Abstract
The water‐permeable skin of amphibians renders them highly sensitive to climatic conditions, and interspecific correlations between environmental moisture levels and rates of water exchange across the skin suggest that natural selection adapts hydroregulatory mechanisms to local challenges. How quickly can such mechanisms shift when a species encounters novel moisture regimes? Cutaneous resistance to water loss and gain in wild‐caught cane toads (Rhinella marina) from Brazil, USA (Hawai'i) and Australia exhibited strong geographic variation. Cutaneous resistance was low in native‐range (Brazilian) toads and in Hawai'ian populations (where toads were introduced in 1932) but significantly higher in toads from eastern Australia (where toads were introduced in 1935). Toads from recently invaded areas in western Australia exhibited cutaneous resistance to water loss similar to the native‐range populations, possibly because toads are restricted to moist sites within this highly arid landscape. Rates of rehydration exhibited significant but less extreme geographic variation, being higher in the native range than in invaded regions. Thus, in less than a century, cane toads invading areas that impose different climatic challenges have diverged in the capacity for hydroregulation.
Keywords: abiotic challenges, Anura, Bufo marinus, physiology, water balance
The invasion of cane toads from South America to Hawai'i and Australia has been accompanied by substantial shifts in the ability of the skin to restrict rates of water flow. Over a timeframe of less than a century, the toad invasion has generated major changes in a fundamental aspect of rates of abiotic exchange between the organism and its environment.
1. INTRODUCTION
The integument of most amphibians is highly permeable to water, restricting the activity of these animals to times and places where water is readily available or can be rapidly replenished (Buckley & Jetz, 2007; Lofts, 2012; Vitt & Caldwell, 2013; Wygoda, 1984). Although an amphibian's behavior, physiology, and morphology all affect the rate at which it exchanges water with the environment (Lofts, 2012; Vitt & Caldwell, 2013), the skin's permeability to water ultimately limits the rate at which water can be gained or lost (Hillyard et al., 1998; McClanahan & Baldwin, 1969; Spotila & Berman, 1976; Tracy, 1975). Some amphibians exhibit “waterproofing” adaptations (such as the skin secretions of Litoria caerulea and Polypedates maculatus, and cocoons of Cyclorana australis) that allow them to survive in arid conditions (Christian & Parry, 1997; Lillywhite et al., 1997), but most amphibians instead exhibit high rates of water flux between the body and the external environment (Lofts, 2012; Spotila & Berman, 1976).
Interspecific correlations between skin permeability and environmental aridity suggest that a species' geographic distribution is constrained by its ability to maintain positive water balance (greater influx of water than efflux) under the conditions it encounters (Seebacher & Franklin, 2011; Tingley et al., 2012). If so, we would predict a biological invasion from one hydric regime to another to impose selection on rates of desiccation and rehydration, and hence on skin permeability. The cane toad (Rhinella marina, formerly Bufo marinus; see Figure 1) provides an ideal model system with which to test that prediction. Native to relatively aseasonal, well‐watered landscapes of South America, the species was translocated to much drier habitats on the Hawai'ian islands (Easteal, 1981; Ward‐Fear et al., 2016) and then Australia (Easteal et al., 1985; Freeland & Martin, 1985). Previous research has documented shifts in the species' morphology, physiology, and behavior coincident with translocation (e.g., Gruber et al., 2017; Hudson et al., 2016, 2017; McCann et al., 2014). We therefore quantified rates of evaporative water loss and gain in cane toads from populations within their native range (Brazil), their translocated range in Hawai'i (USA), and their current range in Australia to explore if skin permeability to water also has changed during translocation and range expansion. Specifically, we expected that colonization of seasonally arid environments in Australia would favor a shift toward higher cutaneous resistance to water flow.
FIGURE 1.

Adult cane toad, Rhinella marina, in arid habitat near Longreach, Queensland. Photograph by R. Shine
2. MATERIALS AND METHODS
2.1. Study species
Cane toads are large “true toads” (family Bufonidae) native to an extensive area of South America (Bessa‐Silva et al., 2020; Zug & Zug, 1979), occurring in a variety of habitats including grassland, woodland, sand dunes, rainforest, mangroves, and anthropogenically disturbed areas. Whereas the dorsal skin is tough and leather‐like, the smoother ventral skin (especially the highly permeable pelvic patch) is responsible for most of the organism's water uptake (Parsons et al., 1993). The species' Latin name Rhinella marina, and one of its common names—the marine toad—come from its putative resistance to higher levels of salinity than are tolerated by most other anurans (Liggins & Grigg, 1985; Uchiyama & Konno, 2006; Wijethunga et al., 2016).
These terrestrial anurans were imported from French Guiana to Puerto Rico to control insect pests in sugar‐cane plantations; from there, 150 toads were translocated to Hawai'i in 1932, and 101 descendants of the Hawai'ian toads were introduced to northeastern Australia in 1935 (Freeland & Martin, 1985; Lever, 2001; Shine, 2018). Both in Hawai'i and Australia, the toads now occupy xeric as well as mesic habitats. In Hawai'i, the toads were released in plantations on leeward as well as windward coasts, but are currently restricted to anthropogenically moistened areas (such as golf courses) in the drier regions (Ward‐Fear et al., 2016). In Australia, toads were released in mesic coastal Queensland (QLD), but have since spread into seasonally arid habitats of Western Australia (WA) and the Northern Territory (NT), as well as colder montane habitats in New South Wales (NSW: for details see Feit et al., 2015; McCann et al., 2014; Newell, 2011; Shine, 2010; Tingley et al., 2012). Cane toads thus occupy a wider range of climatic conditions in Australia than in their native range (Tingley et al., 2014).
2.2. Sampling locations
We collected adult toads (both males and females, ranging from 43 to 313 g; mean = 109.9 g ± 33.5 g SD) from locations in their native range (Brazil), in Hawai'i (USA), and in Australia (see Table 1). All toads were collected by hand at night, placed in damp cloth bags, and kept in a moist, cool environment. Following capture, toads were transported to local laboratory facilities for the experiments.
Table 1.
Collection sites of cane toads used for the current study, with data on year of cane toad introduction and annual rainfall
| Country | State | Location | Year of introduction | Mean annual rainfall (monthly range) | Mean temperature (monthly range) |
|---|---|---|---|---|---|
| Brazil | PA | Alter do Chão | Native range | 1,991 mm (34–346) | 25.9°C (25.1–26.9°C) |
| AM | Manaus | Native range | 2,145 mm (56–295) | 27.4°C (26.9–28.2°C) | |
| USA | HI | Hilo | 1932 | 3,459 mm (177–397) | 23.1°C (21.7–24.6°C) |
| Kailua‐Kona | 1932 | 862 mm (55–88) | 23.5°C (22.0–24.9°C) | ||
| Australia | WA | Kununurra | 2011 | 720 mm (0–186) | 28.8°C (23.3–32.6°C) |
| Oombulgurri | 2013 | 718 mm (0–181) | 29.4°C (24.3–32.9°C) | ||
| NT | Leaning Tree Lagoon | 2006 | 1,500 mm (1–364) | 27.2°C (23.9–29.4°C) | |
| Katherine | 2010 | 1,009 mm (0–250) | 27.5°C (22.1–31.6°C) | ||
| QLD | Charters Towers | 1953 | 692 mm (8–142) | 23.2°C (17.3–27.4°C) | |
| Townsville | 1935 | 1,111 mm (9–275) | 24.1°C (19.0–27.6°C) | ||
| NSW | Brooms Head | 2005 | 1,471 mm (49–188) | 19.2°C (13.8–23.6°C) | |
| Tabbimoble | 2010 | 1,558 mm (52–193) | 19.4°C (14.0–23.6°C) |
PA = Alter do Chão, Pará, AM = Manaus, Amazonas, HI = Hawai'i, WA = Western Australia, NT = Northern Territory, QLD = Queensland, NSW = New South Wales. Dates of toad introduction from Lever (2001) and Urban et al. (2007). Climatic data (from climate‐data.org) show mean annual values, and range of mean monthly values for each site.
Toads from the native range were collected in Manaus, Amazonas (AM) and Alter do Chão, Pará (PA) in Brazil during January and February 2015, a warm and wet time of year (Table 1). We collected toads on the island of Hawai'i (HI, United States of America) during June and July 2015, from sites in the extreme east (Hilo) and extreme west (Kailua‐Kona) of the island. The windward eastern side of the island is humid and warm (Hawai'i wet; HW), whereas the leeward western side is much drier (Hawai'i dry; HD) due to a rain shadow effect coupled with highly porous volcanic soils (Ward‐Fear et al., 2016) (Table 1, Figure 2). However, the toads inhabit moist areas in both of these regions because of anthropogenically provided water (Ward‐Fear et al., 2016). In Australia, we collected toads from eight sites. Two sites were in Western Australia (Oombulgurri sampled in November 2014, Kununurra in October 2015), in the extreme west of the species' range close to the invasion front (<2 years post‐colonization) where the climate is hot (annual average close to 30°C) year‐round, and seasonally arid. Another two sites were in the Northern Territory (Katherine, Leaning Tree Lagoon, both in August 2015), where the climate is less harsh (longer wet season) than the Western Australian sites. One Queensland site (Townsville in September 2015) experiences cooler but seasonally arid conditions, whereas the other (Charters Towers, also in September 2015) is drier for much of the year and very hot in summer. Lastly, the two sites in New South Wales (Brooms Head and Tabbimoble, both in October 2015) are close to the current southeastern invasion front, where the climate is cooler and generally moist (see Table 1, Figure 2 and Kosmala et al., 2017 for details of site locations, invasion history, climatic conditions, and sample sizes). Climate data were sourced from Climate‐Data.org.
FIGURE 2.
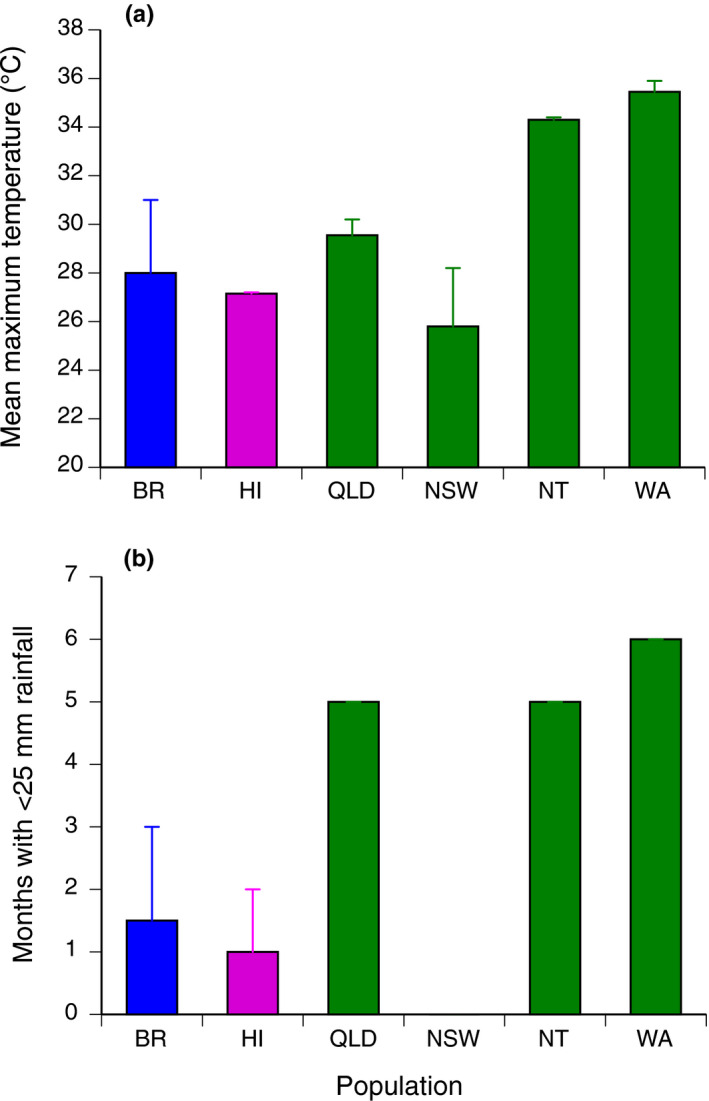
Climatic conditions of the collection sites. (a) Mean maximum temperature, and (b) number of months with less than 25 mm of rainfall (locations with higher bars have more months of very low rainfall, showing dry conditions). BR = Brazil, HI = Hawai'i, QLD = Queensland, NSW = New South Wales, NT = Northern Territory, WA = Western Australia
2.3. Husbandry
After capture, we allowed the toads to acclimate in laboratory conditions for 2 weeks, fed them crickets and mealworms, and provided ad libitum access to water and shelter. The room was set to 25°C, with a 12:12 hr light cycle (for details of methods see Kosmala et al., 2017). All animals were housed and tested in their respective countries of collection, in near‐identical conditions. Prior to measurements, we emptied the toads' bladders by gently applying pressure to the abdomen until urine was released.
2.4. Rates of evaporative water loss and cutaneous resistance
We used a closed system with a positive airflow, with silica gel cylinders at the intake and humidity probes at the outflow of the system (a similar system is described in detail in Young et al., 2005). Dry airflow (<1% relative humidity) was adjusted to 1 L/min, and we recorded baseline humidity values for the system for at least 20 min (after humidity values stabilized) prior to the toads being introduced to the system (average relative humidity 0.048% ± 0.064). Animals were held within an airtight cylindrical container of 1‐L capacity. After a toad was placed into the container, we waited for it to adopt a water‐conserving posture (WCP; with limbs folded beneath the body: see Withers et al., 1984), then recorded temperature and humidity of air that had passed across the toad's body for at least 20 min (as long as the toad remained in WCP). We then removed the animal from the container, and we continued to record temperature and humidity within the airflow chamber for another 20 min to ensure that baseline values had not changed. After it was removed from the airflow system, the toad was weighed and placed in a water tub in its home cage to rehydrate. We also measured rates of evaporative water loss of agar models (3% concentration) to measure the rate of evaporation from a free water surface (i.e., without cutaneous resistance), so that we could calculate individual cutaneous resistance to water loss (method validated by Spotila & Berman, 1976 and Christian et al., 2017). The agar models were made using molds taken from cane toads in WCP and were placed in the chambers such that the ventral portion was hidden (as is the case for a toad in WCP). Molds were made from 21 cane toads collected from the Darwin region, ranging in size from 9.5 to 365 g, and the water loss from the corresponding agar models was used to calculate total resistance, which corresponds to the boundary layer resistance of a toad of the same size. A regression equation was calculated for the relationship between toad mass and the total resistance of the agar models (resistance = 0.7222 + 0.0104 X mass; F 1,19 = 46.8, p < .001; r 2 = 71.1%), and this relationship was used to calculate the boundary layer resistance for each toad. The variability around the regression line was due, in part, to variability in the relationship between mass and surface area, resulting from differences in body condition. The mean boundary layer resistance was 1.9 s/cm (SD = 0.35), and the range was 1.4 (from the smallest toad) to 4.0 s/cm (from the largest toad).
2.5. Rates of rehydration
Following our measures of water flow, these animals were then used in studies to assess effects of desiccation on locomotor performance (Kosmala et al., 2017), which included experimental dehydration in dry air. Prior to all experiments, toads were kept in water for 2 hr to ensure full hydration. The toads were then weighed (after emptying the bladder: see above), placed in desiccating conditions, and reweighed frequently until they reached the desired reduction in mass. Once it reached 70% of its initial body mass, the toad was weighed, placed in a container with ~0.5 mm depth of water, and reweighed every 2 min (after pat‐drying with paper towel) for 14 min to measure rates of rehydration (McClanahan & Baldwin, 1969).
2.6. Statistical analyses
Sites were grouped to form the populations as follows: BR = Alter do Chão and Manaus (Brazil), HI = Hilo and Kailua‐Kona (Hawai'i), WA = Kununurra and Oombulgurri (Western Australia), NT = Katherine and Leaning Tree Lagoon (Northern Territory), QLD = Charters Towers and Townsville (Queensland), and NSW = Brooms Head and Tabbimoble (New South Wales). In addition to examining effects of location, we tested if sex or body mass had an influence on either cutaneous resistance or rehydration. If either sex or body mass had an effect, we used ANCOVA with the additional parameter in question as a covariate; otherwise we used ANOVA.
Cutaneous resistance was not related to toad body mass (r 2 = 0.05, p = .19), and we therefore performed a simple ANOVA with location (country/state) as the factor. Rehydration rate was however significantly affected by a toad's mass (see below). Therefore, we used ANCOVA with mass as a covariate to assess differences in rehydration rate among locations (country/state).
Post hoc tests (Tukey's test) were performed to locate significant differences among groups indicated by ANOVA and ANCOVA. Values of rehydration rate and body mass were ln‐transformed prior to analyses to meet assumptions of normality, homoscedasticity, and homogeneity of variance. Data on cutaneous resistance, rehydration rate, and body mass were not strongly co‐linear (all Pearson's r < 0.31), and residuals from analyses did not violate assumptions of homoscedasticity. Analyses were performed using JMP 11 (SAS Institute, Cary, NC), using an alpha level of p < .05.
3. RESULTS
Preliminary analyses using site of collection as a factor in ANOVA revealed no significant differences in cutaneous resistance among sites within each broader location (country or state), and thus we combined sites within these broader locations for further analysis. Likewise, cutaneous resistance and rehydration rate did not differ between sexes (both F < 1.55, both p > .22) and thus sex was excluded from further analyses.
There was a weak but statistically significant negative correlation between cutaneous resistance and rehydration rate (Spearman r = −0.19, p = .03).
3.1. Geographic variation in cutaneous resistance
ANOVA with collecting location (country or state) as the factor showed significant geographic variation in cutaneous resistance (F 5,158 = 18.94, p < .001; Tukey post hoc tests identified two groups, with NSW and QLD similar to each other, and both significantly higher than the other four regions). Thus, cutaneous resistance to water loss was relatively low in cane toads from the native range and Hawai'i, high in relatively mesic areas of eastern Australia (NSW and QLD), but low in populations from seasonally arid sites within western Australia (NT and WA) (Table 2, Figure 3a).
Table 2.
Sample sizes, body masses, rates of water loss, skin resistance, and rehydration rates of cane toads used in experimental protocols of the current study (mean ± SD for applicable variables)
| Country | State | Location | Number of toads | Mass (g) | SVL (mm) | Collection date | Mean temperature a (°C) | Mean rainfall a (mm) | Rate of water loss (mg/min) | Skin resistance (s/cm) | Rehydration rate (g.mins−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brazil | PA | Alter do Chão | 17 | 110.2 ± 23.6 | 109.1 ± 7.3 | Feb 2015 | 26.1 | 7.7 | 7.2 ± 1.4 | 2.9 ± 2.1 | 0.6 ± 0.2 |
| AM | Manaus | 13 | 76.6 ± 24.2 | 97.6 ± 2.7 | Jan 2015 | 25.6 | 3.8 | 5.9 ± 0.8 | 2.8 ± 2.1 | 0.5 ± 0.2 | |
| USA | HI | Hilo | 11 | 104.1 ± 13.7 | 109.1 ± 4.6 | Jun 2015 | 25.2 | 1.4 | 10.0 ± 1.5 | 3.3 ± 1.0 | 0.4 ± 0.2 |
| Kailua‐Kona | 15 | 125.8 ± 31.4 | 110.4 ± 8.2 | Jun 2015 | 26.8 | 2.0 | 10.4 ± 1.5 | 4.7 ± 2.5 | 0.3 ± 0.1 | ||
| Australia | WA | Kununurra | 17 | 90.6 ± 21.8 | 105.3 ± 5.9 | Oct 2015 | 30.1 | 0.4 | 10.2 ± 2.0 | 2.8 ± 1.0 | 0.4 ± 0.1 |
| Oombulgurri | 6 | 104.8 ± 13.1 | 122.1 ± 5.6 | Nov 2014 | 32.9 | 3.9 | 10.9 ± 1.4 | 2.4 ± 2.1 | 0.5 ± 0.1 | ||
| NT | Leaning Tree Lagoon | 10 | 143.7 ± 24.0 | 118.0 ± 4.1 | Aug 2015 | 23.6 | 0.0 | 10.7 ± 1.4 | 4.5 ± 1.6 | 0.5 ± 0.2 | |
| Katherine | 16 | 130.9 ± 58.4 | 112.3 ± 11.8 | Aug 2015 | 23.3 | 0.0 | 9.9 ± 2.0 | 3.9 ± 2.1 | 0.4 ± 0.1 | ||
| QLD | Charters Towers | 20 | 114.1 ± 31.1 | 110.1 ± 19.2 | Sept 2015 | 25.9 | 0.1 | 8.8 ± 1.7 | 6.3 ± 2.1 | 0.4 ± 0.1 | |
| Townsville | 17 | 92.3 ± 16.4 | 103.8 ± 6.7 | Sept 2015 | 23.1 | 0.0 | 8.1 ± 1.6 | 6.4 ± 1.7 | 0.4 ± 0.1 | ||
| NSW | Brooms Head | 10 | 120.1 ± 23.4 | 108.6 ± 8.4 | Oct 2015 | 20.5 | 0.7 | 9.7 ± 2.0 | 6.5 ± 2.6 | 0.4 ± 0.1 | |
| Tabbimoble | 12 | 113.1 ± 29.3 | 106.2 ± 6.5 | Oct 2015 | 20.0 | 1.2 | 8.9 ± 1.6 | 6.3 ± 1.7 | 0.5 ± 0.1 |
PA = Alter do Chão, Pará, AM = Manaus, Amazonas, HI = Hawai'i, WA = Western Australia, NT = Northern Territory, QLD = Queensland, NSW = New South Wales.
During month of collection.
FIGURE 3.
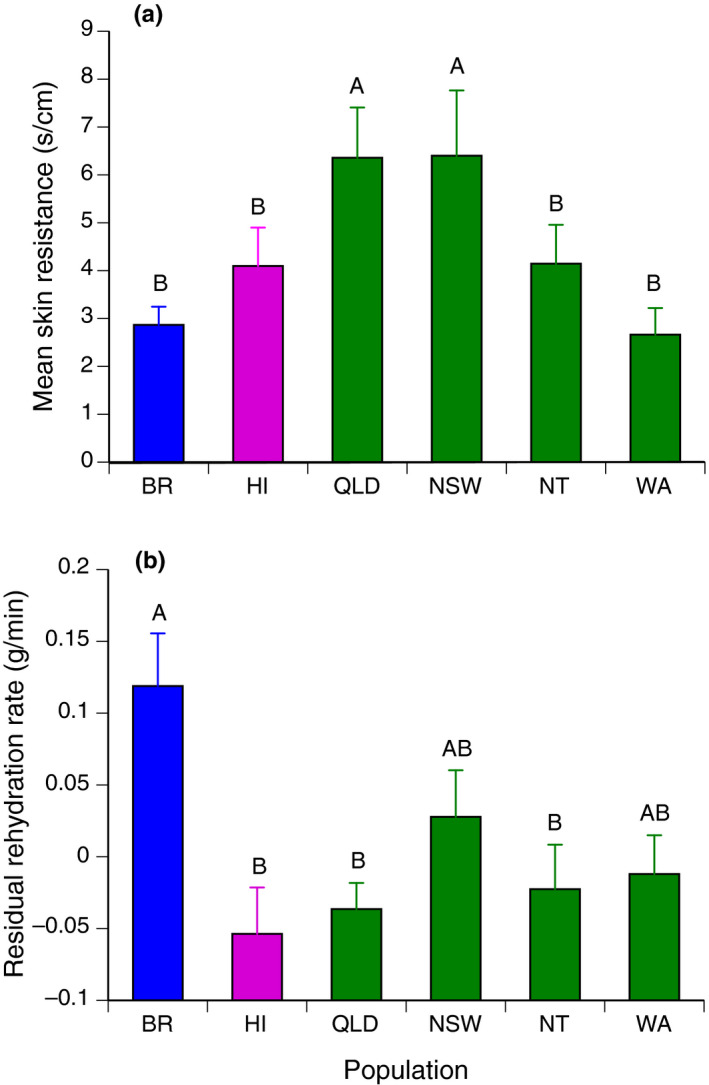
Values of (a) cutaneous skin resistance (s/cm) (±SE), and (b) residual rehydration rates (g/min) (±SE) of cane toads collected in native and invasive populations. BR = Brazil, HI = Hawai'i, QLD = Queensland, NSW = New South Wales, NT = Northern Territory, WA = Western Australia. Letters indicate groups obtained by post hoc (Tukey) test; different letters indicate significant differences. Residual rehydration rate values are adjusted for toad body mass
There was a significant negative correlation between average cutaneous resistance at each site and the mean temperature recorded at the site (Spearman r = −0.78, p = .003), but no significant correlation between average cutaneous resistance and average rainfall (Spearman r = −0.05, p = .88).
3.2. Geographic variation in rates of rehydration
ANCOVA indicated that rehydration rates were positively related to body mass (F 1,124 = 17.65, p < .0001) and also significantly related to population of origin (F 5,124 = 3.70, p < .004). The interaction between mass and location was not significant (F 5,124 = 0.37, p = .87). An ANOVA on the residuals of the regression of rehydration rates on body mass indicated that rehydration rates for Brazilian toads were higher than for all populations in either of the other two countries (Figure 3b).
There were no significant correlations between average rehydration rate at each site and the mean temperature or rainfall (both Spearman r < 0.42, p > .18).
4. DISCUSSION
The rate at which an anuran loses water from its body is a critical aspect of its biology, restricting the times and places where it can be active (Hillyard et al., 1998; McCann et al., 2014; Seebacher & Franklin, 2011; Toledo & Jared, 1993; Young et al., 2005). We found differences in aspects of hydric biology across populations of cane toads. Toads from two regions in eastern Australia (QLD and NSW) exhibited cutaneous resistances significantly higher than seen in the native range of the species (Brazil), or in the “stepping stone” populations in Hawai'i (USA) from which the eastern‐Australian populations were founded in 1935. Surprisingly, however, northwestern‐Australian populations (the most recently invaded sites: NT and WA) had cutaneous resistance levels similar to those of conspecifics in the native range. Our results for Northern Territory toads were slightly higher than those of Young et al. (2005) (cutaneous resistance = 1.7 ± 0.7 s/cm).
What mechanisms underlie these shifts in hydroregulatory abilities of cane toads across northern Australia? First, the differences in cutaneous resistance might be the result of evolutionary changes that have occurred as the toads have adapted to the novel environments they have encountered during the westwards invasion of northern Australia. Another possibility is that, rather than genetic differences, the differences among populations are the result of developmentally plastic responses to local environments. A third possibility is that toads from eastern Australia may have exhibited an acute (temporary) physiological response that affected their cutaneous resistance during the period they were measured. Acute changes in water flux have been documented in toads in response to external agents (Dohm et al., 2001) and to pharmacological agents (biochemical blockers and stimulants) that affect cutaneous blood flow (Burggren & Vitalis, 2005; Hillyard et al., 1998). Acute changes to resistance can occur in frogs in response to environmental temperature (Buttemer & Thomas, 2003; Tracy et al., 2006). Because we relied on measurements from field‐caught animals, we cannot tease apart the degree to which geographic divergence was driven by evolved (heritable) changes versus developmentally plastic or acute responses to local environments. To distinguish among these possibilities, additional field sampling and studies on offspring raised under standardized conditions would be needed.
If the changes in resistance are an evolutionary response, then it could have occurred by one of two patterns: either the change to high cutaneous resistance occurred in Queensland but the trend reversed as the toads moved west over 80 years, or, alternatively, the westbound colonists retained the ancestral state (of low cutaneous resistance) while the toads from eastern Australia evolved high cutaneous resistance later, after the westwards spread of toads had already commenced. Two patterns suggest that the former scenario is more plausible. First, the toad invasion front expanded very slowly in the decades immediately post‐translocation (Freeland & Martin, 1985; Kearney et al., 2008; Phillips et al., 2007; Urban et al., 2007), consistent with the time needed for adaptation of traits (such as cutaneous resistance) to deal with novel climatic challenges (e.g., Aikio et al., 2010; Kowarik, 1995). Second, if high cutaneous resistance did not evolve in Queensland toads until after the westwards invasion had begun, we would have expected low cutaneous resistance in toads from the southern (New South Wales) front due to retention of the ancestral condition (the western and southern fronts expanded at about the same time: Urban et al., 2007). Instead, toads from southern (New South Wales) populations had high cutaneous resistance, like Queensland conspecifics.
In contrast to cutaneous resistance, rates of rehydration showed a simpler pattern. The rates at which toads regain water have decreased over the course of the toad invasion, with native‐range (Brazilian) animals taking up water more rapidly than did those from either Hawai'i or Australia. Thus, mesic conditions within the native range appear to have favored a system of both gaining and losing water rapidly. In contrast, more arid conditions in Hawai'i and Australia have resulted in toads exhibiting greater resistance to water loss in some invaded sites but not others; and a lower rate of rehydration relative to Brazilian toads.
The rate of hydration also depended on body mass, reflecting allometric effects. Relative to internal volume, larger toads have a relatively smaller surface area (across which they gain water) than do smaller conspecifics (McClanahan & Baldwin, 1969; Tracy, 1975; Withers et al., 1984).
Interspecific comparisons suggest that species of anurans exposed to desiccating conditions (e.g., arid or arboreal habitats) tend to exhibit higher cutaneous resistance to water loss (Spotila & Berman, 1976; Tingley et al., 2012; Titon & Gomes, 2017; Wygoda, 1984; Young et al., 2005). This pattern is consistent with the ancestral condition of low cutaneous resistance to evaporative water loss seen in toads from Brazil and the wetter site in Hawai'i. Although the low resistance to water loss of toads from dry sites in Hawai'i (HD) does not fit well with that trend, toads in these sites actually live in anthropogenically moistened habitats where water is freely available (Ward‐Fear et al., 2016). Similarly, although many sites within the range of toads in the Northern Territory and Western Australia are semi‐arid, the toads survive the dry season by inhabiting areas with reliable moisture (Feit et al., 2015; Tingley & Shine, 2011; Tracy et al., 2014). Counter‐intuitively, then, toads in very arid landscapes may live in microhabitats where water is freely available (Tingley & Shine, 2011).
When toads were translocated from Hawai'i to Queensland in 1935, they encountered a climate where precipitation is hotter and more seasonal than in the native range (Table 1; Tingley et al., 2014). However, the toads' subsequent invasion into seasonally arid habitats in the western part of Australia was not accompanied by an increase in cutaneous resistance. In addition to inhabiting wetter sites within this arid location (Feit et al., 2015; Tingley & Shine, 2011; Tracy et al., 2014), a high rate of evaporative water loss may benefit toads in this region because of the extremely high ambient temperatures experienced through most of the year (Figure 2; and see Kosmala et al., 2017 and McCann et al., 2018 for climatic data). Among the 12 study sites, skin resistance was negatively correlated with average temperature. Thus, evaporative cooling (Borgnakke & Sonntag, 2016; Buttemer & Thomas, 2003; Tracy et al., 2006) might enable toads to deal with otherwise‐lethal heat loads (Kosmala et al., 2020). Analogously, Gila monsters increase cutaneous and cloacal evaporative water loss in hot weather (DeNardo et al., 2004). A feedback between body temperature and rates of evaporative water loss (cooler cane toads lose water less rapidly: Malvin & Wood, 1991) might reduce the hydric costs of evaporative cooling.
Our results add two additional variables—rates of water gain and cutaneous resistance to water loss—to the list of functional traits that exhibit geographic variation within cane toads, including across different sites within their invaded range within Australia (e.g., Hudson et al., 2016; Kosmala et al., 2017; McCann et al., 2018). These data demonstrate that anurans can adjust fundamental aspects of their interactions with environmental factors rapidly, if the taxon in question is exposed to abiotic challenges that differ strongly from those which it experiences in the native range.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Georgia Karoline Kosmala: Conceptualization (equal); investigation (equal); methodology (equal); writing–original draft (equal). Gregory P. Brown: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal). Richard Shine: Conceptualization (equal); formal analysis (equal); funding acquisition (equal); resources (equal); supervision (equal); writing–review and editing (equal). Keith Christian: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); supervision (equal).
ACKNOWLEDGEMENTS
We thank Dr. Igor Kaefer, Seu Deco, and the Shine Lab for assistance with collecting toads. We thank Dr. Fernando Ribeiro‐Gomes and Dr. Bill Mautz for logistic assistance. We thank Melanie Elphick for editorial assistance and figure preparation. Collection and transport of toads in Brazil were approved by permit # 7015‐1. Transport of toads in Hawai'i was approved by permit #EX‐15‐12. All procedures were approved by the University of Sydney Animal Ethics Committee (Approval #2014/703). Funding was provided by the Australian Research Council (FL120100074) and the Capes Foundation within the Ministry of Education, Brazil (grant #BEX /13734‐13‐0).
Kosmala GK, Brown GP, Shine R, Christian K. Skin resistance to water gain and loss has changed in cane toads (Rhinella marina) during their Australian invasion. Ecol Evol. 2020;10:13071–13079. 10.1002/ece3.6895
DATA AVAILABILITY STATEMENT
Data are available at Dryad https://doi.org/10.5061/dryad.ttdz08kwc.
REFERENCES
- Aikio, S. , Duncan, R. P. , & Hulme, P. E. (2010). Lag‐phases in alien plant invasions: Separating the facts from the artefacts. Oikos, 119(2), 370–378. 10.1111/j.1600-0706.2009.17963.x [DOI] [Google Scholar]
- Bessa‐Silva, A. , Vallinoto, M. , Sampaio, I. , Flores‐Villela, O. A. , Smith, E. N. , & Sequeira, F. (2020). The roles of vicariance and dispersal in the differentiation of two species of the Rhinella marina species complex. Molecular Phylogenetics and Evolution, 145, 106723 10.1016/j.ympev.2019.106723 [DOI] [PubMed] [Google Scholar]
- Borgnakke, C. , & Sonntag, R. E. (2016). Fundamentals of thermodynamics (9th ed.). Wiley Global Education. [Google Scholar]
- Buckley, L. B. , & Jetz, W. (2007). Environmental and historical constraints on global patterns of amphibian richness. Proceedings of the Royal Society B, 274(1614), 1167–1173. 10.1098/rspb.2006.0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren, W. W. , & Vitalis, T. Z. (2005). The interplay of cutaneous water loss, gas exchange and blood flow in the toad, Bufo woodhousei: Adaptations in a terrestrially adapted amphibian. Journal of Experimental Biology, 208, 105–112. 10.1242/jeb.01349 [DOI] [PubMed] [Google Scholar]
- Buttemer, W. A. , & Thomas, C. (2003). Influence of temperature on evaporative water loss and cutaneous resistance to water vapour diffusion in the orange‐thighed frog (Litoria xanthomera). Australian Journal of Zoology, 51, 111–118. 10.1071/ZO02057 [DOI] [Google Scholar]
- Christian, K. , & Parry, D. (1997). Reduced rates of water loss and chemical properties of skin secretions of the frogs Litoria caerulea and Cyclorana australis . Australian Journal of Zoology, 45, 13–20. 10.1071/ZO96046 [DOI] [Google Scholar]
- Climate‐data.org , (2016). Climate Data for Cities Worldwide. http://en.Climate‐data.org (accessed 28 March, 2016).
- DeNardo, D. F. , Zubal, T. E. , & Hoffman, T. C. (2004). Cloacal evaporative cooling: A previously undescribed means of increasing evaporative water loss at higher temperatures in a desert ectotherm, the Gila monster Heloderma suspectum . Journal of Experimental Biology, 207, 945–953. 10.1242/jeb.00861 [DOI] [PubMed] [Google Scholar]
- Dohm, M. R. , Mautz, W. J. , Looby, P. G. , Gellert, K. S. , & Andrade, J. A. (2001). Effects of ozone on evaporative water loss and thermoregulatory behavior of marine toads (Bufo marinus). Environmental Research, 86, 274–286. 10.1006/enrs.2001.4276 [DOI] [PubMed] [Google Scholar]
- Easteal, S. (1981). The history of introductions of Bufo marinus (Amphibia: Anura): A natural experiment in evolution. Biological Journal of the Linnean Society, 16, 93–113. 10.1111/j.1095-8312.1981.tb01645.x [DOI] [Google Scholar]
- Easteal, S. , van Beurden, E. K. , Floyd, R. B. , & Sabath, M. D. (1985). Continuing geographical spread of Bufo marinus in Australia: Range expansion between 1974 and 1980. Journal of Herpetology, 19, 185–188. 10.2307/1564171 [DOI] [Google Scholar]
- Feit, B. , Dempster, T. , Gibb, H. , & Letnic, M. (2015). Invasive cane toads’ predatory impact on dung beetles is mediated by reservoir type at artificial water points. Ecosystems, 18, 826–838. 10.1007/s10021-015-9865-x [DOI] [Google Scholar]
- Freeland, W. J. , & Martin, K. C. (1985). The rate of range expansion by Bufo marinus in northern Australia, 1980–84. Wildlife Research, 12, 555–559. 10.1071/WR9850555 [DOI] [Google Scholar]
- Gruber, J. , Brown, G. , Whiting, M. J. , & Shine, R. (2017). Geographic divergence in dispersal‐related behaviour in cane toads from range‐front versus range‐core populations in Australia. Behavioral Ecology and Sociobiology, 71, 38 10.1007/s00265-017-2266-8 [DOI] [Google Scholar]
- Hillyard, S. D. , Hoff, K. V. S. , & Propper, C. (1998). The water absorption response: A behavioral assay for physiological processes in terrestrial amphibians. Physiological Zoology, 71, 127–138. 10.1086/515900 [DOI] [PubMed] [Google Scholar]
- Hudson, C. M. , Brown, G. P. , & Shine, R. (2017). Evolutionary shifts in anti‐predator responses of invasive cane toads (Rhinella marina). Behavioral Ecology and Sociobiology, 71, 134 10.1007/s00265-017-2367-4 [DOI] [Google Scholar]
- Hudson, C. M. , McCurry, M. R. , Lundgren, P. , McHenry, C. R. , & Shine, R. (2016). Constructing an invasion machine: The rapid evolution of a dispersal‐enhancing phenotype during the cane toad invasion of Australia. PLoS One, 11, e0156950 10.1371/journal.pone.0156950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney, M. , Phillips, B. L. , Tracy, C. R. , Christian, K. A. , Betts, G. , & Porter, W. P. (2008). Modelling species distributions without using species distributions: The cane toad in Australia under current and future climates. Ecography, 31, 423–434. 10.1111/j.0906-7590.2008.05457.x [DOI] [Google Scholar]
- Kosmala, G. , Brown, G. P. , Christian, K. , & Shine, R. (2017). Locomotor performance of cane toads differs between native‐range and invasive populations. Royal Society Open Science, 4, 170517 10.1098/rsos.170517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmala, G. , Brown, G. P. , & Shine, R. (2020). Colonisation history affects heating rates of invasive cane toads. Scientific Reports, 10, 12553 10.1038/s41598-020-69529-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowarik, I. (1995). Time lags in biological invasions with regard to the success and failure of alien species In Pysek P., Prach K., Rejmanek M., & Wade M. (Eds.), Plant invasions: General aspects and special problems (pp. 15–38). SPB Academic Publishing. [Google Scholar]
- Lever, C. (2001). The cane toad. The history and ecology of a successful colonist. Westbury Academic and Scientific Publishing. [Google Scholar]
- Liggins, G. W. , & Grigg, G. C. (1985). Osmoregulation of the cane toad, Bufo marinus, in salt water. Comparative Biochemistry and Physiology A, 82(3), 613–619. 10.1016/0300-9629(85)90442-6 [DOI] [PubMed] [Google Scholar]
- Lillywhite, H. B. , Mittal, A. K. , Garg, T. K. , & Agrawal, N. (1997). Integumentary structure and its relationship to wiping behaviour in the common Indian tree frog, Polypedates maculatus . Journal of Zoology (London), 243, 675–687. 10.1111/j.1469-7998.1997.tb01969.x [DOI] [Google Scholar]
- Lofts, B. (2012). Physiology of the Amphibia (Vol. 3). Elsevier. [Google Scholar]
- Malvin, G. M. , & Wood, S. C. (1991). Behavioral thermoregulation of the toad, Bufo marinus: Effects of air humidity. Journal of Experimental Zoology Part A, 258, 322–326. 10.1002/jez.1402580307 [DOI] [PubMed] [Google Scholar]
- McCann, S. , Greenlees, M. J. , Newell, D. , & Shine, R. (2014). Rapid acclimation to cold allows the cane toad to invade montane areas within its Australian range. Functional Ecology, 28, 1166–1174. 10.1111/1365-2435.12255 [DOI] [Google Scholar]
- McCann, S. , Kosmala, G. , Greenlees, M. , & Shine, R. (2018). Physiological plasticity in a successful invader: Rapid acclimation to cold occurs only in cool‐climate populations of cane toads (Rhinella marina). Conservation Physiology, 6, cox072 10.1093/conphys/cox072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClanahan, L. , & Baldwin, R. (1969). Rate of water uptake through the integument of the desert toad, Bufo punctatus . Comparative Biochemistry and Physiology, 28, 381–389. 10.1016/0010-406X(69)91351-6 [DOI] [PubMed] [Google Scholar]
- Newell, D. (2011). Recent invasions of World Heritage rainforests in north‐east New South Wales by the cane toad Bufo marinus . Australian Zoologist, 35, 876–883. 10.7882/AZ.2011.041 [DOI] [Google Scholar]
- Parsons, R. H. , McDevitt, V. , Aggerwal, V. , Le Blang, T. , Manley, K. , Kim, N. , Lopez, J. , & Kenedy, A. A. (1993). Regulation of pelvic patch water flow in Bufo marinus: Role of bladder volume and ANG II. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 264(6), R1260–R1265. 10.1152/ajpregu.1993.264.6.R1260 [DOI] [PubMed] [Google Scholar]
- Phillips, B. L. , Brown, G. P. , Greenlees, M. , Webb, J. K. , & Shine, R. (2007). Rapid expansion of the cane toad (Bufo marinus) invasion front in tropical Australia. Austral Ecology, 32, 169–176. 10.1111/j.1442-9993.2007.01664.x [DOI] [Google Scholar]
- Riddell, E. A. , Apanovitch, E. K. , Odom, J. P. , & Sears, M. W. (2017). Physical calculations of resistance to water loss improve predictions of species range models: Comment. Ecology, 98, 2962–2964. 10.1002/ecm.1240 [DOI] [PubMed] [Google Scholar]
- Seebacher, F. , & Franklin, C. E. (2011). Physiology of invasion: Cane toads are constrained by thermal effects on physiological mechanisms that support locomotor performance. Journal of Experimental Biology, 214, 1437–1444. 10.1242/jeb.053124 [DOI] [PubMed] [Google Scholar]
- Shine, R. (2010). The ecological impact of invasive cane toads (Bufo marinus) in Australia. Quarterly Review of Biology, 85, 253–291. 10.1086/655116 [DOI] [PubMed] [Google Scholar]
- Shine, R. (2018). Cane toad wars. University of California Press. [Google Scholar]
- Spotila, J. R. , & Berman, E. N. (1976). Determination of skin resistance and the role of the skin in controlling water loss in amphibians and reptiles. Comparative Biochemistry and Physiology Part A, 55, 407–411. 10.1016/0300-9629(76)90069-4 [DOI] [PubMed] [Google Scholar]
- Tingley, R. , Greenlees, M. J. , & Shine, R. (2012). Hydric balance and locomotor performance of an anuran (Rhinella marina) invading the Australian arid zone. Oikos, 121, 1959–1965. 10.1111/j.1600-0706.2012.20422.x [DOI] [Google Scholar]
- Tingley, R. , & Shine, R. (2011). Desiccation risk drives the spatial ecology of an invasive anuran (Rhinella marina) in the Australian semi‐desert. PLoS One, 6, e25979 10.1371/journal.pone.0025979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley, R. , Vallinoto, M. , Sequeira, F. , & Kearney, M. R. (2014). Realized niche shift during a global biological invasion. Proceedings of the National Academy of Sciences USA, 111, 10233–10238. 10.1073/pnas.1405766111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titon, B. , & Gomes, F. R. (2017). Associations of water balance and thermal sensitivity of toads with macroclimatic characteristics of geographical distribution. Comparative Biochemistry and Physiology Part A, 208, 54–60. 10.1016/j.cbpa.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Toledo, R. C. , & Jared, C. (1993). Cutaneous adaptations to water balance in amphibians. Comparative Biochemistry and Physiology Part A, 105, 593–608. 10.1016/0300-9629(93)90259-7 [DOI] [Google Scholar]
- Tracy, C. R. (1975). Water and energy relations of terrestrial amphibians: Insights from mechanistic modeling In Gates D. M., & Schmerl R. M. (Eds.), Perspectives of biophysical ecology (pp. 325–346). Springer. [Google Scholar]
- Tracy, C. R. , Christian, K. A. , Betts, G. , & Tracy, C. R. (2006). Body temperature and resistance to evaporative water loss in tropical Australian frogs. Comparative Biochemistry and Physiology A, 150, 102–108. 10.1016/j.cbpa.2006.04.031 [DOI] [PubMed] [Google Scholar]
- Tracy, C. R. , Tixier, T. , Le Nöene, C. , & Christian, K. A. (2014). Field hydration state varies among tropical frog species with different habitat use. Physiological and Biochemical Zoology, 87, 197–202. 10.1086/674537 [DOI] [PubMed] [Google Scholar]
- Uchiyama, M. , & Konno, N. (2006). Hormonal regulation of ion and water transport in anuran amphibians. General and Comparative Endocrinology, 147(1), 54–61. 10.1016/j.ygcen.2005.12.018 [DOI] [PubMed] [Google Scholar]
- Urban, M. , Phillips, B. L. , Skelly, D. K. , & Shine, R. (2007). The cane toad's (Chaunus marinus) increasing ability to invade Australia is revealed by a dynamically updated range model. Proceedings of the Royal Society B, 274, 1413–1419. 10.1098/rspb.2007.0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitt, L. J. , & Caldwell, J. P. (2013). Herpetology: An introductory biology of amphibians and reptiles. Academic Press. [Google Scholar]
- Ward‐Fear, G. , Greenlees, M. J. , & Shine, R. (2016). Toads on lava: Spatial ecology and habitat use of invasive cane toads (Rhinella marina) in Hawai’i. PLoS One, 11, e0151700 10.1371/journal.pone.0151700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijethunga, U. , Greenlees, M. , & Shine, R. (2016). Living up to its name? The effect of salinity on development, growth and phenotype of the "marine" toad (Rhinella marina). Journal of Comparative Physiology B, 186, 205–213. 10.1007/s00360-015-0944-2 [DOI] [PubMed] [Google Scholar]
- Withers, P. C. , Hillman, S. S. , & Drewes, R. C. (1984). Evaporative water loss and skin lipids of anuran amphibians. Journal of Experimental Zoology Part A, 232, 11–17. 10.1002/jez.1402320103 [DOI] [Google Scholar]
- Wygoda, M. L. (1984). Low cutaneous evaporative water loss in arboreal frogs. Physiological Zoology, 57, 329–337. 10.1086/physzool.57.3.30163722 [DOI] [Google Scholar]
- Young, J. E. , Christian, K. A. , Donnellan, S. , Tracy, C. R. , & Parry, D. (2005). Comparative analysis of cutaneous evaporative water loss in frogs demonstrates correlation with ecological habits. Physiological and Biochemical Zoology, 78, 847–856. 10.1086/432152 [DOI] [PubMed] [Google Scholar]
- Zug, G. R. , & Zug, P. B. (1979). The marine toad, Bufo marinus: A natural history resumé of native populations. Smithsonian Contributions to Zoology, 284, 1–58. 10.5479/si.00810282.284 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at Dryad https://doi.org/10.5061/dryad.ttdz08kwc.


