Abstract
Objective
To explore the risk factors for idiopathic REM sleep behavior disorder (RBD) in a community population in Beijing.
Methods
Participants aged 55 years and above were recruited from the Beijing Longitudinal Study on Aging II cohort. We identified individuals with possible RBD (pRBD) using the validated RBD Questionnaire–Hong Kong in 2010. A series of environmental, lifestyle, and other potential risk factors were assessed via standardized questionnaires in 2009. Multivariable logistic regression analysis was performed to investigate the association between the studied factors and pRBD.
Results
Of 7,225 participants who were free of parkinsonism and dementia, 219 (3.0%) individuals were considered as having pRBD. Participants with pRBD reported more nonmotor and motor symptoms of Parkinson disease (PD) with adjusted odds ratios (ORs) ranging from 1.10 to 4.40. Participants with pRBD were more likely to report a family history of parkinsonism or dementia (OR 3.03, 95% confidence interval [CI] 1.23–7.46). There was a significant association between pRBD and self-reported hyperlipidemia (OR 1.51, 95% CI 1.09–2.10), ever smoking (OR 1.79, 95% CI 1.20–2.65), prior carbon monoxide (CO) poisoning (OR 2.30, 95% CI 1.39–3.83), and nonoccupational exposure to pesticides (OR 2.21, 95% CI 1.40–3.50).
Conclusion
Our study replicated previously reported associations between pRBD and hyperlipidemia, smoking, pesticide exposure, and several prodromal PD symptoms. We also found previously unreported links with a positive family history of parkinsonism or dementia and CO poisoning. Risk factor profiles for pRBD partially resemble those defined for PD, but also differ in distinct ways.
REM sleep behavior disorder (RBD) is characterized by dream-enacting behaviors associated with the loss of normal muscle atonia during REM sleep on polysomnography (PSG).1 Previous longitudinal studies have revealed that patients with idiopathic RBD (iRBD) have an increased risk of developing neurodegenerative diseases, mainly α-synucleinopathies, including Parkinson disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy.2–4 More than 80% of patients with iRBD will develop one of these neurodegenerative diseases after a decade or more.3 Considering the strong association between iRBD and synuclein-mediated neurodegenerative diseases, research on risk factors for iRBD is important to help reveal the etiology and pathogenesis of α-synucleinopathies, as well as to develop preventive strategies. Understanding RBD risk factors will also be helpful to identify those at risk of developing neurodegenerative synucleinopathies.
Recently, several large population-based studies have explored risk factors for RBD. Most of these studies used questionnaires as screening tools for diagnosing RBD, given that PSG is time-consuming, costly, and infeasible in some clinical centers. These studies reported that RBD not only shared risk factors for PD and dementia, such as head injury, lower education, occupational pesticide exposure, and farming occupation, but also had an intriguingly unique risk profile including cigarette smoking and alcohol use.5–9 However, previous studies have failed to examine several important factors such as carbon monoxide (CO) poisoning, occupational exposure to toxicants except for pesticides, family history of parkinsonism or dementia, and other prodromal symptoms of PD in detail. To better understand the RBD risk factor profiles, in the first wave of follow-up of the Beijing Longitudinal Study on Aging II (BLSA II) cohort, we screened for possible RBD (pRBD) among 7,225 Chinese adults aged ≥55 years using the validated RBD Questionnaire–Hong Kong (RBDQ-HK), and assessed its associations with environmental, lifestyle, and clinical potential risk factors.
Methods
Standard protocol approvals, registrations, and patient consents
Informed consent was obtained from all participants in the study. The Research Ethics Committee of Xuanwu Hospital of Capital Medical University approved the protocols of this study.
Participants/BLSA II cohort
The participants in our study were enrolled from the BLSA II cohort. This prospective, community population–based cohort was established to investigate the health care of older people in the community in Beijing. Detailed information on BLSA II has been published previously.10 The baseline survey was conducted from July to November 2009, and 3 waves of follow-up visits were conducted in 2010 (1-year follow-up), 2013 (4-year follow-up), and 2019 (10-year follow-up). The current study was based on the data obtained at baseline, with supplemental data obtained at 1 year follow-up. BLSA II used a multistage cluster random sampling method to select a representative community cohort of Beijing residents aged 55 and older. Three urban districts and 1 rural county were randomly selected from 18 administrative districts or counties in Beijing. Subsequently, 31 community units were selected randomly, followed by a full selection of households within each community unit. Residents aged ≥55 years who had lived in the selected communities for longer than 1 year were invited to participate.
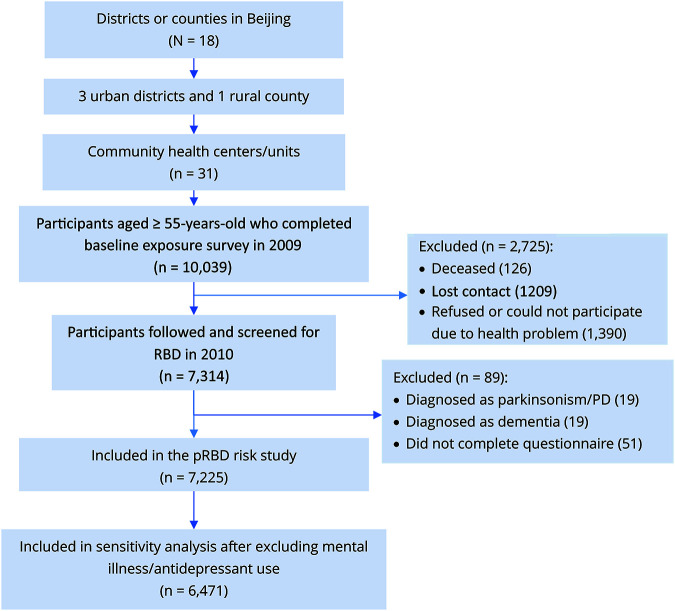
A total of 10,039 participants (6,153 women and 3,886 men) were recruited in the baseline survey, and 7,314 people participated continuously in the 1-year follow-up study. These 7,314 participants constituted the sample for the current pRBD risk study. All the participants received a face-to-face interview. In the baseline survey, information was collected on demographic and socioeconomic characteristics, history of chronic disease, medication use, environmental exposures, and lifestyle behaviors. At the 1-year follow-up visit, participants provided additional information on nonmotor and motor symptoms of PD, including RBD status. We excluded 51 participants who did not provide information on studied exposures or RBD and 38 participants who self-reported being diagnosed with dementia or parkinsonism/PD. In the end, 7,225 participants were included in the final analysis (figure).
Figure. Flow chart of the study.
PD = Parkinson disease; pRBD = possible REM sleep behavior disorder; RBD = REM sleep behavior disorder.
Assessment of pRBD
The presence of pRBD symptoms was defined using the RBDQ-HK in the 1 year follow-up interview. The RBDQ-HK is a self-administered questionnaire comprising 13 questions for the diagnosis of RBD.11 The questionnaire is composed of factor 1 (Q1–Q5, and Q13, dream-content factor) and factor 2 (Q6–Q12, behavioral/dream-enactment factor). Both the overall questionnaire and its subscale (factor 2 of RBDQ-HK) have been validated as having good specificity (86.9% and 81.3%, respectively) and sensitivity (82.2% and 87.9%, respectively) in a large PSG-based study.11 In another validation study in East China, the factor 2 subscale of RBDQ-HK was found to have higher accuracy than the overall scale as a tool to screen for RBD in different clinical populations (including PD and obstructive sleep apnea), with the best cutoff value located at 7/8 (at which the sensitivity was 90% and the specificity was 82%).12 Therefore, pRBD status in the current study was considered as a score ≥8 in the factor 2 subscale of RBDQ-HK, without further confirmation by PSG.
Assessment of exposures/potential risk factors
A standardized structured questionnaire was designed to assess the presence of a variety of environmental and lifestyle risk factors during the baseline interview. All investigators who administered the questionnaire received training for its application. Basic demographic information including age, sex, occupation, education level, and marital status was collected. Educational levels were categorized in the BLSA II as illiteracy, primary school, middle school, and bachelor's degree and above. Total years of education were calculated according to the information provided by participants. Marital status was categorized into single/never married, currently married, widowed, and divorced.
Weight and height were measured by trained physicians and nurses. Body mass index (BMI) was calculated as weight (kg)/height (m2). Participants with BMI ≥30 kg/m2 were considered as being obese, according to WHO-defined international BMI cut points.13 Medical histories of diabetes, hypertension, hyperlipidemia, stroke, coronary heart disease, and mood disorders including depression and anxiety were obtained via self-report. For participants having the aforementioned comorbid conditions, the current use of medications (e.g., antidiabetics, calcium channel blockers, β-blockers, statins, antiplatelets, antidepressants, and benzodiazepines) was also recorded.
Coffee and tea use were assessed with variables that included ever use, frequency of intake (0 = never/rarely use, less than weekly; 1 = occasionally, at least once a week; 2 = regularly, at least once a day), and duration of use. Smoking was measured for smoking status (never smoker, past smoker, or current smoker), duration of smoking, and total pack-years. Passive smoking (defined as living with smokers) was assessed among never smokers. Alcohol consumption was assessed via self-reported drinking status (never, past, or current drinker), drinking frequency (0 = did not drink last year, 1 = occasional drinker, 2 = regular drinker), and duration of alcohol drinking. The average weekly hours spent doing exercise were assessed through a 0–4 Likert scale, ranging from 0 (never) to 4 (more than 20 hours per week).
Histories of occupational exposure to pesticides (herbicides and insecticides) and nonoccupational exposure to pesticides (including household pesticide use) were assessed. Occupational exposure to other toxicants (defined as work exposure for more than 6 months), including chemical solvents, heavy metals, and industrial aerosols, was assessed by a yes/no question. Histories of prior traumatic head injury and CO poisoning resulting in a loss of consciousness were also queried.
Prodromal symptoms of PD were evaluated in the 1-year follow-up interview using a set of established questions. Constipation, olfactory dysfunction, and daytime sleepiness were assessed by single questions, including “Do you have constipation, such that you defecate less than 3 times weekly?” “Do you suffer from a loss of sense of smell, or a significantly decreased sense of smell?” “Do you have trouble staying awake during the daytime?” Depression was defined using the 15-item Geriatric Depression Scale (GDS-15), and a score >7 indicated depressive mood.14 Mini-Mental State Examination (MMSE) was employed to measure cognitive performance. We used an 8-item PD screening questionnaire to identify the subtle motor symptoms of PD. This PD screening questionnaire, which was modified from a well-validated 9-item questionnaire,15 queried symptoms of smaller handwriting, softer voice, inexpressive face, clumsy arms/legs, reduced arm swing, tremor, shuffling gait, and falls. Family history of parkinsonism or dementia was also questioned at the 1-year follow-up visit.
Statistical analysis
Statistical analysis was carried out using SPSS version 18.0 (SPSS, Inc., Chicago, IL). We conducted between-group comparisons of baseline characteristics using Student t test or Wilcoxon rank sum test for continuous variables and χ2 test for categorical variables. Multivariable logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) to measure differences in the prevalence of pRBD across all the exposures. Two multivariate models were fitted: model 1 was only adjusted for age and sex; in model 2, based upon the known potential confounders and candidate risk factors in model 1, we adjusted for age, sex, marital status, rural living, family history of parkinsonism/dementia, BMI, smoking status, alcohol drinking status, tea use, head injury, CO poisoning, pesticide exposure, and occupational exposure to chemical solvents, heavy metals, and industrial aerosols. To avoid overadjustment, in the analyses of prodromal PD symptoms in relation to pRBD risk, we only adjusted for age and sex in the model.
To account for the potential influence of mental illness and antidepressant use, which might be related to antidepressant-associated RBD rather than typical RBD, we conducted a sensitivity analysis excluding those with mental illness (mainly mood disorder) and a medication history of antidepressant use. In this analysis, we excluded participants who screened positive on the GDS-15 or who self-reported physician diagnosis of mood disorder (anxiety or depression).
Data availability
The source data of the BLSA II cohort are not publicly available due to privacy regulations in China. Only members of BLSA II–approved project teams are allowed to access the raw data.
Results
Simple multivariate analysis (age- and sex-adjusted only; model 1)
Sociodemographic variables
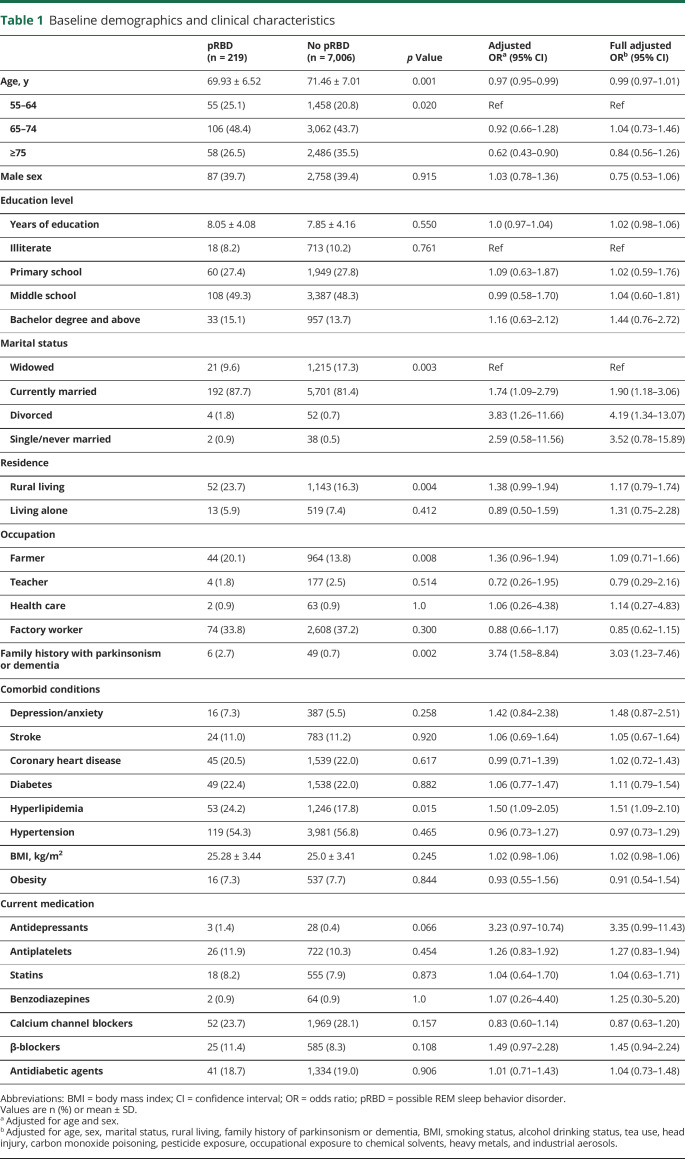
Of the 7,225 included participants who were free of PD/parkinsonism and dementia, 2,845 were male and 4,380 were female (table 1). A total of 219 participants screened positive for pRBD according to the factor 2 subscale of RBDQ-HK. The crude pRBD prevalence was 3.03% (95% CI 2.64%–3.43%). Participants with pRBD were younger than controls (mean age 69.93 vs 71.46 years; OR 0.97, 95% CI 0.95–0.99) and there was a lower prevalence of pRBD in the ≥75 age group (OR 0.62, 95% CI 0.43–0.90). A total of 39.7% of participants with pRBD were male, compared to 39.4% of controls (OR 1.03, 95% CI 0.78–1.36). Those with pRBD were more likely to be currently married (OR 1.74, 95% CI 1.09–2.79) or divorced (OR 3.83, 95% CI 1.26–11.66). Although low education was associated with an increased risk of dementia, we found no evidence of an association between pRBD and education level or total years of schooling. Moreover, we examined different occupational groups that have been linked to PD. Only farming had a borderline relationship with pRBD (OR 1.36, 95% CI 0.96–1.94; adjusted p = 0.087). Similarly, there was a borderline relationship between rural residency and pRBD (OR 1.38, 95% CI 0.99–1.94; adjusted p = 0.059).
Table 1.
Baseline demographics and clinical characteristics
Prodromal symptoms of PD and family history
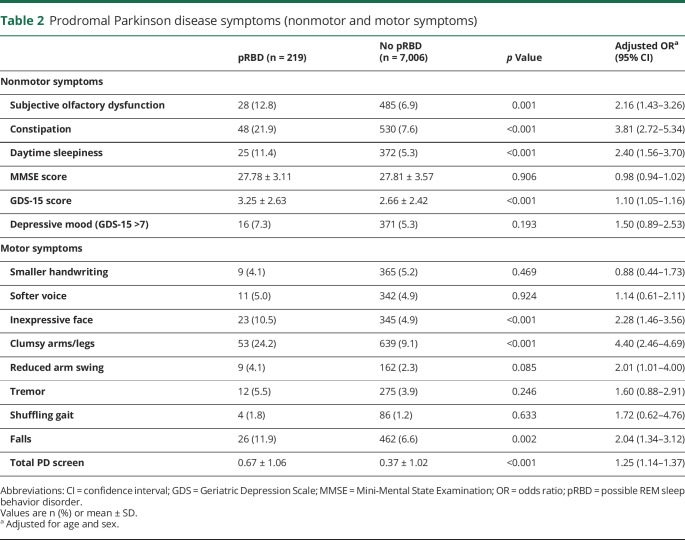
Participants with pRBD were more likely to report other PD nonmotor and motor symptoms (table 2). We found an increased prevalence of subjective olfactory dysfunction (OR 2.16, 95% CI 1.43–3.26), constipation (OR 3.81, 95% CI 2.72–5.34), and daytime sleepiness (OR 2.40, 95% CI 1.56–3.70) among participants with pRBD. Those with pRBD also scored higher on the GDS-15 scale than controls (OR 1.10, 95% CI 1.05–1.16). There was no significant relationship between MMSE score and pRBD. In motor screening, participants with pRBD were more likely to report the occurrence of inexpressive face (OR 2.28, 95% CI 1.46–3.56), reduced arm swing (OR 2.01, 95% CI 1.01–4.0), clumsy arms/legs (OR 4.40, 95% CI 2.46–4.69), and falls (OR 2.04, 95% CI 1.34–3.12). The total self-reported motor symptoms were endorsed more frequently in the pRBD group (OR 1.25, 95% CI 1.14–1.37). Of particular note, participants with pRBD were more likely to report a family history of parkinsonism/dementia (OR 3.74, 95% CI 1.58–8.84) than controls.
Table 2.
Prodromal Parkinson disease symptoms (nonmotor and motor symptoms)
Environmental and lifestyle variables, comorbidity, and medication history
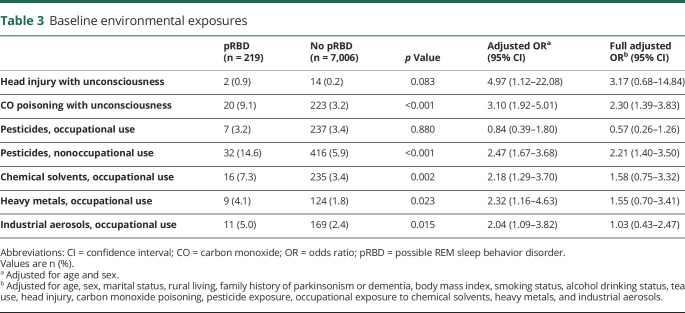
We found a significantly higher occurrence of prior CO poisoning with unconsciousness in the pRBD group compared with controls (OR 3.10, 95% CI 1.92–5.01). Participants with pRBD were also more likely to report having had a head injury resulting in unconsciousness than controls (OR 4.97, 95% CI 1.12–22.08), but statistical power was limited because prior head injuries with unconsciousness were rare in both groups (table 3). We found those with pRBD had a higher prevalence of nonoccupational pesticide exposure than controls (OR 2.47, 95% CI 1.67–3.68). Although occupational exposure to pesticides did not differ between the groups, there was an association between pRBD and occupation exposure to chemical solvents (OR 2.18, 95% CI 1.29–3.70), heavy metals (OR 2.32, 95% CI 1.16–4.63), and industrial aerosols (OR 2.04, 95% CI 1.09–3.82).
Table 3.
Baseline environmental exposures
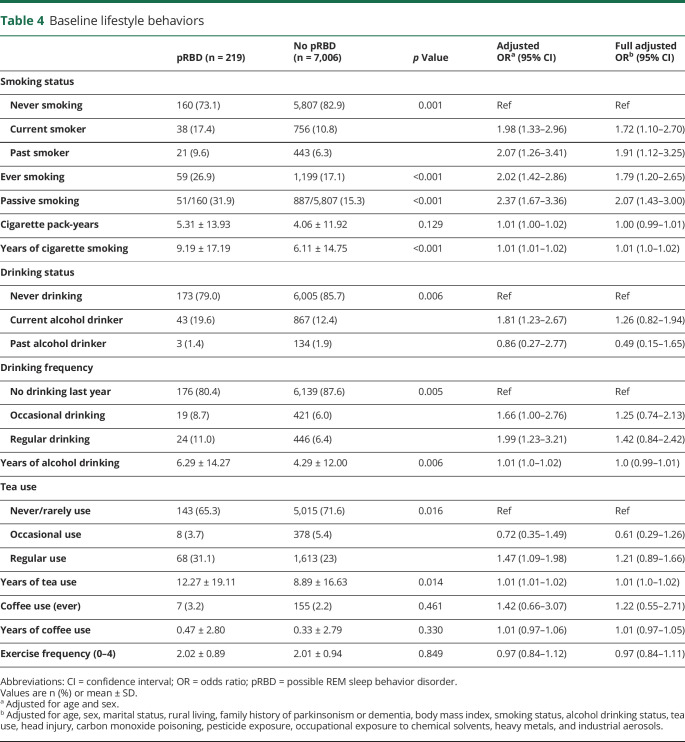
In the current study, we found higher cigarette consumption in participants with pRBD compared with controls (26.9% vs 17.1%; OR 2.02, 95% CI 1.42–2.86). Passive smoking was also more frequently reported in participants with pRBD among the nonsmokers (OR 2.37, 95% CI 1.67–3.36). Participants with pRBD had a longer duration of smoking than controls (9.19 ± 17.19 vs 6.11 ± 14.75 years; OR 1.01, 95% CI 1.01–1.02), but the total pack-years did not differ between the 2 groups. Compared with controls, participants with pRBD were more likely to be current alcohol drinkers (OR 1.81, 95% CI 1.23–2.67) or regular drinkers (OR 1.99, 95% CI 1.23–3.21), and also had a longer duration of alcohol use (6.29 ± 14.27 vs 4.29 ± 12.0 years; OR 1.01, 95% CI 1.0–1.02). Although coffee use has been reported previously as a protective factor in studies of PD,16,17 we found no association between pRBD and coffee intake. However, coffee use was uncommon in both groups (overall prevalence 2.24%). In contrast, there was an association between pRBD and regular tea use (OR 1.47, 95% CI 1.09–1.98) and duration of tea use (OR 1.01, 95% CI 1.01–1.02).
Physician-diagnosed hyperlipidemia was more frequently reported among participants with pRBD compared with controls (OR 1.50, 95% CI 1.09–2.05). However, we found no differences in other comorbid conditions. There was a nonsignificant trend for pRBD participants toward a more frequent use of antidepressants (OR 3.23, 95% CI 0.97–10.74; adjusted p = 0.056).
Full multivariate analysis (model 2)
In the full multivariable logistic regression model (model 2), the association of pRBD with marital status remained. Self-reported hyperlipidemia (OR 1.51, 95% CI 1.09–2.10) and a positive family history of parkinsonism/dementia (OR 3.03, 95% CI 1.23–7.46) also remained risk factors of pRBD in the fully adjusted model. Both current (OR 1.72, 95% CI 1.10–2.70) and past (OR 1.91, 95% CI 1.12–3.25) smoking remained significantly associated with pRBD, as did passive smoking (OR 2.07, 95% CI 1.43–3.0) and smoking duration (OR 1.01, 95% CI 1.0–1.02) (table 4). Furthermore, the significant associations with prior CO poisoning (OR 2.30, 95% CI 1.39–3.83) and nonoccupational exposure to pesticides (OR 2.21, 95% CI 1.40–3.50) remained.
Table 4.
Baseline lifestyle behaviors
Sensitivity analysis
To further explore pRBD in the absence of mental illness, we removed all participants with the GDS-15 score >7, as well as those who reported physician-diagnosed anxiety or depression or who were taking antidepressants. Of the 6,471 remaining participants, 191 participants (2.95%) screened positive for pRBD. The results of risk factors were similar to those of the 2 primary multivariable analyses (tables e1–e4 available from Dryad, doi.org/10.5061/dryad.2jm63xskt).
Discussion
In this community-based study, we found that a positive family history of parkinsonism or dementia, self-reported hyperlipidemia, smoking, nonoccupational pesticide exposure, and CO poisoning with unconsciousness are potential risk factors for pRBD. pRBD was also strongly associated with several prodromal PD symptoms.
In our Beijing community population cohort, we found a prevalence of 3.03% for pRBD using the validated RBDQ-HK. This result was similar to a recent epidemiologic study in a national cohort with 30,097 participants in Canada, which reported a pRBD prevalence of 3.18%.7 However, 2 other population-based studies that used PSG to diagnose RBD reported a prevalence of 1.06%–1.15% for iRBD.8,18 This implies that RBD mimic conditions are probably included in the pRBD group in our study. For this reason, we used the term possible RBD rather than probable RBD with caution in the current study. We found no difference in pRBD risk between sexes, similar to several previous population-based studies that also found no significant sex predilection in RBD.5,6,8 This suggests that the strong male predominance observed in sleep clinics may reflect a selection bias related to RBD, because men with RBD may display more aggressive and violent dream-enacting behaviors than women.19 In our study, we also found that male participants with pRBD reported more “sleep-related injuries” and “attempts to assault/injure” than female participants with pRBD (table e-5 available from Dryad, doi.org/10.5061/dryad.2jm63xskt).
Because RBD is the most specific prodromal marker of PD and DLB, studying the risk factors of RBD provides a window to understand the etiology and identify individuals at risk of neurodegenerative synucleinopathies in their prodromal stages. Consistent with PD/DLB, we found that participants with pRBD were more likely to report a family history of parkinsonism or dementia, which may suggest that common genetic or environmental factors are involved in the development of RBD and neurodegenerative synucleinopathies. This observation is consistent with a recent case–control–family study of iRBD, which reported that first-degree relatives of patients with iRBD had a higher prevalence of clinician-diagnosed PD and dementia.20 Furthermore, in another multicenter study, a family history of dementia was found to be associated with higher RBD phenoconversion risk.21 With regards to the genetic contribution, several recent genetic studies on RBD have confirmed that RBD has a genetic background that partially overlaps with PD/DLB.22,23
We identified a strong relationship between pRBD and several other nonmotor symptoms of PD, including subjective hyposmia, constipation, and daytime sleepiness. The same relationship has been reported previously in large epidemiologic studies on RBD risk.5,6,8,9 Among these nonmotor prodromal PD markers, constipation appeared to have the strongest association, followed by daytime sleepiness, and then subjective hyposmia. Interestingly, this finding is broadly consistent with the Braak hypothesis, which postulates that α-synuclein pathology may start from the enteric nervous system and subsequently undergo transsynaptic propagation through the vagal nerve to the caudal brainstem, and eventually ascend to the midbrain and cortical areas.24–26 Consistent with this hypothesis, a pathologic study has also reported positive α-synuclein immunostaining in colonic biopsies from patients with iRBD.27 Thus the association between RBD and other PD nonmotor symptoms might be caused by the shared pathologic basis underlying synuclein-mediated neurodegeneration.
In the present study, several motor symptoms of PD were also associated with pRBD, including clumsy arms/legs, inexpressive face, reduced arm swing, and falls. Several case–control studies have also demonstrated that subtle motor impairment can be detected in patients with PSG-confirmed iRBD.28–30 Of note, we found no association between pRBD and tremor symptoms, which may be explained by the previous finding that patients with PD with RBD are more likely to have a specific motor subtype with akinetic–rigidity predominance.31 Our results should be interpreted with caution because all motor symptoms were screened using a questionnaire, without objective examination. Furthermore, the 8-item PD screening questionnaire that was used needs to be validated in both clinical and community settings.
In our study, we report a novel result of a significant association between CO poisoning and pRBD. CO intoxication has been reported to induce parkinsonism by targeting a striatal-related neuronal network; this has been confirmed in both a SPECT study and in vivo animal model.32,33 In a population-based cohort study, CO intoxication was also a risk factor for PD.34 Our similar finding in participants with pRBD might suggest a common pathophysiologic pathway linking CO intoxication with RBD and PD. It is possible that CO poisoning may also damage the REM sleep circuit located in the pontine tegmentum and ventromedial medulla, and hence increase the risk of RBD. We noted that a relatively high prevalence of prior CO intoxication was reported in our cohort. This is due in large part to the wide use of coal-fired heating systems in Beijing before 2010.35 CO poisoning was self-reported and not confirmed via medical records in the current study. Future prospective studies are warranted to evaluate the temporal relationship between clinician-diagnosed CO intoxication and RBD development.
Pesticide exposure is a well-established risk factor for PD and is reported to be more common in patients with iRBD than in individuals without RBD.36 Our study revealed a significant association between pRBD and nonoccupational pesticide use. Although the dose–effect relationship between pesticide exposure and RBD risk remains unclear, our finding suggests that nonoccupational pesticide use may also contribute to PD-associated synucleinopathy early in the disease process. It should be noted that occupational exposures to other toxicants, such as chemical solvents, heavy metals, and industrial aerosols, were associated with a higher risk of pRBD in the age- and sex-adjusted model. Because chronic exposure to metals and solvents has been repeatedly implicated in the development of PD,37,38 future studies are needed to clarify the potential roles of specific toxicant exposure in RBD.
Unlike patients with PD and DLB, patients with RBD had no reduction in coffee use and had an increased likelihood of smoking, which has been confirmed in several large epidemiologic studies.7–9,36 In our cohort, there was also no association of coffee intake with pRBD, although the statistical power was limited because of the low prevalence of coffee use in the Chinese population. However, we revealed a strong association between pRBD and cigarette smoking (including current and past smoking). Notably, passive smoking was also associated with pRBD among nonsmokers, which further supported the robust relationship. This finding is intriguing, considering that smoking is a well-known protective factor for PD. A speculative explanation is that smoking might selectively protect nigral dopaminergic neurons, but not the nondopaminergic neurons in extranigral structures involved in prodromal synucleinopathies.21 This finding also suggests that RBD may have a unique risk profile distinct from PD.
Self-reported hyperlipidemia was associated with a higher pRBD risk in our study, even after adjusting for statin use and other cardiovascular/cerebrovascular risk factors including hypertension, diabetes, smoking, alcohol drinking, and BMI (adjusted OR 1.79, 95% CI 1.23–2.60). A previous community-based study in Shanghai, China, also showed that self-reported hyperlipidemia was associated with an increased risk of pRBD.5 The pathophysiologic link remains unclear, but experimental studies indicate that cerebral hypoperfusion can promote α-synuclein aggregation.39,40 Considering that patients with RBD also exhibit α-synuclein aggregates in postmortem pathology,41 it is possible that neurovascular dysfunction associated with vascular risk factors may play a role in RBD pathogenesis. Because hyperlipidemia was not diagnosed by the lipid profile test in this study, further studies with laboratory lipid tests are necessary to explore the association between hyperlipidemia and RBD.
Some limitations of this study should be noted. First, the diagnosis of pRBD was made by a validated questionnaire, but was not confirmed by PSG. Moreover, we did not collect information regarding several mimics of RBD symptoms, including obstructive sleep apnea, restless leg syndrome, and nocturnal epilepsy. Therefore, as with all large-scale studies on pRBD, there are doubtless false-positive cases in the pRBD group. We tried to attenuate this by using the factor 2 subscale of the RBDQ-HK questionnaire, which queries only dream enactment and has a higher sensitivity and specificity than the overall scale.12 Second, environmental and lifestyle exposure information was evaluated via questionnaires, which implies a potential recall bias in the current study. Third, our original cohort had an oversampling of female participants, possibly due to our aged population, which resulted in female predominance in the pRBD group. This selection bias may limit the generalizability to the general elderly population. Because iRBD is diagnosed in sleep centers much more commonly in men, it remains unclear whether risk factor profiles are the same for male and female patients with RBD. However, in a prospective epidemiologic study, male and female patients had a similar risk of neurodegeneration in iRBD.21 Fourth, the relatively high dropout rate from the baseline cohort may have resulted in a sampling bias. We compared demographic information between the dropouts and those remaining at the 1-year follow-up, and found no significant differences (table e-6, available from Dryad, doi.org/10.5061/dryad.2jm63xskt). The main advantages of our study are the large sample size, a comprehensive assessment of risk factors, the systematic population-based sampling at baseline, and the high response rate from the participants. We also adjusted for important confounding variables, including mental illness, through sensitivity analysis.
This study has replicated previous findings that smoking, pesticide exposure, hyperlipidemia, and marital status are associated with pRBD. We also found a previously unreported link with CO poisoning. Participants with pRBD are also more likely to report a positive family history of parkinsonism/dementia and other prodromal PD symptoms, further confirming the close relationship between RBD and neurodegenerative synucleinopathies. Future studies are warranted to evaluate the risk factors associated with neurodegeneration in patients with PSG-confirmed iRBD.
Acknowledgment
The authors thank Jing An for her contribution to database management on the BLSA II cohort and the participants of the BLSA II cohort study.
Glossary
- BLSA II
Beijing Longitudinal Study on Aging II
- BMI
body mass index
- CI
confidence interval
- CO
carbon monoxide
- DLB
dementia with Lewy bodies
- GDS-15
15-item Geriatric Depression Scale
- iRBD
idiopathic RBD
- MMSE
Mini-Mental State Examination
- OR
odds ratio
- PD
Parkinson disease
- pRBD
possible REM sleep behavior disorder
- PSG
polysomnography
- RBD
REM sleep behavior disorder
- RBDQ-HK
RBD Questionnaire–Hong Kong
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study funding
Study funded by the National Key R&D Program of China (2017YFC0909100, 2018YFC1312001, 2017YFC0840105, and 2017YFC1310200), Beijing Municipal Administration of Hospitals' Mission Plan (SML20150803), Beijing Municipal Science & Technology Commission (Z171100000117013), and National Science and Technology Major Project (2017ZX09304018).
Disclosure
H. Zhang, Z. Gu, and C. Yao report no disclosures relevant to the manuscript. Y. Cai reports a grant from the National Key R&D Program of China. Y. Li reports no disclosures relevant to the manuscript. W. Mao reports a grant from the National Science and Technology Major Project, China. E. Xu reports a grant from the National Key R&D Program of China. R.B. Postuma reports grants from Fonds de la Recherche en Sante Quebec, the Canadian Institute of Health Research, the Parkinson Society of Canada, the Weston-Garfield Foundation, the Michael J. Fox Foundation, and the Webster Foundation; and Consultancies for Biotie and Roche. P. Chan reports grants from the Beijing Municipal Administration of Hospitals, Beijing Municipal Science & Technology Commission, China Ministry of Science and Technology, and the Michael J. Fox Foundation. Go to Neurology.org/N for full disclosures.
References
- 1.Schenck CH, Bundlie SR, Patterson AL, Mahowald MW. Rapid eye movement sleep behavior disorder: a treatable parasomnia affecting older adults. JAMA 1987;257:1786–1789. [PubMed] [Google Scholar]
- 2.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009;72:1296–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med 2013;14:744–748. [DOI] [PubMed] [Google Scholar]
- 4.Iranzo A, Fernandez-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One 2014;9:e89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma JF, Qiao Y, Gao X, et al. A community-based study of risk factors for probable rapid eye movement sleep behavior disorder. Sleep Med 2017;30:71–76. [DOI] [PubMed] [Google Scholar]
- 6.Wong JC, Li J, Pavlova M, et al. Risk factors for probable REM sleep behavior disorder: a community-based study. Neurology 2016;86:1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao C, Fereshtehnejad SM, Keezer MR, Wolfson C, Pelletier A, Postuma RB. Risk factors for possible REM sleep behavior disorder: a CLSA population-based cohort study. Neurology 2018;92:e475–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haba-Rubio J, Frauscher B, Marques-Vidal P, et al. Prevalence and determinants of rapid eye movement sleep behavior disorder in the general population. Sleep 2018;41:zxs197. [DOI] [PubMed] [Google Scholar]
- 9.Shrestha S, Kamel F, Umbach DM, et al. Factors associated with dream enacting behaviors among US farmers. Parkinsonism Relat Disord 2018;57:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Z, Guan S, Ding H, et al. Prevalence and incidence of frailty in community-dwelling older people: Beijing Longitudinal Study of Aging II. J Am Geriatr Soc 2016;64:1281–1286. [DOI] [PubMed] [Google Scholar]
- 11.Li SX, Wing YK, Lam SP, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK). Sleep Med 2010;11:43–48. [DOI] [PubMed] [Google Scholar]
- 12.Shen SS, Shen Y, Xiong KP, et al. Validation study of REM Sleep Behavior Disorder Questionnaire–Hong Kong (RBDQ-HK) in east China. Sleep Med 2014;15:952–958. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 14.Weng BK. Reliability and validity of GDS and GHQ short form for the aged. Chin J Psychiatry 1999;1:41–43. [Google Scholar]
- 15.Duarte J, Claveria LE, de Pedro-Cuesta J, Sempere AP, Coria F, Calne DB. Screening Parkinson's disease: a validated questionnaire of high specificity and sensitivity. Mov Disord 1995;10:643–649. [DOI] [PubMed] [Google Scholar]
- 16.Benedetti MD, Bower JH, Maraganore DM, et al. Smoking, alcohol, and coffee consumption preceding Parkinson's disease: a case-control study. Neurology 2000;55:1350–1358. [DOI] [PubMed] [Google Scholar]
- 17.Liu R, Guo X, Park Y, et al. Caffeine intake, smoking, and risk of Parkinson disease in men and women. Am J Epidemiol 2012;175:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang SH, Yoon IY, Lee SD, Han JW, Kim TH, Kim KW. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep 2013;36:1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Zhang J, Li Y, et al. Gender differences in REM sleep behavior disorder: a clinical and polysomnographic study in China. Sleep Med 2015;16:414–418. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zhang J, Lam SP, et al. A case-control-family study of idiopathic rapid eye movement sleep behavior disorder. Ann Neurol 2019;85:582–592. [DOI] [PubMed] [Google Scholar]
- 21.Postuma RB, Iranzo A, Hogl B, et al. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann Neurol 2015;77:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gan-Or Z, Mirelman A, Postuma RB, et al. GBA mutations are associated with rapid eye movement sleep behavior disorder. Ann Clin Transl Neurol 2015;2:941–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krohn L, Ozturk TN, Vanderperre B, et al. Genetic, structural and functional evidence link TMEM175 to synucleinopathies. Ann Neurol 2019;87:139–153. [DOI] [PubMed] [Google Scholar]
- 24.Angot E, Steiner JA, Hansen C, Li JY, Brundin P. Are synucleinopathies prion-like disorders? Lancet Neurol 2010;9:1128–1138. [DOI] [PubMed] [Google Scholar]
- 25.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 26.Hogl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration: an update. Nat Rev Neurol 2018;14:40–55. [DOI] [PubMed] [Google Scholar]
- 27.Sprenger FS, Stefanova N, Gelpi E, et al. Enteric nervous system alpha-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology 2015;85:1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber TR, Lawton M, Rolinski M, et al. Prodromal parkinsonism and neurodegenerative risk stratification in REM sleep behavior disorder. Sleep 2017;40:zsx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iranzo A, Stefani A, Serradell M, et al. Characterization of patients with longstanding idiopathic REM sleep behavior disorder. Neurology 2017;89:242–248. [DOI] [PubMed] [Google Scholar]
- 30.Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain 2012;135:1860–1870. [DOI] [PubMed] [Google Scholar]
- 31.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry 2008;79:1117–1121. [DOI] [PubMed] [Google Scholar]
- 32.Kent M, Creevy KE, Delahunta A. Clinical and neuropathological findings of acute carbon monoxide toxicity in chihuahuas following smoke inhalation. J Am Anim Hosp Assoc 2010;46:259–264. [DOI] [PubMed] [Google Scholar]
- 33.Sun TK, Chen YY, Huang SH, et al. Neurotoxicity of carbon monoxide targets caudate-mediated dopaminergic system. Neurotoxicology 2018;65:272–279. [DOI] [PubMed] [Google Scholar]
- 34.Lai CY, Chou MC, Lin CL, Kao CH. Increased risk of Parkinson disease in patients with carbon monoxide intoxication: a population-based cohort study. Medicine 2015;94:e869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue J, Sun Q, Wang Y, Gao G, Shi L, Yu T. Features of carbon monoxide poisoning in China. Iran J Public Health 2013;42:1192–1193. [PMC free article] [PubMed] [Google Scholar]
- 36.Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology 2012;79:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorell JM, Johnson CC, Rybicki BA, et al. Occupational exposures to metals as risk factors for Parkinson's disease. Neurology 1997;48:650–658. [DOI] [PubMed] [Google Scholar]
- 38.Hageman G, van der Hoek J, van Hout M, et al. Parkinsonism, pyramidal signs, polyneuropathy, and cognitive decline after long-term occupational solvent exposure. J Neurol 1999;246:198–206. [DOI] [PubMed] [Google Scholar]
- 39.Kim T, Vemuganti R. Mechanisms of Parkinson's disease-related proteins in mediating secondary brain damage after cerebral ischemia. J Cereb Blood Flow Metab 2017;37:1910–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unal-Cevik I, Gursoy-Ozdemir Y, Yemisci M, et al. Alpha-synuclein aggregation induced by brief ischemia negatively impacts neuronal survival in vivo: a study in [A30P]alpha-synuclein transgenic mouse. J Cereb Blood Flow Metab 2011;31:913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 2013;12:443–453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The source data of the BLSA II cohort are not publicly available due to privacy regulations in China. Only members of BLSA II–approved project teams are allowed to access the raw data.