Abstract
Background and purpose:
Irregularities of angiogenesis may participate in the pathogenesis of diabetes complications. Pramlintide is an amylin analogue administered for the treatment of type 1 and type 2 diabetes. The present investigation aimed at surveying the effect of pramlintide on angiogenesis-related markers in human umbilical vein endothelial cells (HUVECs).
Experimental approach:
The proliferation of cells was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) method. The effect of pramlintide on migration was estimated by Transwell® assay. in vitro evaluation of angiogenesis was performed by tube formation assay. The secretion of vascular endothelial growth factor (VEGF) to the supernatant of HUVECs was measured by an enzyme- linked immunosorbent assay (ELISA) kit. All experiments were performed in triplicate.
Findings / Results:
Pramlintide exhibited no inhibitory effect on HUVECs proliferation. It significantly increased cell migration at the concentration of 1 μg/mL. Pramlintide (1 μg/mL) also enhanced average tubules length, size, and the mean number of junctions. However, there was not any significant change in VEGF release from HUVECs.
Conclusion and implications:
Findings of this research revealed the effect of pramlintide on angiogenesis- related markers via enhancing migration and tubulogenesis in vitro, suggesting a worthwhile proposition for further clinical researches on improving vascular complications and healing of diabetic wounds.
Keywords: Angiogenesis, Cell migration, Diabetes mellitus, HUVEC, Pramlintide, VEGF
INTRODUCTION
Diabetes mellitus is a complicated endocrine disorder and the fourth leading cause of death in the world. This common health problem affects about 422 million persons (8.5% of the adult population) worldwide and its prevalence is estimated to increase to 642 million in the next 25 years (1,2). Vascular complications of diabetes are important reasons for mortality and morbidity in diabetic patients (3). Endothelial dysfunction and impaired angiogenesis are considered as the main pathological features of diabetic vascular problems (4). Angiogenesis refers to the development of new blood vessels from preexisting vessels (5). This process occurs during some normal and pathological conditions through a series of organized stages. During angiogenesis, various regulating factors are involved in the stimulation of endothelial cells, degradation of the capillary basement membrane, capillary sprouting, migration, tube formation, and finally in maturation and stabilization of the new vessel (6). Diabetes is associated with abnormal angiogenesis in a tissue-specific manner.
Enhanced angiogenesis is a main pathological characteristic in diabetic retinopathy and reduced angiogenesis contributes to the impaired wound healing, peripheral artery diseases, and various ischemic conditions (7).
Pramlintide is an injectable antihyperglycemic drug, approved for the treatment of types 1 and 2 diabetic patients who take insulin and do not reach the desired glucose levels (8). It regulates blood glucose levels through slowing gastric emptying, stimulating satiety and decreasing secretion of glucagon. Pramlintide is a synthetic analog of human amylin and functions as an amylin agonist (9). Amylin or islet amyloid polypeptide (IAPP), is a peptide hormone that acts as a part of the endocrine system of the pancreas in controlling postprandial blood glucose along with insulin (10).
Amylin indeed belongs to the calcitonin gene peptide superfamily, whose members are calcitonin, calcitonin gene-related peptide (CGRP), adrenomedullin, and amylin with some similar biological effects due to the similarity in their structures and receptors (11). There are pieces of evidence that most members of this superfamily have important effects on angiogenesis. Adrenomedullin functions as an angiogenic growth factor and inhibits apoptosis in endothelial cells (12). Calcitonin promotes several stages of angiogenesis via a direct effect on endothelial cells and CGRP also stimulates angiogenesis and facilitates wound healing (13,14).
Regarding the role of calcitonin superfamily peptides on angiogenesis and cross-reactivity between their receptors, the aim of this study was to evaluate the effect of pramlintide as an amylin analog on angiogenesis in human umbilical vein endothelial cells (HUVECs).
MATERIALS AND METHODS
Chemicals
Pramlintide was provided by AstraZeneca Co. (Cambridge, UK). Dulbecco’s modified eagle’s medium (DMEM), fetal bovine serum (FBS), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit were purchased from Bioidea Co. (Tehran, I.R. Iran). Transwell® inserts were obtained from SPL Life Sciences Co., Ltd. (Gyeonggi-do, Korea). Geltrex was purchased from Gibco- BRL, Life Technologies Inc. (California, USA). Calcein acetoxymethyl dye was prepared from Santa Cruz Biotechnology Inc. (Santa Cruz, Canada). Recombinant human vascular endothelial growth factor (VEGF) and the enzyme-linked immunosorbent assay (ELISA) kit for its evaluation were purchased from PeproTech, Inc. (New Jersey, USA).
Cell culture
HUVECs (Pasteur Institute, Tehran, I.R. Iran) were cultured in 75 cm2 flasks in DMEM supplemented with 10% FBS and 100 U/mL penicillin-streptomycin at 37 °C in a humidified 5% CO2 incubator. All experiments were performed in 2 to 4 passages.
MTT assay
The viability of HUVECs was assessed using the MTT method (15). Briefly, the cells were seeded at 1 × 105 cells/well in the 96-well plates. After 24 h plating, HUVECs were treated with pramlintide at 1, 2, 5, 10, and 20 μg/mL and then incubated for an additional 24 h. After that, MTT reagent (10 μL) was added and cultivated for 4 h at 37 °C. The formazan crystals resulting from MTT reaction with living cells were dissolved in dimethyl sulfoxide and absorbance was measured using a microplate reader/spectrophotometer (Bio- Tek, PowerWave XS, USA) at 570 nm. The cells without any exposure to pramlintide were considered as control. The viability of treated HUVECs was evaluated by comparison of the absorbance of each sample with control. All experiments were done in triplicate.
Cell migration assay
Cell migration was evaluated using Transwell® inserts with 8 μm pore sizes. In brief, HUVECs were treated with vehicle or pramlintide (1 and 10 μg/mL) or with VEGF (10 ng/mL, as the standard control) for 24 h and then added to the upper chamber (3 × 104 cells/well). The desired chemo-attractant (growth medium supplemented with 20% FBS) was placed into the lower chamber. After 4 h incubation, the media and non-migrated cells were removed and migrating cells were fixed with 70% ethanol and stained with 0.2% crystal violet for 10 min. The numbers of migrated cells toward the chemo-attractant were counted using an inverted microscope (Nikon Instruments, New York, USA) (16).
Capillary tube formation assay
For evaluation of the effect of pramlintide on tube formation, the Geltrex™ reduced growth factor basement membrane matrix was used. Briefly, the ice-cold 24-well plates were coated with Geltrex™ (10 ng/mL) and incubated at 37 °C for 30 min. HUVECs were plated (1.2 × 105/well) in the absence or presence of the pramlintide (1 and 10 μg/mL) or VEGF (2 ng/mL) on the polymerized Geltrex™ surface layer in DMEM. After 24 h incubation at 37 °C / 5% CO2, the cells were washed with Hanks balanced salt solution and stained with calcein acetoxymethyl dye. Photographs of each well were taken with a Nikon Coolpix camera attached to an inverted fluorescent microscope. The average tubules length, size and the mean number of junctions were measured in 5 high power fields of each well using AngioQuant software (MATLAB, Inc. Tampere, Finland) (17).
VEGF secretion assay
VEGF concentration was measured in supernatants of treated HUVECs by an ELISA kit according to the protocol provided by the manufacturer. The amount of VEGF released in the supernatants was calculated using a standard curve and expressed in pg/mL (18).
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). For statistical analysis, one-way analysis of variance (ANOVA) followed by Tukey post-hoc test was done using SPSS software version 18.0. The P-value < 0.05 was considered statistically significant.
RESULTS
Effect of pramlintide on HUVECs viability
The viability of HUVECs was assessed by the MTT method after 24 h exposure to pramlintide. Treatment with pramlintide at the concentration range of 1-20 μg/mL caused no inhibitory effect on HUVECs proliferation (Fig. 1). Since there were no significant differences between various concentrations, pramlintide was used at the concentrations of 1 and 10 μg/mL in further studies.
Fig. 1.
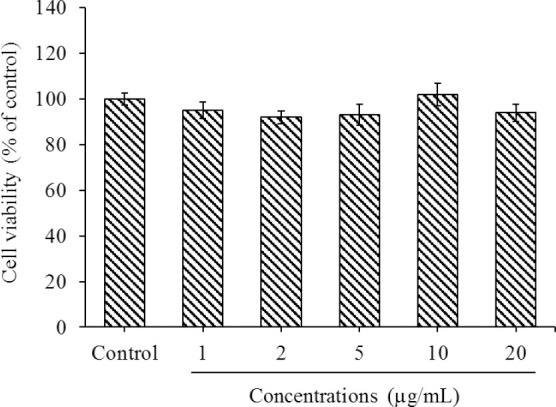
Effect of pramlintide on HUVECs viability evaluated by MTT method. Cells were treated without or with pramlintide (1-20 μg/mL) for 24 h. Data are shown as means ± SEM from triplicate experiments. HUVECs, human umbilical vein endothelial cells; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Effect of pramlintide on HUVECs migration
The effect of pramlintide on the migration of endothelial cells was evaluated by Transwell® assay. As shown in Fig. 2, administration of pramlintide at 1 μg/mL significantly increased cell migration compared to the control after 24 h incubation (P < 0.05). However, this result was significantly less than the VEGF effect (P < 0.05).
Fig. 2.

Effect of pramlintide on HUVECs migration assessed by Transwell® inserts. The 24 h-treated cells without or with pramlintide (1 and 10 μg/mL) or VEGF (10 ng/mL) were added to the upper chamber, incubated for 4 h and the number of cells in the lower chamber was counted. Data are shown as means ± SEM from triplicate experiments. *P < 0.05 and ***P < 0.001 show significant differences in compared to the control group (untreated cells); #P < 0.05 and ##P < 0.01 versus VEGF group. HUVECs, human umbilical vein endothelial cells; VEGF, vascular endothelial growth factor.
Effect of pramlintide on tube formation of HUVECs
Tube formation assay on Geltrex™ basement membrane matrix was used for in vitro evaluation of angiogenesis. Figure 3A represents the tubular structures in normal untreated HUVECs. Treatments with VEGF (Fig. 3B) and pramlintide at 1 μg/mL (Fig. 3C) promoted capillary tube formation compared to normal cells. Quantification of tube formation also exhibited angiogenic effects of VEGF (2 ng/mL) and pramlintide (1 μg/mL) after 24 h treatment in HUVECs (Figs. 4A-C). The highest average tubules length, size, and the mean number of junctions were observed in VEGF-treated cells compared to the control cells (P < 0.001). Pramlintide at 1 μg/mL also caused a significant enhancement in average tubules length, andsize and the mean number of junctions in comparison with normal untreated HUVECs (P < 0.001). Treatment with 10 μg/mL failed to increase the average tubules length size and number of junctions compared with 1 μg/mL of pramlintide (P > 0.05).
Fig. 3.
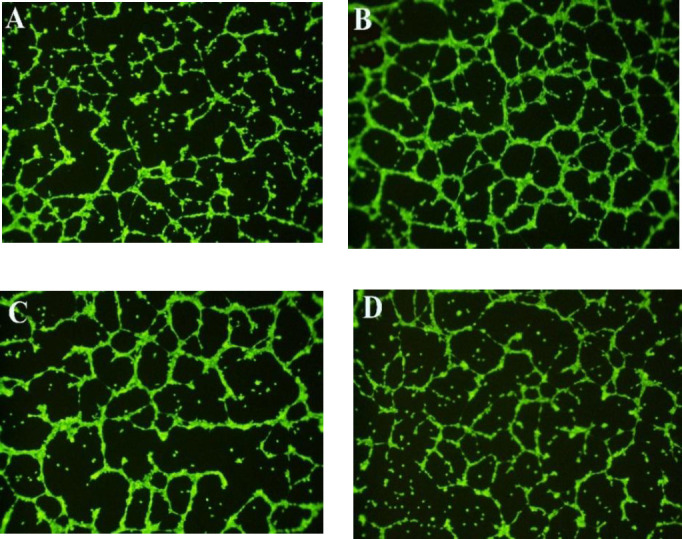
Representative fluorescence photomicrographs of HUVECs tube formation on Geltrex™ (×10). (A) Normal HUVECs were seeded on Geltrex™ and treated with (B) VEGF (2 ng/mL) or (C and D) pramlintide at 1 or 10 μg/mL, respectively, for 24 h. HUVECs, human umbilical vein endothelial cells.
Fig. 4.
Effect of pramlintide on capillary tube formation on Geltrex™. HUVECs were seeded on Geltrex™ and treated without or with pramlintide (1 and 10 μg/mL) or VEGF (2 ng/mL) for 24 h. Quantitation of tube formation was done by calculating the average (A) tubules length, (B) tubules size, and (C) mean number of junctions. Data are shown as means ± SEM from triplicate experiments. ***P < 0.001 indicates significant differences compared to the control group (untreated cells); ##P < 0.01 and ###P < 0.001 versus VEGF group. HUVECs, human umbilical vein endothelial cells; VEGF, vascular endothelial growth factor.
Effect of pramlintide on VEGF secretion
The effect of pramlintide on VEGF secretion in the cell culture supernatants was tested using an ELISA kit. No significant changes were observed after the administration of pramlintide in VEGF release into the supernatants of HUVECs (Fig. 5).
Fig. 5.

Effect of pramlintide on VEGF secretion in HUVECs determined by ELISA. Cells were treated without or with pramlintide (1 and 10 μg/mL) for 24 h and the supernatants were examined for VEGF. Data are shown as means ± SEM from triplicate experiments. HUVECs, human umbilical vein endothelial cells; VEGF, vascular endothelial growth factor; ELISA, enzyme-linked immunosorbent assay.
DISCUSSION
Pramlintide is a new glucose-lowering agent which has been effective in reducing glycosylated hemoglobin and improving glycemic control in diabetic patients in combination therapy with mealtime insulin. Unlike amylin which has an extreme toxic amyloidogenicity effect and is unsuitable for clinical use, pramlintide is a non-aggregating peptide that prevents amyloid formation (19).
Angiogenesis shows a critical role in the pathogenesis of some major complications of diabetes.
In this study, pramlintide, as an amylin analogue, promoted angiogenesis markers by increasing migration and tube formation in HUVECs, however, no significant changes were observed in the VEGF releaseed into the supernatants of HUVECs.
Amylin belongs to a superfamily of closely related genes with similar biological activity profile and cross-reactivity between receptors, which are distributed in many tissues, such as brain, gastrointestinal, cardiovascular, endothelial, and smooth muscle tissues (20). The main receptors for these structurally related peptides include calcitonin receptor, calcitonin receptor-like receptor (CRLR), and receptor- activity-modifying proteins (RAMPs) that are co-expressed in different proportions in various tissues. It is proven that some of these receptors such as RAMPs have a critical role in angiogenesis during wound healing (21). However, the role of amylin in angiogenesis is not fully understood, unlike other members. It is well known that adrenomedullin is a modulator of growth and movement, protector from apoptosis, and a potent angiogenic growth factor in endothelial cells via stimulation of Akt and mitogen-activated protein kinase and upregulation of VEGF expression through CRLR/RAMP2-CRLR/RAMP3 receptors (12). Calcitonin promotes proliferation, migration, invasion, and tube formation in endothelial cells in a concentration-dependent manner and similarly to VEGF (13). CGRP also increases VEGF expression and stimulates proliferation, migration, and angiogenesis of endothelial cells through activation of adenosine monophosphate-activated protein kinase (AMPK)-endothelial nitric oxide synthase (eNOS) and focal adhesion kinase pathways. The effects of CGRP on migration and tube formation have been equal or more potent than that of the VEGF effect (14).
In the present study, pramlintide (1-20 μg/mL) had no significant effect on HUVECs proliferation though no inhibitory effect on cell viability was observed. Our results also revealed that pramlintide 1 μg/mL but not 10 |ig/mL promoted migration and capillary tube formation. In a recent investigation, pramlintide promoted neurogenesis and prevented the mitochondrial-dependent apoptosis with an optimal effect at 250-300 nM (about 1-1.2 μg/mL) (22). Low concentration of a human amylin fragment (3 μM) has also been associated with stimulating VEGF secretion from endothelial cells and preserving the survival of neuron-like cells however it has shown toxic effects on cell proliferation at its high concentration (20 μM) (23). Moreover, amylin has been related to vascular damages dependent on its aggregated form. It seems that the diverse effects of amylin may be mediated by various amylin receptors.
Our results showed the activity of pramlintide on increasing HUVECs migration at 1 μg/mL which was significantly less than VEGF effect (P < 0.05). While CGRP (1 nM) as a member of amylin superfamily has shown a similar effect to that of VEGF (20 ng/mL) on HUVECs migration in the study of Tuo et al. (14).
In this study, no significant change was found in VEGF secretion after exposure of HUVECs to pramlintide. Since the upregulation of VEGF expression and also its release have been reported by other members of amylin superfamily, evaluation of VEGF mRNA expression using reverse transcription polymerase chain reaction (RT-PCR), along with peptide measurements by more accurate methods such as western blotting is recommended.
Regarding our results, significant differences were observed between two concentrations of pramlintide on average tubules length and size and the number of junctions and it could be proposed that pramlintide may have a concentration- dependent effect on angiogenesis with proangiogenic effects at lower concentrations.
Some possible mechanisms for enhancing angiogenesis by pramlintide may be considered based on its potential pharmacological activities and the cellular signaling pathways of pramlintide and amylin. Wu et al. have shown the effect of pramlintide in reducing the expression of some matrix metalloproteinases including MMP3, MMP9, and MMP13 enzymes, and improving neuronal cell survival (22). Specific MMPs such as MMP-2 are involved in enhancing angiogenesis by degrading the capillary basement membrane (24).
Finally, it could be assumed that probable antioxidant effect of pramlintide may be an indirect mechanism for its possible pro- angiogenic effect. Pramlintide has reduced oxidized low-density lipoprotein and nitrotyrosine and prevented from the reduction in the total radical-trapping antioxidant parameter in plasma samples of diabetic patients (25). Pramlintide has shown a helpful effect on antioxidant enzymes such as superoxide dismutase and glutathione peroxidase and on the stress marker heme oxygenase-1. It also resulted in a significant decrease in the production of reactive oxygen species and lipid peroxidation in a neuronal model of oxidative stress in vitro (26).
Although enhancement of angiogenesis may be helpful in improving of such diabetes complications such as wound healing and ischemic conditions but may aggregate other problems like diabetic retinopathy. Concerning the tissue-specific manner of angiogenesis in diabetes, more investigations should be performed to elucidate the effect of pramlintide on angiogenesis in different tissues.
CONCLUSION
In conclusion, the findings of the present study showed the stimulating effect of pramlintide on some angiogenesis markers through promoting migration and tubulogenesis. in vivo and clinical evaluations are needed to determine the potential therapeutic value of pramlintide for improving neovascularization in vascular complications and healing of diabetic wounds.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest in this study.
AUTHORS’ CONTRIBUTION
L. Safaeian contributed to the concept, design, definition of intellectual content, manuscript preparation, and editing. G. Vaseghi, M. Mirian, and M. Dehghani performed the experimental studies, data acquisition, and statistical analysis.
ACKNOWLEDGMENTS
This study was financially supported by the Vice-Chancellery of Research and Technology of Isfahan University of Medical Sciences, Isfahan, I.R. under Grant No. 196157.
REFERENCES
- 1.World health statistics, World Health Organization. Geneva: WHO Press; 2018. p. 7. [Google Scholar]
- 2.International Diabetes Federation IDF Atlas. 7th edition. [Accessed 2019]. Available from: http://www.diabetesatlas.org .
- 3.Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum. Indian J Endocrinol Metab. 2016;20(4):546–551. doi: 10.4103/2230-8210.183480. DOI: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng R, Ma JX. Angiogenesis in diabetes and obesity. Rev Endocr Metab Disord. 2015;16(1):67–75. doi: 10.1007/s11154-015-9310-7. DOI: 10.1007/s11154-015-9310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin AM. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res. 2007;74(2-3):172–183. doi: 10.1016/j.mvr.2007.05.006. DOI: 10.1016/j.mvr.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuazo-Gaztelu I, Casanovas O. Unraveling the role of angiogenesis in cancer ecosystems. Front Oncol. 2018;8:248–260. doi: 10.3389/fonc.2018.00248. DOI: 10.3389/fonc.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23(2):117–145. doi: 10.1002/med.10024. DOI: 10.1002/med. 10024. [DOI] [PubMed] [Google Scholar]
- 8.Edelman S, Maier H, Wilhelm K. Pramlintide in the treatment of diabetes mellitus. BioDrugs. 2008;22(6):375–386. doi: 10.2165/0063030-200822060-00004. DOI: 10.2165/0063030-200822060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Hollander P, Maggs DG, Ruggles JA, Fineman M, Shen L, Kolterman OG, et al. Effect of pramlintide on weight in overweight and obese insulin-treated type 2 diabetes patients. Obes Res. 2004;12(4):661–668. doi: 10.1038/oby.2004.76. DOI: 10.1038/oby.2004.76. [DOI] [PubMed] [Google Scholar]
- 10.Ratner RE, Dickey R, Fineman M, Maggs DG, Shen L, Strobel SA, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med. 2004;21(11):1204–1212. doi: 10.1111/j.1464-5491.2004.01319.x. DOI: 10.1111/j.1464-5491.2004.01319.x. [DOI] [PubMed] [Google Scholar]
- 11.Hasbak P, Opgaard OS, Eskesen K, Schifter S, Arendrup H, Longmore J, et al. Investigation of CGRP receptors and peptide pharmacology in human coronary arteries Characterization with nonpeptide antagonist. J Pharmacol Exp Ther. 2003;304(1):326–333. doi: 10.1124/jpet.102.037754. DOI: 101152/physrev000372003. [DOI] [PubMed] [Google Scholar]
- 12.Ribatti D, Nico B, Spinazzi R, Vacca A, Nussdorfer GG. The role of adrenomedullin in angiogenesis. Peptides. 2005;26(9):1670–1675. doi: 10.1016/j.peptides.2005.02.017. DOI: 10.1016/j.peptides.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Chigurupati S, Kulkarni T, Thomas S, Shah G. Calcitonin stimulates multiple stages of angiogenesis by directly acting on endothelial cells. Cancer Res. 2005;65(18):8519–8529. doi: 10.1158/0008-5472.CAN-05-0848. DOI: 10.1158/0008-5472.CAN-05-0848. [DOI] [PubMed] [Google Scholar]
- 14.Tuo Y, Guo X, Zhang X, Wang Z, Zhou J, Xia L, et al. The biological effects and mechanisms of calcitonin gene-related peptide on human endothelial cell. J Recept Signal Transduct Res. 2013;33(2):114–123. doi: 10.3109/10799893.2013.770528. DOI: 10.3109/10799893.2013.770528. [DOI] [PubMed] [Google Scholar]
- 15.Akbari V, Sadeghi HM, Jafarian-Dehkordi A, Abedi D, Chou CP. Improved biological activity of a single chain antibody fragment against human epidermal growth factor receptor 2 (HER2) expressed in the periplasm of Escherichia coli. Protein Expr Purif. 2015;116:66–74. doi: 10.1016/j.pep.2015.07.005. DOI: 10.1016/j.pep.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro cell migration and invasion assays. J Vis Exp. 2014;88:1–8. doi: 10.3791/51046. DOI: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dana N, Haghjooy Javanmard Sh, Rafiee L. Antiangiogenic and antiproliferative effects of black pomegranate peel extract on melanoma cell line. Res Pharm Sci. 2015;10(2):117–124. [PMC free article] [PubMed] [Google Scholar]
- 18.Liegl R, Koenig S, Siedlecki J, Haritoglou C, Kampik A, Kernt M. Temsirolimus inhibits proliferation and migration in retinal pigment epithelial and endothelial cells via mTOR inhibition and decreases VEGF and PDGF expression. PLoS One. 2014;9(2):e88203,1–10. doi: 10.1371/journal.pone.0088203. DOI: 10.1371/journal.pone.0088203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai Y, Ma JX, Guo J, Wang J, Zhu M, Chen Y, et al. Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009;219(4):446–454. doi: 10.1002/path.2611. DOI: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 20.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84(3):903–934. doi: 10.1152/physrev.00037.2003. DOI: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 21.Kurashige C, Hosono K, Matsuda H, Tsujikawa K, Okamoto H, Majima M. Roles of receptor activity- modifying protein 1 in angiogenesis and lymphangiogenesis during skin wound healing in mice. FASEB J. 2014;28(3):1237–1247. doi: 10.1096/fj.13-238998. DOI: 10.1096/fj. 13-238998. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Song Y, Li S, Liu X, Hua W, Wang K, et al. Pramlintide regulation of extracellular matrix (ECM) and apoptosis through mitochondrial-dependent pathways in human nucleus pulposus cells. Int J Immunopathol Pharmacol. 2017;31(1):1–14. doi: 10.1177/0394632017747500. DOI: 10.1177/0394632017747500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caruso G, Fresta C, Lazzarino G, Distefano DA, Parlascino P, Lunte SM, et al. Sub-toxic human amylin fragment concentrations promote the survival and proliferation of SH-SY5Y cells via the release of VEGF and HSPB5 from endothelial RBE4 cells. Int J Mol Sci. 2018;19(11):E3659,1–18. doi: 10.3390/ijms19113659. DOI: 10.3390/ijms19113659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9(2):267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. DOI: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceriello A, Piconi L, Quagliaro L, Wang Y, Schnabel CA, Ruggles JA, et al. Effects of pramlintide on postprandial glucose excursions and measures of oxidative stress in patients with type 1 diabetes. Diabetes Care. 2005;28(3):632–637. doi: 10.2337/diacare.28.3.632. DOI: 10.2337/diacare.28.3.632. [DOI] [PubMed] [Google Scholar]
- 26.Patrick S, Corrigan R, Grizzanti J, Mey M, Blair J, Pallas M, et al. Neuroprotective effects of the amylin analog, pramlintide, on Alzheimer’s disease are associated with oxidative stress regulation mechanisms. J Alzheimers Dis. 2019;69(1):157–168. doi: 10.3233/JAD-180421. DOI: 10.3233/JAD-180421. [DOI] [PMC free article] [PubMed] [Google Scholar]



