Dawes P, Fortnum H, Moore DR, et al. Hearing in middle age: a population snapshot of 40–69 year olds in the UK. Ear Hear 2014;35:e44–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akeroyd MA. Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. Int J Audiol 2008;47:S53–S71. [DOI] [PubMed] [Google Scholar]
- 28.Sindhusake D, Mitchell P, Smith W, et al. Validation of self-reported hearing loss. The blue mountains hearing study. Int J Epidemiol 2001;30:1371–1378. [DOI] [PubMed] [Google Scholar]
- 29.UKB_WCSGAX: UK Biobank 500K Samples Genotyping Data Generation by the Affymetrix Research Services Laboratory Vol 9. Available at: http://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/affy_data_generation2017.pdf, Accessed September 3, 2018 [Google Scholar]
- 30.UKB_WCSGAX: UK Biobank 500K Samples Processing by the Affymetrix Research Services Laboratory Vol 8. Available at https://www.ukbiobank.ac.uk/wp-content/uploads/2014/04/Affymetrix-UKB_WCSGAX-Lab-Process-1.pdf. Accessed September 3, 2018 [Google Scholar]
- 31.Bycroft C, Freeman C, Petkova D, et al. Genome-wide Genetic Data on ∼500,000 UK Biobank Participants: bioRxiv [online serial]. Available at: biorxiv.org/content/early/2017/07/20/166298.abstract. September 3, 2018 [Google Scholar]
- 32.Genotyping and quality control of UK Biobank, a large-scale, extensively phenotyped prospective resource [online]. Available at: ukbiobank.ac.uk/wp-content/uploads/2014/04/UKBiobank_genotyping_QC_documentation-web.pdf. Accessed September 3, 2018.
- 33.Lyall DM, Cullen B, Allerhand M, et al. Cognitive test scores in UK Biobank: data reduction in 480,416 participants and longitudinal stability in 20,346 participants. PLoS One 2016;11:e0154222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc 1996;91:444–455. [Google Scholar]
- 35.Rees JMB, Wood AM, Burgess S. Extending the MR-Egger method for multivariable mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat Med.2017;36:4705–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement 2015;11:70–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis DA, Campbell MJ, Terry RD, Morrison JH. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer's disease: a quantitative study of visual and auditory cortices. J Neurosci Off J Soc Neurosci 1987;7:1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gates GA, Anderson ML, Feeney MP, McCurry SM, Larson EB. Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch Otolaryngol Neck Surg 2008;134:771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards Jerri D, Lister Jennifer J, Elias Maya N, et al. Auditory processing of older adults with probable mild cognitive impairment. J Speech Lang Hear Res 2017;60:1427–1435. [DOI] [PubMed] [Google Scholar]
- 40.Idrizbegovic E, Hederstierna C, Dahlquist M, Kämpfe Nordström C, Jelic V, Rosenhall U. Central auditory function in early Alzheimer's disease and in mild cognitive impairment. Age Ageing 2011;40:249–254. [DOI] [PubMed] [Google Scholar]
- 41.Sharma M, Purdy SC, Humburg P. Cluster analyses reveals subgroups of children with suspected auditory processing disorders. Front Psychol 2019;10:2481. Available at: ncbi.nlm.nih.gov/pmc/articles/PMC6872645/. Accessed April 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farah R, Schmithorst VJ, Keith RW, Holland SK. Altered white matter microstructure underlies listening difficulties in children suspected of auditory processing disorders: a DTI study. Brain Behav 2014;4:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filshtein TJ, Brenowitz WD, Mayeda ER, et al. Reserve and Alzheimer's disease genetic risk: effects on hospitalization and mortality. Alzheimers Dement 2019;15:907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mener DJ, Betz J, Yaffe K, et al. Apolipoprotein E allele and hearing thresholds in older adults. Am J Alzheimers Dis Other Demen 2016;31:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurniawan C, Westendorp RGJ, de Craen AJM, Gussekloo J, de Laat J, van Exel E. Gene dose of apolipoprotein E and age-related hearing loss. Neurobiol Aging 2012;33:2230.e7–2230.e12. [DOI] [PubMed] [Google Scholar]
- 47.Pimenova AA, Raj T, Goate AM. Untangling genetic risk for Alzheimer's disease. Biol Psychiatry 2018;83:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lahoz C, Schaefer EJ, Cupples LA, et al. Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis 2001;154:529–537. [DOI] [PubMed] [Google Scholar]
- 49.Beecham GW, Hamilton K, Naj AC, et al. Genome-wide association meta-analysis of neuropathologic features of Alzheimer's disease and related dementias. PLoS Genet 2014;10. Available at: ncbi.nlm.nih.gov/pmc/articles/PMC4154667/. Accessed July 26, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis A, McMahon CM, Pichora-Fuller KM, et al. Aging and hearing health: the life-course approach. Gerontologist 2016;56(suppl 2):S256–S267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Researchers can apply to UK Biobank to access the data used in this study (ukbiobank.ac.uk/).