Abstract
Background and purpose:
Polysaccharide sulfate is a major active phytochemical constituent of Caulerpa racemosa, whereas the Eleutherine americana (Aubl) Merr has antioxidant properties. The aim of this research was to investigate the combined effect of polysaccharide sulfate that was isolated from C. racemosa and E. americana on the macrophage activity.
Experimental approach:
The phenolic contents and antioxidant activities of E. americana extracts in water and various ethanol concentrations were studied using the Folin-Ciocalteu and 2,2-diphenyl-1-picryl-hydrazyl- hydrate (DPPH) methods, respectively. Polysaccharide sulfate was isolated from C. racemosa by precipitation method. To assess the macrophage activity, mice were treated orally for 14 days with either a combination of polysaccharide sulfate and E. americana 96% ethanol extract at a specific ratio or with each extract alone. Macrophages were isolated and the phagocytic activity was measured by assessing the ability of the macrophages to phagocytose latex particles and nitric oxide (NO) levels were assessed using a colorimetric assay.
Findings / Results:
The E. americana crude extract in water exhibited the highest yield (13.04%), compared with the extract in 96% ethanol, which had the highest phenolic content (6.37 ± 0.16 mg/g gallic acid equivalent) and the strongest antioxidant activity (IC50, 22.63 ± 1.09 μg/mL). The combination of extracts, when both extracts were administered at 65:65 mg/kg BW, resulted in the highest increases in phagocytosis activity (62.73 ± 5.77%) and NO levels (16.43 ± 1.37 μmol/L).
Conclusion and implications:
The results of this study confirmed the non-specific immunostimulant properties of the combination of polysaccharide sulfate and E. americana and justified their use in traditional medicine. The observed increase in macrophage activity appeared to be correlated with the increased ability of mice to fight infection.
Keywords: Antioxidant, Caulerpa racemosa, Eleutherine americana (Aubl) Merr, Phagocytic, Phenolic content, Polysaccharide sulfate
INTRODUCTION
Infectious diseases remain an important public health issue worldwide. Antibiotics are often specific for the types of bacteria they can treat and, in general, cannot be easily interchanged between infection types. When antibiotics are used correctly, they are generally safe, with few side effects (1,2).
However, the inappropriate use of antibiotics to treat infectious diseases has resulted in a major increase in multidrug resistance (MDR) among bacteria. Microorganisms that have been reported to have developed MDR include Escherichia coli (3), Klebsiella pneumoniae (4), Staphylococcus aureus (5), Streptococcus pneumonia (6), Salmonella enterica serovar Typhi (7), Shigella species (8), and Neisseria gonorrhea (9), which are all considered to be significant agents of human infection.
The use of antibiotics can also endanger the natural microbial flora in the body, which represent essential components of the body’s self-defense (immune) system (10). This situation has resulted in the search for alternative substances that can be used to treat infections, with minimal negative side effects, including those with immunostimulant properties.
Immunostimulants are natural or synthetic products, associated with a variety of chemical characteristics and mechanisms of action, which is capable of activating various components of the human immune system to protect against infections caused by bacteria and viruses. These products can also be used to counter the effects of various diseases that pathologically reduce immune system capabilities (11). Two natural sources of substances with immunostimulant activities that are abundant in South Sulawesi are the seaweed Caulerpa racemosa (C. racemosa) and the bulb of Eleutherine americana (Aubl) Merr (E. Americana).
In South Sulawesi, C. racemosa is known as lawi-lawi and is intensively cultivated in some areas, including the Takalar District (12). The primary chemical component in C. racemosa, polysaccharide sulfates (13,14), has been shown to display antiviral activity towards measles (15), herpes simplex virus (16), human immunodeficiency virus (HIV) (17), dengue virus, and respiratory syncytial virus (18). The polysaccharide sulfates from the edible green alga C. racemosa var peltata has been shown to have immunostimulatory effects by increasing the levels of tumor necrosis factor (TNF)-α, interleukin-1β (H-1β), nitric oxide (NO), and reactive oxygen species (ROS), in a dose- dependent manner, in macrophage cells (19,20).
The bulb of E. americana, Dayak union, is traditionally used in the Jeneponto District to treat measles that is known locally as campak. Preliminary research indicated that treatment with E. americana extract, at 0.08 mg/30 g BW, was able to reduce the germinal diameter of lymph nodes and increase serum immunoglobulin G (IgG) levels in mice (21). The IC50 for the antioxidant activity of an E. americana extract in 70% ethanol was 31.97 μg/mL (strong activity), as assessed by the 2,2- diphenyl-1-picrylhydrazyl (DPPH) assay (22).
The aim of the present study was to develop an herbal product, by combining the polysaccharide sulfates derived from C. racemosa with the antioxidants properties from E. americana, to achieve a synergistic effect and increase the immune power, as determined by evaluating the effects of treatment on the phagocytotic capacity of macrophages and the level of NO. This study also determined the effects of various polarity solvents on the phenolic contents and antioxidant capacities of E. americana and examined the correlations between these two characteristics.
MATERIALS AND METHODS
Materials
RPMI 1640 medium, fetal bovine serum (FBS), penicillin-streptomycin, amphotericin B, 4-(2-hydroxyethyl)-1-piperazineethane- sulfonic acid, and phosphate-buffered saline (PBS) pH 7.4 were purchased from Gibco® (USA). Ethanol, methanol, Giemsa, barium chloride (BaCl2), Folin-Ciocalteu, 1,1- diphenyl-2-picryl hydrazyl (DPPH), cetylpyridinium chloride, sodium acetate, cysteine, ethylenediaminetetraacetic acid (EDTA), crude papain, sodium chloride, sodium carbonate, potassium sulfate, and latex beads were obtained from Sigma-Aldrich (USA).
Sample collection and preparation
The green alga, C. racemosa, was collected from the coast of Takalar, Indonesia, on the Flores Sea, Indonesia. The alga was cleaned under running water, cut into small pieces, and oven-dried at 60 °C. The dried C. racemosa was extracted via an enzymatic technique, using papain, as described by Rodrigues et al. with some modifications (23). The samples were first ground and then rehydrated in 100 mM sodium acetate (pH 5.0), containing cysteine and EDTA (5 mM), to which a solution of crude papain (30 mg/mL) was added. The mixture was heated to 60 °C for 6 h, to extract the polysaccharide, and then the mixture was strained and centrifuged (5,000 rpm, 4 °C, 30 min). The supernatant was added to 16 mL of 10% cetylpyridinium chloride to precipitate the polysaccharide sulfate (25 °C, 24 h). The precipitate was cleaned with 0.05% cetylpyridinium chloride, dissolved in a 100:15 mixture of 2 M sodium chloride and ethanol, and precipitated through the addition of ethanol (4 °C, 24 h). After precipitation, the polysaccharide sulfates were centrifuged, washed twice with 80% ethanol, and washed with 96% ethanol. Finally, the polysaccharide sulfates were oven-dried (60 °C, 4 h).
Fresh bulbs of E. americana were collected from a local market in Gowa, Indonesia. The bulbs were air-dried, after the hand-picked removal of unwanted particles, chopped into small pieces, and ground into a coarse powder using a blender (Miyako, Indonesia). The dried bulbs were extracted in various polarity solvents, including water and 30, 50, 70, and 96% ethanol, using a sonicator for 72 h. Then, the samples were filtered and concentrated using rotary evaporation (Büchi Rotavapor, Germany).
Testing polysaccharide sulfates
The polysaccharide sulfate contents in the C. racemosa extracts were analyzed for sulfate groups (-SO3H) using a BaCl2-gelatin method (24). In a typical procedure, the gelation solution (0.3%) was prepared in hot water (60-70 °C) and incubated at 4 °C overnight. Then 2.0 g of BaCl2 was dissolved in the gelatin solution and incubated for 2 h, at 25 °C. Approximately 0.5 mL of polysaccharide sulfate solution (10.0 mg/mL) was combined with 3.8 mL of 0.5 M HCl and 1.0 mL of BaC2-gelatin reagent, and the mixture was incubated for 15 min. The released Ba-sulfate concentration was measured at γ 360 nm by ultraviolet-visible (UV-Vis) spectrophotometry (Agilent 8453, USA), using potassium sulfate to generate the standard curve. This experiment was performed in triplicate.
Determination of phenolic content
The phenolic contents of the E. americana extracts were determined using the Folin- Ciocalteu method (25). A 0.1 mL volume of 50% Folin-Ciocalteu was added to 0.1 mL of each extract (10 mg/10 mL). A 0.1 mL volume of 7.5% sodium carbonate was added to the mixture, and the mixture was filtered and water was added to bring the total volume of 10 mL. The mixture was incubated at room temperature for 5 min. The absorption of the mixture was recorded using a spectrophotometer (Agilent 8453, USA) at γ 730 nm. A standard curve was produced using several concentrations of gallic acid. The phenolic contents were expressed as mg/g gallic acid equivalent (mg/g GAE). This experiment was performed in triplicate.
Determination of antioxidant activity
The DPPH free radical scavenging activity was evaluated using the method proposed by Liaudanskas et al. (25). Ten mg of each E. americana extract was dissolved in 10 mL of ethanol (1,000 μg/mL). Stock solutions of each extract at volumes of 2.5, 5.0, 10.0, 20.0, and 40.0 μL were added to the wells of a microplate, 75 μL of 240 μM DPPH was added to each well using a multichannel micropipette, and the volume of each well was brought to 200 μL using ethanol. The microplate was incubated at 37 °C for 30 min, and absorbance was recorded at 515 nm using a microplate reader (Biotek ELx808, USA). The antioxidant activity of the E. americana extracts is given as the half- maximal inhibitory concentration (IC50).
Animals
All animal experiments were conducted with local ethical approval from the Faculty of Medicine, Hasanuddin University (Ethics No. 169/H4.8.4.5.31/PP36-KOMETIK/2017). Male 8-10-week-old Balb/c mice (20-30 g) were housed in a 12/12-h light/dark cycle unit, with free access to food and water.
Preparation of sheep red blood cell suspension antigen
Sheep red blood cells were used as antigenic materials. Sheep red blood cell suspensions, 20% in PBS pH 7.4, were used to challenge the animals throughout the study.
Experimental design
The mice were randomly divided into 6 groups, 3 each. Group I, received polysaccharide sulfate 130 mg/kg BW; group II, received E. americana 130 mg/kg BW; groups III-V, received the combination of polysaccharide sulfate and E. americana (30 and 100; 100 and 30; 65 and 65 mg/kg BW); and group VI, vehicle (0.5% sodium carboxymethylcellulose). All animals were treated orally with extracts or vehicle control daily for 14 days. On day 8, all groups were challenged with 0.1 mL of 20% sheep red blood cell suspension, intraperitoneally (i.p). On day 15, the mice were sacrificed by cervical dislocation under anesthetic conditions.
Isolation and preparation of macrophages
Resident peritoneal macrophages were collected by washing out the mouse peritoneal cavities with cold PBS. The macrophage cells (1.5 × 105 cell/mL), attached to glass coverslips (d = 13 mm), were placed into 24-well tissue culture plates and cultured in RPMI medium, supplemented with 10% FBS, 1% penicillin- streptomycin, and 0.5% amphotericin B. Cultures were incubated a 37 °C cell culture incubator, with 5% CO2, for 24 h.
Observation of macrophage phagocytosis activity
Phagocytosis activity was observed using latex beads suspended in PBS. A 200 μL volume of suspended latex beads (d = 2.0 μm) was placed in each well and incubated for 4 h, in 5% CO2, at 37 °C. The medium was removed from the well, leaving the macrophage cells adhered to the coverslips, which were washed 3 times with PBS and dried at room temperature. The coverslip was then immersed in cold methanol, stained with 20% Giemsa, and washed with distilled running water. The number of macrophages engaged in the phagocytosis of latex beads was counted under a microscope, using 20× magnification. Phagocytosis activity was provided as the percentage of macrophage cells observed to be phagocytosing latex beads.
Calculation of NO concentrations
The NO level in the culture supernatant was measured after 24 h incubation. Briefly, 100 μL of supernatant was incubated with 2% (w/v) sulfanilamide in 10% (v/v) o-phosphoric acid, for 15 min. Then, 50 μL of 0.2% (w/v) N-(1- naphthyl)-ethylenediamine dihydrochloride was added, and the mixture was incubated for an additional 15 min at room temperature. The absorbance of the sulfanilamide-N-(1-naphthyl)- ethylenediamine dihydrochloride complex was recorded at 570 nm using a microplate reader (Biotek ELx808, USA). The quantification of nitrite in the sample was standardized against 0-100 μmol/L of NaNO2 (26).
Statistical analysis
The results are expressed as the mean ± standard deviation (SD) from 3 independent experiments which were analyzed by one way ANOVA followed by Tukey’s post hoc test. P < 0.05 was considered as statistically significant.
RESULTS
Extract yields
The extraction of the dried E. americana bulb was performed using the maceration technique at room temperature. Maceration allows the extraction of all components, without heat, which can damage chemical components. The solvents used in this study, based on polarity, were water and 30, 50, 70, and 96% ethanol, which allowed the bioactive compounds to be extracted according to polarity. The yields for the dried extracts in each solvent are presented in Table 1. The results showed a pattern for percent yield, as follows: water > 30% ethanol > 50% ethanol > 70% ethanol > 96% ethanol. These results indicated that the E. americana bulb primarily contains polar compounds.
Table 1.
The yields of Eleutherine americana extracts prepared using different extraction solvents.
| Extraction solvent | Dried bulb (g) | Dried extract (g) | Yield (%) |
|---|---|---|---|
| Water | 50 50 50 | 6.52 5.24 | 13.04 |
| Ethanol (30%) | 50 50 | 4.45 4.13 2.41 | 10.48 8.90 8.26 |
| Ethanol (50%) | 4.82 | ||
| Ethanol (70%) | |||
| Ethanol (96%) |
Approximately 51.90 kg of wet C. racemosa was collected for this study. After cleaning and oven drying, the final dry powder mass was 520.00 g (yield = 1.00%). Estimation of the polysaccharide sulfates contents showed that 1 mg of C. racemosa contains 171.2 μg polysaccharide sulfates.
Total polyphenol content
The total phenolic content was determined in E. americana ethanolic extracts, with the highest phenolic content observed in the extract prepared with 96% ethanol (6.37 ± 0.16 mg/g GAE), followed by 70% ethanol (4.72 ± 0.05 mg/g GAE), and 50% ethanol (3.42 ± 0.37 mg/g GAE), whereas water and 30% ethanol were the lowest levels of 1.00 ± 0.13 and 0.96 ± 0.06 mg/g GAE, respectively (Table 2). The total polyphenol content of the extracts decreased with the increasing polarity of the solvent, indicating that E. americana bulbs primarily contain semi-polar and/or non-polar compounds.
Table 2.
Total phenolic concentrations and IC50 values of the Eleutherine americana bulb extracts prepared using different extraction solvents.
| Treatment | Total phenolic concentration (mg/g GAE) | IC50 (μg/mL) |
|---|---|---|
| Water | 1.00 ± 0.13 | 195.20 ± 11.42 |
| 30% Ethanol | 0.96 ± 0.06 | 70.95 ± 1.89 |
| 50% Ethanol | 3.42 ± 0.37 | 40.55 ± 19.03 |
| 70% Ethanol | 4.72 ± 0.05 | 38.99 ± 2.67 |
| 96 %Ethanol | 6.37 ± 0.16 | 22.63 ± 1.09 |
| Vitamin C | - | 1.00 ± 0.13 |
GAE, gallic acid equivalent.
Antioxidant activity
The DPPH assay was used to determine the antioxidant activity of the different E. americana extracts. All of the extracts showed concentration-dependent increases in the IC50 values (Fig. 1). Antioxidant activity depends on the total polyphenolic compound concentration.
Fig. 1.
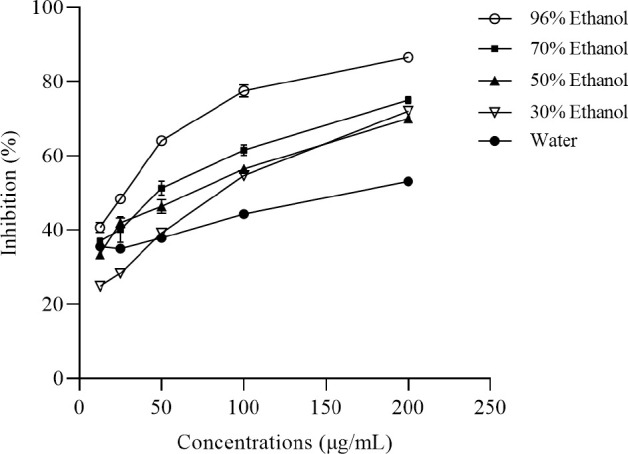
The DPPH radical scavenging activity of Eleutherine americana in various polarity solvents. The absorbance of the DPPH solution alone at 517 nm was 0.821 ± 0.011 (experimental control). DPPH, 2,2-diphenyl-1-picryl-hydrazyl-hydrate.
The antioxidant capacities of the E. americana extracts were expressed as the IC50 values of the extracts. During the DPPH assay, the 96% ethanol extract had the highest antioxidant, and the remaining extracts showed the following pattern: 70% ethanol > 50% ethanol > 30% ethanol > water (Table 2). The antioxidant activities of the extracts decreased as the polarity of the solvent increased, indicating that semi-polar and/or non-polar compounds play roles in antioxidant activity.
Correlation
Furthermore, the total phenolic contents and the antioxidant activities of the extracts (IC50) were found to be highly correlated (correlation = - 0.730), with higher polyphenol levels correlating with higher antioxidant activity.
Macrophage phagocytosis activity
The phagocytic capacity of peritoneal macrophages is expressed as the means ± SD of the percentage of phagocytosing macrophages (Fig. 2). There were significant differences in the phagocytosis activity between all the treated groups and control. The use of either 130 mg/kg BW of polysaccharide sulfate or E. americana increase the phagocytosis activity to 41.33 ± 1.53 and 49.67 ± 1.53%; their combination at 30:100 and 100:30 mg/kg BW were 54.73 ± 0.06 and 52.33 ± 0.58%; and the highest activity, 62.73 ± 1.05%, was found at a combination of 65:65 mg/kg BW. This result indicated a synergistic effect between polysaccharide sulfate and E. americana on the immune system.
Fig. 2.
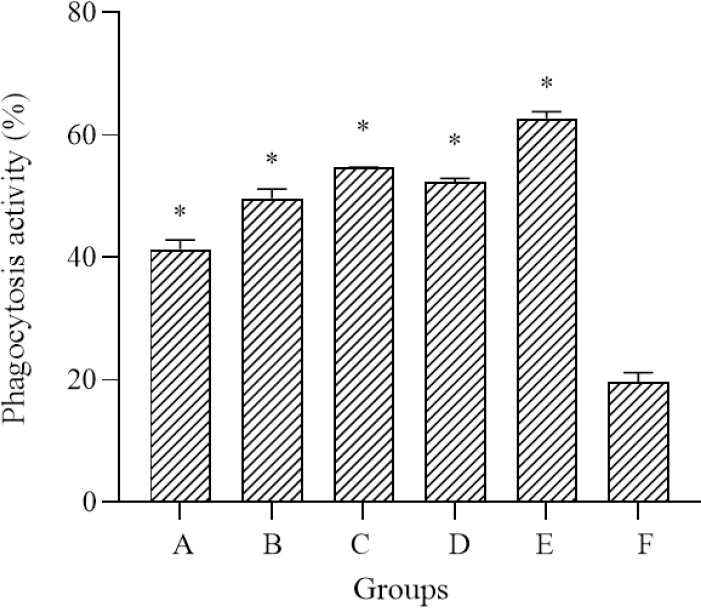
The effects of polysaccharide sulfates and E. americana, either alone or in combination, on the phagocytosis activity of peritoneal macrophages. After incubation at 37 °C for 4 h, the numbers of macrophages engaged in the phagocytosis of latex beads were counted under a microscope, using 20× magnification. *P < 0.05 represents significant differences compared to the control group. E. Americana; Eleutherine americana; BW, body weight; A, polysaccharide sulfate 130 mg/kg BW; B, E. americana 130 mg/kg BW; C, Polysaccharide sulfate and E. americana 30:100 mg/kg BW; D, polysaccharide sulfate and E. americana 100:30 mg/kg BW; E, polysaccharide sulfate and E. americana 65:65 mg/kg BW; F, negative control.
Nitric oxide concentration
In the present study, polysaccharide sulfate and E. americana, both alone and in combination, were evaluated for their abilities to inhibit NO production in peritoneal macrophages. Nitrite accumulation in cells increased following sheep red blood cell suspension treatments. The lowest level of NO was observed for the negative control (2.24 ± 0.38 μmol/L). Polysaccharide sulfate alone, E. americana alone, and their combination at 30:100; 100:30, and 65:65 mg/kg BW increased the NO accumulation in peritoneal macrophages compared with the negative control group (P < 0.05). A combination of 65:65 mg/kg BW indicated the highest ability to increase NO levels to 19.48 ± 1.53 μmol/L (Fig. 3).
Fig. 3.
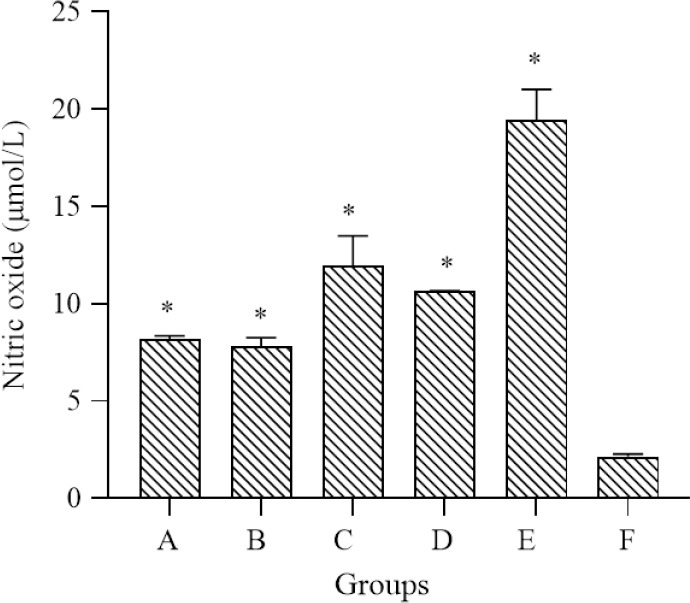
The inhibitory effects of polysaccharide sulfates and E. americana, either alone or in combination, on nitric oxide production by peritoneal macrophages after incubation at 37 °C for 4 h. *P < 0.05 represents significant differences compared to the control group. E. Americana; Eleutherine americana; BW, body weight; A, polysaccharide sulfate 130 mg/kg BW; B, E. americana 130 mg/kg BW; C, Polysaccharide sulfate and E. americana 30: 100 mg/kg BW; D, polysaccharide sulfate and E. americana 100:30 mg/kg BW; E, polysaccharide sulfate and E. americana 65:65 mg/kg BW; F, negative control.
DISCUSSION
This article discusses the synergistic effects of polysaccharides that isolated from C. racemosa and E. americana on the phagocytic activity of macrophage, which is characterized by an increased ability of macrophages to eliminate pathogens. The combination of polysaccharide sulfate and E. americana is based on the fact that C. racemosa containing polysaccharides have already been reported as immunostimulants (19), and E. americana contains high polyphenol content and high antioxidant power (22) whose potential in boosting immune system has been proven. The combination of these two ingredients is expected to have a synergistic effect so that it can reduce the dose given compared to giving alone. This study also reports on how to obtain polysaccharide sulfates and the best solvents used to extract E. americana, which is characterized by the highest content of polyphenols and antioxidants.
The primary phenolic compounds in E. americana extract belong to the flavonoid group, which are powerful antioxidants with immunomodulatory effects (27,28). Phenolic compounds are characterized by the presence of one or more hydroxyl (R-OH) groups bound to benzene. The OH group is polar and easily dissolves in polar solvents. However, increasing the number of carbon chain complex compounds can reduce polarity (29,30). E. americana bulbs are primarily composed of polar compounds, as demonstrated by the higher yield observed for the water-based extract compared with ethanol-based extracts Table 1, which is similar to the results reported by Agustin et al., who found the highest yield with water. Most polyphenol compounds are polar to semi-polar; therefore, the highest polyphenol contents and antioxidant activities are likely to be observed for extracts containing semi-polar solvents, such as ethanol. The addition of water to ethanol can increase the polarity (31).
The combination of polysaccharide sulfate and E. americana has not previously been examined. Polysaccharide sulfates not only provide structural support for the cell membrane but also have bioactive therapeutics effects on the immune system (32). Hao et al. found that treatment with C. racemosa extracted in water boosted the immune system by increasing NO, ROS, TNF-a, and IL-6 levels in RAW264.7 macrophage cells, in a dose- dependent manner (19). The compounds reported from E. americana were eleutherin, eleutherinol, isoeleutherin, di-hydroeleutherinol, and hongconin inhibit RAW 264.7 lipopolysacharide-activated mouse macrophage cell (33). C. racemosa polysaccharides have been reported to increase the percentages of CD3+, CD4+, natural killer (NK), and CD4+/CD8+ cells in female Kunming mice (34).
The high levels of phenolic contents and the strong antioxidant potential of E. americana bulbs led us to examine the effect of E. americana extracts on NO production. Both polysaccharide sulfates and E. americana, alone and in combination, increased the NO production of peritoneal macrophages. This effect indicates that the presence of the extract may be responsible for this activity. NO is a multifunctional signaling molecule and at high concentrations is involved in immune responses by cytokine-activated macrophages (35). In addition, NO has been shown to have positive effects on the cardiovascular system and act as a potent neurotransmitter. However, NO can also act as a proinflammatory mediator that induces inflammation during abnormal situations (36,37).
Immune cells are particularly sensitive to oxidative stress because of the high polyunsaturated fatty acid contents in their plasma membranes, which results in the increased production of ROS; therefore, the immune system generally has higher antioxidant concentrations (38). We predicted that the antioxidant effect of E. americana could supply an endogenous antioxidant, protecting immune cells, such as macrophages.
A synergistic effect was demonstrated between polysaccharide sulfates and E. americana, in the present study, with the optimal combination observed at both extracts were administered at 65:65 mg/kg BW. This combination may represent an ideal natural immunomodulator that deserves further study for development as a potential therapeutic agent.
CONCLUSION
In conclusion, to optimize the phenolic content and antioxidant capacity of E. americana extracts, 96% ethanol should be used. Treatment with a combination of polysaccharide sulfates derived from C. racemosa and 96% ethanol extract of E. americana, at both extracts were administered at 65:65 mg/kg BW, was able to improve macrophage function in mice. The present study provided a novel perspective, examining the nutraceutical use of medicinal plants as adjuvant therapies with immunostimulant effects. Further studies focusing on how polysaccharide sulfates and E. americana modulate the immune system are warranted.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest in this study
AUTHORS’ CONTRIBUTION
E. Pakki, R. Tayeb, I.A. Ridwan, and L. Muslimin conceived and designed the experiments. E. Pakki, R. Tayeb, and U. Usmar performed the experiments and analyzed the data. E. Pakki, R. Tayeb, and L. Muslimin wrote the manuscript.
ACKNOWLEDGMENTS
We thank members of the Department of Biopharmacy at Hasanuddin University for the use of the animal facilities.
REFERENCES
- 1.Van der Meer JWM. The infectious disease challenges of our time. Front Public Health. 2013;1:7–8. doi: 10.3389/fpubh.2013.00007. DOI: 10.3389/fpubh.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spigaglia P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther Adv Infect Dis. 2016;3(1):23–42. doi: 10.1177/2049936115622891. DOI: 10.1177/2049936115622891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kallau NHG, Wibawan IWT, Lukman DW, Sudarwanto MB. Detection of multi-drug resistant (MDR) Escherichia coli and tet gene prevalence at a pig farm in Kupang, Indonesia. J Adv Vet Anim Res. 2018;5(4):388–396. doi: 10.5455/javar.2018.e289. DOI: 10.5455/javar.2018.e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassetti M, Righi E, Carnelutti A, Graziano E, Russo A. Multidrug-resistant Klebsiella pneumoniae: challenges for treatment, prevention and infection control. Expert Rev Anti Infect Ther. 2018;16(10):749–761. doi: 10.1080/14787210.2018.1522249. DOI: 10.1080/14787210.2018.1522249. [DOI] [PubMed] [Google Scholar]
- 5.Assis LM, Nedeljkovic M, Dessen A. New strategies for targeting and treatment of multi-drug resistant Staphylococcus aureus. Drug Resist Updat. 2017;31:1–14. doi: 10.1016/j.drup.2017.03.001. DOI: 10.1016/j.drup.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Arushothy R, Ramasamy H, Hashim R, Raj ASS, Amran F, Samsuddin N, et al. Multidrug-resistant Streptococcus pneumoniae causing invasive pneumococcal disease isolated from a paediatric patient. Int J Infect Dis. 2020;90:219–222. doi: 10.1016/j.ijid.2019.10.037. DOI: 10.1016/j.ijid.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Mutai WC, Muigai AWT, Waiyaki P, Kariuki S. Multi-drug resistant Salmonella enterica serovar Typhi isolates with reduced susceptibility to ciprofloxacin in Kenya. BMC Microbiol. 2018;18(1):e187,1–5. doi: 10.1186/s12866-018-1332-3. DOI: 10.1186/s12866-018-1332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puzari M, Sharma M, Chetia P. Emergence of antibiotic resistant Shigella species: a matter of concern. J Infect Public Health. 2018;11(4):451–454. doi: 10.1016/j.jiph.2017.09.025. DOI: 10.1016/j.jiph.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Martin I, Sawatzky P, Allen V, Lefebvre B, Hoang L, Naidu P, et al. Multidrug-resistant and extensively drug-resistant Neisseria gonorrhoeae in Canada, 2012-2016. Can Commun Dis Rep. 2019;45(2-3):45–53. doi: 10.14745/ccdr.v45i23a01. DOI: 10.14745/ccdr.v45i23a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon MY, Yoon SS. Disruption of the gut ecosystem by antibiotics. Yonsei Med J. 2018;59(1):4–12. doi: 10.3349/ymj.2018.59.1.4. DOI: 10.3349/ymj.2018.59.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bascones-Martinez A, Mattila R, Gomez-Font R, Meurman JH. Immunomodulatory drugs: oral and systemic adverse effects. Med Oral Patol Oral Cir Bucal. 2014;19(1):e24–e31. doi: 10.4317/medoral.19087. DOI: 10.4317/medoral.19087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukarramah M, Wahyuni W, Emilia E. Low fat high protein sausage made from lawi-lawi (Caulerpa racemosa) as a Makassar healthy culinary innovation and alternative foods for children with obesity. Hasanuddin Stud J. 2017;1(1):50–55. [Google Scholar]
- 13.Ji H, Shao H, Zhang C, Hong P, Xiong H. Separation of the polysaccharides in Caulerpa racemosa and their chemical composition and antitumor activity. J Appl Polym Sci. 2008;110(3):1435–1440. DOI: 10.1002/app.28676. [Google Scholar]
- 14.Ribeiro NA, Abreu TM, Chaves HV, Bezerra MM, Monteiro HSA, Jorge RJB, et al. Sulfated polysaccharides isolated from the green seaweed Caulerpa racemosa plays antinociceptive and anti-inflammatory activities in a way dependent on HO-1 pathway activation. Inflamm Res. 2014;63(7):569–580. doi: 10.1007/s00011-014-0728-2. DOI: 10.1007/s00011-014-0728-2. [DOI] [PubMed] [Google Scholar]
- 15.Moran-Santibanez K, Cruz-Suarez LE, Ricque-Marie D, Robledo D, Freile-Pelegrin Y, Pena-Hernandez MA, et al. Synergistic effects of sulfated polysaccharides from Mexican seaweeds against measles virus. Biomed Res Int. 2016;2016:e8502123,1–11. doi: 10.1155/2016/8502123. DOI: 10.1155/2016/8502123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Wang X, Wu H, Liu R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar Drugs. 2014;12(9):4984–5020. doi: 10.3390/md12094984. DOI: 10.3390/md12094984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadi A, Moghadamtousi SZ, Abubakar S, Zandi K. Antiviral potential of algae polysaccharides isolated from marine sources: a review. Biomed Res Int. 2015;2015:e825203,1–10. doi: 10.1155/2015/825203. DOI: 10.1155/2015/825203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Wang SX, Guan HS. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar Drugs. 2012;10(12):2795–2816. doi: 10.3390/md10122795. DOI: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao H, Fu M, Yan R, He B, Li M, Liu Q, et al. Chemical composition and immunostimulatory properties of green alga Caulerpa racemosa var peltata. Food Agr Immunol. 2019;30(1):937–954. DOI: 10.1080/09540105.2019.1646216. [Google Scholar]
- 20.Hao H, Han Y, Yang L, Hu L, Duan X, Yang X, et al. Structural characterization and immunostimulatory activity of a novel polysaccharide from green alga Caulerpa racemosa var peltata. Int J Biol Macromol. 2019;134:891–900. doi: 10.1016/j.ijbiomac.2019.05.084. DOI: 10.1016/j.ijbiomac.2019.05.084. [DOI] [PubMed] [Google Scholar]
- 21.Carmelita A. Effect of ethanol extract of Dayak onion (Eleutherine palmifolia (L) Merr) on Balb/c mice against prevention of decreased germinal center diameter in lymph nodes and serum IgG levels. J Biosains Pascasarjana. 2016;18(1):1–12. [Google Scholar]
- 22.Pratiwi D, Wahdaningsih S, Isnindar I. The test of antioxidant activity from bawang mekah leaves (Eleutherine americana Merr) using DPPH (2,2- diphenyl-1-picrylhydrazyl) method. Trad Med J. 2013;18(1):9–16. DOI: 1022146/tradmedj7755. [Google Scholar]
- 23.Rodrigues J, Quinderé A, Queiroz IN, Coura C, Benevides N. Comparative study of sulfated polysaccharides from Caulerpa spp (Chlorophyceae) Biotechnological tool for species identification. Acta Sci Biol Sci. 2012;34(4):381–389. DOI: 104025/actascibiolsciv34i48976. [Google Scholar]
- 24.Bhadja P, Tan CY, Ouyang JM, Yu K. Repair effect of seaweed polysaccharides with different contents of sulfate group and molecular weights on damaged HK- 2 cells. Polymers (Basel) 2016;8(5):e188,1–14. doi: 10.3390/polym8050188. DOI: 10.3390/polym8050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mindaugas L, Zymonė K, Viškelis J, Klevinskas A, Janulis V. Determination of the phenolic composition and antioxidant activity of pear extracts. J Chem. 2017;2017:e7856521,1–9. DOI: 10.1155/2017/7856521. [Google Scholar]
- 26.Hiransai P, Tangpong J, Kumbuar C, Hoonheang N, Rodpech O, Sangsuk P, et al. Anti-nitric oxide production, anti-proliferation and antioxidant effects of the aqueous extract from Tithonia diversifolia. Asian Pac J Trop Biomed. 2016;6(11):950–956. DOI: 10.1016/j.apjtb.2016.02.002. [Google Scholar]
- 27.Chabib L, Muhtadi W, Rizki M, Rahman R, Suhendri M, Hidayat A. Potential medicinal plants for improve the immune system from Borneo Island and the prospect to be developed as nanomedicine. MATEC Web of Conferences. 2018;154:e04006,1–6. DOI: 10.1051/matecconf/201815404006. [Google Scholar]
- 28.Paramita S, Nuryanto M. Anti-inflammatory activity of bawang Dayak (Eleutherine bulbosa (Mill Urb)) ethanol bulb extracts. J Vocation Health Stud. 2018;2(2):51–55. DOI: 1020473/jvhsV2I2201851-55. [Google Scholar]
- 29.Cutrim CS, Cortez MAS. A review on polyphenols: classification, beneficial effects and their application in dairy products. Int J Dairy Technol. 2018;71(3):564–578. DOI: 10.1111/1471-0307.12515. [Google Scholar]
- 30.Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci. 2007;30(18):3268–3295. doi: 10.1002/jssc.200700261. DOI: 10.1002/jssc.200700261. [DOI] [PubMed] [Google Scholar]
- 31.Agustin AR, Faika S, Ju YH. Influence of extracting solvents on its antioxidant properties of bawang Dayak (Eleutherine palmifolia L Merr) Int J Chem Petrochem Tech. 2016;6(2):1–10. [Google Scholar]
- 32.Ramesh HPF, Tharanathan RN. Carbohydrates-the renewable raw materials of high biotechnological value. Crit Rev Biotechnol. 2003;23(2):149–173. doi: 10.1080/713609312. DOI: 10.1080/713609312. [DOI] [PubMed] [Google Scholar]
- 33.Insanu M, Kusmardiyani S, Hartati R. Recent studies on phytochemicals and pharmacological effects of Eleutherine Americana Merr. Procedia Chem. 2014;13:221–228. DOI: 10.1016/j.proche.2014.12.032. [Google Scholar]
- 34.Shen W, Wang H, Guo G, Tuo J. Immunomodulatory effects of Caulerpa racemosa var peltata polysaccharide and its selenizing product on T lymphocytes and NK cells in mice. Sci China C Life Sci. 2008;51(9):795–801. doi: 10.1007/s11427-008-0106-9. DOI: 10.1007/s11427-008-0106-9. [DOI] [PubMed] [Google Scholar]
- 35.Nonnenmacher Y, Hiller K. Biochemistry of proinflammatory macrophage activation. Cell Mol Life Sci. 2018;75(12):2093–2109. doi: 10.1007/s00018-018-2784-1. DOI: 10.1007/s00018-018-2784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, Vanhoutte PM, Leung SWS. Vascular nitric oxide: beyond eNOS. J Pharmacol Sci. 2015;129(2):83–94. doi: 10.1016/j.jphs.2015.09.002. DOI: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammo- pharmacology. 2007;15(6):252–259. doi: 10.1007/s10787-007-0013-x. DOI: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 38.Ahmadinejad F, Geir Moller S, Hashemzadeh- Chaleshtori M, Bidkhori G, Jami MS. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants (Basel) 2017;6(3):e51,1–15. doi: 10.3390/antiox6030051. DOI: 10.3390/antiox6030051. [DOI] [PMC free article] [PubMed] [Google Scholar]


