Abstract
We examined the naming speed performance of 18 typically achieving and 16 dyslexic adults while simultaneously recording eye movements, articulations and fMRI data. Naming speed tasks, which require participants to name a list of letters or objects, have been proposed as a proxy for reading and are thought to recruit similar reading networks in the left hemisphere of the brain as more complex reading tasks. We employed letter and object naming speed tasks, with task manipulations to make the stimuli more or less phonologically and/or visually similar. Compared to typically achieving readers, readers with dyslexia had a poorer behavioural naming speed task performance, longer fixation durations, more regressions and increased activation in areas of the reading network in the left-hemisphere. Whereas increased network activation was positively associated with performance in dyslexics, it was negatively related to performance in typically achieving readers. Readers with dyslexia had greater bilateral activation and recruited additional regions involved with memory, namely the amygdala and hippocampus; in contrast, the typically achieving readers additionally activated the dorsolateral prefrontal cortex. Areas within the reading network were differentially activated by stimulus manipulations to the naming speed tasks. There was less efficient naming speed behavioural performance, longer fixation durations, more regressions and increased neural activity when letter stimuli were both phonologically and visually similar. Discussion focuses on the differences in activation within the reading network, how they are related to behavioural task differences, and how progress in furthering the understanding of the relationship between behavioural performance and brain activity can change the overall trajectories of children with reading difficulties by contributing to both early identification and remediation processes.
Keywords: fMRI, eye tracking, speech recording, reading network, dyslexia
fMRI, eye tracking and speech recording was combined to examine the mechanisms underlying reading. Compared to controls, readers with dyslexia had a poorer behavioural performance and increased activation in the reading network. Whereas increased network activation was positively associated with performance in dyslexics, it was negatively related to performance in typically achieving readers.
Graphical Abstract
Graphical Abstract.
Introduction
The neural basis of individual differences in reading ability is poorly understood due to the complexity of the components involved in reading (He et al., 2013). Even though most theories of reading include word reading as a key component (Kirby and Savage, 2008; Perfetti and Stafura, 2014), there is a significant complexity in the processes which underlie it. Therefore, to simplify the study of the neural processes that support reading, functional magnetic resonance imaging (fMRI) can be used to examine the brain regions involved in rapid serial naming, or naming speed (NS), in which participants are asked to name out loud sets of stimuli as quickly and as accurately as possible (Wiig et al., 2002; Misra et al., 2004; Breznitz, 2005; Gonzalez-Gerriod et al., 2011; Cummine et al., 2014; Al Dahhan et al., 2020).
NS is described as a ‘microcosm’ of the foundational processes that are necessary for fluent word reading (Wolf and Bowers, 1999), and the pattern of brain activation during these NS tasks coincides with regions of the left-hemisphere dominant neural reading network consisting of occipitotemporal, temporoparietal and inferior frontal areas (Misra et al., 2004; Price and Mechelli, 2005; Shaywitz and Shaywitz, 2008; Cummine et al., 2014; Norton et al., 2015; Al Dahhan et al., 2020). This reading network includes a dorsal stream, which links orthographic information to sublexical phonological representations, and a ventral stream, which recognizes whole words and their meanings (Pugh et al., 2001; Cohen et al., 2008; Price, 2012). The dorsal stream moves anteriorly from the visual cortex towards the parietal lobe and frontal regions and consists of the angular gyrus, superior temporal gyrus and supramarginal gyrus (SMG); the ventral stream transmits information ventro-laterally and anteriorly and encompasses the fusiform gyrus (FG), inferior occipitotemporal regions and the middle temporal gyrus (MTG) (Pugh et al., 2000; Borowsky et al., 2006). Greater activity is found within this network for alphanumeric stimuli than for non-alphanumeric stimuli; alphanumeric stimuli recruit areas in both the dorsal and ventral pathways and non-alphanumeric stimuli primarily recruit areas in the ventral pathway (Misra et al., 2004; Cummine et al., 2014; Al Dahhan et al., 2020).
As children develop reading skills, there is a gradual decrease in brain activity during reading in right hemisphere areas involved in visual memory and an increase in activity within the left-hemisphere reading network (Turkeltaub et al., 2003). Within this network, skilled readers have greater neural activity in the occipitotemporal regions, which is responsible for the fluent and automatic identification of visually presented words (Shaywitz et al., 2006; Norton and Wolf, 2012). For readers with dyslexia, the functioning of this posterior reading system is disrupted which may contribute to why they cannot recognize words automatically (Dehaene et al., 2005). Lower activation in the dorsal temporoparietal system compared to typical readers may indicate impairments in phonological processing, particularly in forming grapheme–phoneme associations, and the hypoactivation found in the ventral occipitotemporal system may indicate a secondary impairment in automatic visual word recognition (Richlan et al., 2011). It has been argued that increased reliance on the inferior frontal regions of the reading network and posterior regions of the right hemisphere compensates for this functional disruption (Price and Mechelli, 2005; Shaywitz and Shaywitz, 2008; Richlan et al., 2011; Norton et al., 2015).
Although the left-hemisphere reading network has been identified in previous research, and studies have shown differences in brain activation between alphanumeric and non-alphanumeric NS tasks for typically achieving readers (Misra et al., 2004; Cummine et al., 2014), including how this network is affected by different stimulus manipulations (Al Dahhan et al., 2020), it is unclear how the reading network differs in readers with dyslexia. To address these important knowledge gaps, and for an in-depth examination of these brain-behaviour group differences, we conducted an fMRI study with simultaneous recordings of eye movements and vocalizations in adult readers with and without dyslexia who were matched on education level. We used video-based eye tracking to measure eye movements, focusing on forward saccades, backwards saccades (regressions) and fixation durations (Rayner, 1997; Starr and Rayner, 2001; Olitsky and Nelson, 2003). Variability in these naming time components and eye movement measures is hypothesized to reflect variability in on-line processing (Rayner, 1997). We also separated vocalizations during NS tasks into articulation times of stimulus names and pause times between articulations, because pause times have been argued to represent the response preparation aspect of executive control (Clarke et al., 2005; Georgiou et al., 2006, 2009; Li et al., 2009; Kirby et al., 2010; Kirby and Savage, 2008).
Combining eye movements with vocalizations allows for a more in-depth examination of how performance is influenced by the coordination of visual and vocal processes, and how this differs between reading abilities. However, speech production during fMRI studies may result in task-related motion artefacts (Soltysik and Hyde, 2006). For this reason, fMRI studies have tended to use covert naming to study reading processes, which presents other concerns. For instance, it is not clear during covert naming that participants are performing the task according to instructions, how accurately they are performing, or whether they are performing the task at all. Al Dahhan et al. (2020) compared overt and covert naming using NS tasks with typically achieving readers and found no differences in behavioural performance, or in the recruitment of areas involved in sensorimotor activity or in the reading network. These findings indicate that speech production motion artefacts and sensorimotor processes were not responsible for differences found between the tasks within the reading network. Therefore, for this study, we examine task performance during overt naming of stimuli.
To investigate the neural processes involved during NS task performance, we first examined the sensorimotor regions involved in the processing and naming of task stimuli. These include oculomotor regions involved in eye-movement control, consisting of frontal, supplementary and parietal eye fields (frontal eye field, supplementary eye field, parietal eye field, respectively), the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex and the caudate nucleus (Connolly et al., 2002; 2005; Ford et al., 2005l Brown et al., 2007; Alahyane et al., 2014); speech regions, consisting of the inferior frontal gyrus (IFG), insula and primary motor cortex (Guenther et al., 2006); and visual areas recruited during reading, consisting of the cuneus, FG and lingual gyrus (Indefrey and Levelt, 2004; Price, 2012). Next, we examine differences in brain activity between alphanumeric and non-alphanumeric task conditions, the effects of stimulus manipulations on brain activity and the relationship between brain activity and behavioural performance. We predict that behavioural performance will be impaired during the naming of object stimuli compared to letter stimuli, and increasing the visual similarity of letters and the phonological similarity of the objects will greatly impair performance. Most importantly, we hypothesize that readers with dyslexia will have poorer behavioural performance on all NS tasks and this will be reflected in an increase in activity within the reading network.
Materials and methods
Participants
Two groups of undergraduate university students matched for education level took part in the study: 16 participants formally diagnosed with dyslexia by qualified professionals (co-authors R.G. and A.H.; mean age = 21.0 years, SD = 3.05; 10 females) recruited from Queen’s University’s Regional Assessment and Resource Centre; and 18 typically achieving readers with no self-reported history of reading disabilities (mean age = 24.1 years, SD = 1.89; 16 females; previously published in Al Dahhan et al., 2020) recruited from the Queen’s University community. Participants presented written informed consent prior to testing, reported English as their first language, were right-handed, as indicated by performance on the Modified Edinburgh Handedness Inventory (Oldfield, 1971), had normal/corrected to normal vision, and no history of head injury or neurological illness. The groups differed on age, F(1,33) = 11.67, P < 0.01, but not sex, χ2(1) = 2.10, P = 0.15.
Reading and cognitive measures
Reading and cognitive measures were previously described in Al Dahhan et al. (2020). A brief description of each measure is provided below; participants’ scores were the number of items correct.
Reading and decoding ability
Reading ability was examined using: Sentence Reading Fluency in which participants read short sentences and indicated whether they were true or false within 3 min; Word Identification, in which participants read up to 106 words with increased difficulty; and Passage Comprehension in which participants read up to 52 passages with incomplete sentences and included a missing word to complete each sentence (Schrank et al., 2014). Decoding ability was measured with performance on Word Attack (Woodcock, 1998) in which participants read up to 45 pseudowords increasing in difficulty.
Phonological awareness
Phonological processing was measured with three tasks from the Comprehensive Test of Phonological Processing (Wagner et al., 1999): Phoneme Elision required participants to separate phonological parts from a spoken word to create another word (34 items), Phoneme Isolation required participants to separate specific sounds in words (32 items) and Word Blending required participants to integrate specific sounds to create words (33 items).
Non-verbal ability
Non-verbal ability was measured using the Matrix Reasoning subtest of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Thirty-five incomplete visual patterns were shown to participants and they were asked to choose one of five possible pieces that would complete the pattern.
Naming Speed tasks
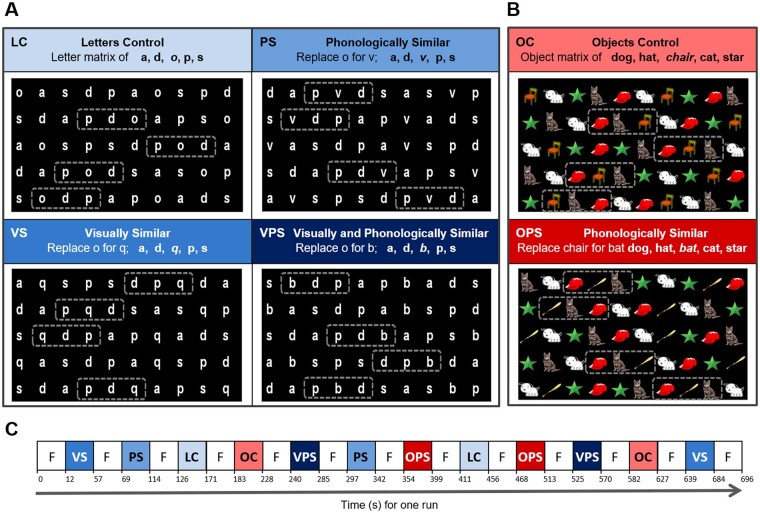
Four adaptations of a letter NS task and two adaptations of an object NS task, with two trials/variants were administered (Fig. 1; see Al Dahhan et al., 2020 for details). Briefly, for the letter NS tasks, the letter o in a control task [letters control (LC); Denckla and Rudel, 1976] with the letter matrix of a, s, o, p, d was substituted with another letter to make the matrix more Phonologically Similar (PS: o replaced with v), Visually Similar (VS: o replaced with q) or Visually and Phonologically Similar (VPS: o replaced with b; Compton, 2003). For the object task conditions, the Object Control task (OC; Denckla and Rudel, 1976), with the stimuli dog, hat, chair, star and cat, and for the Object Phonologically Similar condition (OPS) chair was switched with bat, to rhyme with cat and hat. There were 50 letters/objects for each task with ten repetitions of the five stimuli placed semi-randomly in five rows (Al Dahhan et al., 2020).
Figure 1.
Naming speed (NS) stimuli. (A) Letter NS tasks. Single letter manipulations to the letter o in the control (LC) task were made to make the letters more phonologically similar (PS), visually similar (VS), or visually and phonologically similar (VPS). (B) Object NS tasks. Single object manipulations to the object chair in the control (OC) task was made to make the objects more phonologically similar (OPS). (C) fMRI block paradigm during one run. The four versions of the letter NS task and two versions of the object NS task, with two trials/version were counterbalanced for order. Dashed boxes indicate regions in which the letters or objects became similar to one another. Figure reprinted from Al Dahhan et al. (2020) with permission from Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Recording of eye movements and speech
As previously described in Al Dahhan et al. (2020), the speech was recorded using an MRI compatible optical microphone and an ASIO compatible sound card at a sampling rate of 24 kHz (Optoacoustics Ltd., Israel). Both articulations and eye positions were recorded continuously and were integrated using Experiment Builder (SR Research Ltd.) (Al Dahhan et al., 2020).
Each task matrix was created using Experiment Builder and was back-projected onto a projection screen (DA-LITE) at the head of the magnet bore, using a Avotec SV-6011 colour LCD Projection System (Florida, USA) and an NEC LT265 DLP video projector (Tokyo, Japan; refresh rate: 60 Hz; resolution: 1024 × 768) (for further details, see Al Dahhan et al., 2020). An EyeLink 1000 fibre optic camera was used to record participant’s eye position (SR Research Ltd., Ottawa, ON); viewing was binocular but all recordings were based on the right eye. The vertical and horizontal location of the right eye was digitized at 500 Hz. Before each fMRI run, the eye tracker was calibrated by instructing participants to fixate on nine randomly presented targets (one central and eight around the periphery). The eye tracker was then validated by repeating the process again to ensure that there was no loss of eye tracking and that there was <1° of average error between each target location and participant’s fixation. The letters were presented on a black background in white print (Angsana New font, size 60), with a 1.7° gap between each letter, 2.4° gap between each row, and 0.47° × 0.63° horizontal and vertical size of each letter stimulus, respectively (Al Dahhan et al., 2020; Fig. 1A). The objects were matched for luminance, 50 cd/m2, with a 1.7° gap between each object, 2.3° gap between each row, and 1.4° × 1.4° vertical and horizontal size of each object stimulus (Al Dahhan et al., 2020; Fig. 1B).
fMRI experimental design
Prior to entering the MRI environment, four practice NS trials were administered to each participant (Al Dahhan et al., 2020). Participants first named the six objects and eight letters for the first two practice trials to establish that they knew the stimulus names, and then they performed a letter and object NS task (20 letters/objects presented in four rows) to establish that they understood the task instructions for the last two practice trials. The reading and non-verbal ability tests were administered within a week after participants took part in the neuroimaging portion of the study.
Imaging data were acquired with participants lying supine in a Siemens 3-Tesla Magnetom Trio system (Erlangen, Germany) with a 32-channel receive-only head coil (Al Dahhan et al., 2020). High resolution T1-weighted whole-brain structural scans were collected using a 3D MP-RAGE sequence (repetition time = 1760 ms; flip angle = 9°; echo time = 2.2 ms; field-of-view = 256 mm × 256 mm; matrix size 256 mm × 256 mm; 1 mm iso-voxel resolution; 176 volumes]. Functional images were collected axial oblique with 40 horizontal slices (3.3 mm thick) covering the whole brain. Functional data were acquired using T2*-weighted echo-planar image volumes sensitive to blood oxygen-level dependent contrast (Ogawa et al., 1990; Kwong et al., 1992) collected in an interleaved fashion (repetition time = 2750 ms, echo time = 30 ms, flip angle = 84°, field-of-view = 211 mm × 211 mm, matrix size 64 × 64, 3.3 mm iso-voxel resolution, 192 volumes). For each participant, four functional runs containing 192 volumes and 2 discarded volumes for T1 saturation effects were collected.
For each functional run, the task conditions (two trials/task) were presented for 45 s, with a 12-s fixation block interleaving each condition (Fig. 1C;Al Dahhan et al., 2020). Each run began and ended with a 12-s fixation period in order to initially allow the fMRI signal to reach steady-state longitudinal magnetization and then allow the haemodynamic response to return towards the baseline, respectively (Al Dahhan et al., 2020).
Data analysis
Behavioural data
Speech and eye movements were both examined using custom software that was created in MatLab (MathWorks Inc., USA; see Al Dahhan et al., 2017, 2020 for further details). Briefly, the performance was only examined for participant’s first run through each task in order to avoid practice effects. Data identified with naming errors or skips were taken out from the analyses (2% of data for controls and 5% of data for dyslexics). Naming errors were manually scored from participants’ recorded responses (see Georgiou et al., 2006; Al Dahhan et al., 2017, 2020). NS efficiency was computed by dividing the number of stimuli named correctly by total naming time. Articulation time was calculated as the mean of correct articulations that and were not preceded by a skipped stimulus, and pause time was the mean duration between two correctly articulated stimuli. Fixation duration was measured as the average length of all fixations for correctly articulated stimuli. The start and end of saccades were defined based on acceleration (8000°/s2) and velocity (30°/s) threshold criteria. Regressions were measured as leftward saccades <30° of visual angle on the horizontal and <10° in amplitude.
fMRI preprocessing
BrainVoyager was utilized for all fMRI preprocessing and statistical analyses (Brain Innovation, Netherlands; see Al Dahhan et al., 2020 for further detail). Briefly, preprocessing steps included: slice scan time correction with cubic spline interpolation, 3D motion correction to the first volume in each run, 3D spatial smoothing with a 6 mm full width half maximum Gaussian kernel (i.e. twice the between-plane distance of 3.3 mm; Skuldarkski et al., 1999; Soltysik and Hyde, 2006) and temporal filtering (high-pass filter with a cut-off of two cycles/run and linear trend removal). Functional data were screened for motion artefacts exceeding 2 mm translation or 2° rotation by examining each run’s motion correction plots. Functional images were coregistered to the structural images, normalized into standard Talairach space (Talairach and Tournoux, 1988), and then these parameters were performed on to the coregistered functional data (Al Dahhan et al., 2020).
fMRI whole brain mean activation analysis
A random-effects multi-subject general linear model (RFX GLM) with Z-normalization and separate participant predictors was developed to identify significant differences in brain activity during the task conditions (Al Dahhan et al., 2020). Functional data from all task conditions were subtracted from fixation (main contrast) to evaluate sensorimotor activity and group-level statistical maps were created at a threshold of P < 0.01, t(17) = 2.90 for controls and P < 0.01, t(15) = 2.95 for dyslexics, then corrected for multiple comparisons across the voxel population using a cluster threshold correction at P < 0.05 (yielding a cluster threshold of 10 contiguous voxels, estimated by BrainVoyagers’s Cluster-level Statistical Threshold Estimator at 1000 iterations). After correcting for multiple comparisons, contrast maps were then generated to compare the task conditions. These contrast maps were overlaid onto an average 3D anatomical scan that was constructed from each individual’s T1 scan separately for each group (Al Dahhan et al., 2020).
fMRI region of interest analyses
Six areas of the left-hemisphere reading network were chosen from the main contrast as 125 contiguous voxels (5 × 5 × 5) in a cubic cluster located at the area of peak activation; the SMG, angular gyrus, IFG, superior temporal gyrus, MTG and FG (Al Dahhan et al., 2020). Further data-driven regions of interests (ROIs) were selected based on task comparisons. ROIs were chosen from the main contrast separately for each group to evaluate how the signal pattern in these areas changes across the tasks and across the two groups, and how activation within the reading network is related to behavioural performance (Al Dahhan et al., 2020).
Statistical analysis
SPSS Statistics v19.0 was utilized to conduct statistical analyses (IBM, Chicago, IL, USA) (Al Dahhan et al., 2020). Paired-samples t-tests were administered to evaluate whether performance on the task conditions differed. Separate analyses were examined for beta weight values (GLM parameter estimates), task performance (NS efficiency), NS components (articulation and pause times) and eye movement measures (fixation durations, and number of saccades and regressions). The relationships between each of the dependent variables were examined through bivariate correlations. All statistical analyses reported were corrected for multiple comparisons.
Data availability
Data presented in this article is available upon request.
Results
Cognitive characteristics of participants
Descriptive statistics for standardized measures of reading and non-verbal ability are presented in Table 1. A MANOVA with the group as a between-subjects factor and reading ability and phonological awareness measures as dependent variables revealed a main effect of group, Wilks’ λ = 0.10, F(6,192) = 117.66, P < 0.001. Univariate ANOVAs indicated that the typically achieving group had significantly better performance than the dyslexic group on each reading and phonological awareness measure (all P’s < 0.001). The control and dyslexic groups did not differ on Matrix Reasoning, F(1,33) = 0.04, P > 0.05, indicating that they had the similar mental ability. Similar results were found after controlling for age.
Table 1.
Descriptive statistics of standardized group performance on reading and non-verbal ability measures and F tests following significant MANOVA analyses results
| Variable | Controls (n = 18) |
Dyslexics (n = 16) |
F(1,33) | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Reading and decoding ability | |||||
| Word identification | 120.50 | 6.38 | 85.00 | 8.87 | 182.43* |
| Passage comprehension | 107.11 | 4.00 | 87.31 | 10.15 | 58.47* |
| Sentence reading fluency | 120.89 | 1.45 | 89.06 | 10.77 | 154.36* |
| Word attack | 90.50 | 2.83 | 79.69 | 10.53 | 17.61* |
| Phonological awareness | |||||
| Phoneme elision | 11.22 | 1.22 | 7.19 | 1.97 | 52.81* |
| Phoneme isolation | 11.28 | 1.56 | 7.06 | 3.04 | 26.68* |
| Word blending | 11.11 | 0.83 | 7.25 | 2.62 | 35.21* |
| Non-verbal ability | |||||
| Matrix reasoning | 72.33 | 7.77 | 72.56 | 8.66 | 0.01 |
Word identification, word attack, sentence reading fluency has a mean standard score of 100 with a standard deviation of 15; passage comprehension, phoneme elision and isolation, and word blending has a mean scaled score of 10 and a standard deviation of 3; and matrix reasoning has a mean T score of 50 and a standard deviation of 10. Wilks’ λ = 0.11, F(6,192) = 57.66 P < 0.001;
P < 0.001.
Behavioural task performance
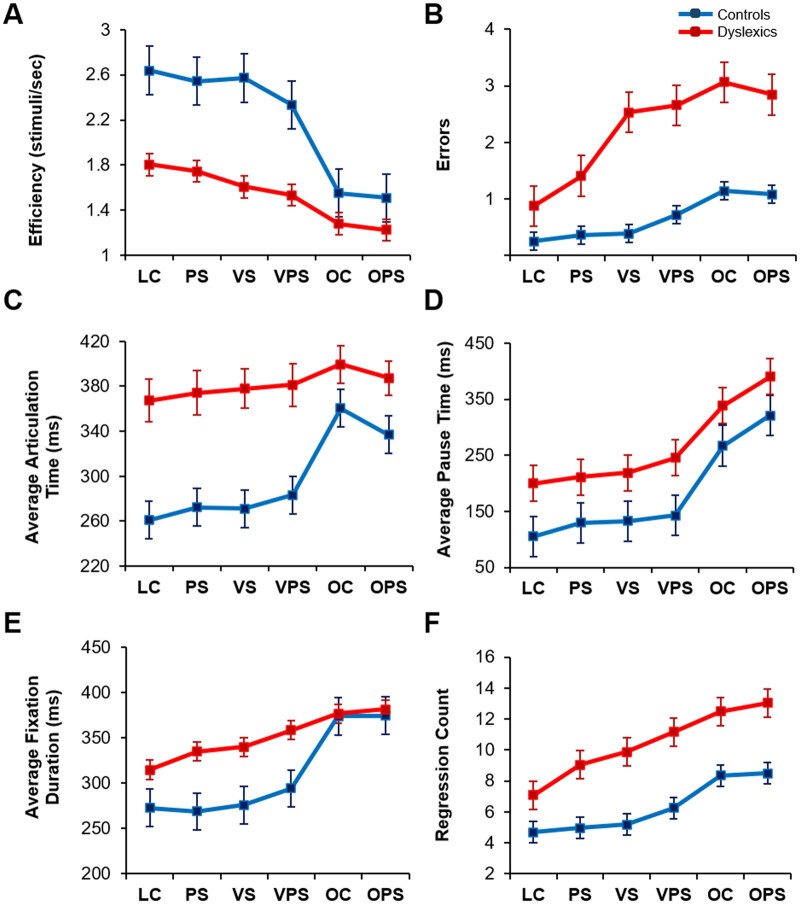
Controls and dyslexic groups differed in NS efficiency (Fig. 2A), errors (Fig. 2B), articulation time (Fig. 2C), pause time (Fig. 2D), fixation duration (Fig. 2E) and regression count (Fig. 2F). A series of group (Control versus Dyslexics) × NS tasks (LC, PS, VS, VPS, OC, OPS) mixed analyses of variance, one for each dependent variable, indicated significant group and task effects for all measures (all P’s < 0.001), and significant group by task interactions for all measures (all P’s < 0.001) except for pause time and regression count (both F’s < 1.05, P > 0.05). Similar results were found after controlling for the effects of age.
Figure 2.
Effect of task version on task performance, NS components and eye movement measures by group. (A) Efficiency score on the NS tasks. (B) Errors/run in naming. (C) Average articulation time per trial. (D) Average pause time per trial. (E) Average fixation duration. (F) Regression count. LC = letters control NS task; NS = naming speed task; OC = object control NS task; OPS = phonologically similar object NS task; PS = phonologically similar NS task; VPS = visually and phonologically similar NS task; VS = visually similar NS task. Standard errors are shown. Control data previously published in Al Dahhan et al. (2020).
To examine the significant group by task interactions, the ANOVAs were repeated separately for each task condition; this follow-up analysis showed no significant interactions (all P’s > 0.05), indicating that the significant interactions were due to different patterns of results in the letter and object tasks (Fig. 2). For the letter task conditions, there were group differences for efficiency, articulation time and fixation duration (all P’s < 0.01), with reduced differences for these measures for the object task conditions (all P’s < 0.05). For errors, the initial interaction was due to task differences among the dyslexics, but not controls (P < 0.05).
For the group effects, one-way ANOVAs using composite scores (raw scores averaged across the six task conditions) indicated that the typically achieving readers were more efficient, made fewer errors, had shorter pause and articulation times and fixation durations, and made fewer regressions than dyslexics (Fig. 2; all P’s < 0.05). For the task effects, paired-samples t-tests using composite scores (raw scores averaged across the four-letter task conditions and the two object task conditions separately) revealed that there were significant differences in performance between the letter and object NS tasks on all measures (all P’s < 0.001); participants were more efficient, made shorter articulation times, pause times and fixations, and fewer regressions and errors on the letter NS tasks than the object NS tasks (Fig. 2). We compared the four-letter NS tasks, averaging across groups; paired-samples t-tests indicated that the visually and phonologically similar task (VPS condition) had the greatest impact on performance: poorer task efficiency (Fig. 2A), increased errors (Fig. 2B), longer articulation times (Fig. 2C), pause times (Fig. 2D) and fixation durations (Fig. 2E), and greater regressions (Fig. 2F) compared to the letters control (LC) condition (all P’s < 0.001). The PS and VS conditions were intermediate in difficulty. For the object task conditions, paired-samples t-tests revealed that there were longer articulation times for OC [Fig. 2C;t (33) = 2.57, P < 0.05, r = 0.72], but longer pause times for OPS [Fig. 2D;t (33) = 4.72, P < 0.001, r = 0.68]. Similar results were found after controlling for the effects of age. This suggests that the slower recognition process (pause time) for OPS allows some preparatory articulation processes to begin during recognition. Correlations between all composite scores are shown in Supplementary Table 1.
Neural activation during task performance
Sensorimotor activation
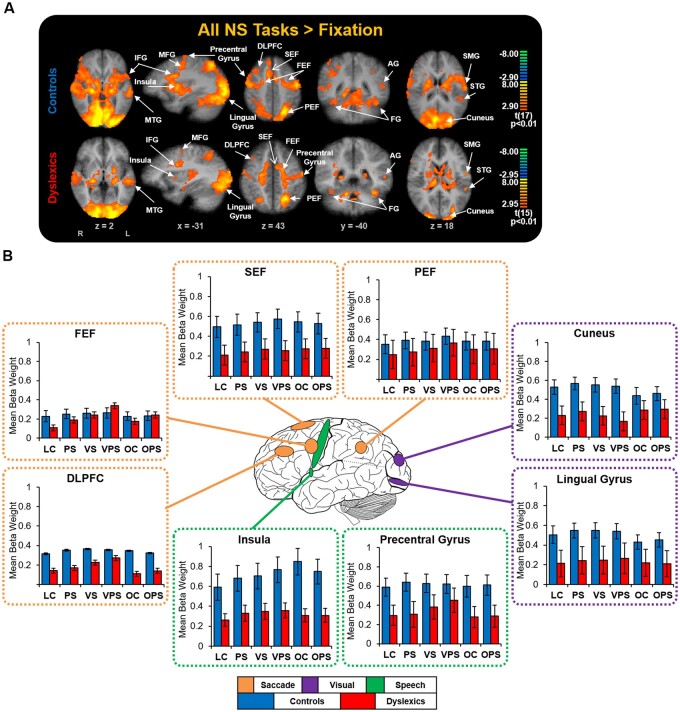
An RFX GLM contrast of all task conditions subtracted from fixation (main contrast) was computed to examine whether the control and dyslexic participant groups recruited critical sensorimotor areas that are necessary to complete the task (Fig. 3A and Supplementary Table 2). For both groups, compared to fixation, activation across the tasks was significantly greater (all P’s < 0.01) in central oculomotor areas that are implicated in eye movement control, consisting of the DLPFC, anterior cingulate cortex, frontal eye field, supplementary eye field, parietal eye field and the caudate nucleus (Connolly et al., 2002, 2005; Ford et al., 2005; Brown et al., 2007; Alahyane et al., 2014); primary speech areas, consisting of the primary motor cortex, IFG and insula (Guenther et al., 2006); and visual regions that are important during reading, consisting of the cuneus, lingual gyrus and the FG (Indefrey and Levelt, 2004; Price, 2012). Each task condition was contrasted with a fixation for each group to establish that specific tasks were not driving these findings and showed similar results. One-way ANOVAs using the average mean beta weights of all task conditions indicated that overall typically achieving readers had more activity in the lingual gyrus and the insula than readers with dyslexia (Fig. 3B; all P’s < 0.05). Similar results were found after controlling for the effects of age.
Figure 3.
Sensorimotor activation during task performance for each group. (A) Contrast map of all NS tasks subtracted from fixation, cluster size corrected at P < 0.05 (10 contiguous voxels). Significant blood oxygen-level dependent activations were observed in all ROIs (‘hot’ colours) in key sensorimotor regions that are involved during the serial processing and naming of letters and objects as well as key regions involved in the reading network, and are labelled. Coordinate values of planes in Talairach space are indicated. (B) Group comparisons of sensorimotor activation among the NS tasks. Standard errors are shown. AG = angular gyrus; DLPFC = dorsolateral prefrontal cortex; FEF = frontal eye fields; FG = fusiform gyrus; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; MTG = middle temporal gyrus; PEF = parietal eye field; SEF = supplementary eye field; SMG = supramarginal gyrus; STG = superior temporal gyrus. Figure adapted from Al Dahhan et al. (2020) with permission from Federation of European Neuroscience Societies and John Wiley & Sons Ltd. Control data previously published in Al Dahhan et al. (2020).
Identifying ROIs
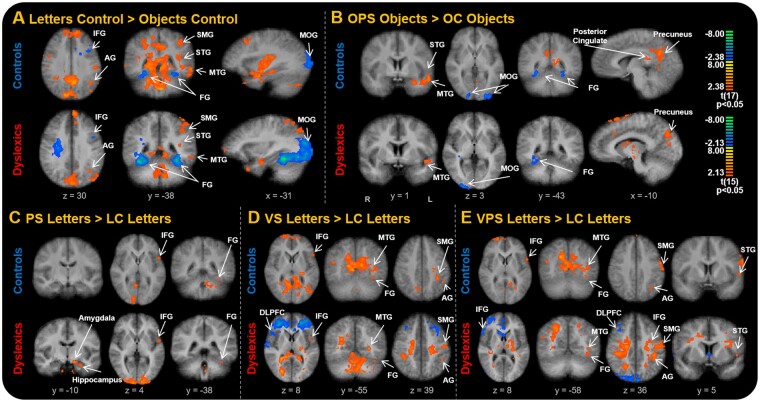
The LC and OC tasks were first compared to examine group differences between alphanumeric and non-alphanumeric stimuli (Fig. 4A), the OPS and OC tasks were then compared (Fig. 4B), and lastly, the LC task was contrasted with the letter tasks (Fig. 4C, D and E). There were many areas of differences, for one or the other or both groups. Areas of the reading network were identified in most of the contrasts, confirming our intention to examine them more carefully. Of the other areas identified, we selected three (amygdala, hippocampus and DLPFC) that consistently showed differences and are of greater interest to understanding reading.
Figure 4.
Task activation differences among stimulus conditions for each group. (A) Contrast of LC and OC NS tasks. (B) Contrast of OPS and OC tasks. (C) Contrast of PS letters and LC letters. (D) Contrast of VS letters and PS letters. (E) Contrast of VPS letters and LC letters. Each cluster map has a cluster size corrected at P < 0.05 (10 contiguous voxels). ROIs are labelled, with significant blood oxygen-level dependent activations shown as ‘hot’ colours or ‘cold’ colours. Coordinate values of planes in Talairach space are indicated. AG = angular gyrus; DLPFC = dorsolateral prefrontal cortex; FG = fusiform gyrus; IFG = inferior frontal gyrus; MOG = middle occipital gyrus; MTG = middle temporal gyrus; LC = letters control NS task; NS = naming speed task; PS = phonologically similar NS task; SMG = supramarginal gyrus; STG = superior temporal gyrus; VS = visually similar NS task; VPS = visually and phonologically similar NS task. Figure adapted from Al Dahhan et al. (2020) with permission from Federation of European Neuroscience Societies and John Wiley & Sons Ltd. Control data previously published in Al Dahhan et al. (2020).
Effect of task manipulations on brain activation in ROIs
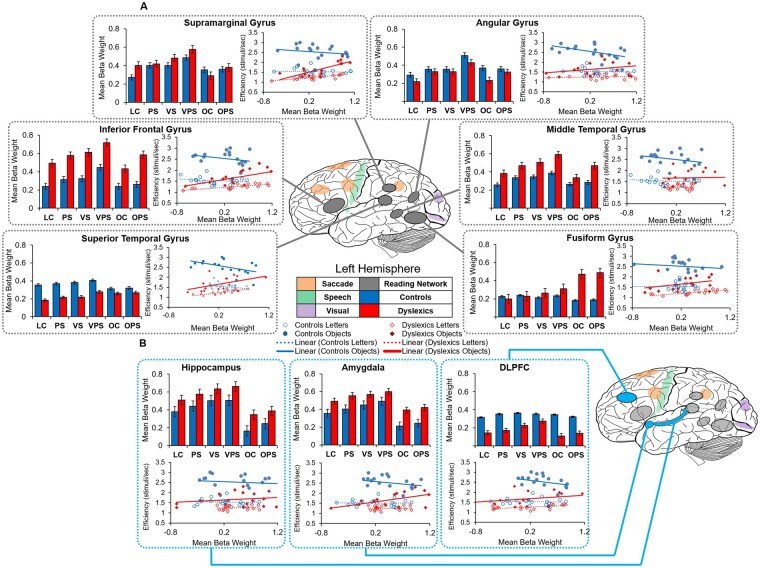
We obtained beta weights from each of the ROIs using the main contrast and conducted a number of group × all task conditions mixed analyses of variance for each region to investigate brain activation differences between the task conditions and examine how they were correlated with behavioural performance (Fig. 5). These analyses showed a significant group effect for the IFG [F(1,33) = 28.71, P < 0.001], MTG [F(1,33) = 34.24, P < 0.001], amygdala [F(1,33) = 69.60, P < 0.001], hippocampus [F(1,33) = 42.67, P < 0.001] and DLPFC [F(1,33) = 8.62, P < 0.01], significant task effects for all reading regions (all P’s < 0.001), amygdala [F(5,160) = 3.10, P < 0.01] and hippocampus [F(5,160) = 11.38, P < 0.001], and a significant group × task interaction for the FG [F(5,160) = 8.75, P < 0.001]. To examine the significant interaction for the FG, we repeated the ANOVA separately for (a) the four letter task conditions and (b) the two object task conditions and found no significant interactions (all P’s > 0.05), indicating that the overall interaction was due to different patterns in the letter and object tasks: for dyslexics’ activation was higher in the object naming than the letter naming tasks (P < 0.05; Fig. 5A) whereas there was no difference for the controls (P > 0.05). Similar results were found after controlling for age.
Figure 5.
Effect of stimulus manipulations on blood oxygen-level dependent activation and task efficiency. (A) Task activation differences within the left-hemisphere reading network between controls and dyslexics. (B) Processing differences between groups. Line graphs show the correlations between activation and NS efficiency in each group. Beta weights were extracted from 125 cubic voxels surrounding the peak activations in regions displaying greater activation during all NS tasks compared to fixation (main contrast) separately for each group. DLPFC = dorsolateral prefrontal cortex; LC = letters control NS task; NS = naming speed task; OC = object control NS task; OPS = phonologically similar object NS task; PS = phonologically similar NS task; VPS = visually and phonologically similar NS task; VS = visually similar NS task. Standard errors are shown. Control data previously published in Al Dahhan et al. (2020).
To examine the effects of task condition, we first compared letter and object naming. Paired-samples t-tests using the mean beta weights showed higher activation during the letter task conditions in the SMG, angular gyrus, IFG, amygdala and hippocampus, and higher activation during the object task conditions in the FG (all P’s < 0.05; Fig. 5). For the letter task condition effects, there was higher activation for each of the reading regions, amygdala, and hippocampus when the stimuli were visually and phonologically similar to one another (VPS condition; P < 0.05), except for the left FG, in which the tasks did not differ (P > 0.05). The only object NS task effects showed greater activation in the MTG and IFG for the OPS task than the OC task (P > 0.05). Similar results were found after controlling for the effects of age.
Relating behavioural performance to brain activation
For both groups, reading ROIs activation levels during both the letter (Table 2) and object (Table 3) NS tasks were correlated with one another and hippocampus activation was positively correlated with both amygdala and DLPFC activation (P < 0.05). Although there was variability among coefficients, in general activation in all ROIs was positively related to efficiency for dyslexics, but negatively related to controls. Regression analyses were conducted to examine the differences between these correlations. Centred neural activation scores from one ROI, group, and their interaction were entered as predictors, with either letter or object NS efficiency as the outcome; we confined our analyses to the efficiency scores because they represent the best single index of performance (see line graphs in Fig. 5). For letter NS efficiency, significant interaction effects were found for the following ROIs: superior temporal gyrus, SMG, IFG, angular gyrus and the amygdala (all betas > 0.80 and P’s ranging from 0.046 to 0.008). For object NS efficiency, significant interaction effects were found for the following ROIs: IFG, superior temporal gyrus, SMG and the FG (all betas > 0.70 and P’s ranging from 0.049 to 0.014). In all cases, the interactions showed greater group differences at low levels of activation and converging differences at higher levels of activation. There was a similar but opposite pattern between groups for articulation time, pause time and fixation duration, which were positively related to activation for controls, but negatively related to dyslexics; the pattern is reversed because high values on these variables represent less efficient responding. Overall, for typically achieving readers, greater activation was associated with longer fixation durations, articulation times, pause times and lower efficiency. For dyslexics, it was the opposite: increased activation was associated with shorter fixation durations, articulation times, pause times and greater efficiency.
Table 2.
Correlations between regions of interest and behaviour for the letter NS tasks
| Controls (n = 18) |
Dyslexics (n = 16) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
| 1. IFG | ||||||||||||||||||
| 2. FG | 0.80*** | 0.54* | ||||||||||||||||
| 3. SMG | 0.50* | 0.74*** | 0.60* | 0.43* | ||||||||||||||
| 4. AG | 0.53* | 0.65** | 0.88*** | 0.79** | 0.27* | 0.32* | ||||||||||||
| 5. MTG | 0.97*** | 0.78*** | 0.49* | 0.51* | 0.81*** | 0.13* | 0.30* | 0.72** | ||||||||||
| 6. STG | 0.56* | 0.50* | 0.62** | 0.51* | 0.42* | 0.25* | 0.72** | 0.57* | 0.79** | 0.80** | ||||||||
| 7. Amygdala | 0.06 | 0.05 | −0.20 | 0.15 | −0.06 | 0.67** | −0.48* | −0.05 | −0.35 | −0.52* | −0.45* | 0.02 | ||||||
| 8. Hippocampus | 0.21 | 0.57* | 0.54* | 0.42* | 0.24 | 0.50* | 0.52* | 0.30 | −0.01 | 0.46* | −0.23 | −0.17 | 0.25 | 0.75** | ||||
| 9. DLPFC | 0.59* | 0.45* | 0.46* | 0.44* | 0.34* | 0.13 | 0.35 | 0.54* | 0.51* | 0.20 | 0.52* | 0.77** | 0.60* | 0.47* | 0.57* | 0.46* | ||
| Letter NS tasks | ||||||||||||||||||
| NS efficiency | −0.24 | −0.10 | −0.18 | −0.76* | −0.06 | −0.58* | −0.24 | −0.14 | −0.16 | 0.45* | 0.34 | 0.74** | 0.26 | 0.03 | 0.47 | 0.52* | 0.27 | 0.38 |
| Articulation time | 0.02 | 0.17 | 0.36 | 0.68* | 0.05 | 0.68* | 0.22 | 0.21 | 0.13 | −0.55* | −0.39 | −0. 0.53* | −0.17 | 0.00 | −0.32 | −0.50* | −0.45* | −0.31 |
| Pause time | −0.02 | −0.02 | −0.11 | 0.40* | 0.04 | 0.12 | −0.06 | −0.09 | −0.27 | −0.48* | −0.28 | −0.72** | −0.32 | −0.14 | −0.58* | 0.49* | −0.03 | −0.38 |
| Fixation duration | 0.18 | 0.16 | 0.24 | 0.58* | −0.14 | 0.52* | 0.23 | −0.06 | −0.38* | −0.31 | −0.36 | −0.52* | −0.00 | 0.36 | 0.14 | −0.28 | −0.21 | 0.06 |
| Regression count | 0.21 | 0.13 | 0.27 | 0.01 | 0.23 | 0.14 | −0.14 | 0.01 | 0.18 | −0.04 | −0.13 | −0.31* | −0.09 | 0.22 | 0.06 | −0.47* | −0.56* | −0.85** |
AG = angular gyrus; DLPFC = dorsolateral prefrontal cortex; FG = fusiform gyrus; IFG = inferior frontal gyrus; MTG = middle temporal gyrus; NS = naming speed; SMG = supramarginal gyrus; STG = superior temporal gyrus.
P < 0.05; **P < 0.01; ***P < 0.001.
Table 3.
Correlations between regions of interest and behaviour for the object NS tasks
| Controls (n = 18) |
Dyslexics (n = 16) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
| 1. IFG | ||||||||||||||||||
| 2. FG | 0.81*** | 0.74** | ||||||||||||||||
| 3. SMG | 0.60** | 0.71** | 0.60* | 0.41* | ||||||||||||||
| 4. AG | 0.57* | 0.65** | 0.91** | 0.80** | 0.27* | 0.26* | ||||||||||||
| 5. MTG | 0.89** | 0.78** | 0.63** | 0.58* | 0.78* | 0.23* | 0.30* | 0.71** | ||||||||||
| 6. STG | 0.47* | 0.67** | 0.81** | 0.72** | 0.47* | 0.25* | 0.69* | 0.69* | 0.79** | 0.78** | ||||||||
| 7. Amygdala | 0.08 | 0.53* | −0.22 | 0.19 | −0.17 | 0.25 | −0.63* | −0.05 | −0.28 | −0.54* | −0.62* | 0.06 | ||||||
| 8. Hippocampus | 0.32 | 0.57* | 0.65** | 0.58* | 0.38 | 0.54* | 0.59** | 0.10 | −0.13 | 0.46* | −0.10 | −0.06 | 0.11 | 0.55** | ||||
| 9. DLPFC | 0.47* | 0.45* | 0.53* | 0.54* | 0.57* | 0.34 | 0.33 | 0.54* | 0.25 | 0.12 | 0.44* | 0.56* | 0.45* | 0.28 | 0.36 | 0.66* | ||
| Object NS tasks | ||||||||||||||||||
| NS efficiency | −0.32 | −0.06 | −0.03 | −0.09 | −0.36 | −0.26 | −0.03 | −0.01 | −0.01 | 0.79** | 0.74** | 0.75** | 0.16 | 0.03 | 0.79** | 0.10 | 0.12 | 0.45* |
| Articulation time | 0.21 | 0.04 | 0.21 | 0.19 | 0.22 | 0.18 | 0.48* | 0.14 | −0.06 | −0.53* | −0.37 | −0.42 | 0.00 | −0.17 | −0.53* | −0.32* | −0.25 | −0.13 |
| Pause time | 0.16 | 0.00 | 0.07 | 0.08 | 0.07 | 0.11 | 0.32 | 0.27 | 0.31 | −0.51* | −0.32 | −0.72** | −0.24 | −0.05 | −0.51* | −.12 | −0.02 | −0.38 |
| Fixation duration | 0.31 | 0.11 | 0.42 | 0.47* | 0.52* | 0.47* | 0.10 | 0.05 | 0.16 | −0.37 | −0.33 | −0.41 | −0.06 | 0.35 | 0.12 | −0.17 | −0.07 | −0.05 |
| Regression count | −0.19 | −0.07 | 0.08 | −0.20 | −0.08 | −0.16 | −0.15 | −0.05 | −0.21 | −0.03 | −0.11 | −0.01 | −0.23 | −0.24 | −0.03 | −0.68** | −0.57* | −0.51* |
AG = angular gyrus; DLPFC = dorsolateral prefrontal cortex; IFG = inferior frontal gyrus; FG = fusiform gyrus; MTG = middle temporal gyrus; NS = naming speed; SMG = supramarginal gyrus; STG = superior temporal gyrus.
P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
To address important gaps in the literature and to build upon previous studies, we employed fMRI, speech recording, and eye tracking in adult readers with and without dyslexia to investigate the mechanisms underlying NS tasks, the brain relationship between alphanumeric and non-alphanumeric task conditions, and the effects task stimulus composition has on performance. Readers with dyslexia had a poorer behavioural performance on all tasks (Fig. 2), and this was reflected in changes in activity within regions of the reading network (Fig. 5). Whereas controls relied on the reading network to complete the tasks and achieved efficiency with less activation, readers with dyslexia had greater bilateral activation, recruited additional regions involved with memory to presumably compensate for their reading disability and employed greater activation to achieve efficiency (Fig. 5), even though that efficiency was less than that shown by the controls. Task manipulations differentially affected performance; there was poorer behavioural performance and greater activation when stimuli were both visually and phonologically similar.
Distinguishing typically achieving readers from readers with dyslexia
Compared to typically achieving readers, readers with dyslexia had a poorer behavioural performance on all tasks: they were less efficient, made more errors, had longer fixation durations, articulation times and pause times, and made more regressions (Fig. 2). These findings replicate previous behavioural studies using these tasks (e.g. Al Dahhan et al., 2014). Longer fixation durations and pause times indicate that encoding and processing alphanumeric stimuli were less automatic for dyslexics, suggesting they have weaker orthographic processing compared to typically achieving readers, resulting in overall decreased task efficiency (Supplementary Table 1; Bowers and Newby-Clark, 2002; Kirby et al., 2010; Yan et al., 2013). These deficits were also likely exacerbated by the speeded requirements of the tasks and the similarity between the stimuli in the matrix, leading to an increase in task difficulty and the inability to efficiently access stimulus representations from lexical stores. These inferences are supported by the differences between groups in correlations between behavioural measures and ROI activation (Tables 2 and 3). For example, activation across all ROIs tended to be positively related to efficiency for dyslexics and negatively related for controls (see Fig. 5). This indicates that the neural processes underlying reading, even in letter naming, are not automatized to the same degree in dyslexics as controls, thus requiring greater activation to complete the tasks.
Previous studies have indicated that, compared to readers who are typically developing, readers with dyslexia demonstrate lower activation in the left FG, which supports skilled orthographic decoding, and in the left temporoparietal regions, which support grapheme-phoneme mapping, and greater activation in the IFG (Price and Mechelli, 2005; Shaywitz and Shaywitz, 2008; Richlan et al., 2011; Norton et al., 2015). In the present study, dyslexics had greater activation than controls in the MTG, IFG, amygdala and hippocampus, and lower activation in the DLPFC, but did not show lower activation in the left FG (Fig. 5). These differences may reflect aspects of processing with which dyslexics struggle, or compensatory processing strategies due to inefficiencies in appropriately activating areas of the reading network (Diehl et al., 2014).
Specifically, greater activation for dyslexics in the IFG may reflect a compensatory strategy to overcome inefficiencies in posterior neural regions that play a role in orthographic and phonological processing (Hoeft et al., 2011); greater activation in this region was associated with more efficient processing for dyslexics (Tables 2 and 3; Fig. 5). However, of the regions in the posterior reading network, only the MTG showed greater activation for dyslexics than controls (Fig. 5A), and greater activation there was not associated with more efficient processing for either group (Tables 2 and 3). A possible explanation for this finding is that this region is involved with orthographic processing, and readers with dyslexia have been found to rely on visualization to compensate for phonological difficulties when processing written information (Bacon et al., 2013; Bacon and Handley, 2014).
Dyslexics also rely on mechanisms involved with memory retrieval to compensate for reading difficulties, which is consistent with greater activation in the amygdala and hippocampus for dyslexics than controls (Fig. 5B;Shaywitz et al., 2006; Shaywitz and Shaywitz, 2008); activation in both areas was positively correlated with letter naming efficiency for dyslexics (Table 2). Overall, these findings align with studies revealing differences in brain-behaviour relationships between typically achieving readers and readers with dyslexia (e.g. Hampson et al., 2006; Hoeft et al., 2007; van der Mark et al., 2011), and imply that reading performance may be more dependent upon specific regions in individuals with dyslexia than in typically achieving readers.
Effect of task condition on behavioural performance and brain activity
Behavioural and fMRI findings in the present study revealed that letter naming performance was more efficient than object naming performance. There was greater activation overall in the ROIs for the letter NS tasks (Fig. 5), suggesting that letter naming was a more automatic process than object naming (Misra et al., 2004; Cummine et al., 2014; Al Dahhan et al., 2020), and that the reading network is more specialized for alphabetic stimuli than for objects. There was greater activation during the letter task conditions in the SMG, angular gyrus, IFG, amygdala and hippocampus compared to the object task conditions, and greater activation during the object task conditions in the FG compared to the letter task conditions (Fig. 5). These findings support the involvement of these areas in the conversion from visual to lexical-semantic to phonological processing for alphabetic stimuli (Jobard et al., 2003; Indefrey and Levelt, 2004; Cattinelli et al., 2013; Taylor et al., 2013; Carreiras et al., 2014; Al Dahhan et al., 2020).
Both groups displayed reduced behavioural performance and greater activation when the letters were both visually and phonologically similar compared to the single letter manipulation task conditions (Fig. 5). Greater speech monitoring and attentional processing were required to complete the tasks when the similarity of the letters in the matrix was greater, as shown by greater activation of the inferior parietal cortex (Fig. 4C, D and E; Christoffels et al., 2007; Binder et al., 2009; Chang et al., 2009; Guenther and Vladusich, 2012; Al Dahhan et al., 2020). These findings provide additional support for the argument that the ability to rapidly access the mental representations of letters is one of the key determiners of letter NS performance (Bowers et al., 1994). For the object task conditions, there were longer articulation times on the OC task, and longer pause times on the OPS task condition (Fig. 2C and D), indicating that more visual and semantic processing is required to differentiate between the objects and prime the articulation of the phonologically similar object names in the OPS task, indicated by greater activation of the posterior cingulate and precuneus (Fig. 4B;Binder et al., 2009). This in turn may have increased the efficiency of processes involved in articulatory recoding and motor speech command execution.
Conclusion
Although neuroimaging research has identified the core networks of language and visual regions that underlie reading (Schlagger and McCandliss, 2007; Price, 2012; Rueckl et al., 2015), and examined how these networks differ in readers with dyslexia (Paulesu et al., 2014; Perfetti and Stafura, 2014; Norton et al., 2015; Pollack et al., 2015), there is little evidence of how fundamental brain processes are impacted in readers with dyslexia in ways that explain how the cognitive processes of reading are affected and how the brain compensates for those disruptions. To address this, this study examined NS performance through fMRI, eye tracking and speech recording to further understand the differences in activation within the reading network that are related to the behavioural task differences identified between typically achieving readers and readers with dyslexia. Even though the two groups differed on all measures of reading and decoding ability, future research should be conducted using community and younger samples, and investigate whether neural activation differences diminish following successful remediation. Further progress in understanding the neural processes that underlie reading can aid in the development of more targeted interventions, and may also enhance early detection of children who are at risk for developing reading difficulties. This has the potential to lead to more effective remediation and could change the outcome trajectories for those with reading deficits.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
An operating grant from the Canadian Institute of Health Research, MOP-FDN-148418, supported this research. Additionally, the Canada Research Chair Program supported DPM.
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
- AC-PC =
anterior commissure-posterior commissure plane
- AG =
angular gyrus
- DLPFC =
dorsolateral prefrontal cortex
- FEF =
frontal eye fields
- FG =
fusiform gyrus
- fMRI =
functional magnetic resonance imaging
- IFG =
inferior frontal gyrus
- LC =
letters control NS task
- MFG =
middle frontal gyrus
- MOG =
middle occipital gyrus
- MP-RAGE =
magnetization-prepared rapid gradient-echo
- MTG =
middle temporal gyrus
- NS =
naming speed
- OC =
object control NS task
- OPS =
phonologically similar object NS task
- PEF =
parietal eye field
- PS =
phonologically similar NS task
- RFX GLM =
random-effects multi-subject general linear model
- ROI =
regions of interest
- SEF =
supplementary eye field
- SMG =
supramarginal gyrus
- STG =
superior temporal gyrus;
- VS =
visually similar NS task
- VPS =
visually and phonologically similar NS task
References
- Alahyane N, Brien DC, Coe BC, Stroman PW, Munoz DP. Developmental improvements in voluntary control of behavior: effect of preparation in the fronto-parietal network. NeuroImage 2014; 98: 103–17. [DOI] [PubMed] [Google Scholar]
- Al Dahhan N, Georgiou GK, Hung R, Munoz D, Parrila R, Kirby JR. Eye-movements of university students with and without reading difficulties during naming speed tasks. Ann Dyslexia 2014; 64: 137–50. [DOI] [PubMed] [Google Scholar]
- Al Dahhan NZ, Kirby JR, Brien DC, Munoz DP. Eye movements and articulations during a letter naming speed task: children with and without dyslexia. J Learn Disabil 2017; 50: 275–85. [DOI] [PubMed] [Google Scholar]
- Al Dahhan NZ, Kirby JR, Chen Y, Brien DC, Munoz DP. Examining the neural and cognitive processes that underlie reading through naming speed tasks. Eur J Neurosci 2020; 51: 2277–98. [DOI] [PubMed] [Google Scholar]
- Bacon AM, Handley SJ. Reasoning and dyslexia: is visual memory a compensated resource? Dyslexia 2014; 20: 330–45. [DOI] [PubMed] [Google Scholar]
- Bacon AM, Parmentier FBR, Barr P. Visuospatial memory in dyslexia: evidence for strategic deficits. Memory 2013; 21: 189–209. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex 2009; 19: 2767–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky R, Cummine J, Owen WJ, Friesen CK, Shih F, Sarty GE. FMRI of ventral and dorsal processing streams in basic reading processes: insular sensitivity to phonology. Brain Topogr 2006; 18: 233–9. [DOI] [PubMed] [Google Scholar]
- Bowers PG, Golden J, Kennedy A, Young A. Limits upon orthographic knowledge due to processes indexed by naming speed. In: Berninger VW, editor. The varieties of orthographic knowledge, Vol 1: Theoretical and developmental issues. Dordrecht, Netherlands: Kluwer; 1994. p. 173–218. [Google Scholar]
- Bowers PG, Newby-Clark E. The role of naming speed within a model of reading acquisition. Read Writ 2002; 15: 109–26. [Google Scholar]
- Breznitz Z. Brain activity during performance of naming tasks: comparison between dyslexic and regular readers. Sci Stud Read 2005; 9: 17–42. [Google Scholar]
- Brown MRG, Vilis T, Everling S. Frontoparietal activation with preparation for antisaccades. J Neurophysiol 2007; 98: 1751–62. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Armstrong BC, Perea M, Frost R. The what, when, where, and how of visual word recognition. Trends Cogn Sci 2014; 18: 90–8. [DOI] [PubMed] [Google Scholar]
- Cattinelli I, Borghese NA, Gallucci M, Paulesu E. Reading the reading brain: a new meta-analysis of functional imaging data on reading. J Neurolinguistics 2013; 26: 214–38. [Google Scholar]
- Chang S-E, Kenney MK, Loucks TMJ, Poletto CJ, Ludlow CL. Common neural substrates support speech and non-speech vocal tract gestures. NeuroImage 2009; 47: 314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffels IK, Formisano E, Schiller NO. Neural correlates of verbal feedback processing: an fMRI study employing overt speech. Hum Brain Mapp 2007; 28: 868–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P, Hulme C, Snowling MJ. Individual differences in RAN and reading: a response timing analysis. J Learn Disabil 2005; 28: 73–86. [Google Scholar]
- Cohen L, Dehaene S, Vinckier F, Jobert A, Montavont A. Reading normal and degraded words: contribution of the dorsal and ventral visual pathways. NeuroImage 2008; 40: 353–66. [DOI] [PubMed] [Google Scholar]
- Compton DL. The influence of item composition on RAN letter performance in first-grade children. J Spec Educ 2003; 37: 81–94. [Google Scholar]
- Connolly JD, Goodale MA, Goltz HC, Munoz DP. fMRI activation in the human frontal eye field is correlated with saccadic reaction time. J Neurophysiol 2005; 94: 605–11. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci 2002; 5: 1345–52. [DOI] [PubMed] [Google Scholar]
- Cummine J, Szepesvari E, Chouinard B, Hanif W, Georgiou GK. A functional investigation of RAN letters, digits, and objects: how similar are they? Behav Brain Res 2014; 275: 157–65. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends Cogn Sci 2005; 9: 335–41. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. Rapid ‘automatized’ naming (R.A.N.): Dyslexia differentiated from other learning disabilities. Neuropsychologia 1976; 14: 471–9. [DOI] [PubMed] [Google Scholar]
- Diehl JJ, Frost SJ, Sherman G, Mencl WE, Kurian A, Molfese P, et al. Neural correlates of language and non-language visuospatial processing in adolescents with reading disability. NeuroImage 2014; 101: 653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MRG, Everling S. Neural processes associated with antisaccade task performance investigated with event-related fMRI. J Neurophysiol 2005; 94: 429–40. [DOI] [PubMed] [Google Scholar]
- Georgiou GK, Parrila R, Kirby J. Rapid naming speed components and early reading acquisition. Sci Stud Read 2006; 10: 199–220. [Google Scholar]
- Georgiou GK, Parrila R, Kirby JR. RAN components and reading development from grade 3 to grade 5: what underlies their relationship. Sci Stud Read 2009; 13: 508–34. [Google Scholar]
- Gonzalez-Gerriod AA, Gómez-Velázquez FR, Zarabozo D, Ruiz-Villeda BA, de la Serna Tuya JM. Rapid automatized naming and lexical decision in children from an electrophysiological perspective. Clin EEG Neurosci 2011; 42: 14–23. [DOI] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang 2006; 96: 280–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH, Vladusich T. A neural theory of speech acquisition and production. J Neurolinguistics 2012; 25: 408–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, et al. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. NeuroImage 2006; 31: 513–9. [DOI] [PubMed] [Google Scholar]
- He Q, Xue G, Chen C, Chen C, Lu Z-L, Dong Q. Decoding the neuroanatomical basis of reading ability: a multivoxel morphometric study. J Neurosci 2013; 33: 12835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, et al. Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci USA 2011; 108: 361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Ueno T, Reiss AL, Meyler A, Whitfield-Gabrieli S, Glover GH, et al. Prediction of children’s reading skills using behavioral, functional, and structural neuroimaging measures. Behav Neurosci 2007; 121: 602–13. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition 2004; 92: 101–44. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metaanalysis of 35 neuroimaging studies. NeuroImage 2003; 20: 693–712. [DOI] [PubMed] [Google Scholar]
- Kirby JR, , Savage RS. Can the simple view deal with the complexities of reading? Literacy 2008; 42: 75–82. [Google Scholar]
- Kirby JR, Georgiou GK, Martinussen R, Parrila R, Bowers P, Landerl K. Naming speed and reading: from prediction to instruction. Read Res Q 2010; 45: 341–62. [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 1992; 89: 5675–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Cutting LE, Ryan M, Zilioli M, Denckla MB, Mahone EM. Response variability in rapid automatized naming predicts reading comprehension. J Clin Exp Neuropsychol 2009; 31: 877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Katzir T, Wolf M, Poldrack RA. Neural systems for rapid automatized naming in skilled readers: unraveling the RAN-reading relationship. Sci Stud Read 2004; 8: 241–56. [Google Scholar]
- Norton ES, Beach SD, Gabrieli JDE. Neurobiology of dyslexia. Curr Opin Neurobiol 2015; 30, 73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton ES, Wolf M. Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities. Annu Rev Psychol 2012; 63: 427–52. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 1990; 87: 9868–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Olitsky SE, Nelson LB. Reading disorders in children. Pediatric Clin North Am 2003; 50: 213–24. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Danelli L, Berlingeri M. Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI studies. Front Hum Neurosci 2014; 8: 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti C, , Stafura J. Word knowledge in a theory of reading comprehension. Scientific Studies of Reading 2014; 18: 22. [Google Scholar]
- Pollack C, Luk G, Christodoulou JA. A meta-analysis of functional reading systems in typically developing and struggling readers across different alphabetic languages. Front Psychol 2015; 6: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage 2012; 62: 816–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Mechelli A. Reading and reading disturbance. Curr Opin Neurobiol 2005; 15: 231–8. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Mental Retard Dev Disabil Res Rev 2000; 6: 207–13. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. Neurobiological studies of reading and reading disability. J Commun Disord 2001; 34: 479–92. [DOI] [PubMed] [Google Scholar]
- Rayner K. Understanding eye movements in reading. Sci Stud Read 1997; 1: 317–39. [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage 2011; 56: 1735–42. [DOI] [PubMed] [Google Scholar]
- Rueckl JG, Paz-Alonso PM, Molfese PJ, Kuo W-J, Bick A, Frost SJ, et al. Universal brain signature of proficient reading: evidence from four contrasting languages. Proc Natl Acad Sci USA 2015; 112: 15510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagger BL, McCandliss BD. Development of neural systems for reading. Annu Rev Neurosci 2007; 30: 475–503. [DOI] [PubMed] [Google Scholar]
- Schrank FA, McGrew KS, Mather N, Woodcock RW. Woodcock-Johnson IV. Rolling Meadows, IL: Riverside Publishing; 2014. [Google Scholar]
- Shaywitz SE, Mody M, Shaywitz BA. Neural mechanisms in dyslexia. Curr Dir Psychol Sci 2006; 15: 278–81. [Google Scholar]
- Shaywitz SE, Shaywitz BA. Paying attention to reading: the neurobiology of reading and dyslexia. Dev Psychopathol 2008; 20: 1329–49. [DOI] [PubMed] [Google Scholar]
- Skuldarkski P, Constable RT, Gore JC. ROC analysis of statistical methods used in functional MRI: individual subjects. NeuroImage 1999; 9: 311–29. [DOI] [PubMed] [Google Scholar]
- Soltysik DA, , Hyde JS. Strategies for block-design fMRI experiments during task-related motion of structures of the oral cavity. NeuroImage 2006; 29: 1260. [DOI] [PubMed] [Google Scholar]
- Starr MS, Rayner K. Eye movements during reading: some current controversies. Trends Cogn Sci 2001; 5: 156–63. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain . Stuttgart: Thieme; 1988. [Google Scholar]
- Taylor JS, Rastle K, Davis MH. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol Bull 2013; 139: 766–91. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci 2003; 6: 767–73. [DOI] [PubMed] [Google Scholar]
- van der Mark S, Klaver P, Bucher K, Maurer U, Schulz E, Brem S, et al. The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. NeuroImage 2011; 54: 2426–36. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Comprehensive test of phonological processing. Austin, TX: PRO-ED; 1999. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Wiig EH, Nielsen NP, Minthon L, McPeek D, Said K, Warkentin S. Parietal lobe activation in rapid, automatized naming by adults. Percept Mot Skills 2002; 94: 1230–44. [DOI] [PubMed] [Google Scholar]
- Wolf M, Bowers PG. The double-deficit hypothesis for the developmental dyslexias. J Educ Psychol 1999; 91: 415–38. [Google Scholar]
- Woodcock R. Woodcock reading mastery tests – revised. Circle Pines, MN: American Guidance Servicesl; 1998. [Google Scholar]
- Yan M, Pan J, Laubrock J, Kliegl R, Shu H. Parafoveal processing efficiency in rapid automatized naming: a comparison between Chinese normal and dyslexic children. J Exp Child Psychol 2013; 115: 579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data presented in this article is available upon request.