Abstract
Objective
To test ketamine infusion efficacy in the treatment of super-refractory status epilepticus (SRSE), we studied patients with SRSE who were treated with ketamine retrospectively. We also studied the effect of high doses of ketamine on brain physiology as reflected by invasive multimodality monitoring (MMM).
Methods
We studied a consecutive series of 68 patients with SRSE who were admitted between 2009 and 2018, treated with ketamine, and monitored with scalp EEG. Eleven of these patients underwent MMM at the time of ketamine administration. We compared patients who had seizure cessation after ketamine initiation to those who did not.
Results
Mean age was 53 ± 18 years and 46% of patients were female. Seizure burden decreased by at least 50% within 24 hours of starting ketamine in 55 (81%) patients, with complete cessation in 43 (63%). Average dose of ketamine infusion was 2.2 ± 1.8 mg/kg/h, with median duration of 2 (1–4) days. Average dose of midazolam was 1.0 ± 0.8 mg/kg/h at the time of ketamine initiation and was started at a median of 0.4 (0.1–1.0) days before ketamine. Using a generalized linear mixed effect model, ketamine was associated with stable mean arterial pressure (odds ratio 1.39, 95% confidence interval 1.38–1.40) and with decreased vasopressor requirements over time. We found no effect on intracranial pressure, cerebral blood flow, or cerebral perfusion pressure.
Conclusion
Ketamine treatment was associated with a decrease in seizure burden in patients with SRSE. Our data support the notion that high-dose ketamine infusions are associated with decreased vasopressor requirements without increased intracranial pressure.
Classification of evidence
This study provides Class IV evidence that ketamine decreases seizures in patients with SRSE.
Refractory status epilepticus (RSE) is defined as the failure of seizures to stop despite administration of appropriately dosed antiepileptic drugs (AEDs). Status epilepticus that continues despite anesthetic agents is called super-refractory status epilepticus (SRSE) and is associated with high morbidity and mortality.1 There are no high-quality data to support the optimal management of SRSE. The most commonly used continuous anesthetics are γ-aminobutyric acid (GABAA) receptor agonists, including midazolam, propofol, and pentobarbital.2 Receptor trafficking occurs in the setting of status epilepticus whereby intrasynaptic membrane GABAA receptors are internalized and NMDA receptors are upregulated. Extrasynaptic GABAA receptors do not endocytose.2 Ketamine works by antagonizing NMDA receptors and inhibiting glutamatergic transmission. Its mechanism of action, combined with a favorable side effect profile, has led to increasing usage in the setting of SRSE.3–9 Available literature on its use consists of case reports or small case series.4–6,8–15 More widespread use of ketamine in patients with brain injury has been limited by concerns over its effect on intracranial pressure (ICP) and cerebral hemodynamics.16,17 Two large systematic reviews support the notion that ketamine does not increase ICP in patients with traumatic and nontraumatic brain injury; however, these data were generated in the operating room and reported only on the safety of ketamine boluses with sparse data available on continuous infusions of higher doses of ketamine.9,18–20 Here, we examine the efficacy of ketamine infusions for the treatment of SRSE and the relationship of high-dose ketamine on systemic and brain physiologic measures.
Methods
Study cohort
We retrospectively identified 261 adult patients with SRSE (>18 years old) admitted to the neurologic intensive care unit at Columbia University Medical Center between January 1, 2009, and December 31, 2018. Status epilepticus was defined as continuous seizure activity for at least 5 minutes. RSE was defined as the failure of seizure control after appropriate dosing of benzodiazepine and 1 AED and SRSE as the state of ongoing status epilepticus despite anesthetic use.1,21,22 Patients were started on ketamine at the discretion of the treating physician. In our intensive care unit, we often start ketamine if the patient is not responding to midazolam. A total of 68 patients (26%) were treated with ketamine infusions. All patients received midazolam infusions in addition to ketamine. In this study, we examine the efficacy of ketamine infusions for the treatment of SRSE (Class IV).
Standard protocol approvals, registrations, and patient consents
The study was approved by the institutional review board of Columbia University Medical Center.
EEG recording and multimodality monitoring (MMM)
Continuous EEG recordings were conducted using the 10–20 system of electrode placement and a digital bedside video monitoring system (XLTEK, Excel-Tech Corp, Natus Medical Incorporated, Oakville, Canada; low-pass filter = 70 Hz, high-pass = 0.1 Hz, sampling rate = 200, 256, or 512 Hz) with 21 EEG channels available for analysis.23 EEG interpretation was conducted by board-certified electroencephalographers. We reviewed all EEG reports including the day prior to starting ketamine, the day of ketamine initiation, the day after ketamine initiation, and the day after termination of ketamine. The presence or absence of seizures was noted, as was seizure type (focal vs generalized).
We reviewed mean arterial pressure (MAP) in all patients. Arterial blood pressure was monitored through an arterial line if clinically indicated (Transpac IV Monitoring Kit, ICU Medical, San Clemente, CA). The arterial line was zeroed at the level of the phlebostatic axis. According to a previously described institutional protocol, invasive neuromonitoring includes measurements of ICP, cerebral perfusion pressure (CPP), and cerebral blood flow (CBF).24–26
Outcome measures
The main outcome measure investigated was seizure control within 24 hours of ketamine initiation, defined as (1) complete seizure cessation or (2) more than 50% reduction of seizure burden. We also evaluated seizure control after ketamine discontinuation, in-hospital mortality, discharge disposition, modified Rankin Scale score,27 and Glasgow Outcome Scale extended score on discharge.28,29 In addition, we studied hospital length of stay, withdrawal of life-sustaining treatment, do not resuscitate status, and whether tracheostomy and percutaneous endoscopic gastrostomy were performed.
Statistical analysis
Variables were compared using the Fisher exact and Mann-Whitney U test for categorical and continuous variables, respectively. All statistical tests were 2-tailed and a p value < 0.05 was considered statistically significant. We used a generalized linear mixed effect model adjusting for repeated measures to study the effect of different doses of ketamine on blood pressure and cerebral multimodality physiologic measures. We calculated Pearson correlation to study the relation between MAP and ketamine dosage. Statistical analyses were performed using R statistical software version 3.4.1 (R Project for Statistical Computing).
Data availability
Datasets are available from the corresponding author for any qualified investigator.
Results
Study cohort
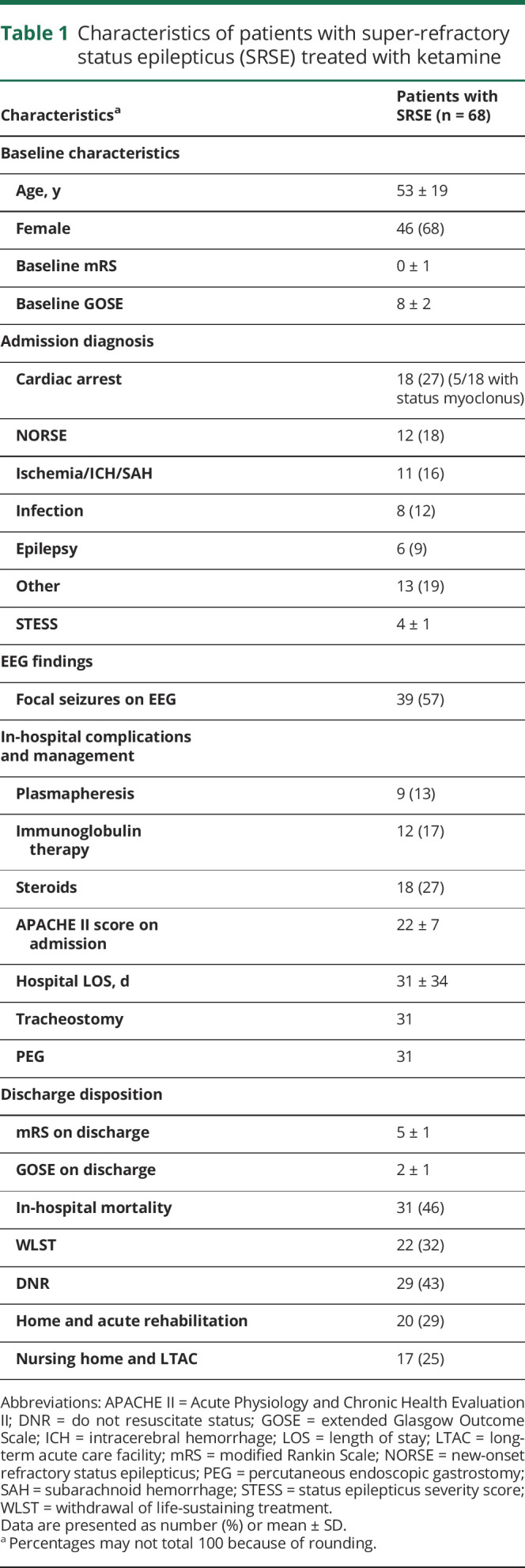
A total of 68 patients were included in the analysis. The average age was 53 ± 19 years and 46 (68%) patients were women. Cardiac arrest was the most common etiology (27%) followed by new-onset refractory status epilepticus (18%). Mean status epilepticus severity score was 4 ± 1, and in-hospital mortality was 46%. Patient characteristics are described in table 1.
Table 1.
Characteristics of patients with super-refractory status epilepticus (SRSE) treated with ketamine
Treatment response to ketamine
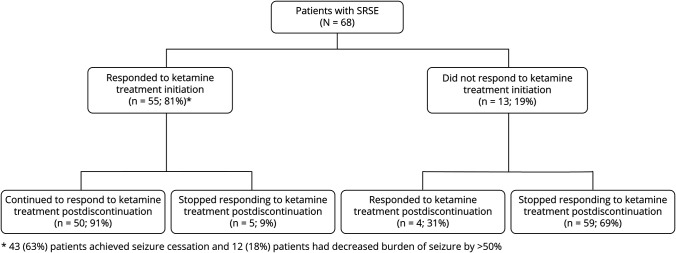
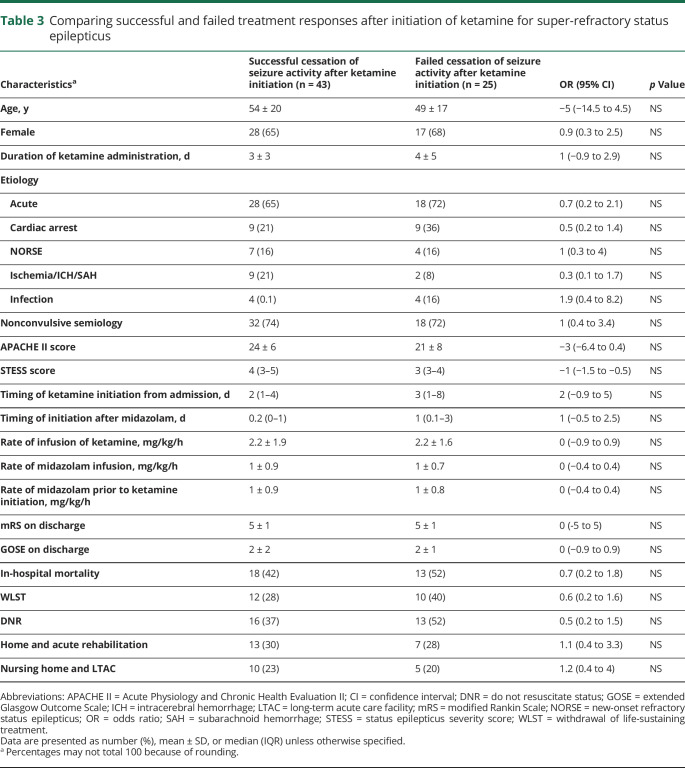
A total of 55 (81%) patients had at least 50% decrease in seizure burden within 24 hours of ketamine initiation and 43 (63%) patients had seizure cessation. After stopping ketamine, 54 (79%) patients achieved at least 50% decrease in seizure burden and 44 (65%) patients had complete seizure cessation (figure 1). Of patients with seizure cessation after starting ketamine, 18 (41%) patients died, and of patients without seizure cessation after stopping ketamine, 13 (54%) patients died. On average, 2 ± 1 concurrent anesthetics (propofol in 36 patients and pentobarbital in 10 patients) were used for SRSE treatment. All patients were treated with midazolam infusions. Ketamine was started at a median of 2 days (1; 4.5) after admission at an average infusion rate of 2.2 ± 1.8 mg/kg/h and maintained for a median duration of 2 (1–4) days (table 2). A comparison between the group of patients that had seizure control and those that failed to be controlled is given in table 3.
Figure 1. Treatment response of patients with super-refractory status epilepticus (SRSE) with ketamine infusion.
Ketamine efficacy in the treatment of SRSE. The figure demonstrates treatment response within 24 hours after initiation of ketamine and after ketamine discontinuation.
Table 2.
Treatment of super-refractory status epilepticus (SRSE) in our cohort
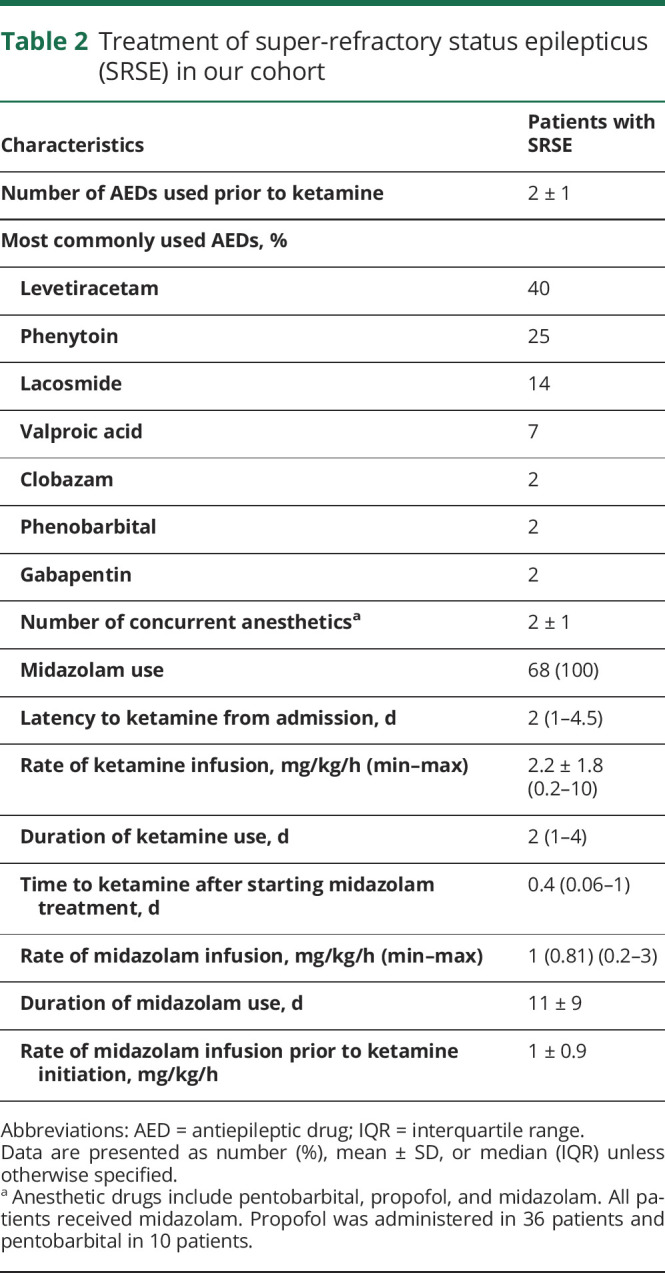
Table 3.
Comparing successful and failed treatment responses after initiation of ketamine for super-refractory status epilepticus
Hemodynamics and cerebral MMM
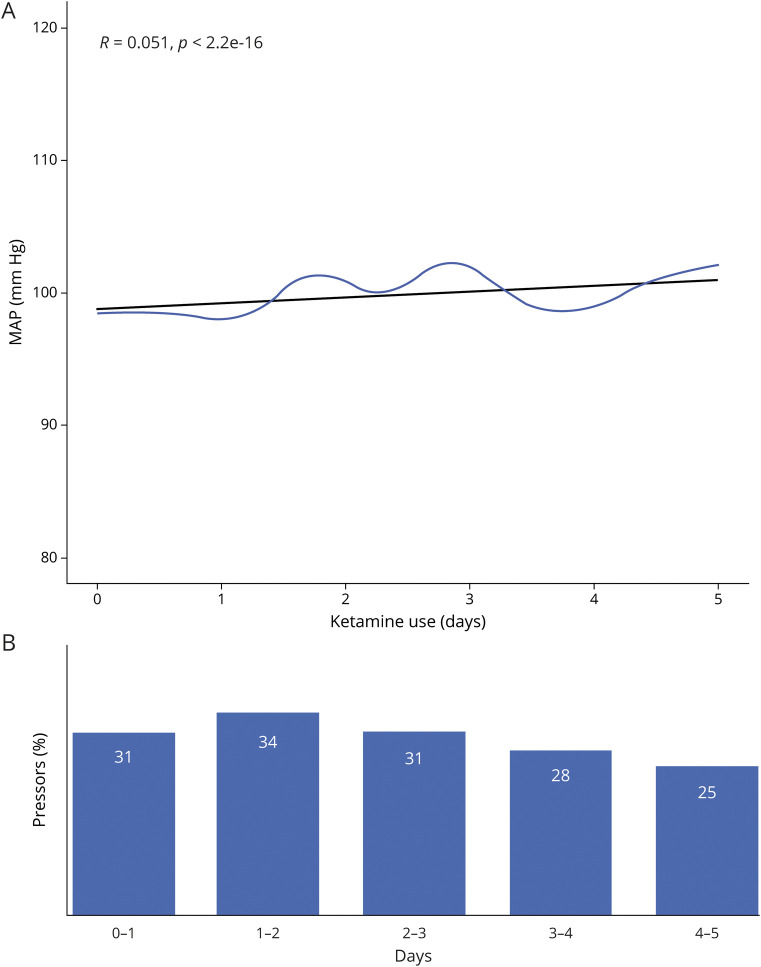
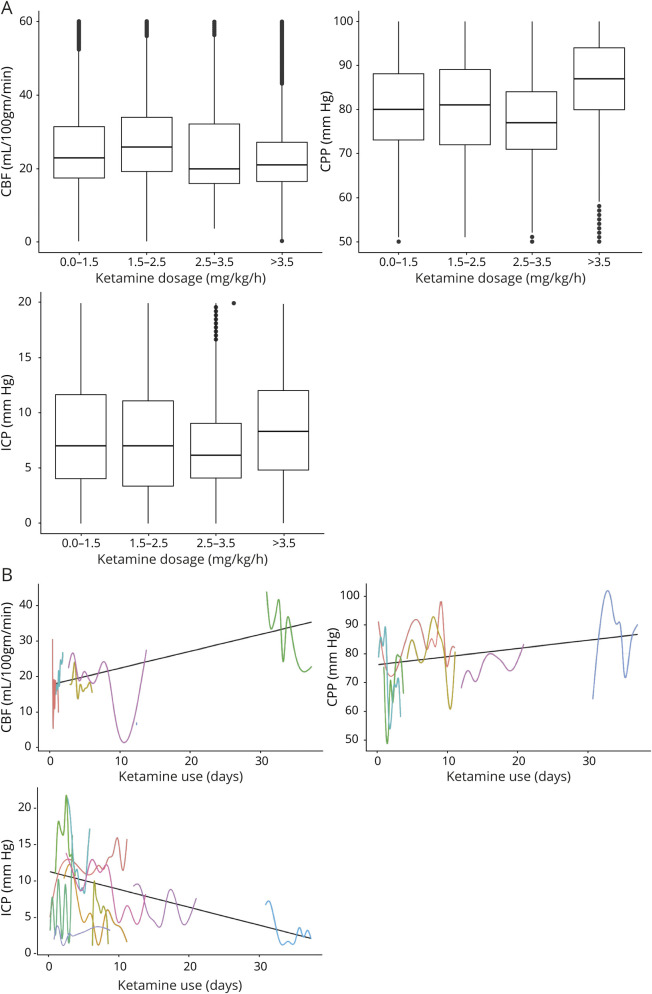
Using a generalized linear mixed effect model, higher dose of ketamine infusion (odds ratio [OR] 1.39, 95% confidence interval [CI] 1.38–1.4) and longer administration time (OR 0.9, 95% CI 0.8–1) were associated with a stable mean MAP and a decrease in vasopressor requirements over time (figure 2). We found no correlation between ketamine dosage and MAP values with Pearson r value 0.05 (p < 0.0001) between the 2 variables. Average MAP was 97 (SD 23). We found no effect of higher dose of ketamine (OR 0.1, 95% CI 0–0.12) and longer administration (OR −0.33, 95% CI −0.32 to −0.34) on ICP, CBF (OR −0.03, 95% CI −0.02 to −0.04) and (OR −0.4, 95% CI −0.44 to −0.44) or CPP (OR −0.13, 95% CI −0.12 to −0.14) and (OR −0.3, 95% CI −0.3 to −0.3) (figure 3).
Figure 2. Average mean arterial pressure (MAP) over time in 68 patients with super-refractory status epilepticus (SRSE) on ketamine infusion.
(A) Average MAP (y-axis) over time (x-axis) (blue line) in patients with SRSE on ketamine infusion for SRSE treatment. No correlation was found, with Pearson r value of 0.051 (black line). (B) Percentage of patients on vasopressors for hemodynamic support from ketamine. Day 1 represents the date of ketamine initiation.
Figure 3. Effect of ketamine infusion on cerebral multimodal monitoring in 11 patients with super-refractory status epilepticus.
(A) Boxplots show the relationship of increasing ketamine doses (x-axis) and mean cerebral blood flow (CBF), cerebral perfusion pressure (CPP), and intracranial pressure (ICP) measurements (y-axis). (B) Average CBF, CPP, and ICP for each patient presented in different colors (x-axis) over time (y-axis) of ketamine administration. Data were computed from 11 patients with ICP monitors and 6 patients with CBF monitors.
Discussion
Our data suggest that ketamine infusions are effective for the treatment of SRSE. In line with other studies, ketamine had no effect on ICP even at prolonged high anesthetic dosages. It was associated with decreased vasopressor requirements. To our knowledge, this is the largest study to evaluate ketamine use in the treatment of SRSE and to examine its effect on cerebral brain physiology.
A number of prior case series studied the role of ketamine for treatment of SRSE.8,9,15 Seizure control ranged between 57% and 91% of cases, with average doses ranging between 1.3 and 2.8 mg/kg/h, with maximum doses as high as 10.5 mg/kg/h. Ketamine was started on average between 5 and 8 days after the onset of status epilepticus.8,9,15 In our study, the treatment response to ketamine, with an average dose of 2.2 mg/kg/h, was 81% after initiation of ketamine. The high response rate might be explained by early initiation of ketamine: 2 days on average after status onset in our study. This correlates well with the Gaspard et al.9 study as treatment was more successful when it was started earlier with higher doses.bib9 Currently, a randomized clinical trial is evaluating the effect of ketamine as a first-line agent for RSE in a pilot study (clinicaltrials.gov/ct2/show/NCT03115489).
Synowiec et al.15 reported weaning off vasopressors in 6 out of 7 patients during ketamine treatment, without clear response in the Gaspard et al.9 study. Differences in these rates likely primarily relate to inconsistencies of blood pressure assessments in retrospective multisite case series. Following our institutional invasive blood pressure monitoring protocol for patients receiving anesthetic drips, we found that MAP is stable after initiation of ketamine with decreased pressor requirements.
Systematic reviews support the notion that ketamine does not increase ICP in traumatic and nontraumatic brain injury. These studies were mostly retrospective and consisted predominantly of patients who received ketamine boluses in the operating room.18,19 In the study by Gaspard et al.,9 7 patients had ICP monitoring; 2 patients with cerebral edema from anoxic brain injury required osmotic therapy while on ketamine and 3 patients were documented to have increased ICP prior to ketamine infusion but did not experience any ICP elevation. Our results show that even at prolonged and high doses of ketamine, ICP elevations were not documented.
There are several limitations to our study. The study is a retrospective, single-center study, creating subject heterogeneity and limiting the generalizability of our findings. Another limitation is that the response of seizures to ketamine infusions could be due to coadministration of additional drugs or occur as a spontaneous improvement from status epilepticus. We have not compared patients who received ketamine with patients who did not. Finally, ketamine was started at the discretion of the treating physician, which may result in a selection bias. The effect of ketamine on treatment of SRSE should be tested in an adequately powered, randomized clinical trial.
This study supports the use of ketamine infusions for the treatment of SRSE and suggests that high doses of ketamine are associated with improved hemodynamics without an increase in ICP. Prospective studies are needed starting ketamine infusions early in the treatment of status epilepticus.
Glossary
- AED
antiepileptic drug
- CBF
cerebral blood flow
- CI
confidence interval
- CPP
cerebral perfusion pressure
- GABAA
γ-aminobutyric acid
- ICP
intracranial pressure
- MAP
mean arterial pressure
- MMM
multimodality monitoring
- OR
odds ratio
- RSE
refractory status epilepticus
- SRSE
super-refractory status epilepticus
Appendix. Authors

Footnotes
Class of Evidence: NPub.org/coe
Study funding
No targeted funding reported.
Disclosures
A. Alkhachroum is supported by the National Center for Advancing Translational Sciences of the NIH under the Miami CTSI KL2 Career Development Award UL1TR002736. C. Der-Nigoghossian, E. Mathews, N. Massad, R. Letchinger, K. Doyle, W-T. Chiu, J. Kromm, C. Rubinos, A. Velazquez, D. Roh, S. Agarwal, S. Park, and E. Connolly report no disclosures relevant to the manuscript. J. Claassen is supported by grant funding from the NIH R01 NS106014 and R03 NS112760 and the DANA Foundation. Go to Neurology.org/N for full disclosures.
References
- 1.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 2.Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol 2006;5:246–256. [DOI] [PubMed] [Google Scholar]
- 3.Borris DJ, Bertram EH, Kapur J. Ketamine controls prolonged status epilepticus. Epilepsy Res 2000;42:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prüss H, Holtkamp M. Ketamine successfully terminates malignant status epilepticus. Epilepsy Res 2008;82:219–222. [DOI] [PubMed] [Google Scholar]
- 5.Sheth RD, Gidal BE. Refractory status epilepticus: response to ketamine. Neurology 1998;51:1765–1766. [DOI] [PubMed] [Google Scholar]
- 6.Ubogu EE, Sagar SM, Lerner AJ, Maddux BN, Suarez JI, Werz MA. Ketamine for refractory status epilepticus: a case of possible ketamine-induced neurotoxicity. Epilepsy Behav 2003;4:70–75. [DOI] [PubMed] [Google Scholar]
- 7.Fang Y, Wang X. Ketamine for the treatment of refractory status epilepticus. Seizure 2015;30:14–20. [DOI] [PubMed] [Google Scholar]
- 8.Sabharwal V, Ramsay E, Martinez R, et al. Propofol-ketamine combination therapy for effective control of super-refractory status epilepticus. Epilepsy Behav 2015;52:264–266. [DOI] [PubMed] [Google Scholar]
- 9.Gaspard N, Foreman B, Judd LM, et al. Intravenous ketamine for the treatment of refractory status epilepticus: a retrospective multicenter study. Epilepsia 2013;54:1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker MC, Smith SJ, Shorvon SD. The intensive care treatment of convulsive status epilepticus in the UK: results of a national survey and recommendations. Anaesthesia 1995;50:130–135. [DOI] [PubMed] [Google Scholar]
- 11.Kofke WA, Bloom MJ, Van Cott A, Brenner RP. Electrographic tachyphylaxis to etomidate and ketamine used for refractory status epilepticus controlled with isoflurane. J Neurosurg Anesthesiol 1997;9:269–272. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh CY, Sung PS, Tsai JJ, Huang CW. Terminating prolonged refractory status epilepticus using ketamine. Clin Neuropharmacol 2010;33:165–167. [DOI] [PubMed] [Google Scholar]
- 13.Kramer AH. Early ketamine to treat refractory status epilepticus. Neurocrit Care 2012;16:299–305. [DOI] [PubMed] [Google Scholar]
- 14.Rosati A, L'Erario M, Ilvento L, et al. Efficacy and safety of ketamine in refractory status epilepticus in children. Neurology 2012;79:2355–2358. [DOI] [PubMed] [Google Scholar]
- 15.Synowiec AS, Singh DS, Yenugadhati V, Valeriano JP, Schramke CJ, Kelly KM. Ketamine use in the treatment of refractory status epilepticus. Epilepsy Res 2013;105:183–188. [DOI] [PubMed] [Google Scholar]
- 16.Himmelseher S, Durieux ME. Revising a dogma: ketamine for patients with neurological injury? Anesth Analg 2005;101:524–534. [DOI] [PubMed] [Google Scholar]
- 17.Aroni F, Iacovidou N, Dontas I, Pourzitaki C, Xanthos T. Pharmacological aspects and potential new clinical applications of ketamine: reevaluation of an old drug. J Clin Pharmacol 2009;49:957–964. [DOI] [PubMed] [Google Scholar]
- 18.Zeiler FA, Teitelbaum J, West M, Gillman LM. The ketamine effect on intracranial pressure in nontraumatic neurological illness. J Crit Care 2014;29:1096–1106. [DOI] [PubMed] [Google Scholar]
- 19.Zeiler FA, Teitelbaum J, West M, Gillman LM. The ketamine effect on ICP in traumatic brain injury. Neurocrit Care 2014;21:163–173. [DOI] [PubMed] [Google Scholar]
- 20.Golub D, Yanai A, Darzi K, Papadopoulos J, Kaufman B. Potential consequences of high-dose infusion of ketamine for refractory status epilepticus: case reports and systematic literature review. Anaesth Intensive Care 2018;46:516–528. [DOI] [PubMed] [Google Scholar]
- 21.Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American Epilepsy Society. Epilepsy Curr 2016;16:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus: report of the ILAE task force on classification of status epilepticus. Epilepsia 2015;56:1515–1523. [DOI] [PubMed] [Google Scholar]
- 23.Claassen J, Velazquez A, Meyers E, et al. Bedside quantitative electroencephalography improves assessment of consciousness in comatose subarachnoid hemorrhage patients. Ann Neurol 2016;80:541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alkhachroum A, Megjhani M, Terilli K, et al. Hyperemia in subarachnoid hemorrhage patients is associated with an increased risk of seizures. J Cereb Blood Flow Metab 2020;40:1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waziri A, Claassen J, Stuart RM, et al. Intracortical electroencephalography in acute brain injury. Ann Neurol 2009;66:366–377. [DOI] [PubMed] [Google Scholar]
- 26.Mikell CB, Dyster TG, Claassen J. Invasive seizure monitoring in the critically-Ill brain injury patient: current practices and a review of the literature. Seizure 2016;41:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom Transient Ischaemic Attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991;54:1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry 1981;44:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow outcome scale and the extended Glasgow outcome scale: guidelines for their use. J Neurotrauma 1998;15:573–585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets are available from the corresponding author for any qualified investigator.