Abstract
This commentary discusses the early licensing and deployment of a COVID-19 vaccine and how accomplishing this goal could compromise ethical principles that guide clinical research.
The current pandemic demands early licensing and deployment of a vaccine against coronavirus disease 2019 (COVID-19) that provides “worthwhile” efficacy (1). However, accomplishing this goal could compromise 2 ethical principles that guide clinical research—scientific validity, which is based on the tradeoff between risk and benefit, and social value, which depends on the short-term and long-term prevention of COVID-19.
Five Western companies are conducting placebo-controlled, phase 3, randomized clinical trials (RCTs) whose primary outcome is the prevention of clinical disease (Table). Each trial will last for up to 2 years and have at least 1 interim analysis. As soon as one of these RCTs establishes vaccine efficacy and provides 2 months of safety data (2), the U.S. Food and Drug Administration (FDA) could within a few days or weeks license the vaccine or provide Emergency Use Authorization (EUA). Deployment could begin immediately after either decision. Early approval is possible because each of these trials has recruited tens of thousands of participants, and the World Health Organization and the FDA (3) require that vaccines show only at least 50% efficacy. These conditions mean that the primary efficacy outcome could be established by some 50 cases in vaccine recipients and 100 cases in placebo recipients (1). Among secondary outcomes are seroconversion rate and geometric mean titers of neutralizing antibodies specific for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Table. Large, Parallel, Double-Blind, Placebo-Controlled, Efficacy Phase 3 Randomized Clinical Trials on COVID-19 Vaccine Candidates Sponsored by Western Companies*.
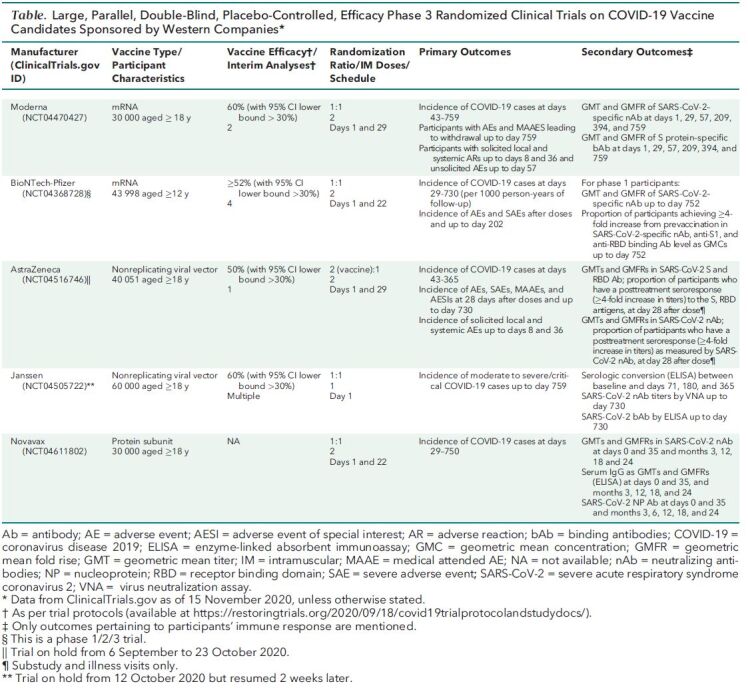
But this scenario will not answer questions about long-term efficacy and safety, which requires more months of data. Moreover, early deployment could interfere with the acquisition of long-term data. In countries where the approved vaccine is deployed and the original trial is continuing, investigators should inform study participants about the approved vaccine's status because this information could affect their willingness to continue participating in the trial (4). Reconsent will be necessary (5), and investigators should tell those who are unwilling to reconsent whether they received vaccine or placebo so that those who received placebo can seek vaccine outside the trial (3). If enough study participants decline to reconsent, the trial might have to be terminated early. If the trial is terminated too early, investigators may not have enough long-term data to identify late-term safety issues, determine how long vaccine efficacy lasts, determine whether waning immunity is associated with reduced levels (or titers) of antibodies that neutralize SARS-CoV-2, and identify the level of neutralizing antibody that correlates with immunity—something that is uncertain to be achieved. Long-term safety considerations are especially important for vaccines that use mRNA technology because their characteristics are less well known.
What is less obvious is that early licensing of any single vaccine might complicate the evaluation of remaining vaccines. Once a vaccine is licensed, new placebo-controlled RCTs of other vaccines will not be acceptable ethically, and noninferiority RCTs will be the most likely alternative (6). The goal of noninferiority trials will be to demonstrate that the immune response (that is, neutralizing antibody titers or levels) of the candidate vaccine is not inferior to that of the approved vaccine within a prespecified margin, which the FDA has established as less than 10% for COVID-19 vaccines (3). Noninferiority trials of vaccines are not new. For example, one systematic review reported that the noninferiority margin was 10% in 74% of such trials and lower than 10% in 22% of them (6).
Another research design that could replace the placebo-controlled RCT is the controlled human challenge trial. In this type of trial, a relatively small number of volunteers are vaccinated with the candidate vaccine and are subsequently challenged with SARS-CoV-2 (7). Whether this trial design will be acceptable to regulatory agencies is unclear, but these trials clearly would be too small to provide reliable safety data.
Another issue is how the existence of an approved vaccine will affect recruitment for clinical trials of the remaining vaccine candidates, no matter what trial design is used. For example, in countries where the approved vaccine is deployed, and especially in countries where it is given without charge to the recipient, it is uncertain how many people would volunteer for a trial of a different vaccine that has not yet shown to protect them from the virus.
To understand how to optimally deploy the different vaccines that we expect will be available, we must know their different characteristics and especially their long-term effects (8). However, early approval and deployment of some vaccines before we know their long-term effects seem inevitable. For example, a recent prediction is that everyone in the United States who wants the vaccine likely could be vaccinated by April 2021 (9). That would be a great achievement. But it would also intensify our concerns about the ethical issues surrounding early vaccine approval and deployment. This possibility makes it even more important for us to plan now for dealing with those issues. A recent poll found that 42% of Americans are unwilling or are unsure they want to be vaccinated (10). Perhaps more people will agree to be vaccinated if we continue to develop vaccines against COVID-19 as well as we have started that effort.
Footnotes
This article was published at Annals.org on 20 November 2020
References
- 1. Krause P , Fleming TR , Longini I , et al; World Health Organization Solidarity Vaccines Trial Expert Group. COVID-19 vaccine trials should seek worthwhile efficacy. Lancet. 2020;396:741-743. [PMID: ] doi: 10.1016/S0140-6736(20)31821-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. U.S. Food and Drug Administration. Emergency use authorization for vaccines to prevent COVID-19. Guidance for industry. October 2020. Accessed at www.fda.gov/media/142749/download on 16 November 2020.
- 3. U.S. Food and Drug Administration. Development and licensure of vaccines to prevent COVID-19. Guidance for industry. June 2020. Accessed at www.fda.gov/media/139638/download on 16 November 2020.
- 4. U.S. Department of Health and Human Services. Protection of Human Subjects. 45 CFR §46.116. Accessed at www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=83cd09e1c0f5c6937cd9d7513160fc3f&pitd=20180719&n=pt45.1.46&r=PART&ty=HTML#se45.1.46_1116 on 16 November 2020.
- 5. Dal-Ré R , Avendaño C , Gil-Aguado A , et al. When should re-consent of subjects participating in a clinical trial be requested? A case-oriented algorithm to assist in the decision-making process. Clin Pharmacol Ther. 2008;83:788-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 6. Donken R , de Melker HE , Rots NY , et al. Comparing vaccines: a systematic review of the use of the non-inferiority margin in vaccine trials. Vaccine. 2015;33:1426-32. [PMID: ] doi: 10.1016/j.vaccine.2015.01.072 [DOI] [PubMed] [Google Scholar]
- 7. Schaefer GO , Tam CC , Savulescu J , et al. COVID-19 vaccine development: Time to consider SARS-CoV-2 challenge studies. Vaccine. 2020;38:5085-5088. [PMID: ] doi: 10.1016/j.vaccine.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Callaghan KP , Blatz AM , Offit PA . Developing a SARS-CoV-2 vaccine at warp speed. JAMA. 2020;324:437-438. [PMID: ] doi: 10.1001/jama.2020.12190 [DOI] [PubMed] [Google Scholar]
- 9. Saplakoglu Y. Coronavirus vaccine could be ready for all Americans by April, Fauci says. LiveScience. 11 November 2020. Accessed at www.livescience.com/fauci-coronavirus-vaccine-available-americans-april.html on 16 November 2020.
- 10. Fisher KA , Bloomstone SJ , Walder J , et al. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann Intern Med. 4 September 2020. [Epub ahead of print].. doi: 10.7326/M20-3569 [DOI] [PMC free article] [PubMed] [Google Scholar]


