Abstract
Carbohydrate antigens are important targets for the immune system, but identification of key glycan antigens is challenging. Direct analysis of glycomes by mass spectrometry is difficult, and detection reagents, such as monoclonal antibodies and lectins, are only available for a small subset of glycans. An alternative approach involves profiling serum anti-glycan antibody populations to identify unique antibodies or changes in antibody subpopulations. Glycan microarray technology allows rapid evaluation of hundreds to thousands of antigen-antibody interactions in a single experiment. This high-throughput format is particularly useful in profiling complex anti-glycan antibodies in serum. Here we elaborate the use of this technology to explore clinically relevant carbohydrate antigens by profiling serum anti-glycan antibodies. Detailed protocols from glycan microarray fabrication to microarray binding assays and analysis of microarray data are presented.
Keywords: Glycan microarray, Carbohydrate array, Anti-glycan antibody, Carbohydrate antigen, Tumor antigen, Serum antibody profile
1. Introduction
Carbohydrates are abundantly present on all cell surfaces, including mammalian cells and pathogen cells. Some of the glycans expressed on tumor cells and pathogens are structurally distinct from normal healthy human glycans [1]. As a result, they can stimulate an immune response which can be harnessed in the diagnosis and treatment of many diseases including cancers and pathogen infections [2, 3]. For example, bacterial carbohydrates that stimulate a neutralizing response can inform vaccine design. However, identification of carbohydrate antigens is extremely challenging due to the complexity of diverse glycan structures in nature, a dearth of structural information on those glycans, and a lack of detecting tools [4, 5].
Antigens, in general, are often identified indirectly by profiling antibody and cellular responses [6]. For example, protein arrays have been used frequently to compare antibody populations before and after infection or vaccination [7]. When antibodies to a particular peptide are detected after immune stimulation, this information is then used to trace the response back to the original antigen. Glycan microarray technology allows analogous evaluations of anti-glycan immune response. On the microarray, a large number of structurally distinct glycans derived from either natural or synthetic sources are immobilized on a glass slide in a spatially defined pattern [8–10]. The source of glycans can be from human, bacteria, virus, or other organisms, and only tiny amounts of material are required. This miniaturized format allows high-throughput screening of hundreds of carbohydrate-protein interactions on a single slide. This technology has been used in many research areas including functional glycomics, drug discovery, and diagnosis [11–14]. One of the applications in vaccine development is discovery of clinically relevant biomarkers by profiling serum anti-glycan antibodies [15]. For example, one can study ligand specificities of the isolated monoclonal antibodies produced in vaccinated or pathogen-infected animals [16, 17]. One can also compare antibody populations of diseased subjects to a group of healthy control individuals to discover disease-specific antigens [for some recent examples, see [18–21]]. Another approach is to evaluate antibody changes in individuals before and after stimulation (e.g., vaccination, pathogen infections) to discover antigens on vaccines or pathogens [for some recent examples, see [22–24]].
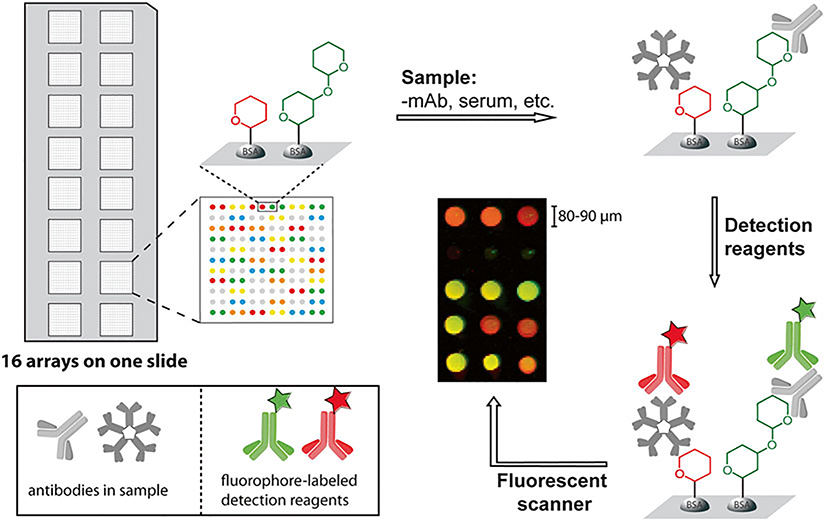
The general approach is relatively straightforward. A slide is first incubated with the sample of interest (e.g., vaccinated or infected sera, monoclonal antibodies). After washing off unbounded samples, the slide is incubated with fluorophore or streptavidin-labeled detection reagents (e.g., fluorophore-labeled anti-human IgG and IgM antibodies) and the captured antibodies on the array are detected with a fluorescent scanner (Fig. 1). Since it is often advantageous to profile many different samples and/or to profile individual samples multiple times under different conditions, many groups use a slide format in which multiple copies of the array are printed on each slide (e.g., 16 arrays/slide, Fig. 1). After physically separating the replicate arrays using a well module, one can carry out multiple array assays on each slide. The protocol described here covers procedures for microarray fabrication, microarray binding assay, and data analysis. In addition, technical challenges and potential pitfalls are also discussed.
Fig. 1. Glycan microarray binding assay.
16 arrays are printed on a single slide with hundreds of BSA-modified neoglycoproteins on one array. Prior to the assay, the slide is fitted with a 16-well module that physically separates the individual arrays. In the binding assay, the slide is first blocked to deactivate reactive functional groups on surface. After blocking, it is incubated with sample of interest, and then the captured antibodies are detected with fluorophore-labeled secondary reagents. Binding is quantitated by a fluorescent scanner. In the example shown, IgG and IgM antibodies are detected with secondary reagents labeled with different dyes
2. Materials
2.1. Reagents and Materials
2.1.1. For Glycan Microarray Construction
Microarray printing pins (Arrayit, SMP2).
SuperEpoxy 2 microarray substrate slides (Arrayit).
384-well low profile microplate, V-bottom.
Thermowell aluminum sealing tape.
Print dye: Alexa Fluor 555 azide.
Glycans for microarray: Glycans used in our lab are either purchased from commercial sources or chemically synthesized in the lab. There are several ways to immobilize glycans onto a solid support. The protocol described here uses neoglycoproteins (proteins in which glycans are covalently linked via a nonnatural linkage) as substrates that are immobilized onto epoxide-modified glass slides. Many natural or synthetic glycans with a free reducing end can be coupled to BSA or HSA in a single step via reductive amination [25]. Our most recent array contains over 500 glycoconjugates, encompassing human glycans, non-human glycans, glycopeptides, and glycoproteins. The glycans are stored at −20 °C until use.
2.1.2. For Glycan Array Profiling of Serum Antibodies
ProPlate multi-array slide module.
ProPlate adhesive seal-strips.
Aluminum foil.
Detecting antibodies: DyLight 549-conjugated Goat Anti-Human IgG and DyLight 649-conjugated Goat Anti-Human IgM.
Reference serum: pooled serum samples from multiple healthy donors to serve as a control.
Serum samples: For case-control study, sera from a group of diseased subjects and a group of healthy controls are recommended; for profiling the same subject at different time points, pre- and post-stimulation (e.g., vaccination, pathogen infection) sera from individual subjects are recommended.
2.2. Equipment
Microarray printer (Biorobotics, MicroGrid II).
Microarray Fluorescent scanner (Molecular Devices, GenePix 4000B).
FLx800 microplate fluorescence reader (BioTek Instruments).
Microscope.
Centrifuge.
Incubator shaker.
Sonicator.
2.3. Software
GenePix Pro 6.0 (Molecular Devices).
Excel.
2.4. Buffer Solutions
Buffer solutions 2–4 should be prepared fresh on the day of experiment.
Phosphate buffered saline (PBS): 10 mM NaHPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 137 mM NaCl, pH 7.4.
Print buffer: 1 × PBS buffer with 2.5 % (v/v) glycerol, 0.0005 % (v/v) Triton-X 100, 0.7 μg/mL print dye (Alexa Fluor 555 azide). Store in the dark to protect the dye.
Blocking buffer: PBS with 3 % (w/v) bovine serum albumin (BSA, globulin free).
Washing buffer: PBS with 0.05 % (v/v) Tween 20 (PBST).
Serum incubation buffer: PBST with 3 % (w/v) BSA and 1 % (w/v) human serum albumin (HSA, globulin free).
Antibody incubation buffer: PBS with 1 % (w/v) BSA and 3 % (w/v) HSA.
3. Methods
3.1. Construction of Glycan Antigen Microarray
3.1.1. Preparing Source Plates for Printing
Dilute glycan antigens with printing buffer containing print dye Alexa 555 (see Note 1) to a final concentration of 125 μg/mL.
Place 8 μL/well (see Note 2) diluted glycan solution into 384-well plates. The layout of 384-well plate depends on the number and configuration of pins used for printing (see step 1 in Subheading 3.1.2 for detail coordination of source plate with pins).
Scan the finished 384-well plates in FLx800 Microplate Fluorescence Reader for any missing samples.
Seal plates with aluminum seal to prevent evaporation and photobleaching of the dye, then cover with lids and store at 4 °C till use.
Spin down the source plate at 1000 rpm for 5 min before printing.
3.1.2. Set Up Robotic Microarray Printer
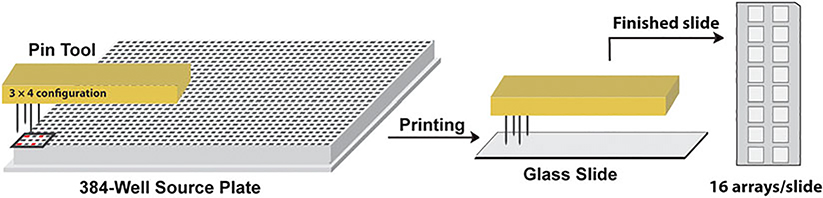
Program the printer so that each slide accommodates 16 arrays and each glycan is printed in duplicate (Fig. 2).
Sonicate printer pins for 15 min in Milli-Q water and blow-dry with argon. Handle with care so as not to touch the pin tips. Tips are delicate, and the quality of pins directly affects the printing quality.
Inspect pins under a microscope for any physical damages, and make sure no debris is trapped inside of pin channels.
Load pins into pin tool.
Load epoxide-coated slides and source plates in the printer.
Humidify the whole printer until humidity reaches 50 − 60 %. Maintain this humidity during the whole printing.
Fig. 2. Coordination of pin configuration with 384-well source plate.
Four pins are arranged in 3 × 4 configuration. Correspondingly, samples in 384-well source plate are arranged so that each sample is distributed in four wells matching with four pins. During printing, four pins are dipped into 384-well source plate, and then deliver sample onto glass slide for printing. The four-pin system allows four arrays to be printed simultaneously. A total of 16 arrays are printed on a single slide
3.1.3. Printing Microarray
Start printing. If possible, printing should be carried out in the dark to minimize photobleaching of the print dye.
During printing, frequently check humidity and pins. Make sure pins are not stuck in the pin tool (see Note 3).
After a print run is complete, scan slides in a fluorescent microarray scanner for any smear, merging spots, and missing spots. Keep record of any defects.
Collect slides in a covered box, and store it in a vacuum-sealed bag at −20 °C. Slides can be stored in this manner for ≥ 6 months without loss of binding capacity or integrity.
3.1.4. Quality Control
In addition to scanning the slides after a print run to detect smears, missing spots, and merged spots, quality of the printed slides could be evaluated by incubating with lectins with known binding specificities (see Note 4). We typically profile a set of 4 lectins on at least one slide from every print batch to assess quality and reproducibility. New data is compared to all previous batches and to known binding preferences.
3.2. Glycan Array Profiling of Serum Antibodies
3.2.1. Preparing Slide Module and Microarray
Remove vacuum-sealed slides from −20 °C freezer and let them warm up to room temperature before opening the package.
Inspect slides for any defects (smear, non-uniformed spots, missing spots, etc.) with fluorescent scanner at PMT 600. Save this pre-scan image as .tiff file for future data analysis. Choose slides with minimum defects for high quality results.
Assemble slide module over the slide to separate 16 printed arrays. The printed area of each array should be aligned in the center of each well. Be careful not to smudge or smear the array surface.
3.2.2. Blocking Glycan Microarray
This process serves dual roles: blocking excess epoxide on slide surface and washing off print dye.
Prepare a fresh solution of blocking buffer: PBS with 3 % BSA (see Note 5).
Gently pipet 200 μL blocking buffer into each well. Make sure not to drop the liquid directly on the printed area. Slowly pipet solution against the corner of well while holding the slide slightly tilted (see Note 6).
Seal the slide module with an adhesive strip to prevent evaporation, and incubate without shaking at room temperature for 2 h, or at 4 °C for overnight (see Note 7).
Discard the blocking solution after removing the strip, and wash the array with 6 × 200 μL/well washing buffer. After each wash cycle, invert the module and tap vigorously on paper towel to remove excess liquid.
3.2.3. Preparing Serum Samples and Reference Sample
All the following steps should be handled in class II biosafety cabinet to prevent bloodborne pathogen infection from serum samples.
Prepare a fresh serum incubation buffer: PBST with 3 % BSA and 1 % HSA (see Note 8).
Dilute serum samples with incubation buffer to an appropriate concentration (1:50 dilution is recommend. However, best condition needs to be optimized for different samples). Mix well by gently tapping the tube rather than vortex.
Prepare a reference sample (1:50 dilution) to serve as a control on each slide. Pooled serum sample could be used as reference. To avoid thaw-freeze cycles, make aliquots of the reference and store at −80 °C. Use a fresh aliquot each time (see Note 9).
3.2.4. Incubating Microarray with Serum Samples
Perform the following steps in class II biosafety cabinet.
- Design layout of the array. Take following factors into consideration to minimize technical errors introduced into experiment:
- Each slide includes a reference control sample;
- Samples are duplicated in wells printed by different pins;
- Samples from the same patient are performed on the same slides.
Pipet 100 μL serum samples and 100 μL reference into their assigned wells. Use care to prevent bubble formation.
Seal the slides and incubate in an orbital shaker at 100 rpm and 37 °C for 4 h.
Discard serum samples and immediately rinse with 200 μL/well washing buffer for three times (see Note 10). Repeat the wash cycle three times, let it sit for 2 min before discarding the solution. After each wash cycle, invert the module and tap vigorously on paper towel to remove excess liquid.
3.2.5. Detecting with Fluorophore-Labeled Secondary Antibodies
Prepare a fresh antibody incubation buffer: PBS with 1 % BSA and 3 % HSA.
Mix DyLight 549 anti-human IgG and DyLight 649 anti-human IgM (1:500 dilutions for both antibodies) in buffer and cover with aluminum foil. Depending on the scanner, two or more detecting antibodies could be used simultaneously (see Note 11).
Pipet 100 μL detecting reagent into each well and seal the module with adhesive strip.
Incubate in an orbital shaker at 100 rpm and 37 °C for 2 h. Cover the module with aluminum foil to prevent photobleaching.
Discard the solution and wash with 7 × 200 μL/well washing buffer. Let the solution sit for 2 min before discarding the solution for the last three wash cycles. After each wash cycle, invert the module and tap vigorously on paper towel to remove excess liquid.
3.2.6. Preparing Slides for Scanning
Carefully remove the slide from the slide module, transfer it into a bath containing washing buffer, and let it soak for 5 min. Make sure the printed surface is facing up. Only hold the slide by the edges to avoid smudge of the printed array surface.
Remove the slide from bath and place it into a 50 mL tube.
Dry the slide by centrifuging at 1000 rpm for 5 min.
Scan the slide in fluorescent scanner at both high and low PMT voltage settings. The scanning resolution is set to 10 μm or finer. Save the generated .tiff files for future data analysis (see Note 12).
3.3. Quantification of Microarray Data
3.3.1. Create a Template for Microarray Quantification and Align It with Microarray Image
Open the image-processing software, GenePix 6.0, and create a GenePix Array List (GAL) file that encodes the size and position of each printed glycan. This will generate a template mask with circular boundaries.
Open the pre-scan image from step 2 in Subheading 3.2.1 and adjust the GAL file so that the mask aligns with the actual spots on the slide. Inspect the whole slide and flag spots as “bad” that are either missing or contain defects in printing. These glycans will be excluded from further analysis. Save these modified settings as a GenePix Setting (GPS) file.
3.3.2. Calculate Signal Intensity for Individual Spot
Open image of the high PMT scan from step 4 of Subheading 3.2.6, and load the GPS file from the previous step. Finely adjust the size and position of the each circle mask so that it fits with individual spot size perfectly (see Note 13).
Use the software to calculate median pixel intensity (MI) and local background pixel intensity (BI) for all of the spots. The signal intensity (SI) of each spot is defined as MI–BI, representing background-corrected signal. Save the result as a GenePix Result (GPR) file and export the data as a .txt file that can be opened in Excel.
Open image of the low PMT scan from step 4 of Subheading 3.2.6, and analyze as above.
In Excel, import .txt result files for both high and low PMT settings. The primary data are derived from the high PMT scan. The low PMT scan is only used to correct those spots that are saturated in the high PMT scan. Use the following equations to calculate signal intensities for each spot (see Note 14 for Correction Factor (CF) calculation):
3.3.3. Determine the Average Signal Intensity for Each Glycan
Average the signal intensity of duplicate spots for each glycan on the same array.
Determine a floor value and adjust all signals below this value to floor value. A floor value 0.5–1.0 times higher than background is recommended so that signal noise close to background is excluded from consideration (see Note 15).
3.3.4. Normalize Slides Using a Reference Serum Sample
If experiments are carried out on different days, the slides used are printed at different times, or different detecting reagents are used, signal intensities may vary between slides. Therefore, signal normalization is recommended when comparing data across different slides. This is done by comparing a reference serum sample on each slide. In our protocol, we selected several glycans on the array that universally bind to serum antibodies (IgG, IgM, and IgA) with mid-intensities (e.g., blood group antigens, alpha-Gal antigens). The median signal for these selected glycans on each slide is adjusted to a standard value. For example, we use 50,000 RFU for IgM and 15,000 RFU for IgG. These values are for comparison purpose only and have no physical meanings.
For the reference sample on each slide, determine the median intensity for the selected glycans. Calculate the ratio of this median value to the standard value. This ratio is used as normalization factor (NF) for this slide.
Normalize all signal intensities on this slide by dividing by NF.
Normalize all slides in the same manner.
3.3.5. Average Signals for Duplicate Serum Samples
In a single experiment, each serum sample is measured in two wells printed by different pins, and each pin prints glycans in duplicate. That is, the same antigen-antibody interaction is measured four times per experiment. This accounts for any printing variabilities within and between pins. Final data are Log-transformed to facilitate analysis of both high signals and low signals on the same scale.
Average each glycan signal resulting from duplicate serum samples.
Apply a Log (base 2) transformation to the final data.
3.4. Post-assay Data Analysis
There are three general strategies used to identify carbohydrate antigens using glycan arrays: identifying glycans bound by monoclonal antibodies, comparing antibody profiles in case subjects versus control subjects, and profiling changes within an individual before and after a stimulus. The approach for evaluating the glycan array data varies based on the type of experiment.
For monoclonal antibodies, one typically compares the data of the antibody of interest to a negative control, such as non-carbohydrate-specific isotype control or buffer. The positive binders for the antibody but not the control provide a list of potential antigen targets [26]. Potential antigens should then be confirmed using other methods.
Case-control studies are used to compare antibody profiles in two different groups to identify specific antibody populations that are unique to one of the groups. This approach is most frequently used to identify disease-specific antigens, although they could be used to identify responses to vaccination or other stimuli [for some recent examples, see [18–21]]. When comparing two groups of people, natural variation between individuals can lead to differences between groups by chance. Therefore, it is important to have enough subjects in each group to identify statistically significant differences and to properly matched subjects. For example, factors such as race, age, gender, and blood type can influence anti-glycan antibody profiles [27–30], and numerous other factors that have not been extensively studied, such as diet, smoking, and season of blood draw, may also affect anti-glycan antibodies. Care should be taken to avoid misinterpretation of the data [31]. Finally, technical variability in the measurement can also affect the results. To minimize the effects of day-to-day technical variability, one should randomize the order in which cases and controls are assayed.
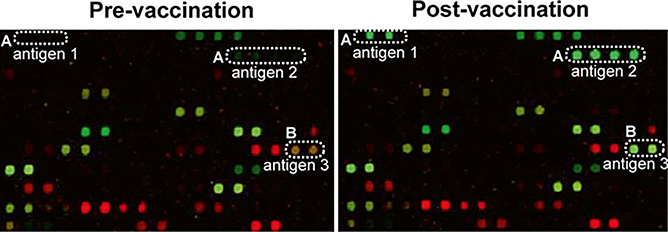
Carbohydrate antigens can also be identified by monitoring changes within individuals over time [for some recent examples, see [22–24]]. For example, one can compare antibody profiles before and after vaccination or acquisition of a disease (see Fig. 3). Since the samples being compared come from the same person, differences due to factors such as genetics, smoking, and diet are typically much smaller than when comparing profiles between individuals. However, technical variability is compounded since one is comparing two different measurements, each with their own error. In addition, one must define the natural biological fluctuations in antibody levels over the relevant time frame. One generally focuses on changes in antibody levels that are larger than the natural variation over time. It is important to note that the immune responses to a stimulus can vary from one patient to another. Hence, during data analysis it may be necessary to evaluate patients in subgroups according to their age, gender, or blood types to identify clinically relevant antigens [32].
Fig. 3.
Example of different types of antibody responses observed before and after vaccination in the same patient. (a) New antibodies to antigen 1 and 2 are produced (from no binding to binding); (b) Existing antibodies to antigen 3 increase in signal (from weak binding to strong binding. IgG signals are shown in green and IgM signals are shown in red
The identified antibody responses provide insights into the corresponding antigen repertoires that trigger antibody response. However, one has to be aware that the structures of glycans on the array may not represent the true antigens present on the cell surface of vaccine or pathogen samples. Fortunately, diversity of glycans on the array can be harnessed to identify the specific motifs interacting with the bound antibodies. A comparison of binding to an antigen of interest to its structurally similar analogs on the array can facilitate rapid identification of epitopes that induce the specific antibody response. For instance, antibody binding to both trisaccharide (Rhaα1–3-Glcβ1–4Glc) and disaccharide (Rhaα1–3-Glc) but not to monosaccharide (Rha) suggests the minimal binding epitope is a disaccharide unit [33]. Once the antigen repertoires are identified, additional follow-up experiments are needed to trace their source of origin and confirm their true presentations on the cell surface.
3.5. Technical Challenges
Several factors can give rise to technical challenges when comparing samples [20, 29, 34]. The most important factor is the quality and reproducibility of the printed slides. Batches printed by different pins or at different times may introduce variation in the measurements. In addition, variation can also arise when using different detection reagents. When multi-well array slides are used, a reference sample can be included in one well on each slide and used to normalize all data on that slide. In this way, signals from different slides can be normalized to the same reference allowing for comparison between different slides. The use of a standard reference sample also allows one to more readily detect printing problems. To further minimize technical variability, serum samples can be run on multiple slides or wells (e.g., samples are assayed in different wells printed by different pins in order to minimize variation between pins). The process used for quantification can also affect the results of measurements. Spot boundaries in the imaging software must be positioned correctly and sized appropriately for precise quantification.
Finally, statistical analysis is an essential tool to determine whether a response is random or specific. Since many individual comparisons are carried out in microarray experiments, one frequently observes statistically significant differences/changes by chance. It is recommended to perform analysis in two stages. First, the analysis method is applied to a training set of patients to identify specific hits. These hits are then verified using a second, separate patient population. This approach helps to minimize false positive results arising from the data analysis [35].
4. Notes
This dye is to help visualize the printed spots. Any dye can be used as long as it meets the following criteria: excitation and emission wavelengths match with scanner; water soluble; compatible with glycans; can be easily washed off; minimum photobleaching over time.
We routinely use 8 μL per sample to print five batches of 30 slides. Less sample volume can be used depending on the number of slides, as long as evaporation problem is minimized.
Pause printing if pins are clogged. Use a forceps to move the pin up and down 20 − 30 times until pin moves freely. Then resume printing.
The protocol for quality check of microarray slides has been published [25].
Use globulin-free BSA and HSA to avoid high background binding to the slide.
Rapid drop of blocking buffer will smear the array and create comet tail. Use of gel-loading pipet tips is recommended for this step.
After adding blocking buffer, keep the module still without shaking. Disturbing the module during blocking will disrupt printed array.
Addition of HSA and BSA serves two purposes: prevent binding of anti-albumin antibodies to the carrier protein core of the neoglycoproteins that can be present in human serum; block nonspecific binding to the array.
Concentration of serum samples needs to be optimized for different samples. However, reference sample is always diluted to 1:50. It is used as a control across different slides.
This quick wash step is to minimize cross-contamination between wells.
Cross-reactivity of the secondary antibody with glycans on the array should be checked by incubating the secondary antibody on the array without any prior serum incubation.
We routinely use combinations of PMT voltages of 520/430. However, these settings need to be adjusted for different scanners. The aim is to detect low intensity signals at high PMT scan, and then acquire data for high intensity signals at low PMT (signal that are saturated at high PMT). At low PMT scan, no spots should be saturated.
This step is critical in data analysis as the size of the spot boundary can dramatically change measured signal intensity, hence, influences experiment results. Only allow spots to be resized to 20 % below the actual print size.
Identify those spots with mid-range intensities (at 30–50 % saturation) at high PMT. For these spots, calculate the intensity ratios at high PMT vs. low PMT. The average ratio is then used as a correction factor (CF). This approach is to extend the dynamic range of the microarray scanner [36].
This step is mainly to adjust for negative values resulting from background correction. Negative values interfere with future mathematic calculations.
Acknowledgement
This work was supported by Intramural Research Program in National Institutes of Health.
References
- 1.Dennis JW, Granovsky M, Warren CE (1999) Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta 1473:21–34 [DOI] [PubMed] [Google Scholar]
- 2.Roy R (2004) New trends in carbohydrate-based vaccines. Drug Discov Today Technol 1:327–336 [DOI] [PubMed] [Google Scholar]
- 3.Lucas AH, Apicella MA, Taylor CE (2005) Carbohydrate moieties as vaccine candidates. Clin Infect Dis 41:705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings RD (2009) The repertoire of glycan determinants in the human glycome. Mol Biosyst 5:1087–1104 [DOI] [PubMed] [Google Scholar]
- 5.Cummings RD, Pierce JM (2014) The challenge and promise of glycomics. Chem Biol 21:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson WH (2006) Antigen arrays for antibody profiling. Curr Opin Chem Biol 10:67–72 [DOI] [PubMed] [Google Scholar]
- 7.Mattoon D, Michaud G, Merkel J et al. (2005) Biomarker discovery using protein microarray technology platforms: Antibody-antigen complex profiling. Expert Rev Proteomics 2: 879–889 [DOI] [PubMed] [Google Scholar]
- 8.Horlacher T, Seeberger PH (2008) Carbohydrate arrays as tools for research and diagnostics. Chem Soc Rev 37:1414–1422 [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Palma AS, Feizi T (2009) Carbohydrate microarrays: Key developments in glycobiology. Biol Chem 390:647–656 [DOI] [PubMed] [Google Scholar]
- 10.Park S, Gildersleeve JC, Blixt O et al. (2013) Carbohydrate microarrays. Chem Soc Rev 42:4310–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin I, Park S, Lee MR (2005) Carbohydrate microarrays: An advanced technology for functional studies of glycans. Chemistry 11: 2894–2901 [DOI] [PubMed] [Google Scholar]
- 12.Rillahan CD, Paulson JC (2011) Glycan microarrays for decoding the glycome. Annu Rev Biochem 80:797–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donczo B, Kerekgyarto J, Szurmai Z et al. (2014) Glycan microarrays: New angles and new strategies. Analyst 139:2650–2657 [DOI] [PubMed] [Google Scholar]
- 14.Geissner A, Anish C, Seeberger PH (2014) Glycan arrays as tools for infectious disease research. Curr Opin Chem Biol 18:38–45 [DOI] [PubMed] [Google Scholar]
- 15.Oyelaran O, Gildersleeve JC (2007) Application of carbohydrate array technology to antigen discovery and vaccine development. Expert Rev Vaccines 6:957–969 [DOI] [PubMed] [Google Scholar]
- 16.Gildersleeve JC, Wang B, Achilefu S et al. (2012) Glycan array analysis of the antigen repertoire targeted by tumor-binding antibodies. Bioorg Med Chem Lett 22:6839–6843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong X, Ma MZ, Gildersleeve JC et al. (2013) Sugar-binding proteins from fish: Selection of high affinity “lambodies” that recognize biomedically relevant glycans. ACS Chem Biol 8:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang CC, Huang YL, Ren CT et al. (2008) Glycan microarray of Globo H and related structures for quantitative analysis of breast cancer. Proc Natl Acad Sci U S A 105: 11661–11666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padler-Karavani V, Hurtado-Ziola N, Pu MY et al. (2011) Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res 71:3352–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob F, Goldstein DR, Bovin NV et al. (2012) Serum antiglycan antibody detection of nonmucinous ovarian cancers by using a printed glycan array. Int J Cancer 130: 138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen K, Gentry-Maharaj A, Burnell M et al. (2013) Microarray glycoprofiling of ca125 improves differential diagnosis of ovarian cancer. J Proteome Res 12:1408–1418 [DOI] [PubMed] [Google Scholar]
- 22.Liao SF, Liang CH, Ho MY et al. (2013) Immunization of fucose-containing polysaccharides from Reishi mushroom induces antibodies to tumor-associated Globo H-series epitopes. Proc Natl Acad Sci U S A 110: 13809–13814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luyai AE, Heimburg-Molinaro J, Prasanphanich NS et al. (2014) Differential expression of anti-glycan antibodies in schistosome-infected humans, rhesus monkeys and mice. Glycobiology 24:602–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell CT, Gulley JL, Oyelaran O et al. (2014) Humoral response to a viral glycan correlates with survival on PROSTVAC-VF. Proc Natl Acad Sci U S A 111:E1749–E1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Gildersleeve JC (2012) General procedure for the synthesis of neoglycoproteins and immobilization on epoxide-modified glass slides. Methods Mol Biol 808:155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Cristina M, Nunziangeli L, Giubilei MA et al. (2010) An antigen microarray immunoassay for multiplex screening of mouse monoclonal antibodies. Nat Protoc 5:1932–1944 [DOI] [PubMed] [Google Scholar]
- 27.Dotan N, Altstock RT, Schwarz M et al. (2006) Anti-glycan antibodies as biomarkers for diagnosis and prognosis. Lupus 15:442–450 [DOI] [PubMed] [Google Scholar]
- 28.Huflejt ME, Vuskovic M, Vasiliu D et al. (2009) Anti-carbohydrate antibodies of normal sera: Findings, surprises and challenges. Mol Immunol 46:3037–3049 [DOI] [PubMed] [Google Scholar]
- 29.Oyelaran O, McShane LM, Dodd L et al. (2009) Profiling human serum antibodies with a carbohydrate antigen microarray. J Proteome Res 8:4301–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muthana SM, Gildersleeve JC (2014) Glycan microarrays: Powerful tools for biomarker discovery. Cancer Biomark 14:29–41 [DOI] [PubMed] [Google Scholar]
- 31.Obukhova P, Piskarev V, Severov V et al. (2011) Profiling of serum antibodies with printed glycan array: Room for data misinterpretation. Glycoconj J 28:501–505 [DOI] [PubMed] [Google Scholar]
- 32.Campbell CT, Gulley JL, Oyelaran O et al. (2013) Serum antibodies to blood group a predict survival on prostvac-vf. Clin Cancer Res 19:1290–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin CE, Broecker F, Oberli MA et al. (2013) Immunological evaluation of a synthetic clostridium difficile oligosaccharide conjugate vaccine candidate and identification of a minimal epitope. J Am Chem Soc 135:9713–9722 [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Cummings RD, Smith DF et al. (2014) Cross-platform comparison of glycan microarray formats. Glycobiology 24:507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor JM, Ankerst DP, Andridge RR (2008) Validation of biomarker-based risk prediction models. Clin Cancer Res 14:5977–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyng H, Badiee A, Svendsrud DH et al. (2004) Profound influence of microarray scanner characteristics on gene expression ratios: Analysis and procedure for correction. BMC Genomics 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]