Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, critical care, mechanical ventilation
Objectives:
The ongoing severe acute respiratory syndrome coronavirus 2 or coronavirus disease 2019 pandemic has demonstrated the potential need for a low-cost, rapidly deployable ventilator. Based on this premise, we sought to design a ventilator with the following criteria: 1) standard components that are accessible to the public, 2) “open-source” compatibility to allow anyone to easily recreate the system, 3) ability to ventilate in acute respiratory distress syndrome, and 4) lowest possible cost to provide adequate oxygenation and ventilation.
Design:
We pursued development of a pneumatic-type ventilator. The basic design involves three electrically controlled solenoid valves, a pressure chamber, the patient breathing circuit, a positive end-expiratory pressure valve, and an electronics control system. Multiple safety elements were built into the design. The user-friendly interface allows simple control of ventilator settings. The ventilator delivers a hybrid form of pneumatic, assist-control ventilation, with predicted tidal volumes of 300–800 mL, positive end-expiratory pressure 0–20 cm H2O, and Fio2 21–100%.
Main Results:
The ventilator was extensively tested with two separate high-fidelity lung simulators and a porcine in vivo model. Both lung simulators were able to simulate a variety of pathologic states, including obstructive lung disease and acute respiratory distress syndrome. The ventilator performed well across all simulated scenarios. Similarly, a porcine in vivo model was used to assess performance in live tissue, with a specific emphasis on gas exchange. The ventilator performed well in vivo and demonstrated noninferior ventilation and oxygenation when compared with the standard ventilator.
Conclusions:
The Portsmouth Ventilator was able to perform well across all simulated pathologies and in vivo. All components may be acquired by the public for a cost of approximately $250 U.S.D. Although this ventilator has limited functionality compared with modern ventilators, the simple design appears to be safe and would allow for rapid mass production if ventilator surge demand exceeded supply.
In late 2019, a novel coronavirus was identified in Wuhan province, China. The virus was later identified as severe acute respiratory syndrome coronavirus-2, or COVID-19, and is the cause of a current worldwide pandemic. Based on the initial Chinese data, 14–17% of hospitalized patients required supplemental respiratory support (1, 2). Italy was one of the first western countries with widespread disease. Their critical care facilities appeared to carry an enormous burden of the patients, with an estimated 16% of actively infected patients requiring admission to an ICU for hypoxic respiratory failure from COVID-19 (3).
This is important for the current crisis, because in the absence of definitive treatment, supportive mechanical ventilation for several days to weeks is the mainstay treatment for severe disease. Currently, there are approximately 62,000 full function ventilators in the United States, with 98,000 basic ventilators and 8,900 in the strategic reserve. The Centers for Disease Control and Prevention estimate that between 2.4 and 21 million Americans will require hospitalization (4). Based on the Italian data (5), the number of patients requiring ventilators will range between 1.4 and 31 patients per ventilator (4). The U.S. Department of Health and Human Services has already started to encourage rationing of ventilator use by eliminating elective surgeries (6). Recent studies similarly project ventilator shortage in the United States (7).
Because of this need, we sought to build a low-cost ventilator for use when surge demand exceeds current capacity. The requirements for this ventilator were as follows:
1) Components must be easily sourced “off-the-shelf” items that are available to the general public.
2) They must have “open-source” compatibility, so the design will be widely available and technically easy to build.
3) Must be able to tolerate a range of ventilation strategies to tolerate high airway pressures associated with acute respiratory distress syndrome (ARDS).
4) A cost containment strategy must be maintained to ensure the ventilator would not be cost-prohibitive.
Although many modern ICU ventilators use a turbine to drive pressure, other types have included a servo control valve, bellows, and pneumatic pressure chambers (8, 9). Considering the technical complexity of the turbine and servo control ventilators, we believed either a bellow- or pneumatic-type ventilator would be the easiest to use with the requirements we established. Initial draft designs resulted in high confidence in the pneumatic model, which we pursued. We hypothesized that the Portsmouth Ventilator would be noninferior to the standard-of-care ventilators, while still meeting our requirements.
Materials and Methods
This ventilator follows the above design and uses a standard ventilator breathing circuit. It incorporates three solenoid valves (Charging, Inspiratory, and Expiratory represented by “C,” “I,” and “E,” respectively, in the above diagram) controlled by a simple microcontroller-driven electronics circuit. The ventilator connects to pipeline gas supply to both air and oxygen. A simple gas blender merges air and oxygen, and can realistically deliver either 100% oxygen, room air (21% oxygen), or a 60% oxygen gas blend to the patient. The gas mixture is delivered to the charging chamber at 50–55 PSI by opening the charging valve. Using a Boyle law relationship (P1V1 = P2V2), the chamber volume at high pressure is discharged into the breathing circuit and patient lungs at a lower pressure and a higher volume (Fig. 1).
Figure 1.
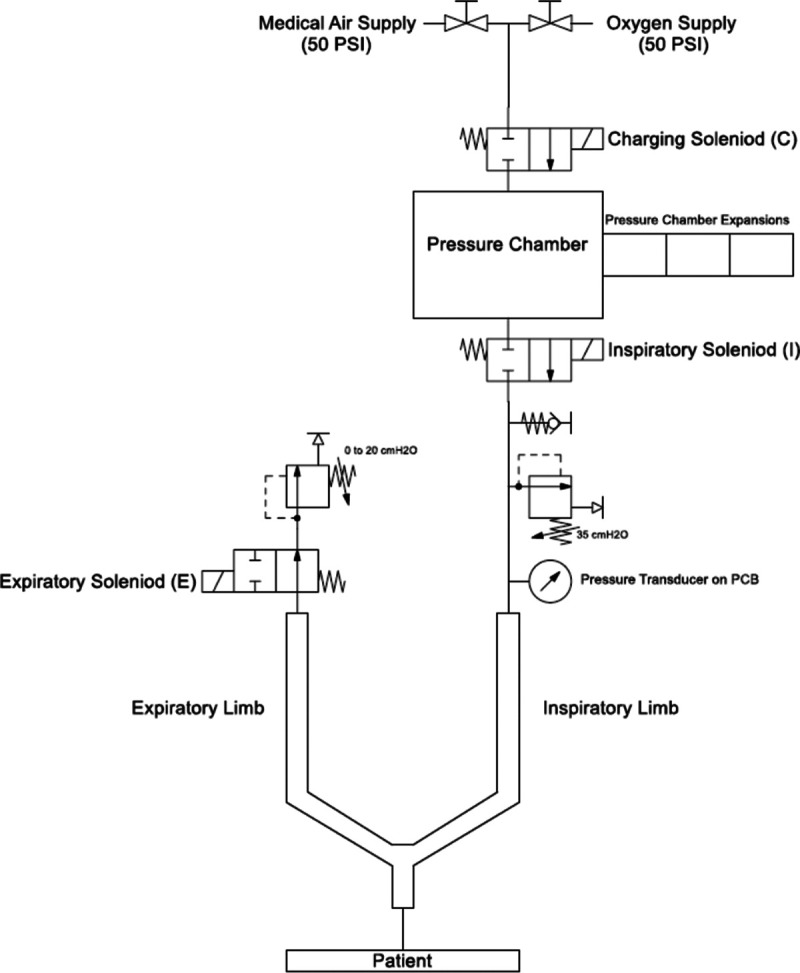
Pneumatic system diagram.
Following inspiration, the inspiratory valve is closed and the expiratory valve is opened. The expiratory valve opens through a positive end-expiratory pressure (PEEP) valve, allowing PEEP (0–20 cm H2O) to be delivered to the patient. The tidal volume on this device is adjusted by adding or removing expansion chambers. Each chamber generates approximately 45-mL additional tidal volume (Table 1). The respiratory rate and inspiratory time are set using attached controls. Additionally, if the patient is spontaneously breathing, the ventilator can detect a respiratory effort by measuring decreased airway pressure and augment by delivering a breath. Sensitivity to the patient’s inspiratory effort is similarly adjusted with controls attached to the microcontroller.
TABLE 1.
Ranges of Values for Performance of the Ventilator
| Parameter | Range |
|---|---|
| Respiratory rate | 4–30 RPM |
| Inspiratory time | 0.5–7.5 s |
| Positive end-expiratory pressure | 0–20 cm H2O |
| Max plateau pressure | 35 cm H2O |
| Tidal volume | 350–800 mL |
| Fio2 | 21–100% |
The breathing circuit is equipped with a 0.5-PSI (35 cm H2O) pressure relief valve to limit airway pressures exceeding 35 cm H2O and a negative pressure relief valve to allow for spontaneous room air inspiration at any point during the respiratory cycle, preventing a negative pressure injury (if the assist-control [AC] portion fails). A pressure transducer continuously monitors airway pressure and displays a green light emitting diode (LED) for airway pressures from 0 to 20 cm H2O, an amber LED for airway pressures from 20 to 30 cm H2O, and a red LED for airway pressures for greater than 30 cm H2O. If a prolonged period of “red” pressures is identified, the ventilator delivers a prolonged expiratory phase. The ventilator will not deliver additional breaths if the airway pressure remains high and will alarm. Alternatively, if the ventilator detects a prolonged period of low airway pressures, it will generate an alarm that indicates either a loss of fresh gas supply or circuit disconnect.
Control System and Components
The hardware and software controlling the ventilator were designed by our group specifically for this project. In an effort to make the ventilator highly scalable and affordable, we chose components that were readily available and inexpensive. The circuit diagram appears in Supplementary Data File 1 (http://links.lww.com/CCX/A440). The ventilator hardware is built on a circuit board with dimensions of about 3 × 3 inches. It is powered by a 120-V wall adapter and has an attached battery backup.
The microcontroller costs under $2 U.S.D at the time of this writing. Gas pressurization and flow are controlled by three 12-V solenoid valves. These valves open and close timed to allow safe and effective ventilation. The timing is adjusted by the user to set respiratory rate, inspiratory time, and sensitivity to spontaneous breathing.
Gas pressure in the airway circuit is measured by a pressure transducer rated for ± 0.5 PSI (24PCEFA6G, Honeywell International, Charlotte, NC) or roughly ± 35 cm H2O. As previously mentioned, the ventilator continuously illuminates LEDs that correspond to specific pressure levels (0–20, 20–30, and 30+ cm H2O), providing the user with visual feedback on airway pressures throughout the respiratory cycle without the need for a display screen. The software and hardware have several dedicated safety features, including alarms for sustained high pressure, circuit disconnect or gas supply failure, and electricity supply failure. In addition to generating visual and auditory alarms, sustained high pressure will trigger ventilation to cease and the expiratory valve to remain open until pressure falls below a specific threshold and then ventilation will resume. Although not adjustable by the user, the overpressure threshold is modifiable in the software.
The code block diagram is in Supplementary Data File 2 (http://links.lww.com/CCX/A441). The main program simply samples airway pressure, illuminates the corresponding LED for the present airway pressure, and monitors for periods of high or low airway pressures. High- and low-priority interrupts are programmed to handle alarms and timing of solenoid opening and closing throughout the respiratory cycle.
Measurements and Results
Simulation Testing
Human lung simulation was achieved with ALS 5000 (IngMar Medical, Pittsburg, PA). Compliance and resistance testing was achieved in a manner similar to the process described by Cristiano et al (11). We compared the Portsmouth Ventilator with both pressure control and volume control with a commercially available ventilator (Dräger Apollo, Dräger, Lubeck, Germany). Three initial trials were completed with the following lung parameters:
1) Resistance 12 cm H2O/L/s and compliance 20 mL/cm H2O.
2) Resistance 12 cm H2O/L/s and compliance 50 mL/cm H2O.
3) Resistance 15 cm H2O/L/s and compliance 50 mL/cm H2O.
After confirmation of acceptable tidal-volume delivery was completed above, ISO Standard 80601-2-12:2020 specifications on volume-control settings were completed for tests 1–7. Additional tests (8–20) were not completed due to the known tidal-volume limitations of the ventilator (cannot deliver tidal volumes less than 300 mL). A modern commercially available ventilator was similarly tested against the standard for comparison. Waveform data from these simulated tests are displayed in Figure 2. Peak inspiratory pressure, plateau pressure, tidal volume delivered, and percent-difference-delivered tidal volume from predicted are summarized in Table 2.
Figure 2.
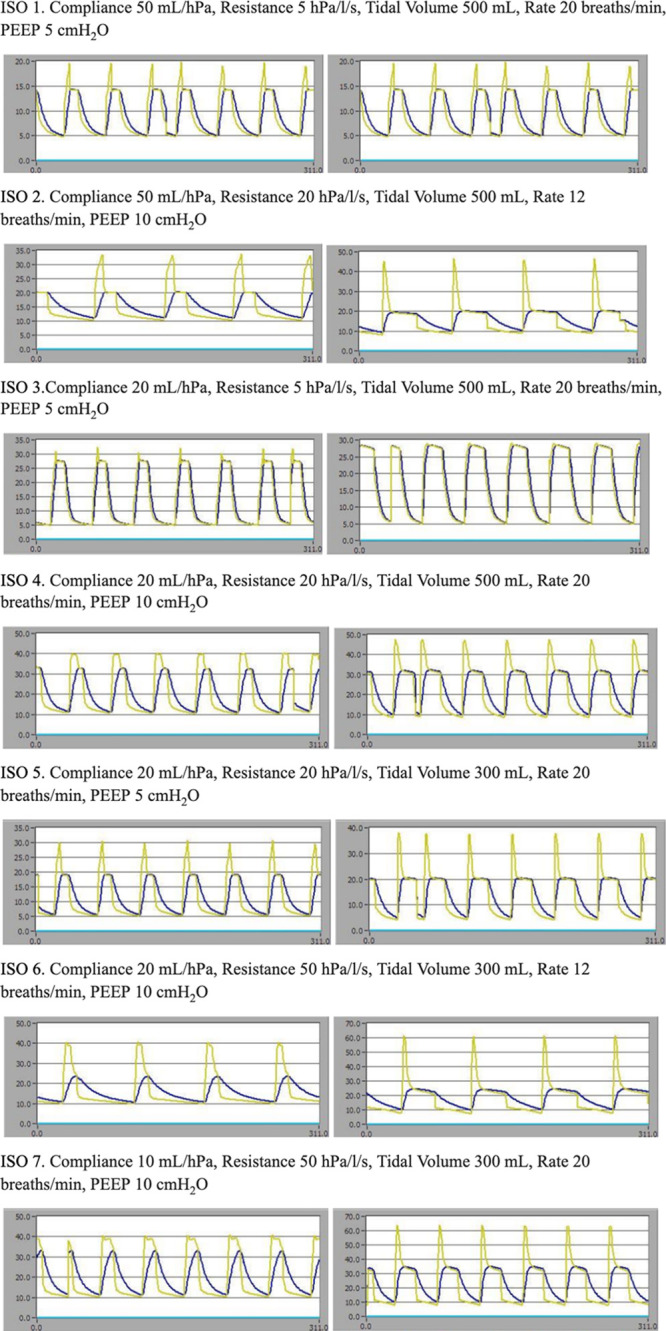
Comparison of performance Portsmouth Ventilator with the Drager Apollo (Drager, Lubeck, Germany) ventilator with ISO test numbers 1–7. Yellow waveform is airway pressure (cm H2O) and blue waveform is tracheal/alveolar pressure. Left column is the standard ventilator and the right column is the Portsmouth Ventilator. PEEP = positive end-expiratory pressure.
TABLE 2.
ISO Tests With Performance Outcomes With Drager Apollo Compared With Portsmouth Ventilator
| Peak Pressure (cm H2O) | Plateau Pressure (cm H2O) | Tidal Volume (mL) | % Difference | |||||
|---|---|---|---|---|---|---|---|---|
| ISO Trial Number | Drager | PV | Drager | PV | Drager | PV | Drager | PV |
| 1 | 19.8 | 21.4 | 15.2 | 17.2 | 482 | 535 | –3.60 | 7.00 |
| 2 | 33.8 | 46 | 27 | 28.2 | 473 | 522 | –5.40 | 4.40 |
| 3 | 32.2 | 28.9 | 21.9 | 25.9 | 490 | 497 | –2.00 | –0.60 |
| 4 | 39.9 | 47.5 | 35.7 | 38.5 | 461 | 477 | –7.80 | –4.60 |
| 5 | 30.5 | 38.1 | 22.3 | 26.1 | 285 | 325 | –5.00 | 8.33 |
| 6 | 40.7 | 61.2 | 31.8 | 37.5 | 275 | 307 | –8.33 | 2.33 |
| 7 | 40.7 | 63.9 | 36 | 45.5 | 246 | 275 | –18.00 | –8.33 |
PV = Portsmouth Ventilator.
We further tested the device with varying degrees of airway resistance and lung compliance to simulate severe ARDS and chronic obstructive pulmonary disease based on existing literature for lung respiratory parameters (12–15). Similarly, we tested extremes of compliance and resistance to validate further the range of pathophysiologic states over which the ventilator can safely operate. This included extremely low compliance with high resistance, extremely high compliance with low resistance, and varying high/low combinations of compliance and resistance. Due to lack of ISO standards for tidal-volume predictability in ventilators that are neither traditional volume control nor pressure control, we set an arbitrary ± 10% range from predicted tidal volume in the trial. This seems clinically appropriate, as it represents less than 1-mL/kg deviation. Because of the large pressure differences in the pulmonary system compared with the pipeline/charging cylinder (20 cm H2O is equivalent to 0.284 PSI), we assumed the predicted tidal volumes would remain nearly constant over a range of compliance, resistance, and PEEP variables.
To be thorough, other examples of ventilator testing were included (16, 17). Based on these, we similarly followed the previously described protocol to perform testing at a resistance of 5 cm H2O/L/s and compliance of 100 mL/cm H2O, resistance of 20 cm H2O/L/s and compliance of 30 mL/cm H2O (ARDS), and resistance of 50 cm H2O/L/s and compliance of 100 mL/cm H2O (obstruction) (16). We followed the additional protocol comparing resistance of 5, 10, and 20 cm H2O with compliances of 30, 70, and 120 mL/cm H2O (17). These data are summarized in Supplementary Data File (http://links.lww.com/CCX/A441).
Testing revealed strongly predictable tidal volumes all within the specified 10% change from baseline. Poorly compliant mechanics were associated with higher plateau pressures and lower tidal volumes, though the ventilator still performed within the standard and was similar to the commercial ventilator.
Other testing is summarized in Supplementary Data File (http://links.lww.com/CCX/A441), which describes the impact of adding pressure chamber expansions to the main pressure chamber on tidal volume and the effect of PEEP on the driving pressure needed to generate these tidal volumes. There was a theoretical concern that the tidal volume delivered might increase in a nonlinear manner due to increased airway pressure, but the stepwise volume increases appear to operate in a linear manner across physiologic pressure ranges (R2 = 0.999). This simulated testing suggests that the proposed mechanism of changing the size of the pressure chamber through the addition or removal of smaller expansion chambers is a reliable and predictable means of modifying the tidal volume that is being delivered to a patient. It also suggests that the Portsmouth Ventilator is able to deliver these tidal volumes at airway pressures that are comparable with other commonly used ventilators.
An additional high-fidelity lung simulator (TestChest, Organis GmbH, Landquart, Switzerland) was used for further simulation testing. ISO standard for volume control ventilators was repeated on the new test lung (tests 1–7) (18). The ventilator was then against the simulated COVID-19 in the lung model. Two models were used: an early model and a late/severe model. The early was characterized by chest wall compliance of 93 mL/hPa, total compliance 52 mL/hPa, and airway resistance 5, whereas the late model had a chest wall compliance of 93 mL/hPa, total compliance 39 mL/hPa, and airway resistance 5. This was similarly tested against the standard ventilator.
In summary, the ventilator performance was similar to existing ventilators across a range of pulmonary mechanics. Changes in PEEP and tidal volumes did not affect predicted tidal volume delivery. Despite changes in airway resistance and compliance, the ventilator was still able to deliver adequate tidal volume breaths. Based on these simulations, the ventilator appeared to be safe for in vivo use.
In Vivo Testing
This study was approved by the Naval Medical Center Portsmouth Institutional Animal Care and Use Committee under protocol number NMCP.2020.0011. A single female 84-kg Yorkshire swine was used for testing. The animal was anesthetized with intramuscular ketamine, acepromazine, and atropine, and placed on 100% Fio2 with 2% isoflurane until intubation. The animal model remained on 100% Fio2 throughout the study period. Following intubation, the animal was transitioned to a total IV anesthetic with fentanyl and propofol. The animal was maintained on a standard veterinary mechanical ventilator throughout induction (Hallowell EMC Model 2000, Hallowell EMC, Pittsfield, MA). The animal was paralyzed with rocuronium that was titrated one of four train-of-four twitches.
Following induction, the animal was maintained on the standard veterinary Hallowell ventilator for 60 minutes. At t = 60, the animal model was transitioned to the Portsmouth Ventilator, and mechanical ventilation was provided for an additional 120 minutes. We collected arterial blood gas measurements and recorded pH, Po2, and Pco2 at t = 0, t = 15, t = 30, t = 45, t = 60, t = 75, t = 90, t = 105, t = 120, t = 135, t = 150, t = 165, and t = 180, where t = 0 corresponds to placement of the arterial line immediately following intubation. Samples from t = 0 to t = 60 reflect standard ventilator function, and all samples beginning at t = 75 reflect the Portsmouth Ventilator. Pulse oximetry and end-tidal CO2 were recorded at these intervals. Airway pressures were monitored and recorded by an external pressure sensor at 60 Hz, in addition to the sensor in the ventilator. After t = 180, the animal was euthanized per standard veterinary protocols.
Throughout the study period, the respiratory parameters of Portsmouth Ventilator were manipulated by the investigators to provide optimal ventilation and then to test its maximal capabilities through “stress-testing” where the respiratory rate was increased sequentially in an effort to determine the threshold at which the ventilator would no longer provide safe or effective ventilation. These parameters were changed every 15 minutes corresponding with the scheduled arterial blood gas analysis. The respiratory parameters that are reported are correlated with the blood gas analysis that was obtained 15 minutes after the ventilator settings were changed (summarized in Table 3). Of note, the animal remained hemodynamically stable throughout the study period.
TABLE 3.
Relevant Ventilator Settings and Measures of Ventilation During Porcine Testing
| Time (min) | Respiratory Rate (Beats/min) | Tidal Volume (mL) | Number of Expansions | Positive End-Expiratory Pressure (cm H2O) | pH | Pao2 (mm Hg) | Etco2 (mm Hg) | Paco2 (mm Hg) | Etco2 to Paco2 Gradient (mm Hg) | Arterial oxygen saturation (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Spontaneous ventilation | ||||||||||
| 0 | 20 | 600 | — | 0 | 7.51 | 355 | 42 | 44.2 | 2.2 | 99 |
| Conventional ventilator | ||||||||||
| 15 | 12 | 500 | — | 0 | 7.49 | 493 | 36 | 48.1 | 12.1 | 100 |
| 30 | 18 | 500 | — | 0 | 7.51 | 481 | 34 | 45.5 | 11.5 | 100 |
| 45 | 18 | 500 | — | 0 | 7.52 | 429 | 33 | 46.6 | 13.6 | 100 |
| 60 | 18 | 500 | — | 0 | 7.5 | 477 | 34 | 47.8 | 13.8 | 100 |
| Portsmouth Ventilator | ||||||||||
| 75 | 18 | — | 4 | 5 | 7.48 | 452 | 42 | 49.3 | 7.3 | 100 |
| 90 | 20 | — | 5 | 5 | 7.48 | 508 | 41 | 50.5 | 9.5 | 100 |
| 105 | 20 | — | 7 | 5 | 7.51 | 392 | 40 | 45 | 5 | 100 |
| 120 | 20 | — | 7 | 5 | 7.5 | 461 | 39 | 45.6 | 6.6 | 99 |
| 135 | 24 | — | 7 | 5 | 7.54 | 482 | 35 | 42.4 | 7.4 | 100 |
| 150 | 24 | — | 7 | 10 | 7.56 | 457 | 34 | 41.2 | 7.2 | 100 |
| 165 | 24 | — | 7 | 10 | 7.57 | 365 | 31 | 38 | 7 | 100 |
| 180 | 30 | — | 7 | 10 | 7.6 | 428 | 29 | 35.5 | 6.5 | 100 |
Etco2 = end-tidal CO2 partial pressure.
During this testing, the Portsmouth Ventilator was able to provide adequate ventilation to the 84-kg swine model. The Etco2-to-Paco2 gradient found while using the Portsmouth Ventilator was significantly lower than the conventional ventilator. The mean difference of these values was significant based on a two-sided t test with p value of less than 0.001. This suggests there was an enhanced open lung ventilation strategy (19) when using the Portsmouth Ventilator when compared with the veterinary ventilator. We theorize that this finding is due to the inability of the veterinary ventilator to administer PEEP. The use of PEEP in modern ventilators has been well documented to improve the gradient and is a critical function (20, 21).
Discussion
The Medicines & Healthcare Products Regulatory Agency in the United Kingdom has released consensus guidelines documenting the minimum acceptable standards that a newly developed ventilator should meet prior to its use on patients who are impacted by the COVID-19 pandemic (22). This is the only guideline published by a major world government, which details the requirements, and we ensured our ventilator met those requirements.
The Portsmouth Ventilator provides a hybrid, pneumatic form of AC ventilation in spontaneously breathing patients. Inspiratory airway pressure in this ventilator is limited to 35 cm H2O by design as a safety mechanism. The system uses a PEEP valve that is commonly available within hospital systems to provide PEEP while using a self-inflating bag respirator, and notably is the only medical component used in the ventilator.
The inspiratory-to-expiratory (I:E) ratio can be adjusted from 1:1 to greater than 1:5 in the setting of very slow respiratory rates. Likewise, the respiratory rate can be set from 4 to 30 breaths/min. The tidal volume of this ventilator can be adjusted from 300- to 800-mL tidal volumes in 45-mL increments.
The Portsmouth Ventilator connects to the wall pipeline air and oxygen supplies using diameter index safety system connectors that are standardized throughout the United States, though these could easily be changed for the local standard connectors wherever this ventilator is needed. By design, this ventilator has a gas reservoir that allows for peak inspiratory flow rates of up to 120 L/min despite the average wall pipeline oxygen supply only providing around 6–10 L/min. The proportioning system is able to provide 50–60% Fio2 in addition to 90–100% Fio2 options through mixing of wall pipeline air and oxygen within the gas blender portion of the ventilator. This ventilator also allows for the use of standard connectors to ISO 5356-1:2015.
A commercially available lithium-polymer battery can provide up to 30 minutes of backup function in case of failure of the main electrical system. This system is powered by a U.S. standard 120-V 3 pin plug (this does not meet the U.K. standard, though could be easily converted to allow for stepping down 240V) using a simple direct current (DC) converter. The circuit can be modified to include an on-board voltage regulator to provide 12V DC with other, more commonly available, power sources such as a standard laptop charger.
The ventilator provides an auditory and visual alarm in the event of gas supply failure by detecting whether minimal inspiratory pressures are not achieved for a designated period of time. It also provides auditory and visual alarms in the setting of electricity supply failure should the battery backup be required. If there is a prolonged period of dangerously elevated airway pressures, the ventilator will enter a fail-safe mode. In this mode, an auditory alarm will sound and the ventilator will open the expiratory valve and not resume ventilation until the airway pressures return to a lower level. The ventilator displays the airway pressure in a categorical fashion, with pressures from 0 to 20 cm H2O powering a green LED, pressures from 20 to 30 cm H2O powering an amber LED, and pressures above 30 cm H2O powering a red LED. Although this does not provide the granularity of a digital display, it is simpler and cheaper, and still provides sufficient information to guide ventilator management.
CONCLUSIONS
The current COVID-19 worldwide pandemic may result in limited healthcare resources. Of those, one of the greatest concerns is the risk of limited ventilators. A simple to build and easy to operate ventilator, made with readily available components, could provide a reliable ventilator solution in the case of surge demand. Similarly, because of relatively low cost (< $250), this could potentially provide a ventilator solution in other resource-constricted environments. The Portsmouth Ventilator has limitations compared with modern ventilators; however, we believe it provides a safe, effective, and rapidly scalable alternative ventilation solution.
ACKNOWLEDGEMENTS
We thank the NASA Langley Research Center team (Mary Stringer, Lisa Scott Carnell, and Corey Diebler) for their efforts in establishing a path for future collaboration to obtain a hardware safety review, and manufacturing and path-to-market plans. We also thank the animal care staff at Naval Medical Center Portsmouth, including the attending Veterinarian MAJ Joanna Fishback, DVM, and CAPT John Devlin, MD, who provided material support for in vivo testing. Similarly, we thank the pharmacy staff and, in particular, Tim Gendron, PharmD, and, finally, significant mentorship and guidance from our departmental and hospital leadership, CDR Jason Longwell, MD, and CAPT Marlisa Elrod, MD/PhD.
Supplementary Material
Footnotes
Drs. Cole, Hughey, and Booth contributed equally to the design, creation, testing, and article creation. HM3 Rector contributed significantly to the technical creation.
The authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government.
We are military service members. This work was prepared as part of our official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
The experiments reported herein were conducted in compliance with the Animal Welfare Act and Regulations and per the principles of the “Guide for the Care and Use of Laboratory Animals,” Institute of Laboratory Animals Resources, National Research Council, National Academy Press, 1996.
This article describes the use of a medical device not yet approved by the FDA for either full approval or emergency use authorization.
REFERENCES
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020; 323:1239–1242 [DOI] [PubMed] [Google Scholar]
- 3.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA. 2020; 323:1545–1546 [DOI] [PubMed] [Google Scholar]
- 4.Truog RD, Mitchell C, Daley GQ. The toughest triage—allocating ventilators in a pandemic. N Engl J Med. 2020; 382:1973–1975 [DOI] [PubMed] [Google Scholar]
- 5.Sorbello M, El-Boghdadly K, Di Giacinto I, et al. The Italian coronavirus disease 2019 outbreak: Recommendations from clinical practice. Anaesthesia. 2020; 75:724–732 [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services. 2020. News Division: Optimizing Ventilator Use During the COVID-19 Pandemic. HHS.gov; Available at: https://www.hhs.gov/about/news/2020/03/31/optimizing-ventilator-use-during-covid19-pandemic.html. Accessed April 1, 2020 [Google Scholar]
- 7.Wells CR, Fitzpatrick MC, Sah P, et al. Projecting the demand for ventilators at the peak of the COVID-19 outbreak in the USA. Lancet Infect Dis. 2020; 20:1123–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thille AW, Lyazidi A, Richard JC, et al. A bench study of intensive-care-unit ventilators: New versus old and turbine-based versus compressed gas-based ventilators. Intensive Care Med. 2009; 35:1368–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams D, Flory S, King R, et al. A low oxygen consumption pneumatic ventilator for emergency construction during a respiratory failure pandemic. Anaesthesia. 2010; 65:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Fire Protection Association. Nfpa 99: Health Care Facilities Code. 2011. 2012 Edition National Fire Protection Association [Google Scholar]
- 11.Galbiati C, Abba A, Agnes P, et al. Mechanical ventilator milano (MVM): A novel mechanical ventilator designed for mass scale production in response to the COVID-19 pandemics. arXiv. 2003–10405Preprint posted online March 23, 2020. arXiv [Google Scholar]
- 12.Arnal JM, Garnero A, Saoli M, et al. Parameters for simulation of adult subjects during mechanical ventilation. Respir Care. 2018; 63:158–168 [DOI] [PubMed] [Google Scholar]
- 13.Davis KA, Peterson HP, Sanders AR, et al. A bench study evaluation of the amount of AutoPEEP when ventilating an electronic lung model simulated to represent normal, ARDS and COPD conditions. Respir Care. 2019; 64Suppl 103239227 [Google Scholar]
- 14.Ferreira JC, Chipman DW, Hill NS, et al. Bilevel vs ICU ventilators providing noninvasive ventilation: Effect of system leaks: A COPD lung model comparison. Chest. 2009; 136:448–456 [DOI] [PubMed] [Google Scholar]
- 15.Beydon L, Svantesson C, Brauer K, et al. Respiratory mechanics in patients ventilated for critical lung disease. Eur Respir J. 1996; 9:262–273 [DOI] [PubMed] [Google Scholar]
- 16.Boussen S, Gainnier M, Michelet P. Evaluation of ventilators used during transport of critically ill patients: A bench study. Respir Care. 2013; 58:1911–1922 [DOI] [PubMed] [Google Scholar]
- 17.L’Her E, Roy A, Marjanovic N. Bench-test comparison of 26 emergency and transport ventilators. Crit Care. 2014; 18:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Organization for Standardization. Medical electrical equipment — part 2-12: Particular requirements for basic safety and essential performance of critical care ventilators. 2020Report No.: ISO 80601-2-12:2020
- 19.Hodgson CL, Tuxen DV, Davies AR, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care. 2011; 15:R133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray IP, Modell JH, Gallagher TJ, et al. Titration of PEEP by the arterial minus end-tidal carbon dioxide gradient. Chest. 1984; 85:100–104 [DOI] [PubMed] [Google Scholar]
- 21.Blanch L, Fernández R, Benito S, et al. Effect of PEEP on the arterial minus end-tidal carbon dioxide gradient. Chest. 1987; 92:451–454 [DOI] [PubMed] [Google Scholar]
- 22.Medicines and Healthcare products Regulatory Agency. Rapidly Manufactured Ventilator System. Report No.: RMVS001, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


