Abstract
Immune checkpoint inhibitors have revolutionized the landscape of cancer treatment. Alongside their many advantages, they elicit immune-related adverse events, including myopathy, which potentially result in substantial morbidity if not recognized and treated promptly. Current knowledge of immune checkpoint inhibitor-associated myopathy is limited. We conducted a 5-year retrospective study of patients with immune checkpoint inhibitor-associated myopathy. Clinical features, survival and ancillary test findings were analysed and compared with those of immune-mediated necrotizing myopathy patients without immune checkpoint inhibitor exposure seen during the same time period. We identified 24 patients with immune checkpoint inhibitor-associated myopathy (median age 69 years; range 28–86) and 38 patients with immune-mediated necrotizing myopathy. Ocular involvement occurred in 9/24 patients with immune checkpoint inhibitor exposure, without electrodiagnostic evidence of neuromuscular transmission defect, and in none of the immune-mediated necrotizing myopathy patients (P < 0.001). Myocarditis occurred in eight immune checkpoint inhibitor-associated myopathy patients and in none of the immune-mediated necrotizing myopathy patients (P < 0.001). Median creatine kinase was 686 IU/l in the immune checkpoint inhibitor cohort (seven with normal creatine kinase) compared to 6456 IU/l in immune-mediated necrotizing myopathy cohort (P < 0.001). Lymphopenia was observed in 18 and 7 patients with and without immune checkpoint inhibitor exposure, respectively (P < 0.001). Myopathological findings were similar between patients with and without immune checkpoint inhibitor exposure, consisting of necrotic fibres with no or subtle inflammation. Necrotic fibres however arranged in clusters in 10/11 immune checkpoint inhibitor-associated myopathy patients but in none of the immune checkpoint inhibitor-naïve patients (P < 0.001). Despite the lower creatine kinase levels in immune checkpoint inhibitor-exposed patients, the number of necrotic fibres was similar in both groups. Immune checkpoint inhibitor-associated myopathy patients had a higher frequency of mitochondrial abnormalities and less number of regenerating fibres than immune-mediated necrotizing myopathy patients (P < 0.001). Anti-hydroxy-3-methylglutaryl-CoA reductase or signal recognition particle antibodies were absent in patients with immune checkpoint inhibitor exposure but positive in two-thirds of immune checkpoint inhibitor-naïve patients. Most patients with immune checkpoint inhibitor-associated myopathy responded favourably to immunomodulatory treatments, but four died from myopathy-related complications and one from myocarditis. Intubated patients had significantly shorter survival compared to non-intubated patients (median survival of 22 days; P = 0.004). In summary, immune checkpoint inhibitor-associated myopathy is a distinct, treatable immune-mediated myopathy with common ocular involvement, frequent lymphopenia and necrotizing histopathology, which contrary to immune-mediated necrotizing myopathy, is featured by clusters of necrotic fibres and not accompanied by anti-hydroxy-3-methylglutaryl-CoA reductase or signal recognition particle antibodies. Normal or mildly elevated creatine kinase level does not exclude the diagnosis.
Keywords: immune checkpoint inhibitor, immune-mediated necrotizing myopathy, myasthenia gravis, myopathy, myositis, necrotizing autoimmune myopathy
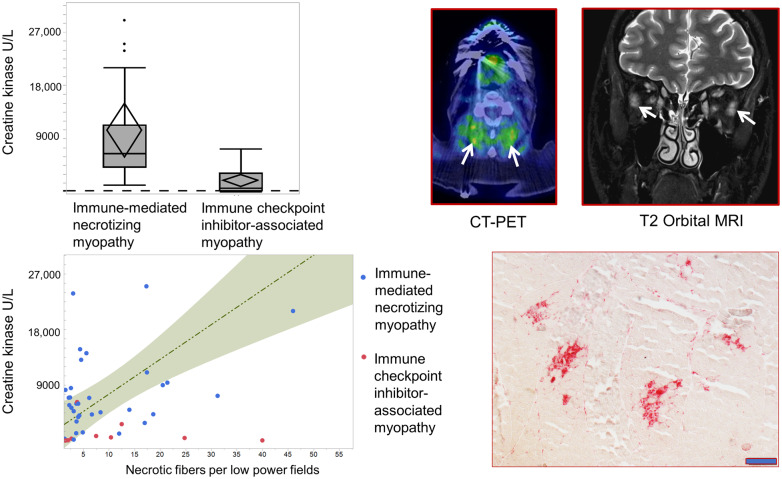
This is a comprehensive analysis of a large cohort of patients with immune checkpoint inhibitor-associated myopathy. Shelly et al. highlight its unique features of ocular involvement and normal creatine kinase levels in some patients, mimicking myasthenia gravis. Multifocal clusters of necrotic fibres are characteristics and occur in even patients without hyperCKemia.
Graphical Abstract
Graphical Abstract.
Introduction
The discovery that cancer cells are capable of hijacking immune checkpoints and evade immune attack has revolutionized cancer immunology and led to novel treatments. Immune checkpoint blockade is a novel cancer therapeutic strategy, in which inhibition of immune suppressor molecules promotes immune attack against cancer cells (Ishida et al., 1992; Leach et al., 1996; Sharma and Allison, 2015). These immune checkpoint inhibitors (ICI) include monoclonal IgG antibodies targeting the programmed cell death-1, nivolumab and pembrolizumab, the programmed cell death ligand-1, atezolizumab, avelumab and durvalumab, and the cytotoxic T-lymphocyte-associated antigen 4, ipilimumab (Bilen et al., 2016).
By unleashing the immune system against cancer, immune checkpoint blockade inevitably causes immune-mediated side effects, or so-called immune-related adverse events (irAEs) (Brahmer et al., 2018). The precise pathophysiology underlying irAEs is not well-understood. Different mechanisms have been postulated, all of which lead to tissue infiltration by highly activated CD4+ or CD8+ T cells (Bilen et al., 2016; Mammen et al., 2019). Although neurological irAEs are not as common as those affecting other organ systems, they can be severe and potentially fatal (Kao et al., 2017). In some cohorts, neuromuscular disorders account for more than 50% of neurological irAEs, including peripheral nerve diseases, neuromuscular junction disorders and myopathies (Suzuki et al., 2017; Kao et al., 2018; Liewluck et al., 2018; Touat et al., 2018; Dubey et al., 2019). Each neuromuscular complication can occur individually or in combination, such as the frequently reported myasthenia gravis-myopathy overlap syndrome. The hyperCKemia, observed in the majority of the reported ICI-associated myasthenia gravis patients, has been attributed to coexisting myopathy or myocarditis (Kao et al., 2018). However, because orbital myositis can occur in ICI-treated patients (Bitton et al., 2019), it can be difficult to ascertain with confidence the coexistence of myasthenia gravis in the absence of electrodiagnostic evidence of defect of neuromuscular transmission. Indeed, even antiacetylcholine receptor binding antibodies can occasionally be detected in the absence of myasthenia gravis (Maddison et al., 2019).
A wide range of immune-mediated myopathies were reported in ICI-treated patients, including polymyositis, dermatomyositis, granulomatous myositis, necrotizing myopathy and non-specific myopathy (Kao et al., 2018). Ocular involvement and multifocal clusters of necrotic fibres are considered unique features, although not universally present, of immune checkpoint inhibitor-associated myopathies (ICIAM) (Liewluck et al., 2018; Touat et al., 2018). It remains debatable whether ophthalmoparesis in ICIAM patients represents orbital myositis or coexisting myasthenia gravis. The current knowledge of ICIAM is limited and mostly derived from individual case reports or small case series. Herein, we present the largest single-centre cohort of ICIAM patients to date. All biopsy-proven patients displayed necrotizing myopathy-like pathology. We then conducted systematic comparison of their clinical, laboratory, electrophysiological and histopathological findings and survival to a cohort of ICI-naive immune-mediated necrotizing myopathy (IMNM) patients seen over the same time period.
Materials and methods
Study population
Institutional Review Board approval was granted by Mayo Clinic. Using electronic data search, we identified patients seen at our institution between January 2015 and December 2019 with a new diagnosis of myopathy while on ICI. We included patients who developed subacute or acute onset weakness of Common Terminology Criteria for Adverse Events version 5.0 (CTCAE) Grade III and above (Puzanov et al., 2017). The myopathy was considered Grade III only if caused limitation of self-care activities of daily living, Grade IV if they were life-threatening and needed urgent intervention and Grade V if directly caused death. In patients presenting with ocular symptoms CTCAE Grade III was considered if ophthalmoparesis, causing double vision in lateral gaze but not in primary gaze, and Grade IV if ophthalmoparesis causing double vision in central gaze or patients required head turning to see beyond central 60°.
A diagnosis of myopathy was further classified as definite, probable and possible. Definite myopathy was designated to patients who had histopathological evidence of myopathy or who fulfilled all three of the following criteria: (i) elevated serum creatine kinase (CK), (ii) electrodiagnostic findings of myopathic motor unit potentials (early recruiting, short-duration, low-amplitude motor unit potentials) and (iii) imaging abnormalities observed in the clinically affected muscle. Probable myopathy was diagnosed if patients fulfilled two of the aforementioned criteria. Patients were categorized as possible myopathy when only one of the three criteria was met. Patients with myopathies from other causes unrelated to ICI were excluded. All ICI-naïve IMNM patients seen during the same time period were identified and served as disease control. Diagnosis of IMNM was based on clinical, serological and pathological findings in keeping with international guidelines (Allenbach et al., 2018b).
Patients’ characteristics
Review of clinical features and laboratory data, including electrodiagnostic findings, were undertaken in correlation with cancer immunotherapy. The included patients were classified into two phenotypes: oculobulbar and generalized myopathy. Oculobulbar myopathy was defined by a combination of the symptoms of ptosis, diplopia, dysarthria or dysphagia of at least CTCAE grade III with no or subtle limb symptoms (CTCAE grade I-II) and the evidence of oculobulbar weakness (by neurologic examination or swallowing studies) with no or mild (MRC grade ≥4) limb weakness. Generalized myopathy was defined by a combination of the symptoms of limb weakness of at least CTCAE grade III and moderate-to-severe limb weakness (MRC grade <4) on neurologic examination. Patients with generalized myopathy may or may not have symptoms or signs of ocular or bulbar muscle involvement. Statin exposure was documented if patients were on any statin therapy at the time of symptom onset.
The diagnosis of myasthenia gravis was reserved for patients who had decrement on repetitive nerve stimulation (RNS) or increased jitter on single-fibre EMG (SFEMG), with or without myasthenia gravis antibodies. Diagnosis of myocarditis was made when patients developed new unexplained dyspnoea, chest pain, syncope, arrhythmia or congestive heart failure, accompanied by histopathological evidence of myocardial inflammation or echocardiographic or cardiac PET imaging abnormalities, consistent with myocarditis. Respiratory muscle involvement was considered if patients developed new-onset dyspnoea accompanied by abnormal pulmonary function tests or diaphragmatic ultrasound, consistent with restrictive lung disease or diaphragmatic weakness (Naddaf and Milone, 2017).
The modified Rankin Scale was used to measure functional outcomes in patients with limb muscle weakness. The scale ranges from 0 (asymptomatic) to 6 (death) (Banks and Marotta, 2007). The reduction of modified Rankin Scale scale by at least one point indicated the favourable outcome. Clinical improvement was defined as improvement of muscle strength (most severely affected muscle) at least one grade on MRC scale. Relapse was defined as recurrence of weakness when not attributable to tumour progression or alternative aetiologies.
Ancillary investigations
Electrophysiological testing
Nerve conduction studies (NCS) and electromyography (EMG) were performed according to standard stimulation and recording techniques for the EMG laboratory at Mayo Clinic, Rochester. Two-hertz RNS was utilized to assess a defect of neuromuscular transmission. RNS was deemed abnormal when a physiological pattern of decrement of >10% was seen (Liewluck et al., 2019). SFEMG abnormality was determined by utilizing quality and cut-off guidelines previously published (Sanders et al., 2019).
Antibodies testing
Acetylcholine receptor (AChR) binding and modulating antibodies were detected by radioimmunoprecipitation assay (RIPA) (normal < 0.02 nmol/l) and by monolayer cultures of human muscle cells and quantitated as percentage loss of binding sites for 125 I-α-bungarotoxin (normal ≤20%), respectively. Striational antibodies were analysed by ELISA immunoassay (normal < 1:120) and diluted to endpoint. Recombinant antigen enzyme-linked immunosorbent assay (QUANTA Lite HMGCR, Inova Diagnostics) detected 3-Hydroxy-3-Methylglutaryl-CoA Reductase (HMGCR-IgG; normal < 20 U) (Musset et al., 2014). Myositis-specific [EJ, Jo-1, MDA5, Mi-2, NXP2, OJ, PL-7, PL-12, signal recognition particle (SRP) and TIF-1γ] and myositis-associated (Ku, PM/Scl-100, SSA, SSB, U1-RNP, U2-RNP and U3-RNP) antibodies were analysed using enzyme immunoassay and RIPA performed by a commercial laboratory (normal <20 U).
Imaging studies
MRI of the head, cervical spine and orbits and whole body [18F] FDG-PET-CT performed at the time of symptom onset or within three months after symptom onset and following treatment were included when available. All examinations were reviewed by a radiologist (FD) blinded to the patients’ clinical state. MRI exams were reviewed for evidence of muscle enlargement, T2 hyperintensity [on any available T2-weighted sequences, included T2 fat-suppressed sequences such as short tau inversion recovery (STIR)], and gadolinium enhancement. Whole body [18F] FDG-PET-CT scans were reviewed for evidence of increased FDG uptake in skeletal muscles including the extraocular, masticator, paraspinal and appendicular musculature. In order to be considered positive, unequivocal signal abnormality, enhancement, and/or FDG uptake had to be present, to a degree and in a distribution that could not simply be considered physiologic.
Histopathology
Patients underwent a muscle biopsy for diagnostic purposes. Most samples were processed but all were analyzed in the Mayo Clinic Muscle Laboratory following standard protocol, including haematoxylin & eosin, modified Gomori trichrome, NADH-tetrazolium reductase, succinate dehydrogenase, cytochrome C oxidase (CCO), ATPase, acid phosphatase, myophosphorylase, oil red O, periodic acid-Schiff, non-specific esterase and Congo red stains.
All slides were analysed by two authors (S.S. and T.L.). We quantified each biopsy for the following features per low power fields (LPF): number of necrotic fibres, regenerating fibres, ragged red fibres, ragged-blue fibres and cytochrome c oxidase-negative fibres. We further divided the pattern of necrotic fibres into (i) multifocal clusters [necrotic fibres distributed unevenly among fascicles, with some normal-appearing fascicles and some fascicles containing groups (at least five fibres) of necrotic fibres], or (ii) even distribution (necrotic fibres present diffusely in most fascicles and not clustering).
Statistical analysis
Categorical variables are reported as numbers or percentages and were compared using the Fisher exact test. Quantitative variables are reported as median and ranges and compared using Mann-Whitney two-tail test. The Kaplan–Meier method was used for survival analysis, with the log-rank test used to compare groups. P < 0.05 was considered significant. Statistical analysis was performed using JMP software (SAS Institute). For survival analysis data cut-off determined was May 1, 2020, time (months) from ICIAM or IMNM diagnosis to death or last follow-up was collected. The following variables were tested as survival predictive factor in ICIAM: using univariate logistic regression analysis age, gender, CK, time to diagnosis, time from symptom onset to treatment, ptosis, ophthalmoparesis, dysphagia or any bulbar involvement, respiratory involvement, intubation, myocarditis, other irAEs, lymphopenia, ICIAM phenotype (oculobulbar and generalized), striational antibodies and severity of the weakest muscle.
Data availability
Results are available upon reasonable request.
Results
Clinical characteristics
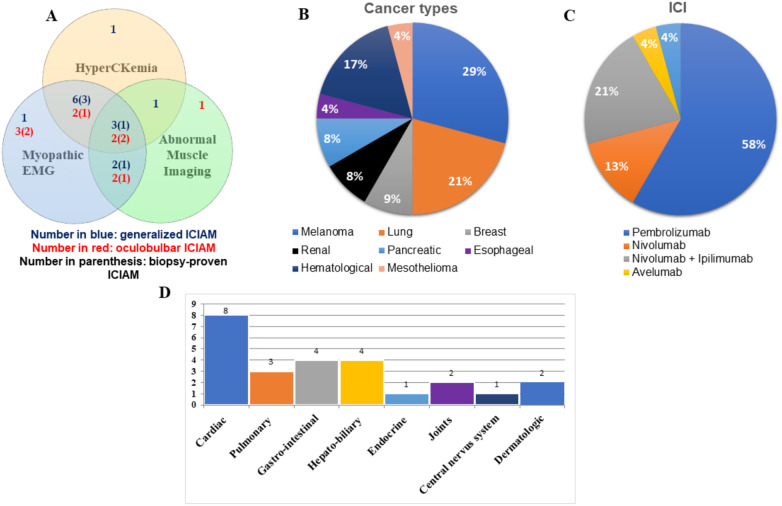
Twenty-four ICIAM (13 definite, 9 probable, 2 possible) and 38 ICI-naïve IMNM patients were included. Figure 1A showed diagnostic features of all ICIAM patients. Among 13 definite ICIAM, 11 patients were myopathologically defined. Three ICIAM patients were previously reported in detail (Liewluck et al., 2018). Patients’ demographics, clinical features and statin exposure are shown in Table 1. ICIAM patients with and without muscle biopsy had similar demographics, phenotype and laboratory values (Supplementary Table 1). Underlying malignancies and immune checkpoint regimens in ICIAM patients are outlined in Fig. 1B and C, respectively. In the IMNM group, six patients had active cancers at the time of the myopathy diagnosis. ICIAM patients first noticed symptoms after 1–18 doses (median two doses) of ICI therapy or 1–609 days (median 35 days) after the first dose of ICI treatment. Most ICIAM patients (19/24) developed initial symptoms within the first three months following ICI initiation. The median time from the last infusion to symptom onset was 8 days (range 0–50 days). The severity of myopathy among 24 ICIAM patients was as follows: CTCAE Grade III (n = 15), Grade IV (n = 5) and Grade V (n = 4). Other coexisting irAEs are shown in Fig. 1D, with myocarditis (n = 8), being the most common concomitant irAEs.
Figure 1.
Characteristics of patients with ICIAM. (A) Diagnostic features of ICIAM in the current cohort. Numbers in red and blue indicate numbers of patients with oculobulbar and generalized phenotype, respectively. Numbers in parenthesis indicate numbers of patients who underwent muscle biopsy and had pathologically defined myopathy. There are two patients with possible ICIAM: one oculobulbar patient with abnormal MRI of extraocular muscles (CK and EMG not performed) and one generalized patient with markedly elevated CK level (EMG and MRI not performed). (B) Cancer types in the current cohort. (C) Immune checkpoint inhibitors used in the current cohort. (D) Coexisting immune-related adverse events in the current cohort.
Table 1.
Demographics, clinical features and laboratory data of ICIAM versus IMNM patients
| ICIAM | IMNM | P value | |
|---|---|---|---|
| Number | 24 | 38 | |
| Age, years (range) | 69 (28–86) | 55 (18–87) | 0.04 |
| Female:male | 7:14 | 19:19 | 0.5 |
| Follow-up time (days) | 396 (11–1506) | 731 (74–3602) | 0.002 |
| Statin use, n (%) | 11 (46) | 22 (58) | 0.29 |
| Clinical phenotype, n (%) | |||
| 1. Oculobulbar | 10 (42) | 0 | <0.001 |
| Ocular involvement | 9 | ||
| Bulbar involvement | 6 | ||
| 2. Generalized | 14 (58) | 38 (100) | <0.001 |
| Ocular involvement | 1 | 0 | |
| Bulbar involvement | 2 | 15 | |
| Symptoms, n (%) | |||
| Myalgia | 13 (54) | 20 (53) | 0.26 |
| Dysphagia | 9 (38) | 12 (31) | 0.4 |
| Respiratory insufficiency | 10 (42) | 10 (26) | 0.23 |
| Signs, n (%) | |||
| Ophthalmoparesis | 6 (25) | 0 | 0.003 |
| Ptosis | 6 (25) | 0 | 0.003 |
| Neck flexor weakness | 8 (33) | 24 (63) | 0.002 |
| Neck extensor weakness | 2 (9) | 8 (21) | 0.19 |
| Hamstrings weaker than quadriceps | 6 (25) | 15 (40) | 0.2 |
| Myocarditis, n (%) | 8 (33) | 0 | <0.001 |
| HyperCKemia, n (%) | 15 (68)a | 37 (100)a,b | <0.001 |
| CK > 1000 IU/l (%) | 10 (45)c | 37 (100) | 0.007 |
| Peak CK, median (range) IU/l | 686 (28–7279) | 6456 (1151–29 000) | <0.001 |
| Aldolase elevation, n (%) | 7/11 (64) | 16/16 (100) | 0.009 |
| With normal CK, n (%) | 2/7 (29)d | 0 | |
| HMGCR-IgG positive, n (%) | 0/13 | 20/38 (52) | <0.001 |
| SRP-IgG positive, n (%) | 0/13 | 4/38 (10) | <0.001 |
| Other myositis-specific antibodies | 0/11 | 2/29e | 0.24 |
| Myositis associated antibodies | 3/11 | 6/29f | 0.35 |
| AChR binding antibody positive, n (%) | 3/17 (18) | 1/3 (33)g | 0.5 |
| AChR modulating antibody positive, n (%) | 2/17 (12) | 1/3 (33)g | 0.4 |
| Striational antibody positive, n (%) | 8/17 (47) | 1/19 (5)g | 0.002 |
| Paraneoplastic antibody positive, n (%) | 3/17 (18)h | 0/18 | <0.001 |
| Lymphopenia, n (%) | 18/24(75) | 7/34 (20) | <0.001 |
| Absolute lymphocyte count, median (range; ×109/l) | 0.72 (0.05–3.5) | 1.38 (0.56–3.4) | <0.001 |
| Elevated TSH, n (%) | 4/24 (19) | 12/28 (42) | 0.14 |
| AST or ALT elevation, n (%) | 15/24 (70) | 32/35 (91) | 0.003 |
AChR = acetylcholine receptor; ALT = amino alanine transferase; AST = aspartate amino transferase; CK = creatinine kinase; HMGCR = 3-hydroxy-3-methylglutaryl-coenzyme-A reductase; ICIAM = immune checkpoint inhibitor-associated myositis; IMNM = immune-mediated necrotizing myopathy; NS = not significant; SRP, signal recognition particle; TSH, thyroid stimulating hormone.
All bold values indicate P-value < or = 0.05.
CK was not available in 2/24 patients (normal <192 in females and <336 in males).
One patient with normal CK was excluded as it was measured after initiation of immunosuppressive therapy.
Oculobulbar (n = 2) and generalized (n = 8) phenotypes.
Both patients had normal AST and ALT.
One IMNM patient with strongly positive HMGCR antibodies also had weakly positive MDA-5 antibody; one double seronegative IMNM patient had weakly positive Mi-2 antibody without cutaneous or pulmonary involvement or myopathological hallmark of dermatomyositis.
Myositis associated antibodies were positive in three ICIAM (one PM/Scl-100, one SSA and one U1-RNP) and six IMNM (one SSA and five SSB) patients.
One patient with pre-existing myasthenia gravis.
One patient with N type VGCC antibodies (0.08 nmol/l; normal <0.03 nmol/l), one patient with GAD65 antibodies (0.13 nmol/l; normal < 0.02 nmol/l) and one patient with ganglionic nicotinic AChR antibodies (0.04 nmol/l; normal < 0.01).
As shown in Table 1, oculobulbar phenotype was observed in 10 ICIAM patients (four ocular only, three bulbar only and three combined ocular and bulbar weakness) and not in any of the IMNM patients (P < 0.001). Mild proximal limb or axial weakness was detected in eight oculobulbar ICIAM patients (four proximal only, two axial only and two combined proximal and axial weakness). The remaining ICIAM and all IMNM patients had a generalized weakness, predominantly affecting proximal limb muscles. One generalized ICIAM patient also had ocular and bulbar weakness. Bulbar weakness occurred in both ICIAM and IMNM in a similar proportion, but ocular involvement was only observed in ICIAM group (P = 0.003). Weakness was non-fatigable in all ICIAM patients and in IMNM patients when tested.
Ancillary investigations
Laboratory studies
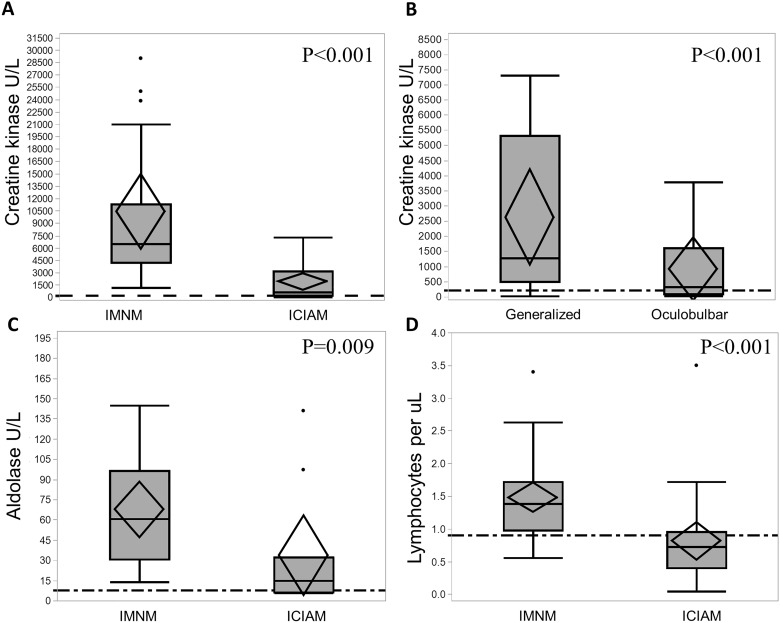
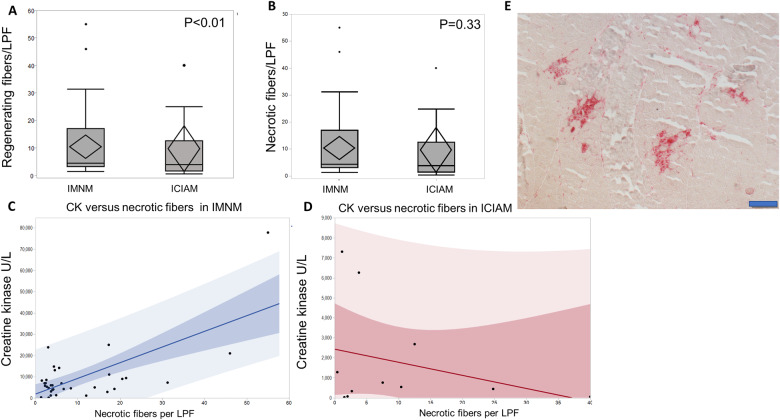
Detailed laboratory findings are shown in Table 1. The CK values were significantly lower (P < 0.001) in ICIAM patients (median 686 IU/l compared to IMNM patients (median 6456 IU/l) (Fig. 2A). The median CK level was not significantly different between pathologically defined ICIAM (493 IU/l) and the remaining ICIAM patients (1062 IU/l; P = 0.87) (Supplementary Table 1). Seven ICIAM patients had normal CK (five oculobulbar and two generalized). A CK < 1000 IU/l was more frequently associated with ICIAM (P = 0.007) and even more so with an oculobulbar phenotype (Fig. 2B). Median CK was 333 IU/l (range 28–3772) in oculobulbar ICIAM and 1284 IU/l (range 30–7303) in generalized ICIAM patients. Aldolase levels correlated with CK values in individual patients (Fig. 2C) except for two ICIAM patients who had elevated aldolase (1.5 and 2 times above upper limit of normal), but normal CK values. Neither of these two ICIAM patients had abnormal liver enzymes. The median time from symptom onset to biopsy was 17 days (range 3–25) in those with normal CK compared to 20 days (range 11–42) in those with hyperCKemia (P = 0.66). One patient with normal CK received 1-week course of prednisone for unrelated reason 30 days prior to CK measurement, while none of the patients with elevated CK levels received any prior immunomodulatory therapy. Thirteen ICIAM patients were tested for anti-HMGCR and anti-SRP antibodies and all were negative. Amongst IMNM patients, 52% were HMGCR and 10% were SRP antibody positive.
Figure 2.
Laboratory data comparison between ICIAM and IMNM patients. (A) Patients with ICIAM have lower CK levels compared to those with IMNM. (B) Among patients with ICIAM, patients with oculobulbar phenotype have lower CK levels than those with generalized weakness. (C) Aldolase levels are significantly higher in the IMNM group compared to the ICIAM group. (D) Patients with ICIAM have lower absolute lymphocyte count compared to those with IMNM.
Lymphopenia was more common in ICIAM patients (18/24) compared to IMNM patients (7/34) (P < 0.001) (Fig. 2D). Pre-ICI lymphocyte count was normal in 10/18 ICIAM patients with lymphopenia at the time of myopathy diagnosis despite 16/18 patients received prior chemotherapy or radiotherapy. After exclusion of eight ICIAM patients with low pre-ICI lymphocyte count, the difference of lymphopenia between ICIAM and IMNM remained significant (P = 0.02).
Striational antibodies were abnormal in 8/17 ICIAM patients tested (median 1:7680; range 1:3840–1:122880; seven patients with titre ≥1:7680) compared to 1/19 of IMNM patients tested (1:15360) (P = 0.002). Six ICIAM patients with striational antibodies had oculobulbar phenotype. CK was normal in three of eight striational antibody-positive ICIAM patients. None of the ICIAM patients with striational antibodies had thymoma. Myocarditis was observed in five and two patients with and without striational antibodies, respectively. The calculated specificity of striational antibodies for ICIAM compared to IMNM was 95% with 47% sensitivity when the AUC = 0.7. The cut-off threshold value was 1:3840.
Three ICIAM patients with striational antibodies were also seropositive for anti-AChR antibodies (Table 2). None of these patients had electrophysiological evidence of neuromuscular transmission defect despite severe respiratory insufficiency, but all had short-duration, low-amplitude motor unit potentials with fibrillation potentials, indicating a myopathic process.
Table 2.
Phenotypic comparison between oculobulbar versus generalized ICIAM patients with acetylcholine receptor antibodies€
|
Oculobulbar ICIAM (
n = 10) |
Generalized ICIAM (
n = 7) |
|||
|---|---|---|---|---|
| AChR-Ab positive (n = 2)a | AChR-Ab negative (n = 8) | AChR-Ab positive (n = 1)b | AChR-Ab negative (n = 6) | |
| AChR binding antibody values (nmol/l) | 11.9 and 0.1 | <0.02 | 1.43 | <0.02 |
| Striational antibody positive, n | 2/2 | 4/8 | 1/1 | 1/4 |
| AChR modulating antibody values (%) | 93 and 0 | <20 | 67 | <20 |
| CK (range, IU/l) | 263–444 | 28–2685 (median 333) | 762 | 611–7307 (median 2924) |
| EMG | 2/2 | 7/8 | 1/1 | 6/6 |
| Myopathic MUP, n | 2/2c | 7/7c | 1/1 | 6/6 |
| Fibrillation potentials, n | 2/2 | 7/7 | 1/1 | 4/6 |
| Abnormal RNS | 0/2 | 0/6 | 0/1 | 0/4 |
| Abnormal SFEMG | 0/0 | 0/2 | 0/0 | 0/0 |
| Abnormal muscle MRI | 1/2d | 4/8 | 0/1e | 2/6 |
| Abnormal muscle biopsy | 1/1f | 5/5 | 1/1g | 3/3 |
| Myocarditis | 1/2 | 3/8 | 1/1 | 2/6 |
| Respiratory failure | 2/2 | 3/8 | 1/1 | 3/6 |
€The number of patients with positive AChR-Ab is low hence it does not have enough power to make any statistical comparison.
AChR = acetylcholine receptor; CK = creatinine kinase; ICIAM = immune checkpoint inhibitor associated myositis; MUP = motor unit potentials; NA = not applicable; NCS = nerve conduction studies; SFEMG = single-fibre EMG.
Normal ranges < 0.02 nmol/l; one patient with ocular weakness and one patient with bulbar weakness.
This patient had axial and proximal weakness without oculobulbar involvement.
Myopathic MUP was observed in axial (n = 6), bulbar (n = 6) and limb (n = 8) muscles.
MRI abnormalities were observed in bulbar and limb muscles.
Normal MRI of brain.
Splenius capitis biopsy compatible with ICIAM.
Deltoid biopsy compatible with ICIAM.
Electrophysiological studies
Electrophysiological evaluation was performed in 21 ICIAM patients (9 oculobulbar and 12 generalized) and 37 IMNM patients. Findings are summarized in Supplementary Table 2. Myopathic motor unit potentials were identified in all ICIAM and IMNM patients, when performed. All seven ICIAM patients without limb weakness and seven ICIAM patients with normal CK also had myopathic motor unit potentials in axial or limb muscles. Fibrillation potentials were observed in all, but four ICIAM patients (one oculobulbar and three generalized). Two ICIAM patients, who did not have fibrillation potentials, underwent muscle biopsy which showed necrotic fibres. Myopathic motor unit potentials were accompanied by fibrillation potentials in all, but one IMNM patient who was on immunosuppressive therapy at the time of the EMG. Myotonic discharges were more common in IMNM (47%) than ICIAM (22%) patients (P < 0.001). Six IMNM (16%) and 12 ICIAM (50%) patients had electrodiagnostic evidence of a length-dependent, sensorimotor axonal peripheral neuropathy (P = 0.01). One ICIAM patient had axonal polyradiculoneuropathy. The higher frequency of peripheral neuropathy in ICIAM group compared to IMNM group remained significance even after exclusion of eight ICIAM patients with prior chemotherapy (P = 0.04). RNS showed no decrement in all 13 (8 oculobulbar and 5 generalized) ICIAM patients performed. Two oculobulbar ICIAM patients underwent SFEMG and both were normal.
Imaging studies
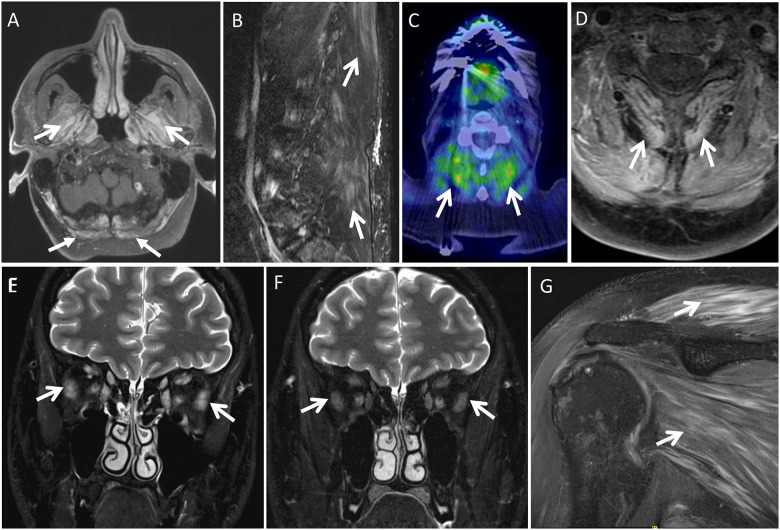
Relevant imaging (MRI or PET) was available in 20/24 ICIAM patients, including 21 MRI and 17 whole body [18F] FDG PET-CT exams. Intramuscular T2 hyperintensity with or without gadolinium enhancement and/or increased FDG uptake in muscle were seen in 11 ICIAM patients (5 oculobulbar and 6 generalized) (Supplementary Table 3), all of whom had myopathic EMG and 6 had hyperCKemia. Paraspinal muscles were abnormal on imaging studies in all but two patients (one oculobulbar and one generalized), with only one patient with such abnormal paraspinal muscles on imaging having neck extensor weakness on examination. In five patients with oculobulbar phenotype, muscles of mastication were abnormal in four and extraocular muscles were abnormal in one (Fig. 3). Extraocular muscle abnormality was noted on MRI dedicated to the orbits, while the other four patients with normal radiological findings of extraocular muscles underwent non-dedicated brain MRI. Three patients also underwent MRI and/or PET after immunomodulatory treatment and all three showed a marked decrease of imaging abnormalities (Fig. 3E and F).
Figure 3.
Imaging in ICIAM. Imaging findings of myopathy in five different patients (A–G). (A) Contrast-enhanced axial fat-suppressed T1-weighted brain MRI at the level of foramen magnum reveals contrast enhancement in the muscles of mastication (arrows) and superior posterior neck muscles (block arrows). (B) Off-midline sagittal fat-suppressed T2-weighted lumbar spine MRI demonstrates mild streaky T2-hyperintensity of the lumbar and visualized lower thoracic posterior paraspinal musculature (arrows). (C) Axial 18F-FDG PET CT fused image at C4–5 shows increased FDG uptake in the posterior paraspinal neck musculature (arrows). (D) In the same patient as C, correlative contrast-enhanced axial fat-suppressed T1-weighted cervical spine MRI demonstrates mildly increased enhancement of this posterior paraspinal neck musculature (arrows). (E,F) Coronal fat-suppressed T2-weighted orbit MRI on initial (E) and follow-up (F) initially shows T2 hyperintensity and mild enlargement of the extraocular muscles bilaterally, which resolves on follow-up (arrows on lateral recti). (G) Oblique coronal T2-weighted fat-suppressed shoulder MRI reveals feather like oedema-type T2-hyperintensity enhancement of the shoulder girdle muscles (arrows).
Among 10 ICIAM patients who had no radiological abnormalities, imaging studies of clinically affected muscles were not performed in 5 patients (2 oculobulbar and 3 generalized phenotypes). The median time from symptom onset to imaging studies of those radiologically abnormal patients was seven days (range 1–39 days) compared to 16 days (range 0–31 days) in those five patients with normal imaging studies of clinically affected muscles (P = 0.9). Myopathic motor unit potentials were observed in all radiologically normal patients. Five patients underwent biopsy of radiologically normal, but electromyographically abnormal muscles, all of which displayed myofibre necrosis.
Muscle pathology
Muscle biopsy slides from 47 patients [11 ICIAM (6 oculobulbar and 5 generalized) and 36 IMNM] were available for review. Several ICIAM patients declined the muscle biopsy because of their terminal illness from cancer itself, severe myopathy or other irAEs requiring ICU care. Data were extracted from biopsy reports of two IMNM patients, whom muscle biopsy slides from other institutes were previously interpreted at our Muscle Lab and slides were no longer available for review. Figure 4 and Table 3 summarize the key pathological findings. All muscle biopsies, including four patients with normal CK (three ICIAM and one IMNM), displayed necrotic and regenerating fibres. The IMNM patient with normal CK underwent muscle biopsy prior to immunomodulatory therapy, and the included CK level was measured after treatment.
Figure 4.
Histopathological comparison between ICIAM and IMNM patients. (A) ICIAM patients have lower number of regenerating fibre per low power field compared to IMNM patients. (B) ICIAM and IMNM patients have similar number of necrotic fibres per low power field (B). (C,D) Correlation between creatinine kinase level and the number of necrotic fibres per low power field demonstrates nonlinear relationship in the ICIAM group and linear relationship in IMNM group. ICIAM patients have relatively similar range of necrotic fibres per low power field but lower CK levels compared to IMNM patients. (E) Acid phosphatase stained section shows macrophages (in red) invading necrotic fibres occurring in clusters (bar 200 μm).
Table 3.
Histopathological characteristics of ICIAM versus IMNM patients
| ICIAM | IMNM | P value | |
|---|---|---|---|
| Number | 11 | 38 | |
| Mean age at time of biopsy | 68 | 60 | 0.08 |
| Clinical phenotype: | |||
| Oculobulbar | 6a | 0 | |
| Generalized | 5 | 38 | <0.001 |
| Distribution of necrotic fibres | |||
| Multifocal clusters | 10/11 | 0/36 | <0.001 |
| Scattered | 1/11 | 36/36b | |
| Necrotic fibres per LPF, median (range) | 3.78 (0.4–40) | 4.7 (1.5–55) | 0.7 |
| Regenerating fibres per LPF, median (range) | 5 (0–14.6) | 8.4 (1–65) | 0.003 |
| Inflammation | 3/11 | 13/38c,d | 0.7 |
| Endomysial | 1/3 | 8/13 | <0.001 |
| Perimysial | 2/3 | 9/13 | 0.4 |
| Invasion of non-necrotic fibre (autoaggression) | 0/11 | 2/38 | 0.36 |
| Non-rimmed vacuoles, n | 2/11 | 14/38 | 0.4 |
| Internalized nuclei, n | 0/11 | 8/38 | <0.001 |
| Fibre splitting, n | 0/11 | 9/28 | <0.001 |
| Fibre type grouping, n | 7/11 | 11/38 | 0.02 |
| Increased connective tissue | 9/11 | 24/38 | 0.4 |
| Endomysial | 3/11 | 1/38 | |
| Perimysial | 6/11 | 23/38 | |
| Cytochrome C oxidase-negative fibres, n | 9/11 | 21/38 | 0.003 |
| Cytochrome C oxidase-negative fibres per LPF, median (range) | 1.3(0–5.4) | 0.31(0–7.5) | <0.01 |
| Ragged-blue fibres, n | 8/11 | 26/38 | <0.01 |
| Ragged-blue fibres per LPF, median (range) | 0.67 (0–1.71) | 0.14 (0–4) | <0.01 |
| Increased acid phosphatase reactivity in connective tissue, n | 8/11 | 3/38e | <0.001 |
| Sarcoplasmic congophilic deposits, n | 1/11 | 2/38 | 0.5 |
LPF = lower power field.
All bold values indicate P-value < or = 0.05.
Muscle biopsies were taken from triceps (n = 3), splenius capitis (n = 1), biceps (n = 1) and deltoid (n = 1). These muscles were not clinically affected but had myopathic changes on needle EMG.
Muscle biopsy slides were not available for review in two patients.
Four patients had endomysial and perimysial inflammation.
Of 13 patient with inflammation, 9 were positive for HMGCR antibodies and the remaining were seronegative.
Two patient were HMGCR antibody positive.
Necrotic fibres were scattered and evenly distributed throughout the sections in all IMNM patients as opposed to multifocal clusters seen in 10 ICIAM patients (P < 0.001, Fig. 4E). A single ICIAM patient with evenly distributed necrotic fibres had dysphagia and mild lower limb weakness for a few months preceding ICI therapy. Multifocal clusters of necrotic fibres had 92% sensitivity and 100% specificity to differentiate ICIAM from IMNM. Few lymphocytes were noted in 3 ICIAM and 12 IMNM patients, but they were too infrequent to diagnose an inflammatory myopathy. ICIAM patients had a significantly lower number of regenerating fibres per LPF and higher number of ragged-blue fibres and cytochrome c oxidase-negative fibres per LPF, while number of necrotic fibres per LPF was not significantly different compared to IMNM patients (Fig. 4A and B). Interstitial histiocytes over-reactive for acid phosphatase in the perimysium were more common in ICIAM (n = 8) than IMNM (n = 3) patients (P < 0.001). CK values directly correlate with the number of necrotic fibres per LPF in IMNM patients, but not in ICIAM patients (Fig. 4C and D). The number of necrotic fibres/LPF, regenerating fibres/LPF and CK values in oculobulbar and generalized ICIAM patients who underwent muscle biopsy were not significantly different (necrosis; P = 0.3, regeneration; P = 0.7, CK; P = 0.4).
Treatment
ICI was discontinued in 22 patients. ICI was not stopped in two patients (CTCAE Grade III) due to the concern for cancer progression. Both patients received concurrent intravenous methylprednisolone (IVMP) or oral prednisone with normalization of CK and resolution of symptoms. One of them was able to discontinue corticosteroids after 2 months, while the other required ongoing therapy with low dose prednisone for 1.5 years until the last follow-up.
Immunomodulatory therapy was introduced in 22/24 patients, including two patients that ICI was not discontinued. Other two patients declined treatment and went into hospice care. Median time from symptom onset to treatment was 7 days (range 0–95 days). Initial treatments included corticosteroid monotherapy in 16 patients (14 IVMP and 2 oral prednisone) and combined therapy in 6 patients (4 IVMP and plasma exchange, 1 IVMP and IVIG and 1 oral prednisone and IVIG). All patients who received an initial 5-day course of IVMP (1 g/day) were subsequently transitioned to oral prednisone. The median duration of treatment was 9 weeks (range 1–112 weeks). Twenty treated patients showed improvement of muscle strength on the exam; however, one subsequently developed aspiration pneumonia and decided to terminate the treatment for myopathy. Weakness was stabilized in the remaining two patients. The median modified Rankin Scale scores before and after treatment were 3 (range 0–5) and 1 (range 0–6), respectively (P = 0.02). Among 14 patients with hyperCKemia, post-treatment CK levels were lower than pre-treatment values in all patients, including eight patients with normalization of CK values. The median time from initiation of immunomodulatory therapy to normal CK was 8 days (range 1–35 days). The patients with persistent hyperCKemia showed a 20–90% reduction (median 37.5%) of CK levels compared to pre-treatment values.
During the follow-up period, eight patients were able to stop immunomodulatory therapy after a median of 5 months of treatment (range 1–24 months) with a median of 23 months of treatment-free (range 0.5–48 months). Sixteen patients required ongoing immunosuppressive therapy with prednisone <20 mg/day (n = 13), cyclophosphamide (n = 2) or rituximab (n = 1). ICI was reinstituted in three patients after the resolution of weakness, one of whom was on low dose prednisone and the others were not on any immunomodulatory therapy at the time of ICI rechallenge. All three patients developed severe irAEs (two recurrent ICIAM and one colitis) after 1–2 doses of ICI.
Survival analysis and outcomes
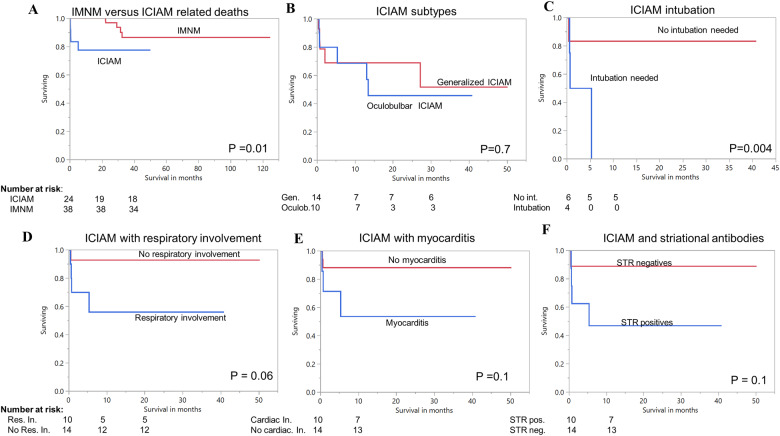
The overall mortality rate among ICIAM patients was 42% (10/24). Five patients died from ICIAM-related complications (respiratory failure in four CTCAE Grade V patients and myocarditis in one CTCAE Grade III patient) and the remaining five patients died from cancer progression (n = 2) or withdrawal/decline of myopathy treatment (n = 3). We included only five patients who died from ICIAM or myocarditis in survival analysis. At data cut-offs, 5/24 ICIAM patients died compared to 4/38 IMNM patients (P = 0.01). The median overall survival was significantly worse in ICIAM compared to IMNM (Fig. 5A). Probability of survival for ICIAM associated deaths at 0.5, 1 and 2 months was 91%, 83% and 78%, respectively. Probability of survival for IMNM patients at 12 and 36 months was 100% and 82.9%. Overall survival (OS) was similar between oculobulbar and generalized ICIAM (P = 0.7; Fig. 5B). Respiratory muscle involvement occurred in 42% (n = 10) of ICIAM patients with nearly half (n = 4) requiring intubation. ICIAM patients with intubation (P = 0.004) had significantly shorter overall survival (median survival of 22 days) but number of patients were too small to draw a definite association. ICIAM patients with striational antibodies, respiratory insufficiency, myocarditis or dysphagia had higher mortality than those without, but none reach statistical significance (Fig. 5D–F).
Figure 5.
Survival analysis in ICIAM. (A) Overall survival analysis for immune check point inhibitor-associated myopathy (ICIAM) versus immune-mediated necrotizing myopathy (IMNM), demonstrating significant lower survival for ICIAM. (B) Survival comparison for ICIAM-related deaths between oculobulbar versus generalized ICIAM subgroup. (C) ICIAM patients who required intubation have reduced survival compared to non-intubated ICIAM patients. (D) There is a trend for lower survival in ICIAM patients with respiratory insufficiency compared to those without. (E) Survival analysis also shows a trend for lower survival in ICIAM patients with myocarditis compared to those without. (F) Survival analysis of patients with positive striational antibodies shows a trend for lower survival compared to seronegative patients. Cardiac In = cardiac involvement; Gen = generalized; Int. = intubation; Neg = negative; Oculob = oculobulbar; Pos. = positive; Res. In. = respiratory involvement; STR = striational antibodies.
Discussion
This study reports the clinical, serological and pathological features of the largest cohort of ICIAM patients to date, consisting of 11 patients with pathologically defined myopathy and 13 patients with a diagnosis of myopathy based on combined laboratory, electrophysiological and/or radiological criteria. ICIAM patients with and without muscle biopsies share a similar demographic, clinical and ancillary test profiles. All pathologically defined patients had necrotizing myopathy-like pathology. A systematic comparison between ICIAM and ICI-naïve IMNM patients discloses several distinctive clinico-sero-pathological characteristics of ICIAM.
Clinical characteristics
Oculobulbar phenotype is a distinctive feature of ICIAM
In most ICIAM patients, myopathy symptoms started shortly after ICI initiation, most commonly after the second dose of ICI. Oculobulbar weakness can be the predominant or sole presentation in nearly half of the ICIAM patients, while the remaining patients had proximal weakness with or without oculobulbar involvement. Bulbar weakness occurred in a similar proportion of ICIAM and IMNM patients, but oculomotor weakness was only observed in ICIAM patients. One-fourth of our ICIAM patients had ophthalmoparesis, which confirm the previous notion of oculomotor involvement as a characteristic feature of ICIAM (Liewluck et al., 2018; Touat et al., 2018; Seki et al., 2019). Ophthalmoparesis is a common manifestation of myasthenia gravis, orbital myositis and certain hereditary myopathies, including mitochondrial myopathies, but is not considered a typical presentation of any other acquired or immune-mediated myopathies (Kono et al., 2012; Allenbach et al., 2014; Kassardjian et al., 2015; Allenbach et al., 2017; Milone, 2017; McNab, 2020).
CK can be normal in oculobulbar ICIAM, which can mimic myasthenia gravis
Half of our oculobulbar ICIAM patients had normal CK levels in contrast to those previously reported ICIAM patients with oculomotor involvement, who had elevated CK levels (Touat et al., 2018; Seki et al., 2019). The presence of myopathic motor unit potentials and fibrillation potentials in clinically affected or unaffected axial and limb muscles, the absence of decrement on RNS or abnormal jitter on SFEMG, and the observed radiological abnormalities in oculobulbar, axial or appendicular muscles signal the underlying myopathic process. This is further supported by the myopathological evidence of necrotic fibres in three oculobulbar ICIAM patients with normal CK levels. Therefore, it is clinically challenging to distinguish oculobulbar ICIAM from myasthenia gravis when CK level is not elevated.
AChR antibodies in ICIAM: coexisting myasthenia or a marker of autoimmunity?
As mentioned earlier, the myasthenia gravis-myopathy overlap syndrome has been increasingly recognized in ICI-treated patients (Suzuki et al., 2017; Kao et al., 2018). Three of our ICIAM patients were positive for AChR antibodies, all of whom had severe respiratory insufficiency. Myopathic motor unit potentials with associated fibrillation potentials, elevated CK levels and necrotic fibres support a diagnosis of myopathy. It is unclear whether these patients had concomitant myasthenia gravis or the presence of AChR antibodies is simply an indicator of nonspecific autoimmunity (Robbins et al., 2019). Although SFEMG was not performed in any of these three patients, none had decrement on RNS, which is a very sensitive diagnostic tool for a defect of neuromuscular transmission during myasthenic crisis (Oh et al., 2019). The normal RNS in these patients with severe respiratory insufficiency suggests that a defect of neuromuscular transmission, if present, is not a major contributing factor to their symptoms. Some of the reported patients with ICI-associated myasthenia gravis and respiratory insufficiency also had no decrement on RNS, one of whom also had normal SFEMG, suggesting that the myopathy might be the main culprit for the muscle weakness in these reported patients (Loochtan et al., 2015; Sciacca et al., 2016; Chang et al., 2017; Gonzalez et al., 2017; Suzuki et al., 2017; Tan et al., 2017; Fellner et al., 2018).
Ancillary investigations
Few characteristic laboratory findings emerge from the current ICIAM cohort.
ICIAM patients had lower CK levels compared to IMNM patients
CK was not universally elevated in all patients, differing from previous reports (Liewluck et al., 2018; Touat et al., 2018; Seki et al., 2019). Seven ICIAM patients had normal CK level, of which three had muscle biopsies showing necrotic fibres. It is known that the number of necrotic fibres correlates with the CK levels in IMNM (Allenbach et al., 2018a). The number of necrotic fibres in these three patients with normal CK was unexpectedly similar to what observed in ICIAM or IMNM patients with hyperCKemia in our cohort. Therefore, the lower CK levels in some ICIAM patients are not due to the lower number of necrotic fibres in affected muscles but could probably be due to a more focal muscle involvement in these patients.
Two of seven ICIAM patients with normal CK had elevated aldolase levels. Such discrepancy of CK and aldolase levels has been reported in some patients with other immune-mediated myopathies (Carter et al., 2001; Nozaki and Pestronk, 2009; Mathur et al., 2014). A prospective study of aldolase and CK levels in ICIAM patients is crucial to verify the diagnostic utility of aldolase measurement in ICIAM patients with normal CK.
Lymphopenia was more frequent in ICIAM compared to IMNM patients
Lymphopenia encountered at the time of muscle weakness was frequent in ICIAM patients. Although most ICIAM patients received chemotherapy or radiotherapy predated ICI, normal lymphocyte count at the time of ICI initiation suggested that post-ICI lymphopenia was a result of ICI, not other preceding cancer therapies. B cell lymphopenia following ICI administration has been linked to an increased risk of developing irAEs, including ICIAM (Das et al., 2018; Mammen et al., 2019); however, none of our patients underwent lymphocyte subset analysis. Though the mechanism of ICI-induced lymphopenia remains unknown, the routine evaluation of lymphocyte count and subset could be helpful to identify patients potentially at high risk of developing ICIAM or other irAEs.
ICIAM as a seronegative IMNM
While our ICIAM patients had necrotizing myopathy-like pathology, none of the patients tested positive for anti-HMGCR or SRP-antibodies. It is possible that the homogenous phenotype of oculobulbar involvement in ICIAM could be a result of not yet discovered antibody. Striational antibodies were positive in nearly half of our ICIAM patients and were reported in 68% of Japanese programmed cell death-1 inhibitor-associated myopathy cohort (Seki et al., 2019). Six of our eight striational antibody-positive patients had a titre ≥ 1:7680. Such a high titre of striational antibodies was reported with a high predictive value for underlying cancers (McKeon et al., 2013). Further studies are required to investigate whether striational antibody positivity can be used as a serologic biomarker for ICIAM.
EMG identified subclinical axial or limb muscle involvement in oculobulbar ICIAM patients
Myopathic motor unit potentials were observed in axial or limb muscles in all ICIAM patients, including the oculobulbar patients with no limb weakness or normal CK. Myopathic changes were accompanied by fibrillation potentials in nearly 85% of patients. EMG can therefore help identify subclinical involvement of axial or limb muscles in oculobulbar ICIAM patients and select the appropriate muscle for biopsy. Several non-myopathic conditions, e.g. deconditioning, malnutrition or myasthenia gravis, may give rise to short-duration, low-amplitude motor unit potentials, mimicking myopathy, but without fibrillation potentials. Muscle biopsy therefore is important to distinguish these pseudomyopathic conditions from myopathy when fibrillation potentials are absent.
Imaging studies identified subclinical cervical paraspinal muscle involvement in ICIAM patients
The systematic assessment of radiological abnormalities in a large cohort of ICIAM patients has not been previously reported. Muscle MRI and PET scans were abnormal in 11/20 patients who had imaging done after symptoms onset. Our result suggests that imaging studies could be utilized as a non-invasive diagnostic tool of ICIAM in conjunction with serologic or electrophysiologic abnormalities, especially when muscle biopsy is not possible. Paraspinal and masticatory muscles were the most common radiologically affected muscles. Radiological abnormalities mirrored the clinical findings in most patients. Interestingly the paraspinal musculature was abnormal on imaging in nearly 50% of patients despite neck extensor weakness identified in only a minority of patients. In comparison to electrophysiological studies, imaging studies are particularly useful in evaluating muscles that are not accessible (e.g. extraocular muscles) or not routinely examined by needle EMG (e.g. muscle of mastication, trapezius or cervical paraspinal muscles) in cases of suspected myopathy. However, needle EMG appears more sensitive than imaging studies when it comes to the evaluation of limb muscles given that all of the radiologically normal ICIAM patients had myopathic changes on needle EMG. Imaging also has sensitivity and specificity limitations, as muscles groups such as extraocular muscles, muscles of mastication and paraspinal muscles can have findings physiologically on imaging. For example, normal extraocular muscles are known to demonstrate physiologic FDG uptake, even to quite prominent degree or robustly enhance on MRI. Thus, as in the example shown in Fig. 3, T2 hyperintensity and/or enlargement of these muscles are more reliable when attempting to identify myopathy. These findings may be seen to better advantage on dedicated orbit MRI than non-dedicated brain MRI.
Multifocal clusters of necrotic fibres are unique to ICIAM
Despite various histopathologically characterized immune-mediated myopathies have been reported in ICIAM patients, all 11 ICIAM patients in our cohort who underwent muscle biopsy had necrotizing myopathy-like pathology. However, by comparing to ICI-naïve IMNM muscle biopsies, we identify multifocal clusters of necrotic fibres as a distinctive pathological feature of ICIAM similar to what previously reported (Seki et al., 2019). Although CK values were significantly lower in ICIAM patients compared to IMNM patients, we observed similar numbers of necrotic fibres between ICIAM and IMNM. Unlike IMNM, the number of necrotic fibres did not correlate with the CK levels in ICIAM. The number of regenerating fibres was significantly lower in ICIAM compared to IMNM, which may suggest an impaired regeneration in ICIAM.
ICIAM muscle biopsies also showed a higher number of cytochrome c oxidase-negative and ragged-blue fibres, which indicate mitochondrial dysfunction, and more frequent interstitial histiocytes compared to IMNM biopsies. The pathological evidence of mitochondrial dysfunction in ICIAM is of uncertain significance. The possibility that the histological signs of mitochondrial dysfunction represent the result of drug toxicity cannot be excluded. Given ophthalmoparesis is a common manifestation of mitochondrial myopathies, whether the mitochondrial changes observed here have any effect on the pathogenesis of extraocular muscle weakness remain to be elucidated. The presence of macrophages in connective tissue elements have been previously reported in ICIAM patients and also in anti-HMGCR antibody-IMNM (Alshehri et al., 2015; Touat et al., 2018; Seki et al., 2019).
Treatment
ICIAM is a steroid-responsive myopathy
All treated patients received steroids, most as a monotherapy. Weakness was improved in most patients and stabilized in a couple of patients after immunomodulatory therapy. In those two patients with CTCAE Grade III, whom ICI was never stopped, symptoms resolved with concurrent immunomodulatory therapy. About one-third of the patients were in remission and was off any immunomodulatory therapy for a median of nearly 2 years, including one patient whom ICI was never discontinued. Therefore, concurrent treatment of ICIAM, while on ICI, may be possible in mild cases, but close follow-up is warranted. The ICI rechallenge in patients with ICIAM has not been widely reported (Delyon et al., 2019). In our cohort, all three patients who were restarted on ICI had serious irAEs necessitating cessation of ICI. Based on our experience, it would be prudent not to reintroduce ICI in patients with ICIAM.
Intubation is a poor prognostic factor in ICIAM
To our knowledge, this study is the first to analyse survival in ICIAM. As expected, ICIAM has an overall worse survival compared to IMNM group. ICIAM patients with severe respiratory failure requiring intubation had a significantly shorter survival. The ICIAM-related mortality rate in our cohort was 20%, which is similar to what reported in World Health Organization (WHO) database of individual safety case reports (Anquetil et al., 2018). However, the WHO database identified coexisting myocarditis as a poor prognostic factor, but authors had only limited access to the detailed medical records of those patients and survival curves or overall survival was not performed. Despite myocarditis occurred in one-third of our patients, myocarditis was not a poor predictor of survival in our study. Two other cohorts of ICIAM estimated mortality rate at 30–50%, but all their patients died from cancer-related causes and no death was associated with ICIAM (Touat et al., 2018; Seki et al., 2019).
Limitations
Patients in this series received non-standardized investigations and treatments, as these were at the discretion of the treating physicians. The exclusion of Grades 1 and 2 ICIAM due to the uncertainty of myopathy diagnosis in those patients could contribute to the bias when compared ICIAM patients to IMNM cohort, especially regarding the disease severity and mortality. Despite these limitations, this study has revealed comprehensive spectrum of the clinical and laboratory features of ICIAM.
Conclusion
ICIAM typically occurs within the first 3 months of ICI initiation and nearly half of the patients had predominant oculobulbar weakness with no or subtle proximal limb involvement. CK levels were normal in one-third of patients, most of which had oculobulbar phenotype. All ICI-treated patients, who develop oculobulbar, axial or limb weakness, should undergo EMG regardless of the CK values. Needle EMG of cervical paraspinals and muscles of mastication should be considered if needle EMG of limb muscles are unrevealing. Positive AChR or striational antibodies should be interpreted cautiously when the patients have severe weakness and no electrodiagnostic evidence of neuromuscular transmission defect. Imaging studies can help evaluate cranial muscles, including for instance extraocular muscles, which are not easily accessible by needle EMG. Concomitant irAEs are not uncommon, especially myocarditis. Intubation is a poor prognostic factor. ICI should be discontinued and immunomodulatory therapy should be commenced promptly. Necrotic fibres arranging in multifocal clusters are distinctive features of ICIAM. Future studies looking into the regenerating capacity and mitochondrial dysfunction of muscle fibres may shed light on the pathomechanism of ICIAM. Lymphocyte count and subset analysis may help identify patients who are at risk of developing ICIAM or other irAEs.
Supplementary material
Supplementary material is available at Brain Communications online.
Competing interest
Dr. Liewluck reports receiving a discretionary fund from the Department of Neurology, Mayo Clinic for unrelated research projects. Dr. Milone reports receiving research funding from a Mayo Clinic benefactor and a discretionary fund from the Department of Neurology, Mayo Clinic for unrelated research projects, and an honorarium to serve as associated editor of Neurology Genetics. Dr. Zekeridou reports a patent on PDE10A-IgG as a biomarker of paraneoplastic neurological autoimmunity. Drs. Shelly, Triplett, Pinto and Diehn report no disclosure.
Supplementary Material
Glossary
- CK =
creatine kinase
- CTCAE =
Common Terminology Criteria for Adverse Events version
- HMGCR =
3-Hydroxy-3-Methylglutaryl-CoA Reductase
- ICIAM =
immune checkpoint inhibitor-associated myopathy
- IMNM =
immune-mediated necrotizing myopathy
- irAEs =
immune-related adverse events
- IVMP =
intravenous methylprednisolone;
- PD-1 =
programmed cell death-1
- RNS =
repetitive nerve stimulation
- SFEMG =
single-fibre EMG
- SRP =
signal recognition particle
References
- Allenbach Y, Drouot L, Rigolet A, Charuel JL, Jouen F, Romero NB, et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine 2014; 93: 150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allenbach Y, Benveniste O, Goebel H-H, Stenzel W. Integrated classification of inflammatory myopathies. Neuropathol Appl Neurobiol 2017; 43: 62–81. [DOI] [PubMed] [Google Scholar]
- Allenbach Y, Arouche-Delaperche L, Preusse C, Radbruch H, Butler-Browne G, Champtiaux N, et al. Necrosis in anti-SRP(+) and anti-HMGCR(+)myopathies: role of autoantibodies and complement. Neurology 2018. a; 90: e507–e517. [DOI] [PubMed] [Google Scholar]
- Allenbach Y, Mammen AL, Benveniste O, Stenzel W, Allenbach Y, Amato A, et al. 224th ENMC International Workshop: clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14-16 October 2016. Neuromuscul Disord 2018. b; 28: 87–99., [DOI] [PubMed] [Google Scholar]
- Alshehri A, Choksi R, Bucelli R, Pestronk A. Myopathy with anti-HMGCR antibodies: perimysium and myofiber pathology. Neurol Neuroimmunol Neuroinflamm 2015; 2: e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anquetil C, Salem J-E, Lebrun-Vignes B, Johnson DB, Mammen AL, Stenzel W, et al. Immune checkpoint inhibitor-associated myositis: expanding the spectrum of cardiac complications of the immunotherapy revolution. Circulation 2018; 138: 743–5. [DOI] [PubMed] [Google Scholar]
- Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007; 38: 1091–6. [DOI] [PubMed] [Google Scholar]
- Bilen MA, Subudhi SK, Gao J, Tannir NM, Tu S-M, Sharma P, et al. Acute rhabdomyolysis with severe polymyositis following ipilimumab-nivolumab treatment in a cancer patient with elevated anti-striated muscle antibody. J Immunother Cancer 2016; 4: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitton K, Michot J-M, Barreau E, Lambotte O, Haigh O, Marabelle A, et al. Prevalence and clinical patterns of ocular complications associated with anti-PD-1/PD-L1 anticancer immunotherapy. Am J Ophthalmol 2019; 202: 109–17. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM; in oration with the National Comprehensive Cancer Network, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36: 1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JD, Kanik KS, Vasey FB, Valeriano-Marcet J. Dermatomyositis with normal creatine kinase and elevated aldolase levels. J Rheumatol 2001; 28: 2366–7. [PubMed] [Google Scholar]
- Chang E, Sabichi AL, Sada YH. Myasthenia gravis after nivolumab therapy for squamous cell carcinoma of the bladder. J Immunother 2017; 40: 114–6. [DOI] [PubMed] [Google Scholar]
- Das R, Bar N, Ferreira M, Newman AM, Zhang L, Bailur JK, et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 2018; 128: 715–20., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delyon J, Brunet-Possenti F, Leonard-Louis S, Arangalage D, Baudet M, Baroudjian B, et al. Immune checkpoint inhibitor rechallenge in patients with immune-related myositis. Ann Rheum Dis 2019; 78: e129. [DOI] [PubMed] [Google Scholar]
- Dubey D, David WS, Amato AA, Reynolds KL, Clement NF, Chute DF, et al. Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology 2019; 93: e1093–e1103. [DOI] [PubMed] [Google Scholar]
- Fellner A, Makranz C, Lotem M, Bokstein F, Taliansky A, Rosenberg S, et al. Neurologic complications of immune checkpoint inhibitors. J Neurooncol 2018; 137: 601–9. [DOI] [PubMed] [Google Scholar]
- Gonzalez NL, Puwanant A, Lu A, Marks SM, Živković SA. Myasthenia triggered by immune checkpoint inhibitors: new case and literature review. Neuromusc Disord 2017; 27: 266–8. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11: 3887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao JC, Liao B, Markovic SN, Klein CJ, Naddaf E, Staff NP, et al. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol 2017; 74: 1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao JC, Brickshawana A, Liewluck T. Neuromuscular complications of programmed cell death-1 (PD-1) inhibitors. Curr Neurol Neurosci Rep 2018; 18: 63. [DOI] [PubMed] [Google Scholar]
- Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015; 72: 996–1003. [DOI] [PubMed] [Google Scholar]
- Kono S, Bunai T, Terada T, Shimoyama K, Konishi T, Shirakawa K, et al. Subacute progressive ophthalmoplegia associated with dermatomyositis. J Neurol 2012; 259: 1982–4. [DOI] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271: 1734–6. [DOI] [PubMed] [Google Scholar]
- Liewluck T, Kao JC, Mauermann ML. PD-1 inhibitor-associated myopathies: emerging immune-mediated myopathies. J Immunother 2018; 41: 208–11. [DOI] [PubMed] [Google Scholar]
- Liewluck T, Laughlin RS, Litchy WJ, Milone M. Neuromuscular transmission defects in myopathies. Muscle Nerve 2019; 60: E8–E9. [DOI] [PubMed] [Google Scholar]
- Loochtan AI, Nickolich MS, Hobson-Webb LD. Myasthenia gravis associated with ipilimumab and nivolumab in the treatment of small cell lung cancer. Muscle Nerve 2015; 52: 307–8. [DOI] [PubMed] [Google Scholar]
- Maddison P, Sadalage G, Ambrose PA, Jacob S, Vincent A. False-positive acetylcholine receptor antibody results in patients without myasthenia gravis. J Neuroimmunol 2019; 332: 69–72. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Rajan A, Pak K, Lehky T, Casciola-Rosen L, Donahue RN, et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann Rheum Dis 2019; 78: 150–2., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur T, Manadan AM, Thiagarajan S, Hota B, Block JA. The utility of serum aldolase in normal creatine kinase dermatomyositis. J Clin Rheumatol 2014; 20: 47–8. [DOI] [PubMed] [Google Scholar]
- McKeon A, Lennon VA, LaChance DH, Klein CJ, Pittock SJ. Striational antibodies in a paraneoplastic context. Muscle Nerve 2013; 47: 585–7. [DOI] [PubMed] [Google Scholar]
- McNab AA. Orbital myositis: a comprehensive review and reclassification. Ophthalmic Plast Reconstr Surg 2020; 36: 109–17. [DOI] [PubMed] [Google Scholar]
- Milone M. Diagnosis and management of immune-mediated myopathies. Mayo Clin Proc 2017; 92: 826–37. [DOI] [PubMed] [Google Scholar]
- Musset L, Miyara M, Benveniste O, Charuel J-L, Shikhman A, Boyer O, et al. Analysis of autoantibodies to 3-hydroxy-3-methylglutaryl-coenzyme A reductase using different technologies. J Immunol Res 2014; 2014: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddaf E, Milone M. Hereditary myopathies with early respiratory insufficiency in adults. Muscle Nerve 2017; 56: 881–6. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Pestronk A. High aldolase with normal creatine kinase in serum predicts a myopathy with perimysial pathology. J Neurol Neurosurg Psychiatry 2009; 80: 904–8. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Jeong D, Lee I, Alsharabati M. Repetitive nerve stimulation test in myasthenic crisis. Muscle Nerve 2019; 59: 544–8. [DOI] [PubMed] [Google Scholar]
- Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, on behalf of the Society for Immunotherapy of Cancer Toxicity Management Working Group, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunotherapy Cancer 2017; 5: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins NM, Mozaffar T, Mammen AL, Liewluck T, Guidon A, Lawson VH, et al. Reader response: pearls & oysters: pembrolizumab-induced myasthenia gravis. Neurology 2019; 93: 183–184. [DOI] [PubMed] [Google Scholar]
- Sanders DB, Arimura K, Cui L, Ertaş M, Farrugia ME, Gilchrist J, et al. Guidelines for single fiber EMG. Clin Neurophysiol 2019; 130: 1417–1439., [DOI] [PubMed] [Google Scholar]
- Sciacca G, Nicoletti A, Rampello L, Noto L, Parra HJS, Zappia M, et al. Benign form of myasthenia gravis after nivolumab treatment. Muscle Nerve 2016; 54: 507–509. [DOI] [PubMed] [Google Scholar]
- Seki M, Uruha A, Ohnuki Y, Kamada S, Noda T, Onda A, et al. Inflammatory myopathy associated with PD-1 inhibitors. J Autoimmun 2019; 100: 105–113. [DOI] [PubMed] [Google Scholar]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348: 56–61. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Ishikawa N, Konoeda F, Seki N, Fukushima S, Takahashi K, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017; 89: 1127–1134., [DOI] [PubMed] [Google Scholar]
- Tan RYC, Toh CK, Takano A. Continued response to one dose of nivolumab complicated by myasthenic crisis and myositis. J Thoracic Oncol 2017; 12: e90–e91. [DOI] [PubMed] [Google Scholar]
- Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Auré K, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018; 91: e985–e994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Results are available upon reasonable request.