Abstract
Colorectal cancer (CRC) is a leading cause of morbidity and mortality. Post-CRC resection complications and lower quality of life (QoL) are associated with a lower long-term survival. Perioperative administration of probiotics/synbiotics might lower prevalence of side effects and improve QoL and survival among CRC patients. Medline, Web of Science, Cochrane database, Embase, and clinical trials registries were searched in January 2020. Altogether, 16 randomized placebo-controlled probiotic/synbiotic clinical trials that included patients undergoing CRC surgery and investigated postoperative complications and QoL side effects were found. Meta-analyses using random-effects model were performed on data from 11 studies to calculate the effects of probiotics/synbiotics on common CRC resection postoperative side effects and complications. Perioperative probiotics/synbiotics administration was associated with lower infection incidence (odds ratio [OR] = 0.34, P < 0.001), lower diarrheal incidence (OR = 0.38, P < 0.001), faster return to normal gut function (mean difference [MD] −0.66 days, P < 0.001), shorter postoperative antibiotics use (MD −0.64 days, P < 0.001), lower incidence of septicemia (OR = 0.31, P < 0.001), and shorter length of hospital stay (MD −0.41 days, P = 0.110). The results support the hypothesis that short-term perioperative administration of probiotics/synbiotics, which are easy to administer, have few side-effects, and are low cost compared with alternatives, might help to alleviate gastrointestinal symptoms and postoperative complications among CRC patients.
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the second most common cause of cancer-related death worldwide. In 2018, CRC accounted for more than 1.8 million incident cases and almost 900,000 deaths (1). The current 5-year relative survival rate of CRC patients in the United States is around 65% (2).
Occurrence of CRC and its treatment go along with symptoms and side effects that negatively impact the quality of life (QoL) of CRC patients. After CRC resection, temporary disturbances in the gastrointestinal tract for about 3–6 months are common, with the most prevalent symptoms after conventional treatment being bowel dysfunction, diarrhea, and lower QoL (3–5). The symptoms often persist in the long run. In a study from Germany (6), 43%, 31%, and 31% of CRC patients still reported problems 1 year after their cancer diagnosis about pain, constipation, and diarrhea, respectively. Moreover, chronic diarrhea is frequently reported by CRC survivors even up to 10 years after treatment (7), as are constant or late impairments in their QoL (8). Efficacy of current treatments such as antidiarrheal medications or bulk-forming agents in alleviating the symptoms is limited.
The term “synbiotics” refers to a combination of live bacteria, known as probiotics and prebiotics, which are mostly carbohydrate-based substances such as starch or dietary fiber that serve to induce growth and activity of beneficial bacteria in the gut (9). In a meta-analysis published in 2016, Wu et al. found that perioperative probiotics/synbiotics supplementation reduced infectious complications and surgery side effects, shortened antibiotics use and hospital stay, and contributed to earlier return of normal gut function and better QoL for patients undergoing a variety of surgeries for different medical reasons (10). However, no meta-analysis was conducted thus far on the effect of synbiotics specifically on patients' postcolorectal resection and relying only on data from randomized controlled trials (RCTs). We, in this study, aim to establish a much-needed evidence base for potential use of synbiotics for improving CRC resection outcomes, symptoms, and potentially even long-term prognosis for patients.
METHODS
This systematic review and meta-analysis protocol was planned, conducted, and reported in adherence to the standards of quality for reporting systematic review and meta-analysis (PRISMA) guidelines (11). The review was registered with PROSPERO International Prospective Register of Systematic Reviews (PROSPERO 2019 CRD42019131206).
Literature search strategy
A systematic literature search was first conducted on February 27–28, 2019, and then repeated with the same search terms and limitations on January 2–3, 2020, on Medline, ISI Web of Science, the Cochrane library, Embase, clinicaltrials.gov, and the International Clinical Trials Registry Platform. Using custom search strategies, the aim of this review was to identify randomized double-blind placebo-controlled probiotic/synbiotic clinical trials including patients undergoing CRC surgery and investigating postoperative complications and QoL measures. Details of the search strategy are provided in the Supplemental Material (see Supplementary Digital Content 1, http://links.lww.com/CTG/A441). Email alerts were set for Medline and ISI Web of Science for new publications that match the search strategy after the first search date. No restrictions such as language or date of publication were set. After removal of duplicates, 2 authors (E.L.A. and P.R.C.) independently screened the titles and abstracts of the identified articles. Both authors also reviewed the full texts of each eligible article and the reference lists of identified studies were searched for additional relevant studies. Disagreements were discussed among all authors until agreement was reached.
Inclusion criteria and outcome measures
Studies were included if they met all of the following inclusion criteria, which were decided on before the search: double-blind placebo-controlled clinical trials with published final results and study population included CRC patients undergoing or having undergone a colorectal resection and they assessed QoL, gastrointestinal function, and other postoperative complications as an outcome. The outcomes of interest of this review were selected on the basis that they are directly related to patient well-being, disease burden, and prognosis in postoperative CRC patients. Therefore, studies investigating only changes in microbiota or inflammatory markers as an outcome were not considered. QoL is usually measured using validated questionnaires; postoperative complications are measured in absolute numbers, using established methodology.
Data extraction and quality assessment
Two authors, E.L.A. and P.R.C., independently selected studies based on the inclusion criteria, and disagreements were discussed until agreement was reached. To assure validity and high quality of the data and subsequent results, the data were extracted independently by E.L.A. and P.R.C., using predefined standardized data extraction forms, and then compared and discussed. The included studies were furthermore independently evaluated for quality by EA and PC based on the US National Institute of Health National Heart, Lung, and Blood Institute (NHLBI) study quality assessment tool for controlled intervention studies (12). The tool comprises 14 yes/no/not applicable criteria assessing randomization, treatment allocation, blinding, similarity of groups at baseline, dropout, adherence, study size and power, intervention protocol, outcome measure assessment, and statistical analysis of study data. Based on these criteria, the risk of bias is estimated, and thereafter, study quality can be rated as good, fair or poor.
Statistical methods: meta-analysis
A meta-analysis was conducted for key health outcomes. Outcomes were chosen for analysis when there were at least 3 studies measuring that outcome with available numerical data of sufficient quality. Meta-analyses were conducted using the metafor package (13) in R 3.6.1 (14). For the analysis of length of hospital stay, the replmiss function in the metafor package was used to fill in missing SD data based on reported P values. Random effects models were used in analyses of all outcome measures of interest to account for in between-study heterogeneity. Metafor was also used to measure between-study heterogeneity using I2 and Cochrane Q. Meta-regression was conducted (rma in metafor) in case of high heterogeneity. It was not possible to assess publication bias using funnel plots due to the small number of studies (<10) in each of the analyses. Statistical significance was set at 2-sided P value <0.05.
RESULTS
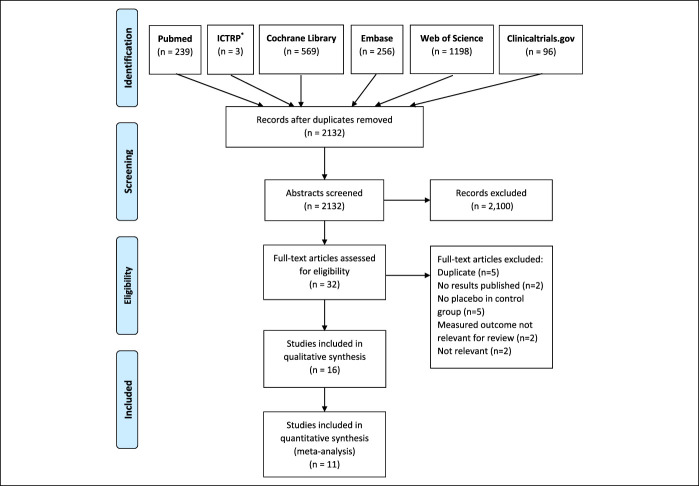
The literature search process, shown in a PRISMA flow diagram in Figure 1, yielded a total of 2,361 records. After exclusion of 229 duplicates, titles and abstracts of 1880 studies were screened for relevance. Of these, 2132 studies were deemed as not relevant for the review topic and, thus, excluded. The full texts of 32 studies were read, of which 16 studies were excluded for not meeting all the inclusion criteria.
Figure 1.
PRISMA flow diagram. *ICTRP, International Clinical Trials Registry Platform.
Of these 16 excluded studies, 5 studies included duplicate data also appearing in other publication and, therefore, were excluded. The final results were not published for 2 trials. In 5 studies, there was no placebo group, the measured outcome was not relevant for this review in another 2 studies, and 2 more studies were deemed not relevant for this review. In total, 16 RCTs were included in the qualitative analysis of the systematic review (15–30), and 11 studies were included in the quantitative meta-analyses (15,17,19–23,25,27,28,30).
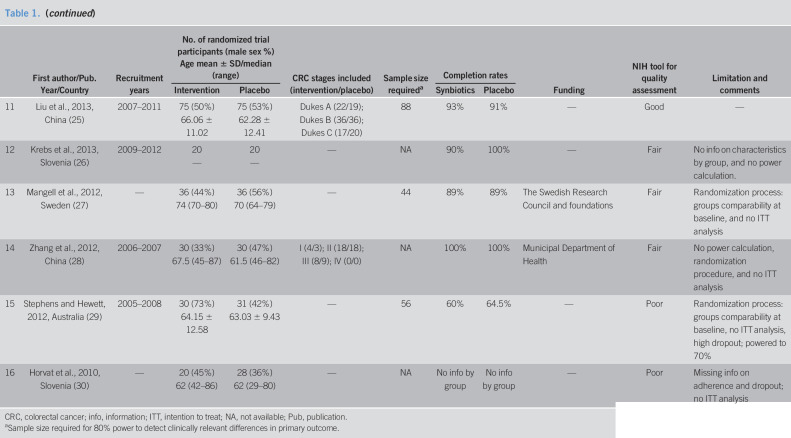
The 16 included studies were published between the years 2010 and 2019 and conducted in 12 different countries in 4 continents (Table 1). Eleven studies reported periods of data collection, which ranged between the years 2005 and 2015. Study sizes ranged from 40 to 164 participants. The mean or median age of participants in most studies was in the 60s, except for the study Lee et al. (24) with a younger population with mean age of 56 years and the study by Mangell et al. (27) with a median age of 70 years among the placebo group and 74 years in the intervention group. Of the 11 studies that included data on calculation of sample size required for 80% power to detect clinically relevant differences in their primary outcome, 3 studies did not reach the required size.
Table 1.
Characteristics of studies included in systematic review
| First author/Pub. Year/Country | Recruitment years | No. of randomized trial participants (male sex %) Age mean ± SD/median (range) |
CRC stages included (intervention/placebo) | Sample size requireda | Completion rates | Funding | NIH tool for quality assessment | Limitation and comments | |||
| Intervention | Placebo | Synbiotics | Placebo | ||||||||
| 1 | Polakowski et al., 2019, Brazil (15) | — | 36 (55%) 60.9 ± 6.7 |
37 (51%) 58.9 ± 6.3 |
I (8/9); II (22/21); III (6/7) | 77 | — | — | — | Fair | No ITT analysis |
| 2 | Golkhalkhali et al., 2018, Malaysia (16) | — | 70 No info |
70 No info |
— | 128 | — | — | University of Malaysia | Fair | No info on sex, intervention included, and omega 3; no ITT analysis |
| 3 | Flesch et al., 2017, Brazil (17) | 2013–2015 | 49 (45%) 64.5 |
42 (37%) 61.1 |
I (14/11); II (20/20); III (14/6); IV (1/5) | 90 | — | — | None | Fair | No ITT analysis |
| 4 | Theodoropoulos et al., 2016, Greece (18) | 2008–2012 | 37 (53%) 66.8 ± 2.2 |
36 (62%) 69 ± 1.4 |
0 (5/8); I (9, 9); II (15/10); III (9/10); IV (0/1) | 66 | 89% | 92% | — | Fair | No ITT analysis |
| 5 | Yang et al., 2016, China (19) | 2011–2012 | 30 (50%) 63.9 ± 12.25 |
30 (40%) 62.2 ± 11.1 |
0–II (23/21); III (7/9) | NA | 71% | 81% | National Natural Science Foundation | Poor | No ITT analysis, no power calculation, and high dropout rate |
| 6 | Tan et al., 2016, Malaysia (20) | — | 20 (55%) 64.3 ± 14.5 |
20 (65%) 68.4 ± 11.9 | I (7/4); II (7/11); III (6/5); IV (0/0) | NA | 100% | 100% | B-Crobes Laboratory Sdn. Bhd. | Fair | No power calculation. Industry funded |
| 7 | Kotzampassi et al., 2015, Greece (21) | 2013–2014 | 84 (68%) 65.9 ± 11.5 |
80 (72%) 66.4 ± 11.9 | — | 416 | 98% | 98% | — | Fair | Study prematurely stopped after 40% enrolment due to efficacy in the primary outcome, and no ITT analysis |
| 8 | Mego et al., 2015, Slovakia (22) | 2011–2013 | 23 (61%) 62 (45–75) |
23 (52%) 64 (42–81) |
Metastatic (stage IV) | 200 | 100% | 100% | Ministry of Education | Fair | Group comparability at baseline; study was prematurely terminated due to slow accrual |
| 9 | Liu et al., 2015, China (23) | 2007–2013 | 66 (53%) 65.6 ± 18.2 |
68 (51%) 60.2 ± 16.2 | Colorectal liver metastases (stage IV) | 240 | 89% | 85% | National Natural Science Foundation | Fair | Study population too small for significant results |
| 10 | Lee et al., 2014, South Korea (24) | 2012–2012 | 33 (54%) 56.36 ± 6.02 | 33 (62%) 56.03 ± 10.86 | II (11/17); III (16/13) | 50 (1-sided α = 10%) | 85% | 97% | Ministry of Science | Poor | Study population too small for significant results, and no ITT analysis |
| 11 | Liu et al., 2013, China (25) | 2007–2011 | 75 (50%) 66.06 ± 11.02 | 75 (53%) 62.28 ± 12.41 | Dukes A (22/19); Dukes B (36/36); Dukes C (17/20) | 88 | 93% | 91% | — | Good | — |
| 12 | Krebs et al., 2013, Slovenia (26) | 2009–2012 | 20 — |
20 — |
— | NA | 90% | 100% | — | Fair | No info on characteristics by group, and no power calculation. |
| 13 | Mangell et al., 2012, Sweden (27) | — | 36 (44%) 74 (70–80) |
36 (56%) 70 (64–79) |
— | 44 | 89% | 89% | The Swedish Research Council and foundations | Fair | Randomization process: groups comparability at baseline, and no ITT analysis |
| 14 | Zhang et al., 2012, China (28) | 2006–2007 | 30 (33%) 67.5 (45–87) |
30 (47%) 61.5 (46–82) |
I (4/3); II (18/18); III (8/9); IV (0/0) | NA | 100% | 100% | Municipal Department of Health | Fair | No power calculation, randomization procedure, and no ITT analysis |
| 15 | Stephens and Hewett, 2012, Australia (29) | 2005–2008 | 30 (73%) 64.15 ± 12.58 |
31 (42%) 63.03 ± 9.43 |
— | 56 | 60% | 64.5% | — | Poor | Randomization process: groups comparability at baseline, no ITT analysis, high dropout; powered to 70% |
| 16 | Horvat et al., 2010, Slovenia (30) | — | 20 (45%) 62 (42–86) |
28 (36%) 62 (29–80) |
— | NA | No info by group | No info by group | — | Poor | Missing info on adherence and dropout; no ITT analysis |
CRC, colorectal cancer; info, information; ITT, intention to treat; NA, not available; Pub, publication.
Sample size required for 80% power to detect clinically relevant differences in primary outcome.
The NHBLI tool for quality assessment lists the following as fatal flaws that lead to significant risk of bias and, thus, a study of poor quality: high dropout rate, high differential dropout rate (15% at most), no intention-to-treat analysis/completers-only analysis. Taking this into account, 1 study was deemed to be of good quality (25), 11 studies with 1 fatal flaw were deemed to be of fair quality (15–18,20–23,26–28), and 4 studies, which had more than 1 flaw considered fatal, were rated as having poor quality (19,24,29,30).
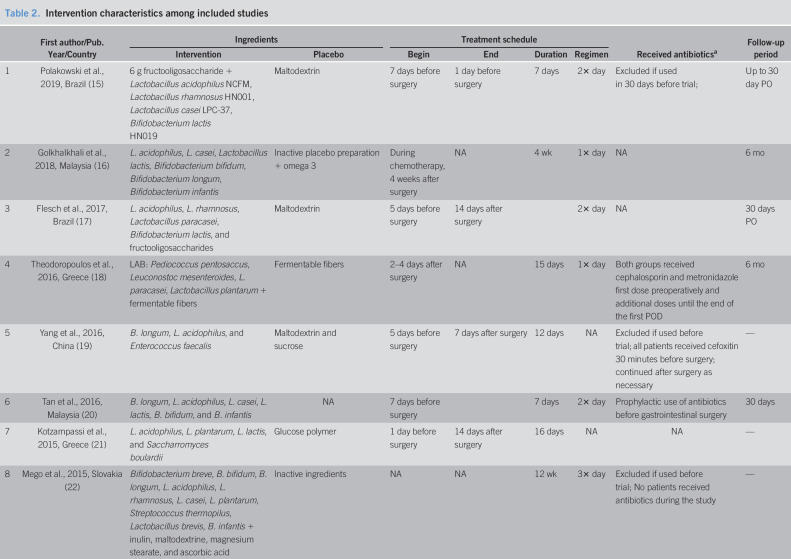
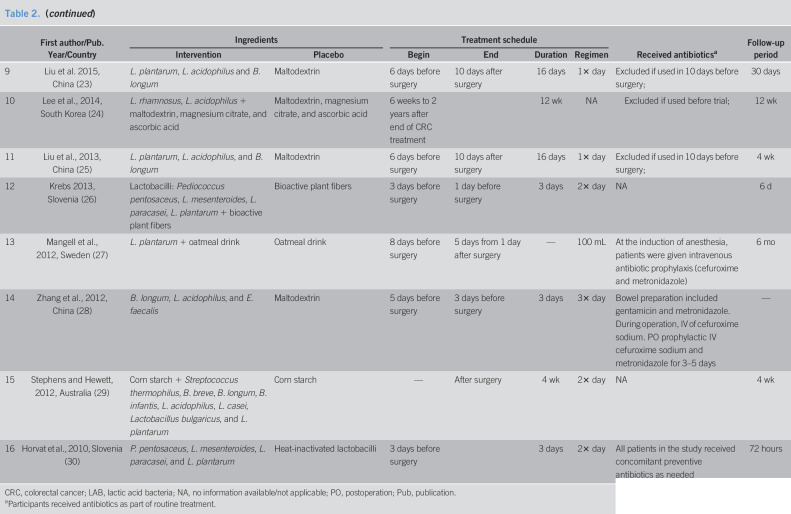
Fifteen of the included RCTs used synbiotics, a combination of live bacteria (probiotics) and mostly carbohydrate-based substances (prebiotics), but specific content varied widely (Table 2). The study by Golkhalkhali et al. (16) used probiotics and not synbiotics and, in addition, the participants in both groups received omega 3 fatty acids. Mangell et al. (27) used only one bacterium, Lactobacillus plantarum, together with an oatmeal drink, whereas Mego et al. (22) used 10 different bacteria together with inulin, maltodextrine, magnesium stearate, and ascorbic acid. Horvat et al. (30) used heat-inactivated lactobacilli as the placebo instead of the carbohydrate-based prebiotics that were used in most other studies. The most commonly used bacteria were Lactobacillus acidophilus (in 12 of the included studies), other various species of Lactobacillus (in all 16 included studies), and Bifidobacterium species (in 10 of the included studies).
Table 2.
Intervention characteristics among included studies
| First author/Pub. Year/Country | Ingredients | Treatment schedule | Received antibioticsa | Follow-up period | |||||
| Intervention | Placebo | Begin | End | Duration | Regimen | ||||
| 1 | Polakowski et al., 2019, Brazil (15) | 6 g fructooligosaccharide + Lactobacillus acidophilus NCFM, Lactobacillus rhamnosus HN001, Lactobacillus casei LPC-37, Bifidobacterium lactis HN019 | Maltodextrin | 7 days before surgery | 1 day before surgery | 7 days | 2× day | Excluded if used in 30 days before trial; | Up to 30 day PO |
| 2 | Golkhalkhali et al., 2018, Malaysia (16) | L. acidophilus, L. casei, Lactobacillus lactis, Bifidobacterium bifidum, Bifidobacterium longum, Bifidobacterium infantis | Inactive placebo preparation + omega 3 | During chemotherapy, 4 weeks after surgery | NA | 4 wk | 1× day | NA | 6 mo |
| 3 | Flesch et al., 2017, Brazil (17) | L. acidophilus, L. rhamnosus, Lactobacillus paracasei, Bifidobacterium lactis, and fructooligosaccharides | Maltodextrin | 5 days before surgery | 14 days after surgery | 2× day | NA | 30 days PO | |
| 4 | Theodoropoulos et al., 2016, Greece (18) | LAB: Pediococcus pentosaccus, Leuconostoc mesenteroides, L. paracasei, Lactobacillus plantarum + fermentable fibers | Fermentable fibers | 2–4 days after surgery | NA | 15 days | 1× day | Both groups received cephalosporin and metronidazole first dose preoperatively and additional doses until the end of the first POD | 6 mo |
| 5 | Yang et al., 2016, China (19) | B. longum, L. acidophilus, and Enterococcus faecalis | Maltodextrin and sucrose | 5 days before surgery | 7 days after surgery | 12 days | NA | Excluded if used before trial; all patients received cefoxitin 30 minutes before surgery; continued after surgery as necessary | — |
| 6 | Tan et al., 2016, Malaysia (20) | B. longum, L. acidophilus, L. casei, L. lactis, B. bifidum, and B. infantis | NA | 7 days before surgery | 7 days | 2× day | Prophylactic use of antibiotics before gastrointestinal surgery | 30 days | |
| 7 | Kotzampassi et al., 2015, Greece (21) | L. acidophilus, L. plantarum, L. lactis, and Saccharromyces boulardii | Glucose polymer | 1 day before surgery | 14 days after surgery | 16 days | NA | NA | — |
| 8 | Mego et al., 2015, Slovakia (22) | Bifidobacterium breve, B. bifidum, B. longum, L. acidophilus, L. rhamnosus, L. casei, L. plantarum, Streptococcus thermopilus, Lactobacillus brevis, B. infantis + inulin, maltodextrine, magnesium stearate, and ascorbic acid | Inactive ingredients | NA | NA | 12 wk | 3× day | Excluded if used before trial; No patients received antibiotics during the study | — |
| 9 | Liu et al. 2015, China (23) | L. plantarum, L. acidophilus and B. longum | Maltodextrin | 6 days before surgery | 10 days after surgery | 16 days | 1× day | Excluded if used in 10 days before surgery; | 30 days |
| 10 | Lee et al., 2014, South Korea (24) | L. rhamnosus, L. acidophilus + maltodextrin, magnesium citrate, and ascorbic acid | Maltodextrin, magnesium citrate, and ascorbic acid | 6 weeks to 2 years after end of CRC treatment | 12 wk | NA | Excluded if used before trial; | 12 wk | |
| 11 | Liu et al., 2013, China (25) | L. plantarum, L. acidophilus, and B. longum | Maltodextrin | 6 days before surgery | 10 days after surgery | 16 days | 1× day | Excluded if used in 10 days before surgery; | 4 wk |
| 12 | Krebs 2013, Slovenia (26) | Lactobacilli: Pediococcus pentosaceus, L. mesenteroides, L. paracasei, L. plantarum + bioactive plant fibers | Bioactive plant fibers | 3 days before surgery | 1 day before surgery | 3 days | 2× day | NA | 6 d |
| 13 | Mangell et al., 2012, Sweden (27) | L. plantarum + oatmeal drink | Oatmeal drink | 8 days before surgery | 5 days from 1 day after surgery | — | 100 mL | At the induction of anesthesia, patients were given intravenous antibiotic prophylaxis (cefuroxime and metronidazole) | 6 mo |
| 14 | Zhang et al., 2012, China (28) | B. longum, L. acidophilus, and E. faecalis | Maltodextrin | 5 days before surgery | 3 days before surgery | 3 days | 3× day | Bowel preparation included gentamicin and metronidazole. During operation, IV of cefuroxime sodium. PO prophylactic IV cefuroxime sodium and metronidazole for 3–5 days | — |
| 15 | Stephens and Hewett, 2012, Australia (29) | Corn starch + Streptococcus thermophilus, B. breve, B. longum, B. infantis, L. acidophilus, L. casei, Lactobacillus bulgaricus, and L. plantarum | Corn starch | — | After surgery | 4 wk | 2× day | NA | 4 wk |
| 16 | Horvat et al., 2010, Slovenia (30) | P. pentosaceus, L. mesenteroides, L. paracasei, and L. plantarum | Heat-inactivated lactobacilli | 3 days before surgery | 3 days | 2× day | All patients in the study received concomitant preventive antibiotics as needed | 72 hours | |
CRC, colorectal cancer; LAB, lactic acid bacteria; NA, no information available/not applicable; PO, postoperation; Pub, publication.
Participants received antibiotics as part of routine treatment.
Treatment with probiotics/synbiotics was initiated 1 to 8 days before surgery in 11 of the studies (15,17,19–21,23,25,27,28,30), while 4 studies (16,18,24,29) started the intervention only after surgery. Duration of treatment ranged between 3 days and 12 weeks. The follow-up period ranged between 72 hours and 6 months, but follow-up duration information was missing for 4 of the studies (19,21,22,28).
The aim of the included RCTs was to investigate whether the use of probiotics/synbiotics perioperatively in CRC patients can lead to better postoperative outcomes. The measured outcomes varied between studies and included postoperative infections, QoL measured using validated questionnaires, gastrointestinal symptoms and time to return to normal gut function, antibiotics use, and duration of hospital stay. When reported by the participating studies, the outcome measures were based on hospital electronic medical records and electronic case reports, providing information on postoperative gut function, occurrence of postoperative infections, antibiotic usage, and length of hospital stay (calculated from operation to discharge). QoL scores were obtained from questionnaires such as the EORTC QLQ and GIQLI. Chemotherapy side effects were also evaluated based on the Common Terminology Criteria for Adverse Events, version 3.0.
Synthesis of the results: meta-analysis
The following 6 clinical postoperative outcomes were included in the meta-analyses: duration of hospital stay, diarrheal incidence, infection incidence, time to return to normal gut function/first postoperative defecation, duration of antibiotics use, and incidence of septicemia (Table 3 and Figure 2). When numerical data for outcomes of interest were missing, the corresponding authors of the included studies were contacted (15–17,20,21,30), but only 1 author responded with the missing information (21). We did not conduct a meta-analysis of QoL score due to high between-study heterogeneity (I2 = 99.2%), in line with the use of 3 different questionnaires to calculate QoL scores by the 4 studies reporting this outcome: GIQLI (18,29), FACT-G total (24), and EORTC QLQ-c30 (16).
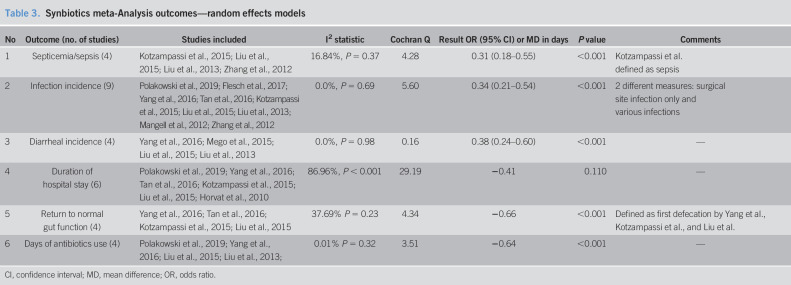
Table 3.
Synbiotics meta-Analysis outcomes—random effects models
| No | Outcome (no. of studies) | Studies included | I2 statistic | Cochran Q | Result OR (95% CI) or MD in days | P value | Comments |
| 1 | Septicemia/sepsis (4) | Kotzampassi et al., 2015; Liu et al., 2015; Liu et al., 2013; Zhang et al., 2012 | 16.84%, P = 0.37 | 4.28 | 0.31 (0.18–0.55) | <0.001 | Kotzampassi et al. defined as sepsis |
| 2 | Infection incidence (9) | Polakowski et al., 2019; Flesch et al., 2017; Yang et al., 2016; Tan et al., 2016; Kotzampassi et al., 2015; Liu et al., 2015; Liu et al., 2013; Mangell et al., 2012; Zhang et al., 2012 | 0.0%, P = 0.69 | 5.60 | 0.34 (0.21–0.54) | <0.001 | 2 different measures: surgical site infection only and various infections |
| 3 | Diarrheal incidence (4) | Yang et al., 2016; Mego et al., 2015; Liu et al., 2015; Liu et al., 2013 | 0.0%, P = 0.98 | 0.16 | 0.38 (0.24–0.60) | <0.001 | — |
| 4 | Duration of hospital stay (6) | Polakowski et al., 2019; Yang et al., 2016; Tan et al., 2016; Kotzampassi et al., 2015; Liu et al., 2015; Horvat et al., 2010 | 86.96%, P < 0.001 | 29.19 | −0.41 | 0.110 | — |
| 5 | Return to normal gut function (4) | Yang et al., 2016; Tan et al., 2016; Kotzampassi et al., 2015; Liu et al., 2015 | 37.69% P = 0.23 | 4.34 | −0.66 | <0.001 | Defined as first defecation by Yang et al., Kotzampassi et al., and Liu et al. |
| 6 | Days of antibiotics use (4) | Polakowski et al., 2019; Yang et al., 2016; Liu et al., 2015; Liu et al., 2013; | 0.01% P = 0.32 | 3.51 | −0.64 | <0.001 | — |
CI, confidence interval; MD, mean difference; OR, odds ratio.
Figure 2.
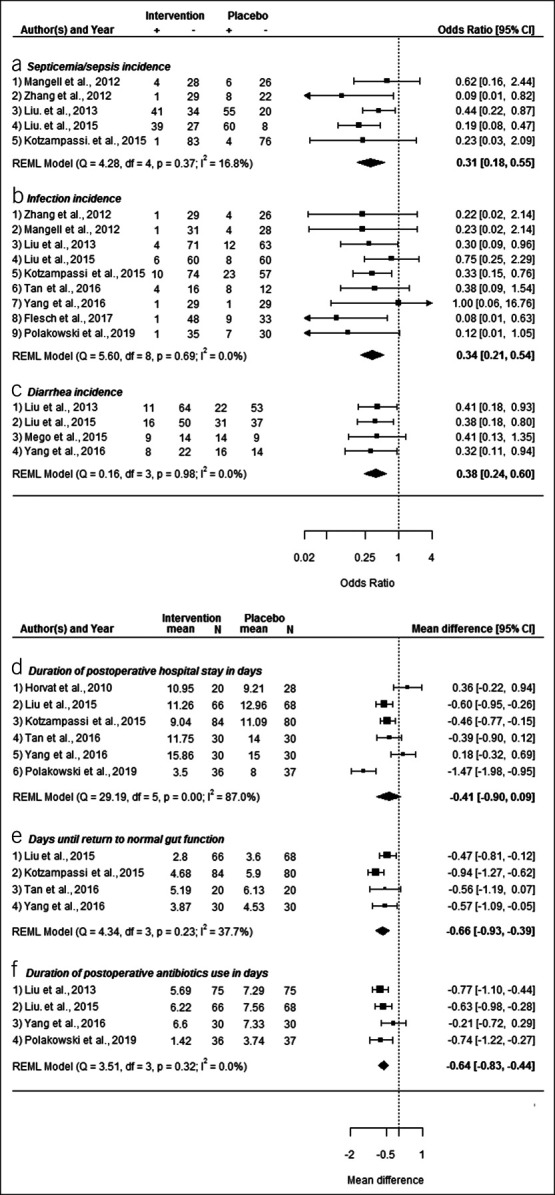
Forest plots of meta-analyses outcomes. CI, confidence interval; REML, restricted maximum likelihood; +, event, −, no event.
Septicemia and sepsis.
Five studies examined the association between synbiotics vs placebo and the occurrence of septicemia or sepsis use after colorectal surgery (21,23,25,27,28). The outcome was defined as “septicemia” by 4 of the studies and as “sepsis” in the study by Kotzampassi et al. (21) All included studies reported improved outcome for the intervention group, although the results were not statistically significant in 2 of the studies (21,27). No between-study heterogeneity was observed (I2 = 16.8%). In the random effects model, use of probiotic/synbiotics was associated with a statistically significant lower incidence of postoperative septicemia or sepsis (odds ratio [OR] = 0.31, 95% confidence interval [CI] 0.18–0.55, P < 0.001) (Figure 2a).
Infection incidence.
Nine studies examined the effect of synbiotics vs placebo on infection incidence after surgery (15,17,19–21,23,25,27,28). No between-study heterogeneity was observed (I2 = 0%). Most included studies regarded several different infections together, and some studies reported separately on infection at the surgical site (17,19,23,28). Meta-analysis (Figure 2b) showed that the use of probiotics/synbiotics was associated with a significant decrease in postsurgery infections (OR = 0.34, 95% CI 0.21–0.54, P < 0.001).
Diarrheal incidence.
Four studies reported numeric results for the association between administration of probiotics/synbiotics supplement vs placebo and diarrheal incidence (19,22,23,25). No between-study heterogeneity was observed (I2 = 0%). Meta-analysis (Figure 2c) indicated a statistically significant lower incidence of diarrhea among those who received synbiotics compared with that of the placebo groups (OR = 0.38, 95% CI 0.24–0.60, P < 0.001).
Duration of hospital stay.
Six studies included enough information to analyze the association between perioperative administration of synbiotics vs placebo and the duration in days of postsurgery hospital stay (15,19–21,23,30). The formula published by Wan et al. (31) was used to estimate mean and SD because only sample size, median, and range metrics were provided in the publications by Polakowski et al. (15) and Tan et al. (20) Meta-analysis (Figure 2d) showed that the mean difference (MD) in days of hospital stay was −0.41 (95% CI −0.90 to 0.09, P = 0.110), indicating a shorter hospital stay for those in the intervention group compared with those in the placebo group, but the result was not statistically significant, and between-study heterogeneity was high (I2 = 87%). Subanalyses excluding a single study at a time did not significantly lower the heterogeneity, and meta-regressions (rma in metaphor) looking at year of publication, location of the study (continent), and the quality assessment score were unable to identify the source of heterogeneity (R2 = 0 for all 3 analyses).
Return to normal gut function and first defecation.
Four studies reported on the impact of synbiotics on the number of days it took for the return to normal gut function (20) or first defecation after colorectal surgery (19,20,23,30). A low between-study heterogeneity was observed (I2 = 37.7%). A meta-analysis (Figure 2e) yielded MD in days of −0.66 (95% CI −0.93 to −0.39, P < 0.001), indicating a statistically significant shorter duration until return to normal gut function among those receiving probiotics/synbiotics.
Days of antibiotics use.
Four studies examined the association between synbiotics and the number of days of antibiotics use after colorectal surgery (15,19,23,25). All these 4 studies excluded participants who have taken antibiotics before inclusion in the trial. No between-study heterogeneity was observed (I2 = 0.0%). Meta-analysis (Figure 2f) yielded a MD in days of −0.64 (95% CI −0.83 to −0.44, P < 0.001), indicating a statistically significant shorter duration of postoperative antibiotics use among those who received synbiotics compared with that of the placebo groups.
DISCUSSION
This is the first systematic review and meta-analysis to include exclusively randomized double-blind placebo-controlled clinical trials focusing on probiotics/synbiotics administered perioperatively to patients undergoing CRC resection. For 6 meta-analyzed postoperative outcomes: diarrheal incidence, infection incidence, time to return to normal gut function, septicemia/sepsis, duration of antibiotics use, and duration of postoperative hospital stay, probiotics/synbiotics improved the outcomes for participants compared with those by placebo, and for the first 5 aforementioned outcomes, the results were statistically significant (P < 0.001).
In a review article from 2012, Peitsidou et al. (32) supported the role of preoperative probiotics/synbiotics interventions in reducing postoperative infectious complications and therapy-induced diarrhea and concluded that such supplements might have potential to improve postsurgical gastrointestinal related QoL. However, the authors also emphasized that evidence at time of publication was not sufficient to recommend such interventions in the context of CRC resection. In 2013, He et al. (33) conducted a meta-analysis including 6 RCTs on the efficacy of perioperative probiotics/synbiotics treatment in patients undergoing colorectal resection for cancer. They found a positive effect of the intervention on diarrhea, symptomatic intestinal obstructions, and operative total infections' incidence. However, no differences were found for septic morbidity, incision infection, perineal infection, intraabdominal infection, anastomotic leak, first defecation time, or length of hospital stay. Since then, 14 more publications reporting results from double-blind placebo-controlled clinical trials on perioperative synbiotics interventions in CRC patients undergoing resection were identified and, thus, included in this analysis (15–25,27,29,30).
Overall, our meta-analyses support the suggestions of previous studies that administration of probiotics/synbiotics around or early after CRC surgery might be beneficial and effective for the recovery of the large bowel. In addition, we found that synbiotics alleviated the risk of some postoperative complications, promoting a better prognosis. Plausible mechanisms potentially at work behind these associations include, among others, beneficial effects on restoration or even amelioration of the intestinal mucosa barrier (34), local immune function restoration (35), and synthesis of B and K vitamins and short-chain fatty acids (36). Long-term prevalence of gastrointestinal symptoms and nonlethal postoperative complications after tumor resection were found to be associated with a lower long-term survival in CRC patients for whom persisting gastrointestinal symptoms emerged as one of the factors that strongly compromised patients' QoL (8,37–40).
The synthesis of current evidence and the much larger number of studies included provide a broader evidence base for the impact of probiotics/synbiotics administration on functional outcomes and postsurgical complications, thus facilitating future informed decision making by clinicians concerning treatment of patients' resection side effects or prevention of postoperative complications and offering immediate translation of scientific research into clinical practice. Traditional treatments of postsurgical symptoms, such as antidiarrheal medications or bulk-forming agents, show only very limited efficacy (7). The results of this meta-analyses suggest that short-term perioperative administration of synbiotics, which are easy to administer, have less side effects, and are low cost compared with other alternatives, might play a role in alleviating gastrointestinal symptoms and postoperative complications of CRC patients (25).
However, there are limitations to this analysis due to the heterogeneity of included confirmatory RCTs about the type of bacteria used for the intervention group, the timing of the synbiotics administration, the duration of synbiotics administration, and the duration of follow-up. Several of the studies did not include information on completion/dropout rates, a few studies did not include power calculation for study population size, and others did not reach the calculated size. In addition, 11 of the studies did not include intention-to-treat analyses, and only 1 of the included studies was deemed to be of “good” quality, whereas others had at least 1 serious flaw that might have led to significant bias.
In conclusion, the findings of this systematic review and meta-analysis support the use of probiotics/synbiotics to alleviate and ease postoperative symptoms, adverse side effects, and complications after colorectal surgery in CRC patients. Further large RCTs could add much needed more comprehensive evidence for clinical practice to elucidate options for improvement of cancer burden, symptoms, QoL, and potentially even longer-term prognosis.
CONFLICTS OF INTEREST
Guarantor of the article: Efrat L. Amitay, MPH, PhD.
Specific author contributions: E.L.A.: study design, literature search, data collection and interpretation, statistical analysis, and manuscript writing; P.R.C.: literature search, data collection and interpretation, and critical manuscript revision; A.G.: data interpretation and critical manuscript revision; D.C.L.: data interpretation and critical manuscript revision; H.B.: study concept, study design, funding, data interpretation, and critical manuscript revision. All authors approved the manuscript version submitted for publication.
Financial support: The study was funded by the German Federal Ministry of Education and Research (BMBF), 01KG1809. The funder had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Potential competing interests: None to report.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A441
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67(3):177–93. [DOI] [PubMed] [Google Scholar]
- 3.Eras Compliance Group. The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: Results from an International Registry. Ann Surg 2015;261(6):1153–9. [DOI] [PubMed] [Google Scholar]
- 4.Crombe T, Bot J, Messager M, et al. Malignancy is a risk factor for postoperative infectious complications after elective colorectal resection. Int J Colorectal Dis 2016;31(4):885–94. [DOI] [PubMed] [Google Scholar]
- 5.Ratjen I, Schafmayer C, Enderle J, et al. Health-related quality of life in long-term survivors of colorectal cancer and its association with all-cause mortality: A German cohort study. BMC Cancer 2018;18(1):1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arndt V, Merx H, Stegmaier C, et al. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: A population-based study. J Clin Oncol 2004;22(23):4829–36. [DOI] [PubMed] [Google Scholar]
- 7.Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. J Natl Compr Canc Netw 2009;7(8):883–93; quiz 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen L, Herrmann A, Stegmaier C, et al. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: A population-based study. J Clin Oncol 2011;29(24):3263–9. [DOI] [PubMed] [Google Scholar]
- 9.Sanders ME, Merenstein DJ, Reid G, et al. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat Rev Gastroenterol Hepatol 2019;16(10):605–16. [DOI] [PubMed] [Google Scholar]
- 10.Wu XD, Liu MM, Liang X, et al. Effects of perioperative supplementation with pro-/synbiotics on clinical outcomes in surgical patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Clin Nutr 2018;37(2):505–15. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NHLBI. Quality Assessment of Controlled Intervention Studies. (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). Accessed May 15, 2019. [Google Scholar]
- 13.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36(3):1–48. [Google Scholar]
- 14.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R foundation for statistical computing; 2015. [Google Scholar]
- 15.Polakowski CB, Kato M, Preti VB, et al. Impact of the preoperative use of synbiotics in colorectal cancer patients: A prospective, randomized, double-blind, placebo-controlled study. Nutrition 2019;58:40–6. [DOI] [PubMed] [Google Scholar]
- 16.Golkhalkhali B, Rajandram R, Paliany AS, et al. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: A randomized controlled trial. Asia Pac J Clin Oncol 2018;14(3):179–91. [DOI] [PubMed] [Google Scholar]
- 17.Flesch AT, Tonial ST, Contu PC, et al. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: A randomized, double-blind clinical trial. Rev Col Bras Cir 2017;44(6):567–73. [DOI] [PubMed] [Google Scholar]
- 18.Theodoropoulos GE, Memos NA, Peitsidou K, et al. Synbiotics and gastrointestinal function-related quality of life after elective colorectal cancer resection. Ann Gastroenterol 2016;29(1):56–62. [PMC free article] [PubMed] [Google Scholar]
- 19.Yang YZ, Xia Y, Chen HQ, et al. The effect of perioperative probiotics treatment for colorectal cancer: Short-term outcomes of a randomized controlled trial. Oncotarget 2016;7(7):8432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan CK, Said S, Rajandram R, et al. Pre-surgical administration of microbial cell preparation in colorectal cancer patients: A randomized controlled trial. World J Surg 2016;40(8):1985–92. [DOI] [PubMed] [Google Scholar]
- 21.Kotzampassi K, Stavrou G, Damoraki G, et al. A four-probiotics regimen reduces postoperative complications after colorectal surgery: A randomized, double-blind, placebo-controlled study. World J Surg 2015;39(11):2776–83. [DOI] [PubMed] [Google Scholar]
- 22.Mego M, Chovanec J, Vochyanova-Andrezalova I, et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement Ther Med 2015;23(3):356–62. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Li C, Huang M, et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: A double-center and double-blind randomized clinical trial. BMC Gastroenterol 2015;15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JY, Chu SH, Jeon JY, et al. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: A double-blind, randomized, placebo-controlled trial. Dig Liver Dis 2014;46(12):1126–32. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZH, Huang MJ, Zhang XW, et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: A double-center and double-blind randomized clinical trial. Am J Clin Nutr 2013;97(1):117–26. [DOI] [PubMed] [Google Scholar]
- 26.Krebs B, Horvat M, Golle A, et al. A randomized clinical trial of synbiotic treatment before colorectal cancer surgery. Am Surg 2013;79(12):E340–2. [PubMed] [Google Scholar]
- 27.Mangell P, Thorlacius H, Syk I, et al. Lactobacillus plantarum 299v does not reduce enteric bacteria or bacterial translocation in patients undergoing colon resection. Dig Dis Sci 2012;57(7):1915–24. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JW, Du P, Gao J, et al. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am J Med Sci 2012;343(3):199–205. [DOI] [PubMed] [Google Scholar]
- 29.Stephens JH, Hewett PJ. Clinical trial assessing VSL#3 for the treatment of anterior resection syndrome. ANZ J Surg 2012;82(6):420–7. [DOI] [PubMed] [Google Scholar]
- 30.Horvat M, Krebs B, Potrc S, et al. Preoperative synbiotic bowel conditioning for elective colorectal surgery. Wien Klin Wochenschr 2010;122(Suppl 2):26–30. [DOI] [PubMed] [Google Scholar]
- 31.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peitsidou K, Karantanos T, Theodoropoulos GE. Probiotics, prebiotics, synbiotics: Is there enough evidence to support their use in colorectal cancer surgery. Dig Surg 2012;29(5):426–38. [DOI] [PubMed] [Google Scholar]
- 33.He D, Wang HY, Feng JY, et al. Use of pro-/synbiotics as prophylaxis in patients undergoing colorectal resection for cancer: A meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol 2013;37(4):406–15. [DOI] [PubMed] [Google Scholar]
- 34.Liu D, Jiang XY, Zhou LS, et al. Effects of probiotics on intestinal mucosa barrier in patients with colorectal cancer after operation: Meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95(15):e3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagnini C, Saeed R, Bamias G, et al. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A 2010;107(1):454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeBlanc JG, Chain F, Martin R, et al. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact 2017;16(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter V, Jansen L, Hoffmeister M, et al. Smoking and survival of colorectal cancer patients: Population-based study from Germany. Int J Cancer 2015;137(6):1433–45. [DOI] [PubMed] [Google Scholar]
- 38.Odermatt M, Miskovic D, Flashman K, et al. Major postoperative complications following elective resection for colorectal cancer decrease long-term survival but not the time to recurrence. Colorectal Dis 2015;17(2):141–9. [DOI] [PubMed] [Google Scholar]
- 39.Walter V, Jansen L, Ulrich A, et al. Alcohol consumption and survival of colorectal cancer patients: A population-based study from Germany. Am J Clin Nutr 2016;103(6):1497–506. [DOI] [PubMed] [Google Scholar]
- 40.Arndt V, Koch-Gallenkamp L, Jansen L, et al. Quality of life in long-term and very long-term cancer survivors versus population controls in Germany. Acta Oncol 2017;56(2):190–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.