Abstract
Aim:
To evaluate the efficacy of locally delivered nanomedicine, vasoactive intestinal peptide in sterically stabilized micelles (VIP-SSM) to the colon and conduct in vitro release studies of a potential oral formulation.
Materials & methods:
Intracolonic instillation of VIP-SSM was tested in a mouse model of dextran sulfate sodium-induced colitis. Based on the effective mouse dose, human equivalent dose containing nanomedicine powder was filled into enteric coated capsules for in vitro release testing.
Results:
Colonic delivery of VIP-SSM significantly alleviated colitis. VIP-SSM containing capsules completely dissolved at colonic pH allowing micelles to reform with active VIP. Capsule formulations exhibited reproducible release profiles when stored up to 6 weeks demonstrating stability.
Conclusion:
VIP-SSM is an effective nanomedicine formulation which appears to have potential for oral treatment of colitis in humans.
Keywords: : colitis, colonic delivery, inflammatory bowel disease, oral capsule form, oral nanomedicine, slc26a3, sterically stabilized micelles, targeted delivery, VIP nanomedicine
Vasoactive intestinal peptide (VIP) is a widely studied endogenous immunomodulatory neuropeptide for its role in inflammatory disorders including inflammatory bowel disease (IBD) [1–5]. In this regard, the beneficial effects of VIP in IBD have shown promise due to its important role in normal physiological processes of the intestine and anti-inflammatory action on immune cells [6,7]. Previous reports have demonstrated altered expression of endogenous VIP in IBD, which may indicate disturbances in the circulating levels, potentially contributing to the underlying pathology [8,9]. Although the potent immunomodulatory properties of VIP have been widely studied, the main drawback in vivo is its rapid degradation due to short half-life and off-target effects after systemic administration. Thus, VIP is far from being used clinically due to the inherent instability and toxicity of the peptide.
The local release of neuropeptides into luminal milieu is likely due to the liberation of these peptides by enteroendocrine cells and enteric neurons [10,11]. The expression of the receptors for VIP in the intestine were traditionally assumed to be located at the basolateral membranes of enterocytes [12]. However, we recently demonstrated that the key receptor of VIP (VPAC1) was luminally expressed in both the human and mouse intestines [13]. The luminal expression of VIP receptors may partake in mediating mucosal protective functions and thereby facilitate alleviation of chemically induced colitis. Therefore, it was important to test if there was a therapeutic role for luminal VIP in the management of colitis.
We and others have also presented evidence of the therapeutic benefit of VIP in alleviating colitis associated inflammation and diarrhea after systemic administration [5,14,15]. Additionally, we demonstrated the superior therapeutic effects of the nanomedicine form of VIP, (VIP in sterically stabilized micelles, VIP-SSM) as compared with the free peptide in mediating these effects at a very low dose (0.25 nmol) when administered in vivo via parenteral route [14]. Being a disease of the gastrointestinal tract and chronic in nature, IBD types both ulcerative colitis and Crohn’s disease, require management with an agent which can be delivered in a less invasive manner.
In this regard, oral administration is the most common route of drug delivery with minimal invasiveness [16]. Thus, patients prefer and show higher compliance to drugs administered via oral route. The luminal receptor for VIP, is a potential target for direct colonic delivery of the peptide during disease state, either via oral route with specific colonic release or in enema formulations which are directly administered to the lumen. Envisioning this, and to mimic luminal delivery in vivo, VIP-SSM nanomedicine was first administered to mice via intracolonic route through the rectal instillation. Mice with dextran sulfate sodium (DSS) induced colitis showed recovery with minimal signs of inflammation when the same dose of 0.25 nmol shown to be effective via parenteral route was directly applied to the colon as a single dose.
Furthermore, the nanomedicine VIP-SSM can be freeze-dried without the addition of cryo-protectants and is stable in the dry form [17]. This advantageous characteristic of the nanomedicine was employed to test its prospects as a solid formulation. Due to the labile nature of both the peptide and the nanomedicine, it was required to be encapsulated to bypass the harsh acidic conditions of the stomach. Therefore, commercially available, enteric coated capsules which resist acidic pH and preferentially dissolve at colonic pH were utilized to fill out the powdered nanomedicine. The effective dose for humans was determined converting the dose for mice, employing a standard pharmacokinetic formula based on allometric scaling [18]. The calculated human equivalent dose based on the effective mouse dose was filled into capsules and tested for its release in vitro. VIP-SSM capsules preferentially dissolved at basic pH releasing nanoparticles and full peptide of VIP as determined by dynamic light scattering and ELISA. Also, storage of these capsules showed reproducible release profiles indicating stability of the formulation in capsules for at least 6 weeks. Thus, these studies demonstrate proof of concept of the potential scale up and reproducibility of a nanomedicine based capsule formulation for oral administration.
In summary, our current study demonstrates for the first time, a novel nanomedicine of VIP, which is efficacious in vivo as an anti-inflammatory and antidiarrheal agent via direct colonic administration. In addition, the clinically relevant oral capsule formulation of the nanomedicine was also tested for its potential to release the nanomedicine in simulated colonic fluids for its potential use in the clinical setting in future as an oral pharmaceutical product.
Materials & methods
Sodium salt of 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N-methoxy (polyethylene glycol)-2000 (DSPE-PEG2000) was purchased from LIPOID GmbH (Ludwigshafen, Germany). Human vasoactive intestinal peptide was synthesized at the Protein Research Laboratory, Research Resource Center, University of Illinois at Chicago (IL, USA). Sterile normal saline (N Saline) was purchased from Baxter (IL, USA), RNeasy mini kits were purchased from Qiagen (CA, USA). All other chemicals unless otherwise specified were of analytical grade and purchased from Sigma-Aldrich (MO, USA).
Mice
Male, 4–8 weeks old C57BL/6 mice were purchased from Jackson laboratories, (ME, USA). All animal studies performed were approved by the Animal Care Committee of the University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center (JBVAMC, IL, USA).
Preparation of VIP-SSM nanomedicine
VIP-SSM was prepared under sterile conditions as described before by simple dissolution method [14]. Briefly, a stock solution of 1.2 mM DSPE-PEG2000 was prepared, vortexed for 1 min and saturated with argon gas to remove air. The resulting lipid solution was kept in the dark at room temperature (RT) for 1 h in a sterile enclosed glass vial. Afterward, the stock solution of micelles was mixed at a 1:10 dilution ratio with a freshly prepared 25 μM solution of VIP in a lamina flow hood to obtain the nanomedicine VIP-SSM at 0.25 nmol in 100 μl of 1 mM SSM; 1 mM solution of SSM was used as vehicle control. All solutions were incubated in the dark at RT for self-assembly and to reach equilibrium. Free peptide solution of 0.25 nmol was prepared just prior to injection. Final nanomedicine solutions were characterized for their size by dynamic light scattering (DLS) with the NICOMP particle sizer (CA, USA) (Supplementary Figure 1) [14]. VIP-SSM has been widely characterized by us for its physicochemical properties including size, peptide association and loading [19], circular dichroism [20,21] and structure using molecular dynamic simulation [22]. The biological activity of VIP and VIP-SSM was tested in vitro, prior to in vivo studies by quantifying the activation of VPAC1 receptor and subsequent release of cyclic adenosine mono phosphate (Supplementary data).
In vivo evaluation of VIP-SSM as a local treatment for the colon after intrarectal instillation
A total of 3.5% w/v DSS (MP Biomedicals, OH, USA) was administered in drinking water for 7 days followed by 5 days tap water. The treatments were administered on day 5 (Figure 1A). Mice were anesthetized with ketamine/xylazine for catheterization (3.5-Fr silicon catheter, 4 cm into the lumen) and treatments were administered intraluminally to the colon via intrarectal instillation. The dose used was based on the previous studies conducted with DSS [14] and 2,4,6-trinitrobenzene sulfonic acid colitis (Supplementary data) in the same strain of mice. Distal colonic tissues were harvested at the end of the study for analysis. Mice were separated into five groups; control, VIP-SSM, DSS, DSS-VIP-SSM and DSS-VIP.
Figure 1. . Intra-rectal administration of a single dose of vasoactive intestinal peptide in sterically stabilized micelles to the colonic lumen improved bodyweight and colonic length of mice after dextran sulfate sodium insult.
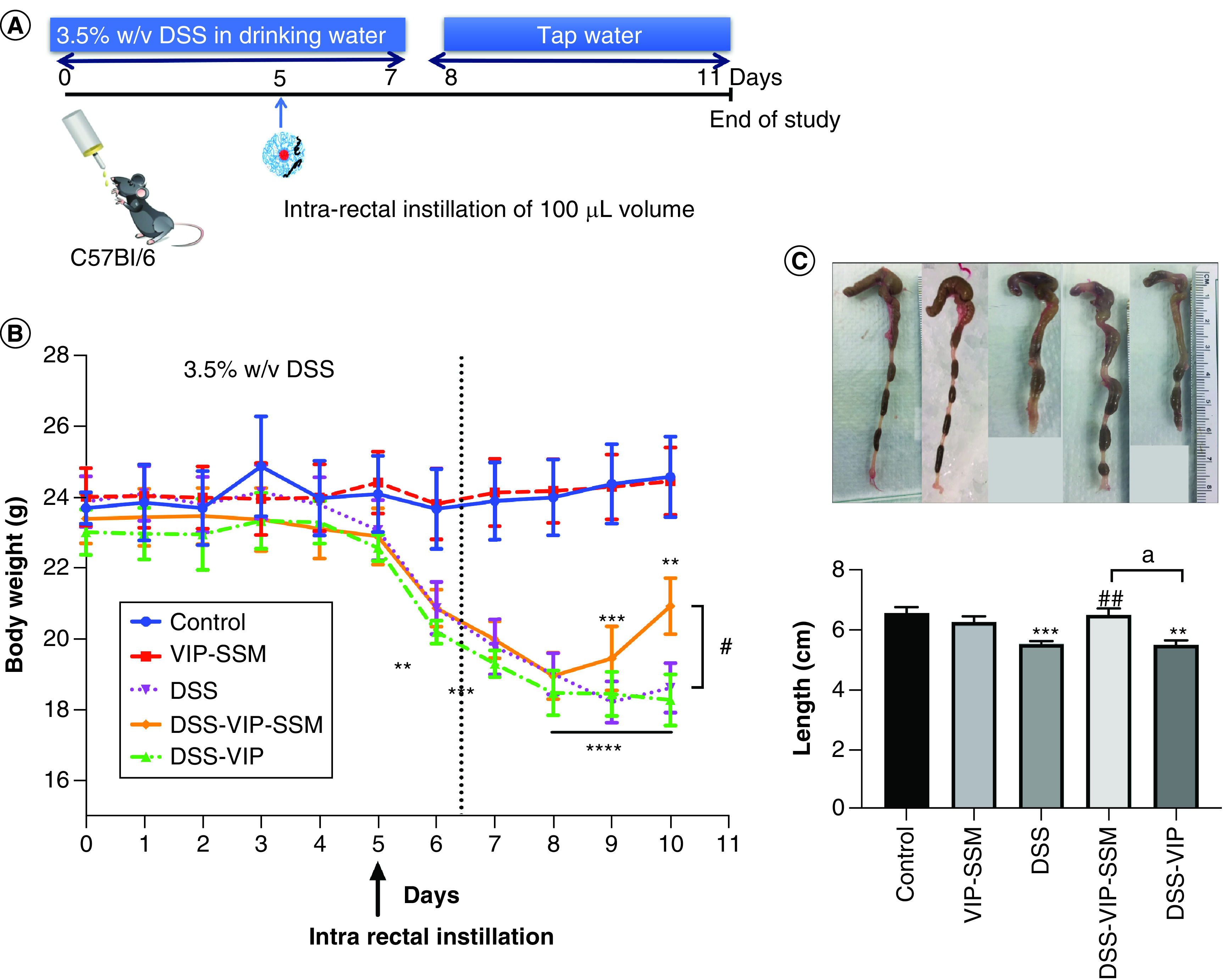
(A) Schematic representation of animal study design for local nanomedicine administration in DSS colitis. (B) Bodyweight change in all treatment groups through the course of the study. (C) Representative photograph of whole excised colons of mice in all groups and graphical representation of average length per group. Data represented as mean ± SEM, n = 6.
**p < 0.005; ***p < 0.0005; ****p < 0.0001 versus control, #p < 0.05; ##p < 0.005 versus DSS; ap < 0.05 DSS-VIP-SSM versus DSS-VIP.
DSS: Dextran sulfate sodium; VIP: Vasoactive intestinal peptide; VIP-SSM: Vasoactive intestinal peptide in sterically stabilized micelles.
Myeloperoxidase assay for distal colonic tissues
A portion of the distal colonic tissues from mice in all groups were used for colorimetric myeloperoxidase assay as described before [23]. Absorbance was measured using a microplate reader (Synergy 4, BioTek, VT, USA) at 450 nm after a detectable color development had occurred at known intervals.
Real time polymerase chain reaction for pro-inflammatory cytokines
Mouse mucosal scrapings from distal colon were collected in Trizol reagent (Qiagen, CA, USA) and extracted to the aqueous phase prior to the use of the RNeasy kit (Qiagen). All distal colonic mucosa were processed with lithium chloride as described before to avoid interaction of DSS with PCR or degradation of RT enzyme [24]. Equal amounts of RNA were reverse transcribed and amplified using Brilliant SYBR green quantitative polymerase chain reaction quantitative polymerase chain reaction (qPCR) master mix kit (Stratagene, CA, USA). Relative expression of genes were calculated according to the ΔΔCt method normalized to internal control glyceraldehyde 3-phosphate dehydrogenase [25]. Cytokines assessed include IL-1β, C-X-C motif chemokine ligand-1 and 2 (CXCL-1, CXCL-2). Mouse primer sequences used are listed in Supplementary Table 1 (Supplementary data).
Hematoxylin and eosin staining
Staining was performed on formalin fixed, paraffin embedded distal colonic tissue sections of 5 μm thickness. First slides were heated to 60°C and immersed in Xylene (Thermo Fisher Scientific, NH, USA) for 20 min. These slides were then gradually immersed in solutions of ethanol of decreasing concentrations (100, 90, 70% v/v ethanol) for rehydration. Finally, slides were immersed in distilled water. Following the rehydration process, slides were stained with hematoxylin and eosin staining kit (Scytek Laboratories, UT, USA) according to the manufacturer’s protocol.
Histopathological scoring
All hematoxylin and eosin stained slides were blinded by assigning random numbers and assessed by a pathologist, according to the following criterion; scores ranging from 0 to 3 were given where 0 indicates no change and 3 indicates maximal change in parameters compared with normal histology [14].
Effect of VIP-SSM on goblet cell number in distal colonic tissue
Paraffin embedded distal colonic tissue sections were stained with periodic acid Schiff staining kit (Sigma-Aldrich) as per the manufacturer’s protocol after deparaffinization as described before [26]. The presence and number of goblet cells were assessed in each group to examine the effects of VIP nanomedicine on goblet cells during colitis as average count of goblet cells per crypt per mouse in at least ten crypts per mouse.
Immunofluorescence staining
Cryopreserved mouse distal colonic tissues were sectioned at 5 μm. These sections were stained for respective proteins (DRA and VPAC1) as described earlier [8]. Primary antibodies used were for the respective proteins of interest (DRA and VPAC1) in 1% v/v normal goat serum at a ratio of 1:100. The primary antibodies were fluorescently labelled with respective secondary antibodies tagged either with fluorophore Alexa fluor 488 (green) or 594 (red) (Invitrogen, CA, USA) at a ratio of 1:100 in 1% normal goat serum. The slides were mounted with 4,6-diamino-2-phenylindole (Invitrogen) to stain nuclei and stored at -20°C until imaged. Images were acquired with the aid of the fluorescent microscope Olympus BX51 or Zeiss Axiocam acc1 (Oberkochen, Germany). ImageJ software was used for quantification of DRA immunofluorescence intensity.
Preparation of capsules & dissolution studies
Based on the effective dose observed in vivo in mice, an empirical pharmacokinetic formula was used to calculate the human equivalent dose (Supplementary data). The nanomedicine was prepared in bulk, freeze dried and fill weights were determined accounting for volume of liquid in the colon and dose for a 60 kg patient [27] (Supplementary data). The nanomedicine was filled into commercially available enteric coated capsules from PCCA (TX, USA) and stored accordingly before performing release studies. Enteric coated capsules were used to protect the nanomedicine from the acidic and harsh environment in the stomach. To determine the release of capsule content (freeze dried VIP-SSM), a dissolution assay was performed modified to laboratory scale. Capsules were immersed with the aid of a sinking device (spring) recommended by the US Pharmacopoeia, in 30 ml of buffer (pH of 6 at 37°C) with constant stirring using a stir-bar. Micelle formation was determined with DLS and amount of active VIP was determined by VIP enzyme immuno assay (RayBiotech, GA, USA) after performing necessary dilutions.
Stability of VIP in nanomedicine capsules compared with capsules with free peptide in an inert diluent
To determine the higher stability of peptide associated with nanomedicine in capsule form a study was conducted with VIP-SSM filled capsules and capsules filled with the same amount of peptide and an inert diluent (lactose) (Supplementary data). Capsules were prepared as before and were filled at the same fill weight. Dissolution was performed as described before and compared for release of full-length (active VIP) from capsules after dissolution.
Storage stability of VIP-SSM capsules
Prepared capsules were stored in air-tight containers in dark in the refrigerator for up to 6 weeks and at each week dissolution was performed as before and the release of full-length VIP was assessed and compared with determine stability of VIP in capsules (Supplementary data).
Statistical analysis
Each in vivo experiment was conducted with six mice per group and in vitro experiments were conducted from at least three independent samples. All data were statistically analyzed and a p-value of 0.05 or less was considered statistically significant. Studies with two or more groups were subjected to one-way analysis of variance (ANOVA) and Tukey’s post hoc test while studies with two groups were analyzed with student’s t-test. For DRA immunofluorescence analysis nested ANOVA was employed to compare values across groups.
Results
Locally administered VIP-SSM via intra-rectal route reduced weight loss in DSS colitis
The therapeutic properties of VIP nanomedicine after a single dose local administration via intrarectal instillation (Figure 1A), was determined by monitoring the bodyweight of mice throughout the duration of the study. As shown in Figure 1B, mice started losing bodyweight from day 6 with DSS. Treatments were administered on day 5 and DSS was continued up to day 7. When mice were switched to tap water, nanomedicine treated mice started showing a faster recovery in average bodyweight compared with the untreated and free peptide treated groups. At the end of the study, mice in the VIP-SSM treated group showed a significant improvement in bodyweight compared with no treatment and free peptide treatment. In line with the above findings, at the end of the study when mice colons were harvested and analyzed for diarrheal phenotype only VIP-SSM mice showed improved stool consistency and recovered colonic length (Figure 1C).
Local administration of VIP-SSM to the colonic lumen alleviated inflammation associated with DSS colitis
The anti-inflammatory effects of VIP-SSM were determined by analyzing the expression levels of mRNA of pro-inflammatory cytokines in the distal colonic mucosa of untreated and treated mice. mRNA levels for cytokines including IL-1β, CXCL-1 and CXCL-2 were significantly up regulated with DSS colitis (Figure 2). The administration of VIP-SSM nanomedicine as a single local dose of 0.25 nmol, significantly attenuated the increased levels of these cytokine transcripts to almost control levels. These effects were not observed with the free VIP peptide confirming the superior effects of the nanomedicine when administered via intrarectal route to the colonic lumen. These results were comparable to those obtained from systemic administration of VIP-SSM [14] and further confirm the potential benefit of local administration of the nanomedicine to manage colitis.
Figure 2. . Locally administered vasoactive intestinal peptide in sterically stabilized micelles nanomedicine significantly reduced mRNA expression of pro-inflammatory cytokines in the distal colonic mucosa.
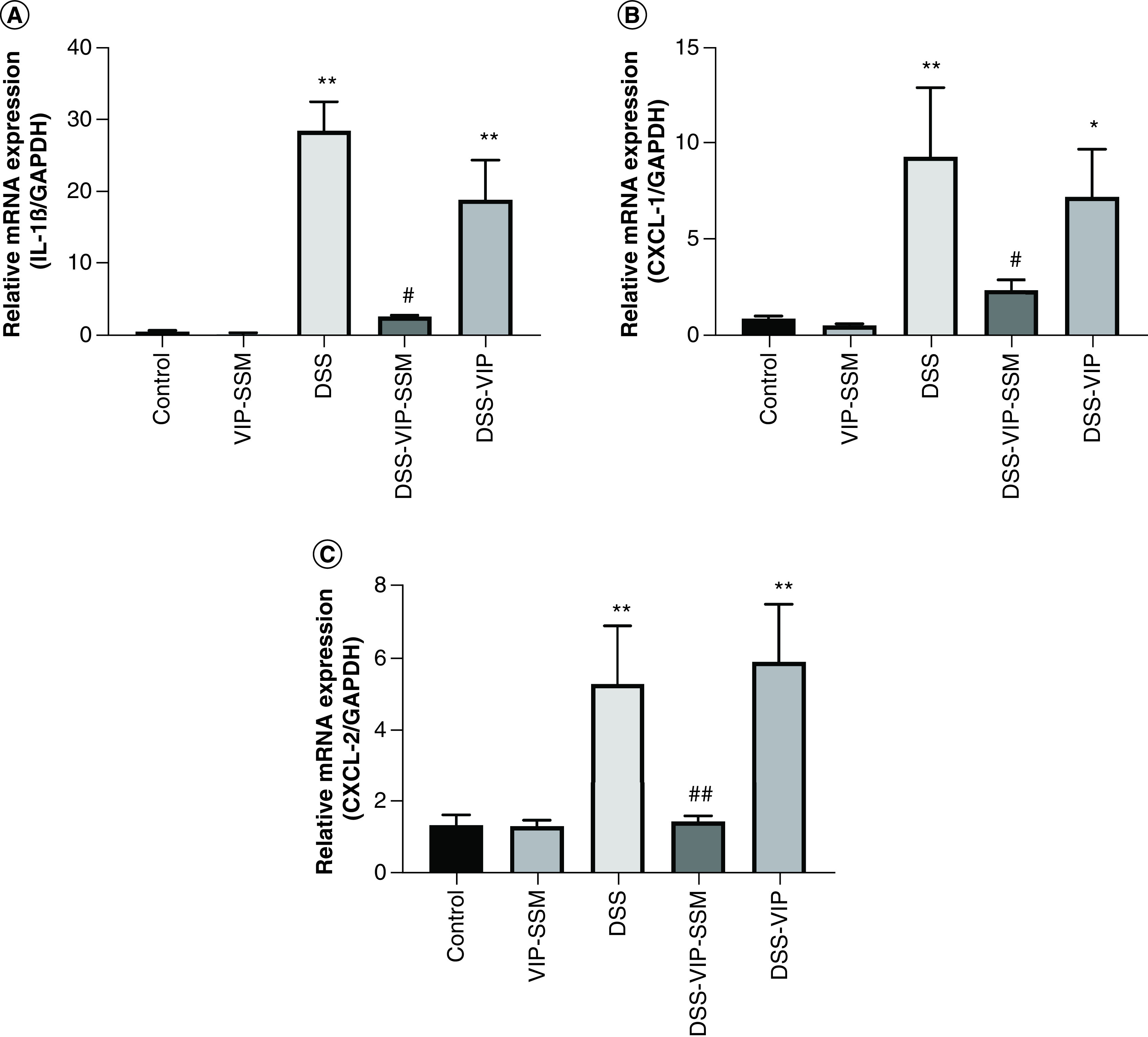
mRNA isolated from mouse distal colonic mucosa was subjected to qPCR with specific primers for (A) IL-1β, (B) CXCL-1 and (C) CXCL-2. Gene expression was normalized to internal control GAPDH. Data represented as mean ± SEM, n = 6.
*p < 0.05; **p < 0.005 versus control; #p < 0.05; ##p < 0.005 versus DSS.
CXCL-1: C-X-C motif chemokine ligand-1; CXCL-2: C-X-C motif chemokine ligand-2; DSS: Dextran sulfate sodium; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
In line with these findings, when distal colonic histology was evaluated for pathological parameters of colitis including epithelial damage, accumulation of inflammatory infiltrate and crypt destruction, VIP-SSM showed a remarkable recovery from diseased state (Figure 3A). This was evident from the significantly low histopathological scores in mice treated with the nanomedicine (Figure 3B). It should be noted that the free peptide had no such effects on the histopathological score and that the total values were similar to the untreated mice. In parallel, myeloperoxidase activity of the distal colon of the VIP nanomedicine group also demonstrated significant amelioration of leukocyte infiltration compared with DSS mice (Figure 3C). Collectively, these results support the conclusion that VIP-SSM, but not free VIP, could successfully alleviate inflammation when administered locally to the colon.
Figure 3. . Locally delivered vasoactive intestinal peptide in sterically stabilized micelles alleviates colonic inflammation and myeloperoxidase activity in dextran sulfate sodium mice.
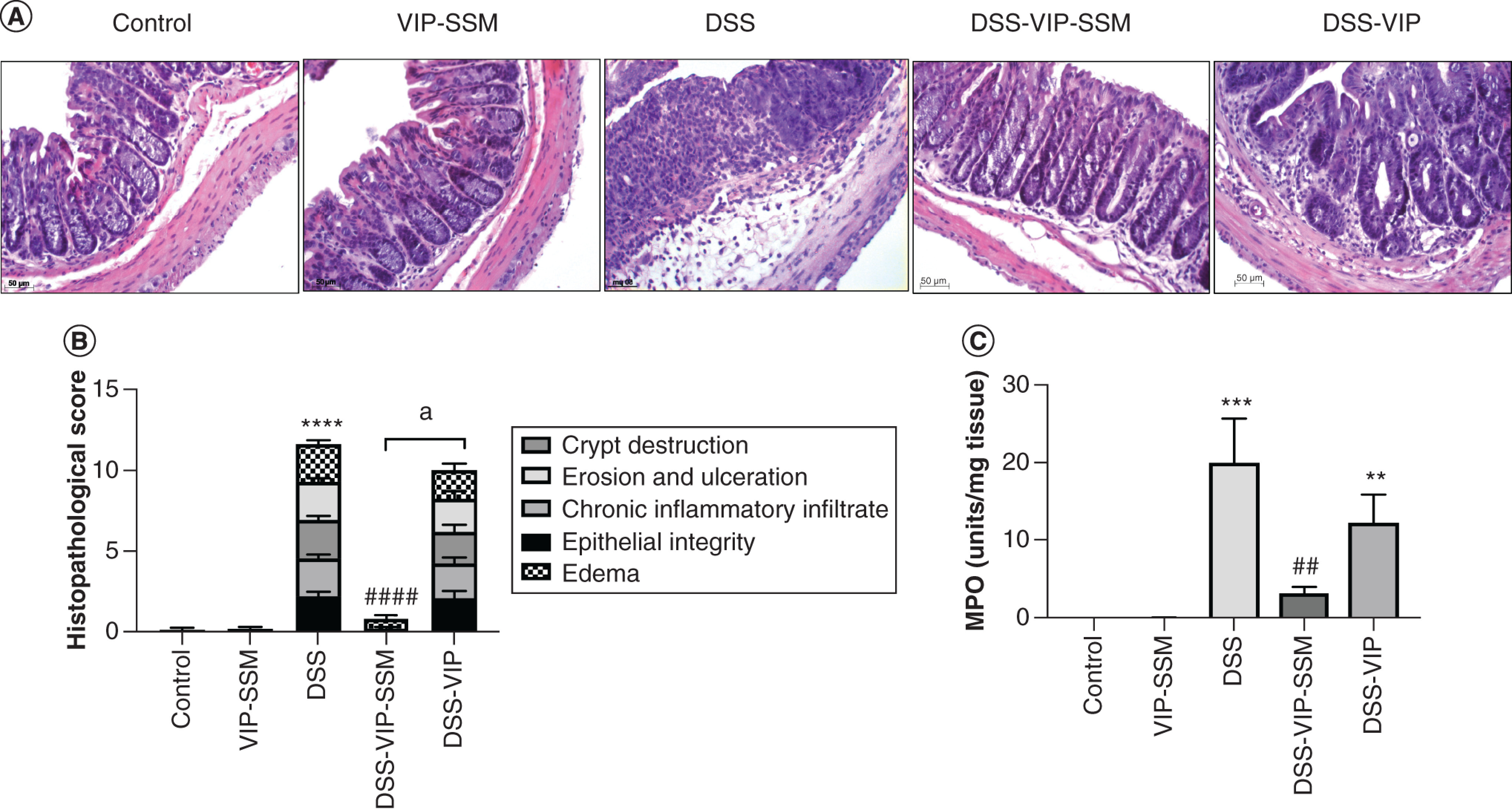
(A) Representative colonic micrographs of all treatment groups after hematoxylin and eosin stain. (B) Graphical representation of histopathological score of average in each group. (C) Bar diagram depicting MPO activity in distal colonic tissues in all treatment groups. Data represented as mean ± SEM, n = 6.
**p < 0.005; ***p < 0.0005; ****p < 0.0001 versus control; ##p < 0.005; ####p < 0.0001 versus DSS, ap < 0.0001 DSS-VIP-SSM versus DSS-VIP.
DSS: Dextran sulfate sodium; MPO: Myeloperoxidase; VIP-SSM: Vasoactive intestinal peptide in sterically stabilized micelles.
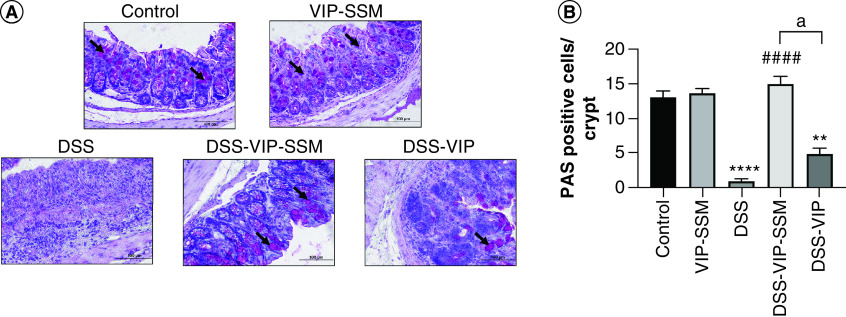
Additionally, the loss of mucus secreting goblet cells which is observed in colitis, represented here by staining for periodic acid Schiff-positive goblet cells in distal colonic tissues were markedly reduced in DSS (Figure 4). This negative effect was also alleviated in mice who received local VIP-SSM nanomedicine showing significantly improved number of goblet cells in nanomedicine treated groups compared with untreated or free VIP treated group.
Figure 4. . Colonic delivery of vasoactive intestinal peptide in sterically stabilized micelles nanomedicine rescued mice from dextran sulfate sodium induced goblet cell loss.
(A) Representative micrographs and (B) graphical representation of distal colonic tissues stained with PAS stain showing goblet cells (indicated with arrow heads). Data represented as mean ± SEM, n = 6.
**p < 0.005; ****p < 0.0001 versus control; ####p < 0.0001 versus DSS, ap < 0.05 DSS-VIP-SSM versus DSS-VIP.
DSS: Dextran sulfate sodium; PAS: Periodic acid Schiff; VIP-SSM: Vasoactive intestinal peptide in sterically stabilized micelles.
Another key parameter assessed was the downregulation of DRA, which is the critical chloride transporter in the distal colon and its downregulation is a hallmark of IBD and the DSS colitis model. This effect of decreased DRA expression was also alleviated with local administration of VIP-SSM only. This is depicted in the immunofluorescence images represented in Figure 5A. The mitigation of colitis induced DRA expression was quantified by DRA immunofluorescence intensity assessment. As shown in Figure 5B, significant attenuation of DSS induced loss of DRA immunoreactivity was observed in VIP-SSM treated mice and not in mice treated with the free peptide. These results show that local administration of the nanomedicine has beneficial effects at the same dose which showed alleviation of inflammation after systemic (ip.) administration in both DSS [14] and 2,4,6-trinitrobenzene sulfonic acid colitis (Supplementary Figure 3).
Figure 5. . Vasoactive intestinal peptide in sterically stabilized micelles intrarectal delivery attenuated dextran sulfate sodium-induced DRA loss in mice distal colon.
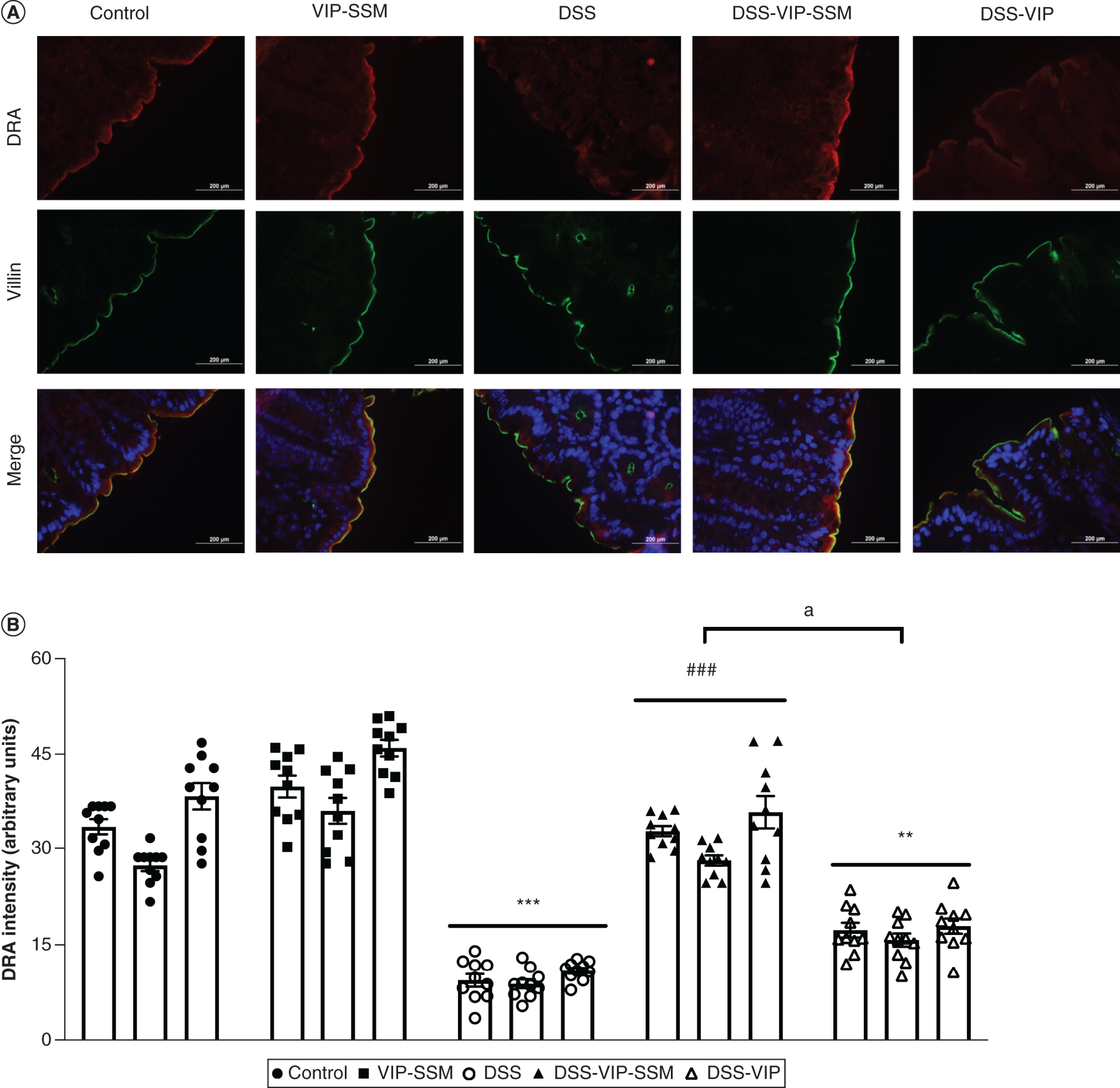
(A) Representative micrographs of fluorescently stained DRA (red) with the apical marker villin (green) and DAPI (blue). (B) Graphical representation of quantified DRA immunofluorescence intensity (arbitrary units) across treatment groups. Data represented as average ± SEM, n = 3 with 10 data points per n.
**p < 0.005; ***p < 0.0005 versus control; ###p < 0.0005 versus DSS, ap < 0.005 DSS-VIP-SSM versus DSS-VIP.
DAPI: 4,6-diamino-2-phenylindole; DSS: Dextran sulfate sodium; VIP-SSM: Vasoactive intestinal peptide in sterically stabilized micelles.
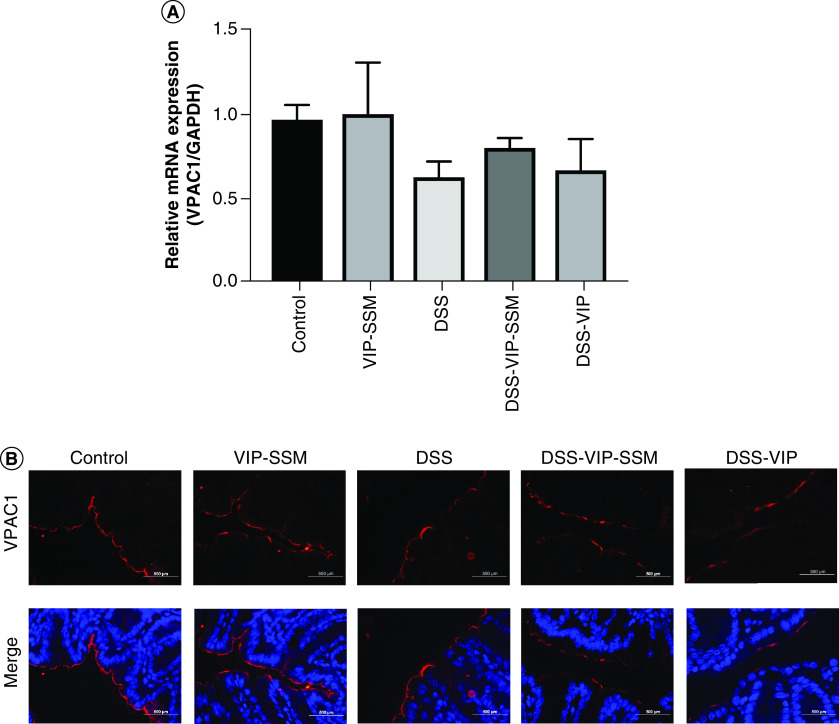
DSS colitis did not alter the expression of VPAC1 levels in the colonic mucosa
The expression of VPAC1 levels in the colonic mucosa was investigated in order to ascertain if DSS colitis could negatively affect its expression under diseased conditions.
VPAC1 mRNA and immunofluorescence studies demonstrated no overall significant difference in its expression across groups in a therapeutic setting in DSS colitis (Figure 6). Thus, targeting VIP-nanomedicine locally to the colon under inflammatory conditions could be an important and safe potential treatment strategy to manage IBD.
Figure 6. . Dextran sulfate sodium model of colitis has negligible effects on VPAC1 expression in the distal colon.
(A) Graphical representation of VPAC1 mRNA in the Distal colon. Gene expression normalized to internal control GAPDH. mRNA isolated from mouse distal colon was subjected to qPCR with specific primers for VPAC1. Data represented as average ± SEM, n = 6. (B) Immunofluorescence staining of VPAC1 (red) and DAPI (blue) in distal colonic tissue sections.
DAPI: 4,6-diamino-2-phenylindole; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; qPCR: Quantitative polymerase chain reaction.
VIP-SSM nanomedicine can be developed into an oral capsule product which releases contents to reform nanomedicine when dissolved at colonic pH
Capsules containing freeze-dried VIP-SSM were filled in the laboratory as depicted in Figure 7A. These capsules started dissolving in the buffer from 10 min after immersion (Figure 7B). The capsule contents gradually dissolved over the next 10 min showing release of contents from the edges of the capsule. After 20 min there was rapid dissolution which resulted in a visibly clear solution at the end of 40 min. These observations indicated that VIP-SSM dissolved in colonic fluids and were completely released from the enteric coated capsules without forming clumps at the interfaces.
Figure 7. . Freeze-dried vasoactive intestinal peptide in sterically stabilized micelles nanomedicine containing capsules completely dissolve in buffer (pH 6) releasing nanomedicine with active vasoactive intestinal peptide.
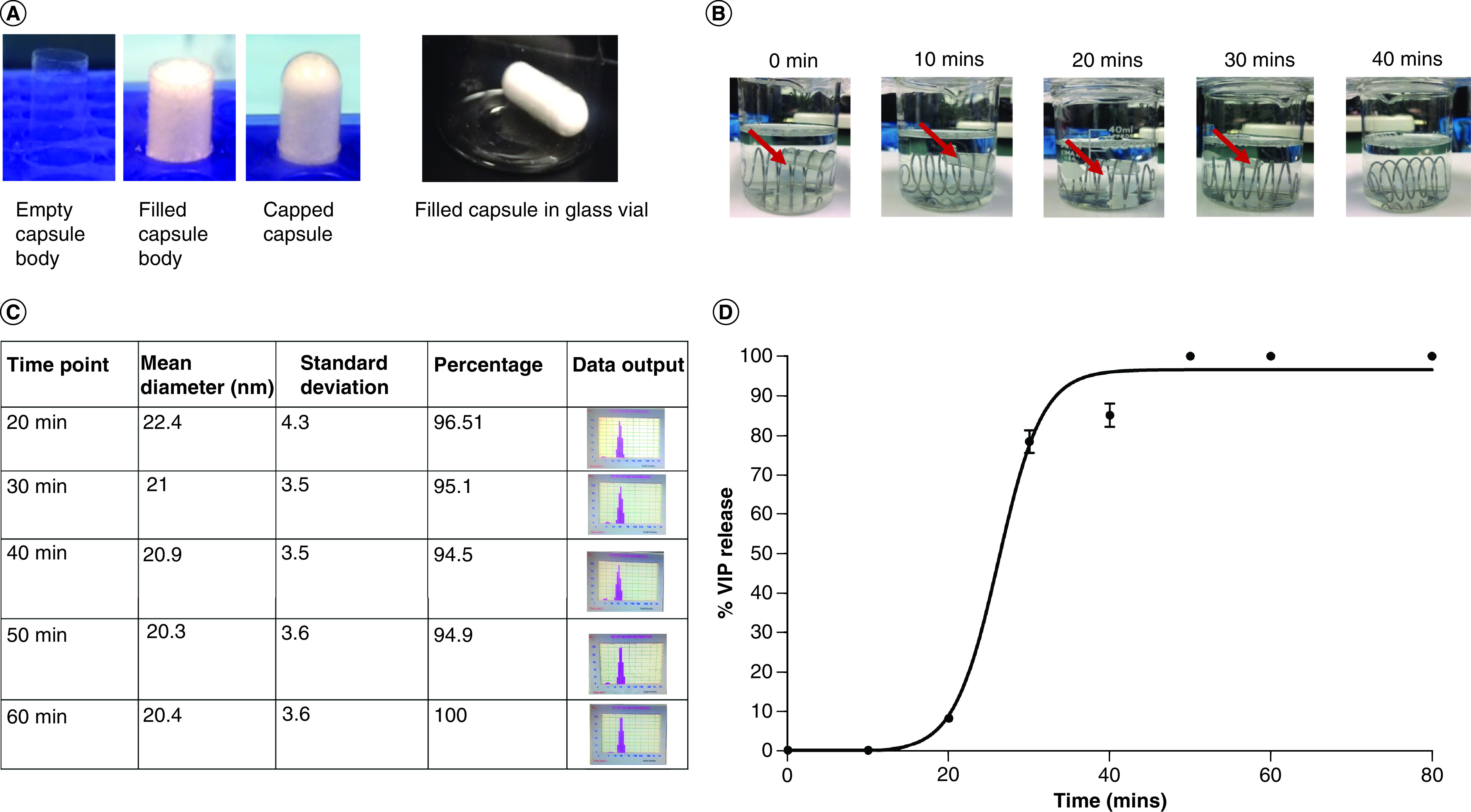
(A) Representative photographs of the capsule filling process for freeze dried VIP-SSM at human equivalent dose. (B) Complete dissolution of VIP-SSM capsules at 40 min in 37°C while continuous stirring. Red arrow points at the capsule. (C) Particle size distribution of dissolution solution at given time points (from 20 min onward where detectable amounts of nanomedicine was released to solution). (D) Percentage release of full-length VIP from capsules as determined by VIP EIA.
EIA: Enzyme immuno assay; VIP-SSM: Vasoactive intestinal peptide in sterically stabilized micelles.
Reformation of micelles after capsule dissolution assessed by dynamic light scattering
All capsules completely dissolved in pH 6 buffer giving rise to a visually clear solution. However, for the nanomedicine to be effective in managing human IBD, as shown in vivo, it is of utmost importance that once dissolved, the nanomedicine is intact with bioactive VIP. Therefore, the reformation of micelles after capsule dissolution was determined by dynamic light scattering. Presence of particles ranging in the size of approximately 15–20 nm was used to infer the presence of micelles in the dissolution contents (since micelles are ∼15 nm). Figure 7C shows the population of particles corresponding to micelles in solution at each time point of analysis, starting from 20 min. Since the capsule contents did not dissolve completely at 10 min and very few micelles were in solution, the aliquot used for DLS analysis was unable to initialize the NICOMP particle sizer. However, starting from 20 min, where the capsule showed significant swelling and dissolution, analysis was possible and over 96% of the particles analyzed were in the size range of micelles (Figure 7C). At 60 min, when all capsule content was released, the majority of the particles were in micellar size range indicating successful reformation of micelles after freeze-dried powder was dissolved in simulated colonic fluid.
Dissolved nanomedicine from capsules contained active VIP as determined by ELISA
The peptide in the capsules, VIP, ideally should be present at similar amounts as in the original powder fills, and in active form after all contents were released out of capsules to the dissolution media, to mediate therapeutic benefits in IBD. Therefore, to understand if capsular contents, once dissolved, would have active VIP, ELISA was performed on aliquots taken at 10-min time intervals. As shown in Figure 7D, each capsule released VIP, starting from 20 min parallel to the findings from micelle reformation assessed by DLS. Beyond 20-min release of VIP from capsules showed an exponential increase fitting a four-parametric sigmoidal curve (Figure 7D). Due to the complete dissolution of capsules after 40-min, the release curve showed a plateau indicating 100% release amount for a given capsule.
Released VIP was intact & associated with micelles showing higher stability
To compare the content stability of capsules filled with VIP-SSM or free peptide in an inert diluent filler, dissolution studies were performed. As shown in Figure 8A, capsules (n = 4) containing VIP-SSM showed a dissolution pattern similar to which was observed previously, in other words, releasing contents exponentially from 20–40 min and then reaching a plateau after all contents were released (Figure 8A). The capsules which had the same fill weight of VIP in lactose initially released VIP faster, but gave rise to lower total levels of VIP in solution at time points starting from 30 min compared with the capsules with VIP-SSM. In the initial phase of release, free VIP containing capsules released contents more rapidly compared with the nanomedicine powder containing capsules, thus, this was reflected in the VIP release profile demonstrating higher VIP at the 20-min time point, compared with capsules with VIP-SSM (Figure 8A). Most importantly, unlike capsules containing VIP-SSM, free peptide containing capsules started showing a relative drop in VIP levels, evidently from 20–30 and 40 min and beyond, suggesting potential degradation when free peptide is in solution. The stability of the peptide in SSM confirms the association of VIP with SSM, thus, resisting autolysis associated with the free peptide. The rate of autolysis is faster at dilute concentrations and can be attributed to the release profile of free VIP from 20–30 min showing a rapid drop in total levels. It could also be explained by the fact that VIP at lower concentrations, in other words, 0.4 μM, is in the monomeric form and not in its micellar form which is more stable [28]. Therefore, the degradation observed becomes less pronounced at higher concentrations. Since the same dose of VIP released from capsules does not give rise to the correct amounts of VIP, the peptide in solution can only resist autolysis if present in the micellar form. Finally, a known concentration of free peptide (10 ng/μl), freshly prepared was used to compare with released peptide from capsules and is shown as a red triangle in Figure 8A.
Figure 8. . Stability of freeze-dried vasoactive intestinal peptide in sterically stabilized micelles nanomedicine capsules.
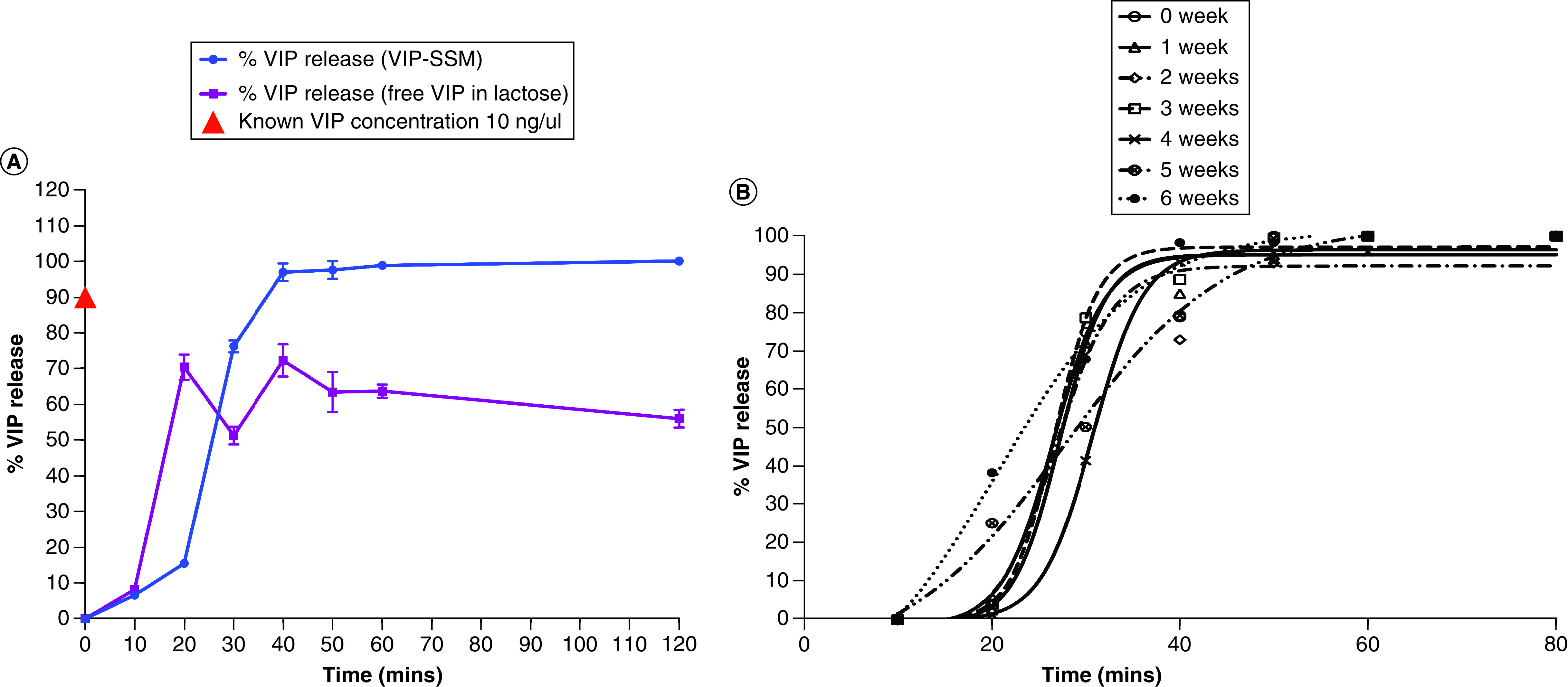
(A) Comparison of percentage of full-length VIP released from capsules containing VIP-SSM nanomedicine (blue) and VIP released from capsules filled with a commercially used diluent filler: lactose (purple). (B) Release profiles of VIP-SSM capsules stored in airtight glass containers at 4°C up to 6 weeks. VIP EIA was used to determine release of full-length VIP peptide. Data points are the average of at least three independent experiments ± SEM.
EIA: Enzyme immuno assay; VIP-SSM: Vasoactive intestinal peptide in sterically stabilized micelles.
VIP-SSM containing capsules can be stored over 6 weeks
Capsules stored for 6 weeks showed very similar outcomes to data presented earlier on freshly prepared capsules, dissolving completely after 40 min. The release of active, full-length VIP after storage at each week was determined and results are depicted in Figure 8B. VIP release from capsules followed a similar pattern to the one observed earlier fitting a four-parametric sigmoidal release. Data at each week of storage did not show a significant difference in releasing active VIP. This shows reasonable evidence of the stability of the nanomedicine, with regard to the active ingredient VIP, in capsules in the freeze-dried form when stored in sealed containers at 4°C for at least up to 6 weeks.
Discussion
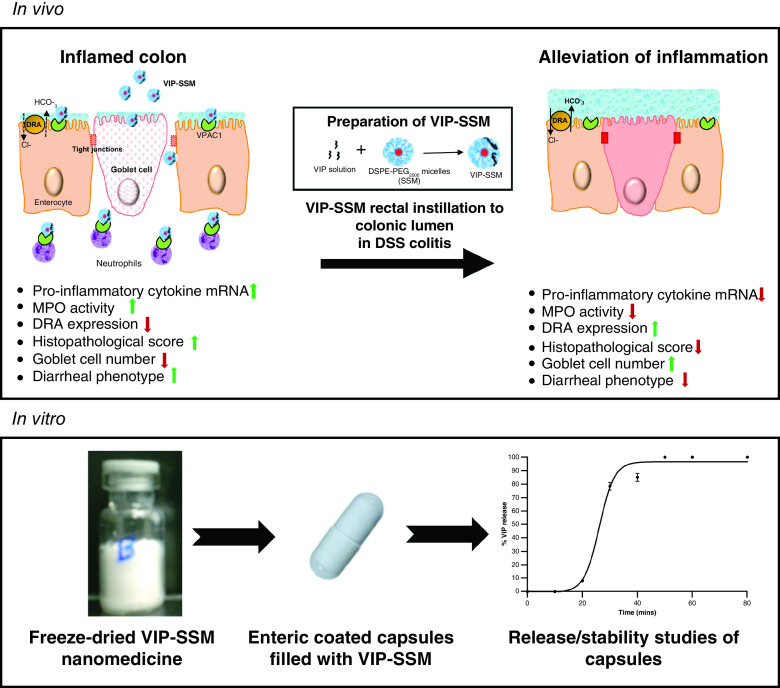
Inflammatory bowel disease is a global health burden which is chronic in nature, affecting millions worldwide [29]. Currently available treatments are aimed at merely relieving symptoms and thus, there is a pressing need for novel therapeutic modalities to manage the disease. Herein we show the effectiveness of VIP-SSM nanomedicine in alleviating DSS colitis and then show prospects of its development as a potential oral formulation.
Peptide drugs have gained substantial attention as therapeutic agents for their high specificity, potency and relatively low toxicity [30,31]. However, due to their excessive susceptibility to enzymatic degradation and low oral bioavailability, peptide drugs are mostly administered by injection. Thus, there are only a very few peptide drugs available in the market as oral dosage forms [32]. There have also been some attempts to deliver peptides as nanomedicines via oral route. In this regard, Insulin [33,34], GLP-1 [35] and a few other peptides have been used as model peptide drugs tested via oral route to be delivered for systemic effects. In addition, notable efforts have also been taken to deliver peptide nanomedicines for local management of diseases in the GI tract including the proresolving peptide Ac2–26 [36] and others [36–39]. However, there is still an important need of urgency for delivering peptides via oral route to improve their clinical use [40].
In the current study, intracolonic administration of VIP-SSM but not the free peptide in DSS colitis had significant beneficial effects as a single dose (Figures 1–6). We have previously shown that VIP-SSM targeted the inflamed site after systemic administration [19], and our current speculation is that when delivered locally, VIP in SSM alone binds to VPAC1 receptor in adequate amounts, also entering lamina propria via damaged mucosa to bind with receptors on the immune cells, to mediate alleviation of inflammation. There are several reasons why the nanomedicine could have shown better effects than the free peptide after luminal delivery: nanoparticles are known to adhere and retain better at the site of inflammation by persorption [41]; the size of the nanomedicine allows passive accumulation at the damaged intestinal mucosa by epithelial retention effect [42]; and finally, the PEG present on the surface of the nanomedicine may allow mucus penetration and interaction with VIP receptors in the colonic mucosa [43]. However, the retentive capacity of the free peptide could be less due to potentially being flushed out by diarrhea and associated movements in the inflamed intestine, instability due to bacterial and enzymatic cleavage and, therefore, would not reach the target receptors at the required dose. Ulcerative colitis is currently managed with enema solutions of drugs such as aminosalicylates [44,45]. In this regard, our current studies show effectiveness of using VIP nanomedicine solution by local instillation as a novel approach for VIP therapy.
However, to manage a GI disease, an oral formulation would be the ideal preferred route to improve patient compliance. Therefore, it was of interest to show the feasibility of the use of VIP nanomedicine in a clinically applicable dosage form for oral use. The methods used in the current study may not have been optimal with regard to determining the dose for human usage, some of which are discussed below. Though conducting the in vivo study after oral administration in mice would have been ideal, but it was not feasible and, therefore, colonic instillation was used as an alternative mode of administration to mimic specific delivery to the colon. To determine the required VIP dose to be filled in the capsules, allometric scaling was employed. Although allometric scaling is used frequently for dose conversions among species, it may not be the best method; however, we utilized this method for its ease of and frequent usage in interspecies dose conversions [18]. Once the mouse to human dose was determined, the concentration of the peptide in the colon was calculated based on the lowest volume which is known to be present in the colon (10 ml). This allowed incorporation of the highest VIP dose. However, when the release studies were performed on laboratory scale, the maximum volume in the colon, which is 30 ml was used to allow complete immersion of the capsule in the fluid.
The presence of VIP associated with SSM in solution after dissolution was determined by assaying the presence of full-length VIP in solution by ELISA. Self-association of the peptide with micelle during nanomedicine preparation results in a conformational change of the peptide from random coil to an alfa helical form [22]. Phospholipid micelles and VIP interact with coulombic coupling and VIP is believed to be present on the interface between the phospholipid head and the PEG corona of the micelle. Therefore, it would have been more relevant to assess the presence of peptides in micellar form by determining its secondary structure in the solution as we did previously by circular dichroism (CD) [17]. However, CD was not feasible to be performed due to the possible interference from the capsule polymer contents, since high purity of the sample is required for CD analysis [46]. However, based on our extensive experience with VIP-SSM, indirect methods of micelle size and VIP activity determinations should lead to accurate conclusions of intact VIP nanomedicine.
For stability studies, the capsules were stored in air-tight glass containers saturated with an inert gas in dark to try and mimic the blister pack wrapping. Capsules stored over time at 4°C for 6 weeks, showed similar micelle forming and VIP release patterns as determined by ELISA and, therefore, showed retention of VIP’s activity, for up to at least 6 weeks (Figure 8B). These studies showed promise and potential for developing VIP-SSM as an oral formulation with favorable activity. However, it would be worthwhile to perform longer term storage studies of these capsules in future, for commercialization of the product.
Although, determination of peptide association with micelles were inferred from conducting ELISA, it is not an ideal method for this purpose; however, the assumption that peptide was still associated with micelles was inferred for the following possible reasons. The labile peptide is known to degrade rapidly in solution by autolysis at physiological pH following first order kinetics giving rise to multiple inactive degradation products [47,48]. Therefore, during the dissolution process, carried out at 37°C under light, we believe that the free peptide would be degraded and the peptide associated with the micelles would resist autolysis to retain its activity (Figure 8A). The stability of the peptide in SSM confirms the association of VIP with SSM, thus, resisting autolysis associated with the free peptide [28]. Finally, the capsules of VIP-SSM were stable for at least 6 weeks when stored appropriately providing important data on shelf-life stability which is important for a commercially viable drug product (Figure 8B).
In summary, we have demonstrated a proof-of-concept that a peptide nanomedicine with immunomodulatory properties is potentially beneficial for managing IBD. In addition, VIP-SSM nanomedicine can be used to deliver active VIP to the target site with minimal side effects. Finally, the formulation is versatile in its capacity to be effective both as a systemic parenteral formulation and a locally delivered oral formulation. Furthermore, for pediatric and geriatric patients with colitis who are unable to take drugs orally, the intracolonic instillation via enema may serve as a better method for treatment. Additionally, these data indicate the potential feasibility of delivering a peptide as nanomedicine in the dry form, in a capsule by oral route, which has not been achieved so far for IBD. Further studies are warranted to explore the clinical applicability of this peptide nanomedicine.
Conclusion
Our data demonstrate that VIP-SSM is a versatile nanomedicine which could be used via multiple parenteral routes of administration for the treatment of IBD. Our studies also demonstrate for the first time that VIP-SSM can be formulated into an oral capsule form for future clinical use in IBD.
Summary Points.
Nanomedicines are superior in treating chronic inflammatory diseases such as inflammatory bowel disease.
Vasoactive intestinal peptide (VIP) receptor, VPAC1, is abundant in the colonic lumen and is not downregulated during colonic inflammation, serving as a target for inflammatory bowel disease therapy.
Targeting VPAC1 in the colon by VIP in sterically stabilized micelles (VIP-SSM) alleviated colitis after a single dose of 0.25 nmol/mouse via direct intracolonic administration.
VIP-SSM, but not the free peptide at the same dose improved reduced bodyweight, colonic histology and increased meyeloperoxidase activity demonstrating its anti-inflammatory efficacy in vivo.
VIP-SSM ameliorated the reduced expression of SLC26A3 (DRA) and improved goblet cell number back to healthy control levels which was not observed with the free peptide thus, showing direct activation of VPAC1 receptor in the colon.
Human equivalent dose containing VIP-SSM was successfully freeze-dried and encapsulated in enteric coated capsules.
These capsules released VIP-SSM nanomedicine with active VIP after dissolution in colonic pH.
VIP-SSM capsules are stable up-to 6 weeks when refrigerated in sealed containers and, therefore, can be potentially developed into a clinically relevant oral formulation for managing colitis in humans.
Supplementary Material
Acknowledgments
Special thanks to G Guzman, Pathologist at the University of Illinois at Chicago and staff at UIC RRC core imaging facility.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/nnm-2020-0280
Author contributions
Initial conceptualization was performed by H Onyuksel and PK Dudeja. Evolution of conceptualization was performed by D Jayawardena. Investigations were performed by D Jayawardena, AN Anbazhagan, S Priyamvada and A Kumar. Resources were provided by PK Dudeja, H Onyuksel and S Saksena. Supervision was performed by PK Dudeja and H Onyuksel. D Jayawardena wrote the original draft. Review and editing were performed by all authors.
Financial & competing interests disclosure
These studies were supported by the NIDDK grants R01 DK54016, R01 DK92441 (PK Dudeja) and the Department of Veterans Affairs BX 002011 (PK Dudeja) and VA SRCS Award (IK6 BX005242, PK Dudeja), BX 002867 (S Saksena), BX004719 (A Kumar), UIC Dean’s Fellowship (D Jayawardena) and TUBITAK Award (H Onyuksel). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR15482 from the National Center for Research Resources, NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All animal studies performed were approved by the Animal Care Committee of the University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center (IL, USA). The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Abad C, Waschek JA. Immunomodulatory roles of VIP and PACAP in models of multiple sclerosis. Curr. Pharm. Des. 17(10), 1025–1035 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Delgado M, Martinez C, Pozo D. et al. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-α and IL-6. J. Immunol. 162(2), 1200–1205 (1999). [PubMed] [Google Scholar]
- 3.Gomariz R, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr. Pharm. Des. 7(2), 89–111 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Smalley S, Barrow P, Foster N. Immunomodulation of innate immune responses by vasoactive intestinal peptide (VIP): its therapeutic potential in inflammatory disease. Clin. Exp. Immunol. 157(2), 225–234 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights the immunomodulatory properties of vasoactive intestinal peptide (VIP) and therefore forms the basis of its use in inflammatory diseases.
- 5.Seo S, Miyake H, Alganabi M. et al. Vasoactive intestinal peptide decreases inflammation and tight junction disruption in experimental necrotizing enterocolitis. J. Pediatr. Surg. 54(12), 2520–2523 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Campbell J, Berry J, Liang Y. Anatomy and physiology of the small intestine. : Shackelford's Surgery of the Alimentary Tract - 2 Volume Set. Elsevier, PA, USA, 817–841 (2019). [Google Scholar]
- 7.Seillet C, Luong K, Tellier J. et al. The neuropeptide VIP confers anticipatory mucosal immunity by regulating ILC3 activity. Nat. Immunol. 21, 168–177 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Jönsson M, Norrgård Ö, Hansson M, Forsgren S. Decrease in binding for the neuropeptide VIP in response to marked inflammation of the mucosa in ulcerative colitis. Ann. NY Acad. Sci. 1107(1), 280–289 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Mazumdar S, Das KM. Immunocytochemical localization of vasoactive intestinal peptide and substance P in the colon from normal subjects and patients with inflammatory bowel disease. Am. J. Gastroenterol. 87(2), 176–181 (1992). [PubMed] [Google Scholar]
- 10.Cassuto J, Fahrenkrug J, Jodal M, Tuttle R, Lundgren O. Release of vasoactive intestinal polypeptide from the cat small intestine exposed to cholera toxin. Gut 22(11), 958–963 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan X, Karpen HE, Stephens J. et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130(1), 150–164 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Dharmsathaphorn K, Harms V, Yamashiro DJ, Hughes R, Binder H, Wright E. Preferential binding of vasoactive intestinal polypeptide to basolateral membrane of rat and rabbit enterocytes. J. Clin. Invest. 71(1), 27–35 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayawardena D, Guzman G, Gill RK, Alrefai WA, Onyuksel H, Dudeja PK. Expression and localization of VPAC1, the major receptor of vasoactive intestinal peptide along the length of the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 313(1), G16–G25 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • The presence of VPAC1 receptors in human and mouse colonic epithelium is characterized. This is the receptor which we target in this manuscript.
- 14.Jayawardena D, Anbazhagan AN, Guzman G, Dudeja PK, Onyuksel H. Vasoactive intestinal peptide nanomedicine for the management of inflammatory bowel disease. Mol. Pharm. 14(11), 3698–3708 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is the first time vasoactive intestinal peptide in sterically stabilized micelles (VIP-SSM) was tested in colitis and the optimal dose for the current study was decided upon.
- 15.Abad C, Martinez C, Juarranz MG. et al. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology 124(4), 961–971 (2003). [DOI] [PubMed] [Google Scholar]; • Describes the beneficial effect of VIP for the first time in Inflammatory bowel disease. Frequent high doses were used for alleviating inflammation.
- 16.Motlekar NA, Youan B-BC. The quest for non-invasive delivery of bioactive macromolecules: a focus on heparins. J. Control. Rel. 113(2), 91–101 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim SB, Rubinstein I, Önyüksel H. Freeze drying of peptide drugs self-associated with long-circulating, biocompatible and biodegradable sterically stabilized phospholipid nanomicelles. Int. J. Pharm. 356(1–2), 345–350 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; • VIP-SSM freeze dried product is characterized, thereby demonstrating the stability of this peptide nanomedicine during the freeze drying process.
- 18.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7(2), 27 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Interspecies dosage conversion is discussed which was employed in the calculation of the human equivalent dose.
- 19.Sethi V, Rubinstein I, Kuzmis A, Kastrissios H, Artwohl J, Onyuksel H. Novel, biocompatible, and disease modifying VIP nanomedicine for rheumatoid arthritis. Mol. Pharm. 10(2), 728–738 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• VIP-SSM nanomedicine was characterized for its physico-chemical properties and demonstrated the stability of this formulation in vivo.
- 20.Banerjee A, Onyuksel H. Peptide delivery using phospholipid micelles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 4(5), 562–574 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Lim SB, Banerjee A, Önyüksel H. Improvement of drug safety by the use of lipid-based nanocarriers. J. Control. Rel. 163(1), 34–45 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Vukovic L, Madriaga A, Kuzmis A. et al. Solubilization of therapeutic agents in micellar nanomedicines. Langmuir 29(51), 15747–15754 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; • VIP-SSM structure was determined by molecular dynamic simulation studies.
- 23.Krawisz J, Sharon P, Stenson W. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: assessment of inflammation in rat and hamster models. Gastroenterology 87(6), 1344–1350 (1984). [PubMed] [Google Scholar]
- 24.Viennois E, Chen F, Laroui H, Baker MT, Merlin D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res. Notes 6(1), 360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3(6), 1101–1108 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Lindén SK, Florin TH, Mcguckin MA. Mucin dynamics in intestinal bacterial infection. PLoS ONE 3(12), e3952 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McConnell EL, Fadda HM, Basit AW. Gut instincts: explorations in intestinal physiology and drug delivery. Int. J. Pharm. 364(2), 213–226 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Önyüksel H, Bodalia B, Sethi V, Dagar S, Rubinsteina I. Surface-active properties of vasoactive intestinal peptide*. Peptides 21(3), 419–423 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12(12), 720–727 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat. Rev. Drug Discov. 4(4), 298 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Lewis AL, Richard J. Challenges in the delivery of peptide drugs: an industry perspective. Ther. Deliv. 6(2), 149–163 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Richard J. Challenges in oral peptide delivery: lessons learnt from the clinic and future prospects. Ther. Deliv. 8(8), 663–684 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 24(12), 2198–2206 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Sarmento B, Martins S, Ferreira D, Souto EB. Oral insulin delivery by means of solid lipid nanoparticles. Int. J. Nanomedicine 2(4), 743 (2007). [PMC free article] [PubMed] [Google Scholar]
- 35.Araújo F, Shrestha N, Shahbazi M-A. et al. The impact of nanoparticles on the mucosal translocation and transport of GLP-1 across the intestinal epithelium. Biomaterials 35(33), 9199–9207 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Li C, Zhao Y, Cheng J. et al. A proresolving peptide nanotherapy for site-specific treatment of inflammatory bowel disease by regulating proinflammatory microenvironment and gut microbiota. Adv. Sci. 6(18), 1900610 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laroui H, Dalmasso G, Nguyen HTT, Yan Y, Sitaraman SV, Merlin D. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology 138(3), 843–853 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 64(6), 557–570 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Des Rieux A, Fievez V, Garinot M, Schneider Y-J, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J. Control. Rel. 116(1), 1–27 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Hamman JH, Enslin GM, Kotzé AF. Oral delivery of peptide drugs. BioDrugs 19(3), 165–177 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Hua S, Marks E, Schneider JJ, Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomedicine 11(5), 1117–1132 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Xiao B, Merlin D. Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease. Expert Opin. Drug Deliv. 9(11), 1393–1407 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Taipaleenmäki EM, Mouritzen SA, Schattling PS, Zhang Y, Städler B. Mucopenetrating micelles with a PEG corona. Nanoscale 9(46), 18438–18448 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Chapman NJ, Brown ML, Phillips SF. et al. Distribution of mesalamine enemas in patients with active distal ulcerative colitis. Mayo Clin. Proc. 67(3), 245–248 (1992). [DOI] [PubMed] [Google Scholar]
- 45.Sandborn W, Tremaine W, Leighton JA. et al. Nicotine tartrate liquid enemas for mildly to moderately active left-sided ulcerative colitis unresponsive to first-line therapy: a pilot study. Aliment. Pharmacol. Ther. 11(4), 663–671 (1997). [DOI] [PubMed] [Google Scholar]
- 46.Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 1(6), 2876 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mody R, Tramontano A, Paul S. Spontaneous hydrolysis of vasoactive intestinal peptide in neutral aqueous solution. Chem. Biol. Drug Des. 44(5), 441–447 (1994). [DOI] [PubMed] [Google Scholar]
- 48.Cui X, Cao D, Qu C, Zhang X, Zheng A. A study of the chemical and biological stability of vasoactive intestinal peptide. Drug Dev. Ind. Pharm. 39(12), 1907–1910 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.