Abstract
This study aimed to explore the dynamic variations in the phenolic and volatile organic compounds of sugarcane vinegar subjected to different production processes. The determination of phenolic and volatile organic compounds was performed by UPLC-MS and solid phase micro extraction (SPME) coupled with gas chromatography combined with mass spectrometry (GC–MS). The complete fermentation process of sugarcane lasted nine days, and production of vinegar of up to 3.04% (w/v), total acids, and 4.1° alcoholicity was accomplished. Various phenolic compounds of sugarcane juice (non-sterilized) and those of alcoholic and acetic acid fermentation were obtained after nine days of fermentation. These were benzoic acid (2.024, 1.002, and 1.027 mg L–1), ferulic acid (0.060, 0.205, and 1.124 mg L–1), quinic acid (0.019, 0.074, and 0.031 mg L–1), chlorogenic acid (0.349, 1.635, and 1.217 mg L–1), apigenin (0.002, 0.099, and 0.004 mg L–1), kaempferol (0.003, 0.336, and 0.003 mg L–1), caffeic acid (−, 0.005, and 0.005 mg L–1), luteolin (0.003, 0.323, and 0.005 mg L–1), and p-coumaric acid (0.018, 0.015, and 0.027 mg L–1). Forty-five volatile organic compounds were also identified. The sugarcane juice can be commercialized as an alternative to wine as it presents characteristics of an alcoholic fermented beverage.
1. Introduction
Vinegar is one of the most popular and valuable food products throughout the globe. Vinegar is a traditional acidic condiment in China and has drawn more attention for its several health benefits. It acts as an antioxidant, antibacterial, anti-inflammatory, and anticancer agent; helps in preventing cardiac disorders; regulates high blood pressure and glucose and lipid metabolism; improves cognition; and promotes weight loss.1−4 It is used as a seasoning and preservative for preparing foods and sometimes as a beverage.5 It is obtained from raw materials containing mainly carbohydrates by a two-step fermentation process: one involves ethanol formation by yeasts (usually Saccharomyces spp.) by the conversion of fermentable sugars (alcoholic fermentation) and the second involves the oxidation of ethanol to acetic acid (acetic acid fermentation or acetification).5−7 Due to its health benefits, the Chinese have developed various types of vinegar products in recent years, like apple cider and other fruits vinegars, which are most popular. Fruit vinegar is brewed with artificially planted or wild-type fruits as raw materials; the production cost of the raw materials determines the scale of research and development and production of vinegar. Recently, new products linked with various fruits have arisen, that is, fruit juice with added vinegar and fruit vinegar, which improve and/or maintain the organoleptic and health-promoting benefits.1,4,8−10
There are few research organizations and/or industries which are involved in the production of sugarcane-based vinegar beverages. Therefore, the agro-food industries are focusing their research and development on unique food products with a higher nutritional value, based on traditional processes.11 The quality of these food products and their acceptance by users depends on their various characteristics, the most important one being aroma. In wine-based vinegars and derived products, aroma is due to the presence of various volatile organic compounds (VOCs) which belong to various chemical classes. The VOCs may come from the products like red wines, fruits, cider, malted barley, honey, and others and/or may be formed during fermentation production and storage.8,10,12−14 VOCs are present to a large extent in fruits and aromatic/medicinal plants and significantly affect the quality of vinegars. Importantly, the production of sugarcane vinegar involves various processes like alcoholic and acetic acid fermentation and fumigation. These processes affect the total phenolic contents of the vinegars.15,16 Few studies have been carried out to determine the levels of the phenolic acids and volatile organic compounds generated during the production of sugarcane vinegar.
Sugarcane is cultivated in about 102 countries of arid and semi-arid areas covering an area of about 24 million hectares.17 In China, sugarcane has been used for sugar production for a long time, but only recently winemaking from sugarcane has drawn the attention of the Chinese researchers/scientists. Sugarcane provides sugar and alcohol and its cultivation originates from Southeast Asia.18 Globally, China is the third largest producer of sugarcane and is also its exporter.19 China’s sugarcane production is forecast at 9.25 million metric tons for the year 2020–2021, up by 450,000 metric tons from the previous year. The predicted increase is mainly due to the expected return of normal atmospheric conditions as well as stable sugarcane prices.20 A total of 9.445 million tons of sugarcane was produced during the year 2018–2019 crushing season. Guangxi province in China is the largest producer of sugarcane (11.54 million mu in 2018–2019), which produces 6.34 million tons of cane sugar, amounting to 67.1% of the country’s total cane sugar production (source: www.chinasugar.org.cn).
Sugarcane juice is a drink which is commonly consumed in various countries and is rich in carbohydrates and various electrolytes.21,22 It has been shown to be more effective as a sports and rehydration drink. Sucrose is the main component of cane juice, is a more suitable carbon source for microbial growth, and thus can be directly used in the fermentation process.21,23 In recent years, due to the promotion of various health care programmes, the use of cane juice to process fruit wine or low-alcohol beverages has received significant attention.4,7
Sugarcane juice is obtained by pressing the fibrous mature stems which are rich in sugar. In addition, it is an excellent medium in the fermentation process in the development of alcoholic drinks,24,25 especially for distilled liquors like cachaca and rum. The direct use of sugarcane juice to produce liquor is still in its infancy, and few researchers have worked on fermented production of low-alcoholic products such as fruit wine. However, there are few reports available on the fermentation of cane juice for the preparation of fermented drinks similar to wine. The fermentation of wine is a combination of complex interactions involving a variety of materials, yeast and specific methods/techniques.26
Aromas and fruity flavors of wine or other alcoholic drinks are derived mainly from the raw materials used in fermentation, although few aromas are also produced during fermentation. Most of the volatile compounds present in grapes are also known to be constituents of various other fruits. However, very little information is available on the volatile compounds of fermented alcoholic drinks made from the sugarcane juice. No reports are available on phenolic and volatile compounds present in sugarcane alcoholic drinks. Therefore, this study was aimed to assess the technical feasibility and determine phenolic and volatile compounds of an alcoholic and acetic acid fermented beverage made from sugarcane juice.
2. Results
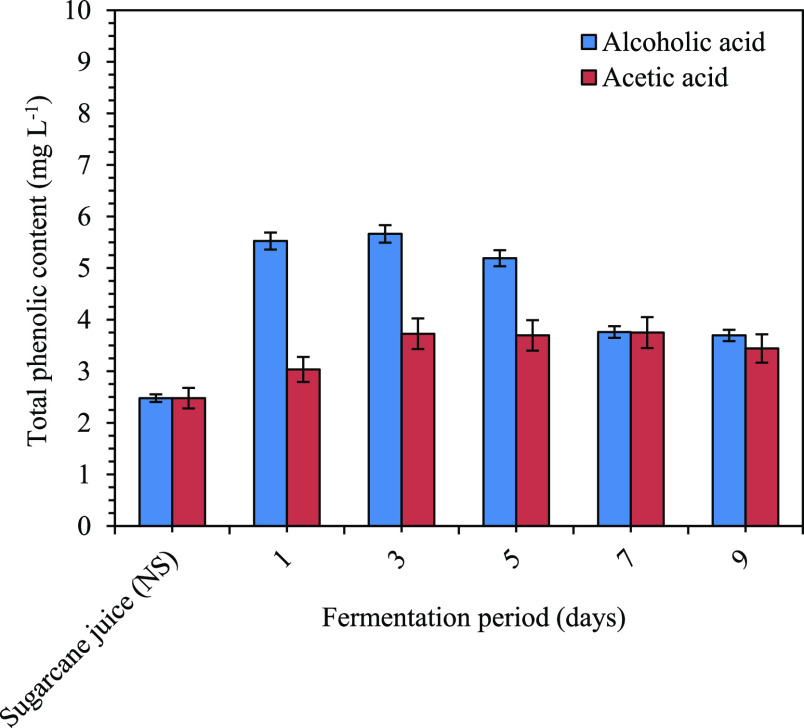
The quantification of phenolic compounds gives an estimate of the content of all compounds belonging to the subclass of phenolic compounds present in a sample (Figure 1). The phenolic compounds which were quantified in the beverage were benzoic acid, ferulic acid, quinic acid, chlorogenic acid, apigenin, kaempferol, caffeic acid, luteolin, and p-coumaric acid (Table 1). Significant differences were observed in the levels of phenolic compounds produced during fermentation. In this study, benzoic acid content was the highest followed by chlorogenic and ferulic acid in sugarcane juice (non-sterilized), alcoholic fermentation was found highest in chlorogenic acid followed by benzoic and kaempferol, and the chlorogenic acid level was found highest followed by ferulic and benzoic acid in acetic acid fermentation. Before fermentation of sugarcane juice (non-sterilized), mainly eight phenolic compounds were identified. During fermentation, nine phenolic acids were identified (Figure 1 and Table 1).
Figure 1.
Changes in total phenolic contents in the extract of sugarcane juice (NS—non-sterilized) and vinegar fermentation during alcoholic and acetic acid fermentation processes. Results are expressed as the mean ± SE (n = 3). Sugarcane juice (0 days, NS—non-sterilized) and alcoholic and acetic acid fermentation.
Table 1. Phenolic Compounds in the Sugarcane Juice (Non-sterilized) and Fermented Sugarcane Juice (Raw Vinegar) (mg L–1)a.
| alcoholic
fermentation (days) |
acetic
acid fermentation (days) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| phenolic acid | sugarcane juice (non-sterilized) | 1 | 3 | 5 | 7 | 9 | 1 | 3 | 5 | 7 | 9 |
| benzoic acid | 2.024 ± 0.018b | 1.188 ± 0.252e | 1.286 ± 0.141c | 0.925 ± 0.232e | 1.016 ± 0.075c | 1.002 ± 0.028d | 0.572 ± 0.044b | 1.075 ± 0.237c | 0.926 ± 0.149d | 0.969 ± 0.032c | 1.027 ± 0.073e |
| ferulic acid | 0.060 ± 0.004b | 0.343 ± 0.068e | 0.264 ± 0.017c | 0.134 ± 0.008e | 0.219 ± 0.015c | 0.205 ± 0.019d | 1.160 ± 0.017b | 1.154 ± 0.057c | 1.340 ± 0.002d | 1.458 ± 0.009c | 1.124 ± 0.063e |
| quinic acid | 0.019 ± 0.002b | 0.104 ± 0.008e | 0.090 ± 0.012c | 0.081 ± 0.012e | 0.096 ± 0.011c | 0.074 ± 0.009d | 0.080 ± 0.005b | 0.036 ± 0.008c | 0.027 ± 0.001d | 0.032 ± 0.002c | 0.031 ± 0.002e |
| chlorogenic acid | 0.349 ± 0.004b | 3.687 ± 0.117e | 3.595 ± 0.054c | 3.589 ± 0.076e | 1.857 ± 0.007c | 1.635 ± 0.071d | 1.185 ± 0.031b | 1.425 ± 0.023c | 1.366 ± 0.033d | 1.258 ± 0.011c | 1.217 ± 0.063e |
| apigenin | 0.002 ± 0.0001d | 0.024 ± 0.0004c,d | 0.053 ± 0.0034d | 0.059 ± 0.0011c | 0.018 ± 0.0032d,e | 0.099 ± 0.0045e | 0.003 ± 0.00d | 0.003 ± 0.0002c | 0.003 ± 0.0001c | 0.003 ± 0.000d | 0.004 ± 0.000c |
| kaempferol | 0.003 ± 0.0005d | 0.081 ± 0.0012c,d | 0.181 ± 0.0133c,d | 0.197 ± 0.0045c | 0.267 ± 0.0179c | 0.336 ± 0.0098c | 0.005 ± 0.0008d | 0.004 ± 0.0003c | 0.002 ± 0.0008c | 0.0000 | 0.003 ± 0.0001c |
| caffeic acid | − | 0.005 ± 0.0005d | 0.006 ± 0.0004d | 0.005 ± 0.0005c | 0.005 ± 0.00e | 0.005 ± 0f | 0.005 ± 0.0003d | 0.006 ± 0.0002c | 0.006 ± 0.0004c | 0.005 ± 0.0007d | 0.005 ± 0.0003c |
| luteolin | 0.003 ± 0d | 0.079 ± 0.0021c,d | 0.175 ± 0.0033c,d | 0.188 ± 0.0007c | 0.264 ± 0.0027c | 0.323 ± 0.0002c | 0.006 ± 0.0004d | 0.004 ± 0.0003c | 0.003 ± 0.0004c | 0.004 ± 0.0002d | 0.005 ± 0.0001c |
| p-coumaric acid | 0.018 ± 0.0033d | 0.014 ± 0.0028c,d | 0.013 ± 0.0021d | 0.013 ± 0.0018c | 0.018 ± 0.0044d,e | 0.015 ± 0.0019e,f | 0.020 ± 0.0050c,d | 0.021 ± 0.0041c | 0.022 ± 0.0011c | 0.020± 0.0044d | 0.027 ± 0.0001c |
“−”: non-detection. Results are expressed as the mean ± SD (n = 3). In each row, different superscript letters indicate significant differences among different production processes.
The phenolic acids in sugarcane vinegar fermentation substrates under different production processes were subjected to principal component analysis (PCA) (Figure 2A). The first three principal components explained 219.91% of the total variation (PC1 = 53.44%, PC2 = 77.44%, and PC3 = 89.03%, respectively). PC3 was highly affected by chlorogenic acid, quinic acid, benzoic acid, apigenin, kaempferol, and luteolin acid. PC 1 and PC2 were primarily correlated with p-coumaric acid, caffeic acid, and ferulic acid. The relatively dispersed distribution of the data points in the PCA plot revealed the differential changing pattern of phenolic acids produced during the sugarcane vinegar fermentation substrates and those produced during alcoholic and acetic acid fermentation production processes. The similarities of phenolic acids in vinegar fermentation substrates were evaluated through hierarchical cluster analysis (HCA) (Figure 2B). Similarly, phenolic acids were also obtained in the extracts of sugarcane vinegar during alcoholic and acetic acid fermentation.
Figure 2.
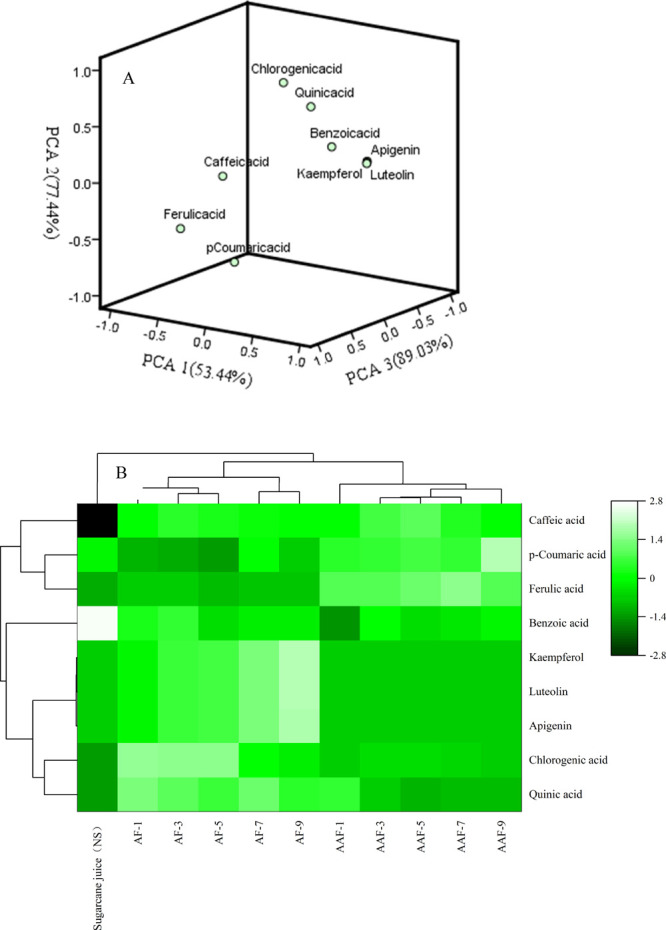
Principal component analyses (PCA – A) and hierarchical cluster (B) of phenolic acid compounds of sugarcane fermentation during the alcoholic and acetic acid fermentation processes. NS – non-sterilized, AF – alcoholic fermentation, AAF – acetic acid fermentation.
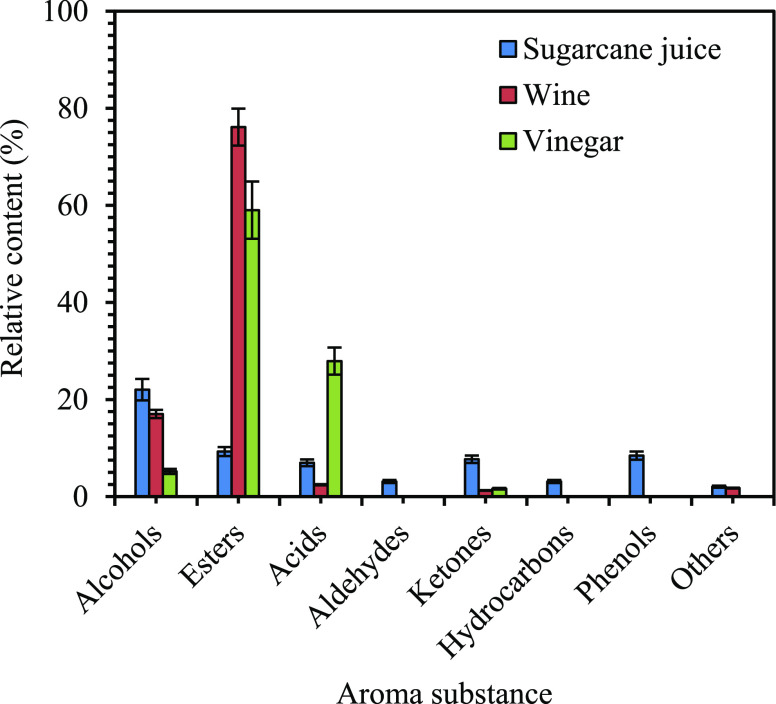
The extracted VOCs of aromatic substances in sugarcane juice, wine, and vinegar were between 95 and 115 species. The relative content (%) of each compound was calculated by the area normalization method, and 28–34 species of high-response substances were screened and identified. It accounts for 61.4–96.22% of the total peak area and is the main aroma substance of the sugarcane fermentation products (Table 2). Twenty-eight major aroma compounds were identified in sugarcane juice, 30 in wine, and 34 in vinegar, and these were alcohols, esters, aldehydes, acids, ketones, hydrocarbons, phenols, and others. There are various kinds of aroma substances which were found in sugarcane juice. The relative content (%) of alcohol in sugarcane is 22.02%. The relative content percentages of various kinds of alcohol were 2-heptanol (10.768%), n-hexanol (4.031%), n-octanol (1.734%), isoamyl alcohol (1.660%), decanol (1.604%), n-pentanol (1.442%), and so forth. This was followed by esters (9.273%). Ethyl lactate has the highest relative content (8.19%), phenols (8.445%) mainly o-methoxyphenol (6.78%) and 4-vinyl-2-methoxyphenol (1.664%), ketones (7.693%), and acids (6.958%). The relative contents of aldehydes and other heterocyclics were about 3.105 and 3.108%, respectively. The relative content percentage of n-decanal was (2.037%); 2,3-dihydrobenzofuran was (1.763%); and 1,3-detertbutyl benzene, the relative content of benzene (1.345%), is relatively large.
Table 2. Volatile Compounds in Sugarcane Juice (Non-sterilized), Wine, and Raw Vinegara.
| relative
content (%) |
|||||
|---|---|---|---|---|---|
| peak number | RT (min) | component | sugarcane juice | wine | vinegar |
| 1 | 3.558 | ethyl acetate | 0.201 ± 0.004i | 0.7325 ± 0.035d,e | 14.163 ± 0.400c |
| 2 | 4.613 | ethyl lactate | 8.19 ± 0.388b | 74.235 ± 0.974a | 43.640 ± 1.296a |
| 3 | 5.709 | octamethylcyclotetrasiloxane | 0.155 ± 0.005i | 0.0205 ± 0.054e | 0.016 ± 0.002 |
| 4 | 5.893 | isobutyl acetate | − | − | 0.068 ± 0.011e |
| 5 | 7.158 | ethyl Isovalerate | − | − | 0.017 ± 0.008e |
| 6 | 7.432 | N-hexanal | − | 0.066 ± 0.008e | 0.056 ± 0.003c |
| 7 | 7.444 | decane | − | − | 0.049 ± 0.000 |
| 8 | 8.062 | isobutanol | − | 1.256 ± 0.055c,d | 0.575 ± 0.026e |
| 9 | 8.364 | isoamyl acetate | − | 0.010 ± 0.001e | 0.496 ± 0.012e |
| 10 | 8.555 | 3-penten-2-one | − | − | 0.274 ± 0.024e |
| 11 | 8.579 | trans-3-penten-2-one | − | 0.182 ± 0.003e | − |
| 12 | 10.473 | 4-methyl-2-heptanone | 1.467 ± 0.202g,h | − | − |
| 13 | 10.808 | isoamyl alcohol | 1.660 ± 0.086g | 15.142 ± 0.583b | 3.703 ± 0.093d |
| 14 | 11.154 | ethyl caproate | − | 0.140 ± 0.039e | 0.0785 ± 0.002e |
| 15 | 11.24 | 6-methyl-2-heptanone | 0.131 ± 0.023i | − | − |
| 16 | 11.362 | 4-ethoxy-2-pentanone | − | 0.033 ± 0.001e | 0.051 ± 0.002e |
| 17 | 11.797 | n-pentanol | 1.442 ± 0.027g,h | 0.009 ± 0.001e | − |
| 18 | 12.446 | octanal | 0.171 ± 0.083i | 0.0085 ± 0.002e | 0.025 ± 0.007e |
| 19 | 12.575 | 3-hydroxy-2-butanone | 5.962 ± 0.514d | − | 0.093 ± 0.002e |
| 20 | 13.246 | 2-hexadecanol | 0.641 ± 0.012h,i | − | − |
| 21 | 13.314 | trans-2-heptenal | − | 0.0125 ± 0.001e | 0.0285 ± 0.005e |
| 22 | 13.378 | 2-heptanol | 10.768 ± 0.072a | 0.0395 ± 0.002e | 0.013 ± 0.000e |
| 23 | 13.626 | methylheptenone | 0.133 ± 0.005i | 0.0055 ± 0.001e | 0.0065 ± 0.001e |
| 24 | 13.835 | ethyl lactate | − | 0.0295 ± 0.000e | 0.099 ± 0.002e |
| 25 | 14.086 | n-hexanol | 4.031 ± 0.082f | 0.0185 ± 0.001e | 0.018 ± 0.000 |
| 26 | 14.091 | 2-acetoxytetradecane | − | − | 0.020 ± 0.000 |
| 27 | 14.736 | nonanal | 0.897 ± 0.478g,h,i | 0.054 ± 0.021e | 0.157 ± 0.046e |
| 28 | 15.206 | 1,3-hexadiene | − | 0.004 ± 0.000 | − |
| 29 | 15.342 | 1,3-di-tert-butylbenzene | 1.345 ± 0.143g,h | 0.214 ± 0.000 | 0.689 ± 0.13e |
| 30 | 15.527 | ethyl caprylate | 0.932 ± 0.687c,d,e | 0.413 ± 0.12e | |
| 31 | 15.785 | acetic acid | 4.949 ± 0.863e | 1.736 ± 0.322c | 27.445 ± 0.362b |
| 32 | 16.569 | decanal | 2.037 ± 0.000 | 0.0285 ± 0.013e | 0.118 ± 0.029e |
| 33 | 16.577 | decanol | 1.604 ± 0.003 | − | − |
| 34 | 17.172 | propionic acid | − | 0.019 ± 0.001 | 0.0505 ± 0.008e |
| 35 | 17.619 | n-octanol | 1.734 ± 0.012g | − | − |
| 36 | 18.432 | methyl benzoate | 0.882 ± 0.031g,h,i | 0.041 ± 0.003e | 0.0385 ± 0.001e |
| 37 | 18.986 | valeric acid | 0.045 ± 0.000 | 0.028 ± 0.000 | 0.217 ± 0.008e |
| 38 | 21.313 | o-methoxyphenol | 6.780 ± 0.108c | − | − |
| 39 | 21.584 | benzyl alcohol | 0.142 ± 0.000 | 0.006 ± 0.000 | − |
| 40 | 22.035 | phenylethanol | 0.538 ± 0.049d,e | 0.876 ± 0.056e | |
| 41 | 23.33 | n-octanoic acid | 0.172 ± 0.027i | 0.646 ± 0.128d,e | 0.187 ± 0.013e |
| 42 | 24.721 | 4-vinyl-2-methoxyphenol | 1.664 ± 0.009g | − | 0.005 ± 0.000 |
| 43 | 26.464 | 2-hydroxycinnamic acid | 1.792 ± 0.000 | − | 0.014 ± 0.000 |
| 44 | 26.465 | 2,3-dihydrobenzofuran | 1.763 ± 0.000 | 0.0115 ± 0.001e | − |
| 45 | 27.679 | 5-hydroxymethyl furfural | − | 0.0205 ± 0.003e | 0.036 ± 0.007e |
Data are represented as mean ± SE. “−” not detected, RT = retention time.
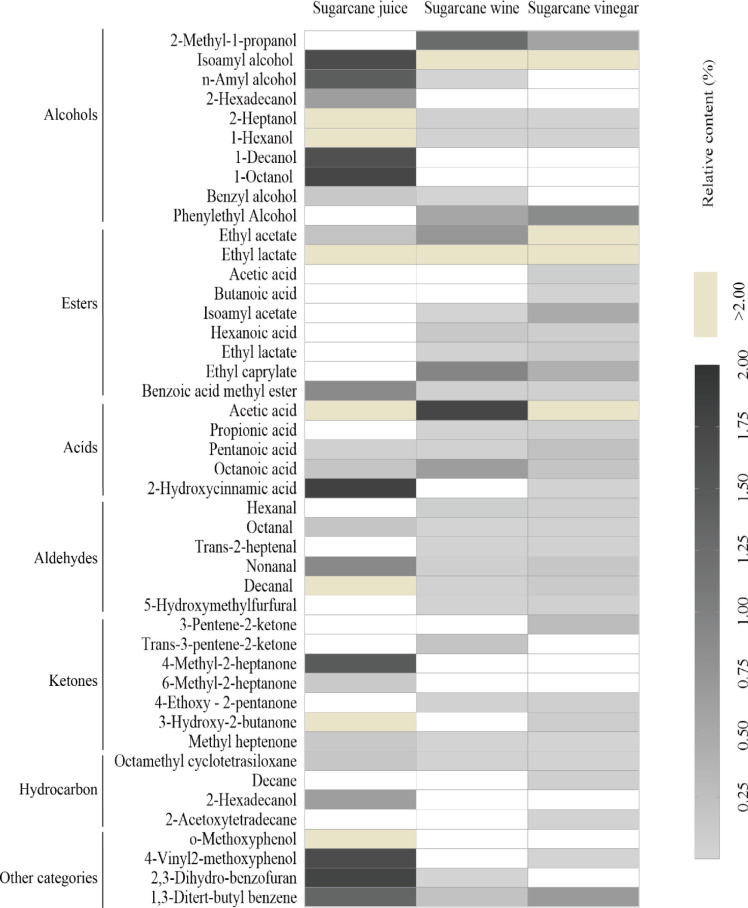
Sugarcane wine has the highest relative content of esters (76.120%), with seven compounds such as ethyl lactate (74.235%); alcohols (17.010%), including isoamyl alcohol (15.142%) and isobutanol (1.256%); other compounds and acids (2.429%), mainly composed of acetic acid (1.736%); and hydrocarbons, and the relative content of others is small. The percentage of aroma substance of esters is 59.014% in raw sugarcane vinegar and mainly includes ethyl acetate (14.163%), ethyl lactate (43.640%), and acids (27.914%), and other components are mainly composed of acetic acid (27.445%), alcohols (5.185%), isoamyl alcohol (3.703%), aldehydes, and ketones. The relative content of hydrocarbons, phenol, and other heterocyclics is relatively small, only 11.626%. The distribution of aroma substances in sugarcane juice (non-sterilized), wine, and vinegar is shown in Figure 3. The concentrations of VOCs in sugarcane juice, wine, and vinegar are presented in Table 2 and Figure 4 as a heatmap. The cluster analysis according to the squared Euclidean distance method was carried out to identify the similarity among the samples.
Figure 3.
Classes of volatile compounds in the sugarcane juice, the sugarcane wine and the sugarcane vinegar. Results are presented as the mean ± SE (n = 3).
Figure 4.
Hierarchical clustering of volatile organic compounds detected in sugarcane juice, wine and vinegar.
Alcohols are the main substances in fermented wines that give the sweetness and flavor to the wine. The alcohol and acid react to generate various esters, which constitute the special aroma of fermented wines. After sugarcane juice is completely fermented with alcohol, the type and the relative content of its alcoholic compounds, namely, n-pentanol, 2-heptanol, n-hexanol, and so forth, were estimated, and it was found that the relative content of isobutanol and isoamyl alcohol gradually decreases. Isoamyl alcohol has an apple brandy aroma and pungent taste.22,26−29 It exists in sugarcane juice in the form of natural esters produced by the metabolism of sugar compounds in sugarcane juice by yeast, and their relative content increases to 13.48% and constitutes the main component of the characteristic flavor of sugarcane wine.
Phenylethanol has a variety of flavors such as rose, violet, jasmine, and so forth. Esters, the main components of fermented wines, are volatile compounds with an aromatic odor and play a key role in the formation of fermented wines.6,29−34 The types and relative content of ester compounds have increased significantly. The types have increased from 3 to 7, and the relative content has increased eight times. The ester compounds include ethyl lactate (74.235%), ethyl caprylate (0.932%), and ethyl acetate (0.732%), while other compounds are isoamyl acetate, ethyl n-hexanoate, ethyl lactate, ethyl octanoate, and so forth. Ester compounds are formed due to the activity of yeast, and together they provide fruity and mellow aroma to sugarcane wine.
Acids are the main substances of wine aroma and help in the formation of esters. The relative content of acidic compounds in sugarcane wine decreased to 4.529%. Among them, the relative content of acetic acid and 2-hydroxycinnamic acid decreased significantly. 2-Hydroxycinnamic acid was not detected in sugarcane wine. Although the relative content of acetic acid was reduced to 1.736%, but it is still the flavoring agent in sugarcane wine. Propionic acid appears in the sugarcane wine after fermentation and is a by-product of yeast protein metabolism. The types and relative content of hydrocarbons, phenols, and other heterocyclics were significantly reduced, especially o-methoxyphenol and 4-vinyl-2-methoxyphenol. The relative contents of 3-dihydrobenzofuran and 1,3-di-tert-butylbenzene were reduced to less than 0.2%. These compounds are closely related to those involved in the Maillard reaction and microbial metabolism of sugarcane juice during fermentation.
Sour taste is the main characteristic of fruit vinegar, and it also determines the flavor and quality of the product. The types and relative content of acids in sugarcane vinegar are higher than wine. There are six types of acids whose relative content is 27.914%, these are acetic acid, propionic acid, valeric acid, n-octanoic acid, 2-hydroxycinnamic acid, and caprylic acid. Acetic acid has a strong vinegar fragrance. The acetic acid content in sugarcane vinegar increases significantly, mainly due to the action of ethanol dehydrogenase of acetic acid bacteria on ethanol during the two-step acetic acid fermentation. Ethanol is first oxidized to form acetaldehyde, and then acetaldehyde is oxidized to form acetic acid. These acids impart the flavor to fruit vinegar.
Esters are important in imparting the aroma characteristics to fruit vinegar. The aromas are floral, fruity, wine, and honey. The types of esters in sugarcane vinegar increased compared with sugarcane wine, and the relative content decreased (59.01%), mainly because the relative content of ethyl lactate decreased, while the relative content of ethyl acetate increased (13.42%). Ethyl acetate has a fruity aroma when isobutyl acetate and ethyl isovalerate are added to it. These ester compounds are used during fermentation of sugarcane by acetic acid bacteria. The relative content and types of alcohol in sugarcane vinegar have been reduced, but they still contribute to the flavor of sugarcane vinegar, and they are mainly isobutanol, isoamyl alcohol (3.703), 2-heptanol, n-hexanol, and so forth. In the process of acetic acid fermentation, the ventilation and aging of the processing technology also reduce the content of some volatile components of raw sugarcane vinegar.
3. Discussion
The Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) have shown that the final product should be one having considerable standards of safety and is an important element for safety purposes.35 The moisture content in sugarcane juice was 860 ± 103 g L–1. This value is in accordance with the guidelines of the Organization for Economic Co-operation and Development (OECD)36 which states that the extracted sugarcane juice has nearly 86% water. The moisture content of the sugarcane juice helps in the fermentation for the preparation of beverages. The nutritional value of sugarcane juice is associated to its higher sugar level37 while its protein and lipid levels are very low. Mineral nutrients such as calcium, potassium, and phosphorus are mainly found in sugarcane juice.17 The method of harvesting and that of the filtration process determines the mineral content of cane juice.38 The changes in the chemical composition of cane juice can occur due to various reasons, namely, due to varietal differences in the crop, soil conditions, climatic changes, harvesting time, and the method of extraction and filtration of the juice.38
The pH of cane juice indicates that the product can be consumed or not.39 The variation in pH of cane juice can be attributed to the harvesting period of the crop and the method of extraction of the juice. The variation in the soluble solid content (SSC) of the juice may be due to environmental conditions, soil profile, harvesting time, crop variety, as well as the way the crop was harvested. The crop with higher soluble solids content was more suitable for fermentation.40 The level of sugarcane juice in a crop will depend on the crop variety, harvesting time, and other factors.36 During fermentation, a declining trend in the pH of cane juice was observed. The pH values were found to be reduced on the third day of fermentation, and then it was found to be more or less constant throughout the fermentation process. A similar trend was seen in Ananas comosus L. Merr. fermented beverage from Angola.41 The important factor in determining the final quality of fermented drink is the presence of volatile acids. The presence of acetic acid is not desirable in alcoholic fermentation because besides changing the flavor and aroma of the drink, it also indicates contamination by acetic acid bacteria.42
In wine, there are two groups of phenolic acids, namely, hydroxybenzoic and hydroxycinnamic acid.43 Gallic acid, a type of phenolic acid, is found in the plants in the form of free acids, esters, catechin derivatives, and hydrolysable tannins.44 Gallic acid and its derivatives showed good biological activity as an antimicrobial, antioxidant, and antidiabetic agent. The content of gallic acid in sugarcane wine is comparable to that of regular wine.45 Tian et al.45 showed that the phenolic compounds, namely, ferulic acid, chlorogenic acid, caffeic acid, and p-coumaric acid of dry wine obtained from grapes were also found in the wine produced in the present study.
The phenolic compounds in vinegar are mainly derived from the raw materials. However, the changes in phenolic contents occurred during the different stages of fermentation in the production of vinegar.46 As expected, the phenolic contents in vinegar fermentation substrates steadily enhanced as alcoholic and acetic acid fermentation progressed. The production of phenolic acids is due to specific chemical changes during acetic acid fermentation.47 The level of phenolic acids, flavonoids, and aroma components changes during vinegar production.16,48,49
In fermented sugarcane juice, forty-five VOCs were quantified. The higher alcohol concentration is the main precursors for the formation of esters and related aromas.42 1-octanol contributes to a fruity aroma in beverages and significantly contributes to their flavor by enhancing sweetness and improving after taste.50 Acetal (1,1-diethoxyethane) is a VOC formed during fermentation in the production of sugarcane wines and plays a significant role in imparting a sweet cookie flavor to the wine.51
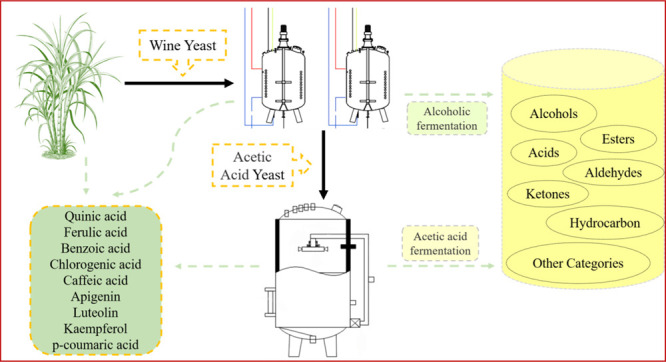
In conclusion, the optimized method based on HS-SPME/GC–MS was found to be a suitable tool for the identification of the volatile organic compounds of sugarcane vinegar obtained from sugarcane juice. A total of forty-five VOCs were quantified. The VOCs of sugarcane vinegar may be influenced by environmental conditions, light intensity, and agronomic methods used for the farming of sugarcane crop. The VOCs impart the flavor and aroma to the beverage. Different phenolic compounds such as benzoic acid, ferulic acid, quinic acid, chlorogenic acid, apigenin, kaempferol, caffeic acid, luteolin and p-coumaric acid were identified, and they were found in concentrations which could be compared with those present in other wine varieties. This method helps to identify the authenticity of composition of wine as well wine-based aromatic vinegars. The findings suggest that sugarcane juice can be used as an alternative to produce wine once it is optimized to have appropriate characteristics for an alcoholic fermented beverage. Therefore, fermented sugarcane juice may eventually find a place in the global agro-industrial sector.
4. Experimental Section
The mature stalks of sugarcane (Saccharum officinarum L. spp. Hybrid) were collected from the experimental area of the Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences (GxAAS), Nanning, Guangxi, China. After harvesting, they were transported to the laboratory, washed, and crushed in the Agricultural Products Processing Research Institute, GxAAS, Nanning, Guangxi, China. The collected sugarcane juice was filtered to remove suspended solids.
4.1. Fermentation Conditions
The alcoholic and acetic acid fermentations were carried out in three steps. The total soluble solid content of sugarcane juice was standardized to 20 °Brix (pH 5.5). The inocula were activated by solubilization of 10 g of yeast in 100 mL of water at 40 °C, and the solution was stirred manually, and then the yeast was added to the wort at 10 gL–1 concentration, as per the manufacturers’ instructions. The fermentation process following inoculation was performed in a container (1000 L) fitted with a hydraulic bung outlet for removing CO2. The vessel was kept under a controlled temperature (27 °C) in an incubator, until the soluble solid content reached 5 °Brix or up to constant. After fermentation, the medium was filtered through a 0.22 μm microporous membrane into glass bottles previously cleaned and sterilized, and the bottles were then kept in a refrigerator under a controlled temperature. Production of vinegar of up to 3.04% (w/v) total acid and 4.1° alcoholicity was accomplished.7,52 In the current study, the fermentation substrates of sugarcane vinegar during different production periods, including raw materials, alcoholic and acetic acid fermentation products (1–9th day) were collected for further analysis.
4.2. Determination of Phenolic Compounds by Ultra-High Pressure Liquid Chromatography Tandem Mass spectrometry (UPLC-MS)
The phenolic compounds were analyzed according to the method53 by using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS), (Waters EVEO TQ-S, Waters Corporation, USA) with an electrospray ionization source. The nebulizer gas was nitrogen, and the collision was argon. MS/MS detection was performed in negative and positive-ion modes and by using multiple reaction monitoring. The mobile phase consisting of deionized water with 0.1% formic acid and acetonitrile with 0.1% formic acid was pumped at a flow rate of 0.25 mL min–1. The gradient elution program was as follows: 95% A from 0 to 0.88 min, 95 to 78% A from 0.88 to 1.28 min, 78% A from 1.28 to 4.48 min, 78 to 55% A from 4.48 to 9.08 min, 55% A from 9.08 to 13.88 min, 55 to 95% A from 13.88 to 14.50 min, and 95% A from 14.50 to 15.40 min. The column temperature was maintained at 40 °C, and the injected sample volume was 2 μL. Individual phenolic compounds were identified by comparing the retention time with respective standards and quantified using external standard methods.
4.3. Determination of Volatile Compounds by SPME
The volatile compounds were extracted by solid phase micro extraction (SPME) and determined by gas chromatography. Sample analysis was performed on a gas chromatograph coupled with a mass spectrometer (Bruker, USA) and a mass detector, as per the method.54 The instrument conditions were as follows: injector temperature 250 °C, detector temperature 300 °C, flow rate of hydrogen as a carrier gas at 3.3 mL min–1, and nitrogen as a make-up gas at 30 mL min–1. The flow rates of detector gas (hydrogen) and air were 40 and 400 mL min–1, respectively. The programmed temperature was 50 °C (3 min), increased to 90 °C at the rate of 5 °C min–1, and held at 230 °C for 7 min at 10 °C min–1. The mass spectra were compared with those of the literature and NIST Standard Reference Database and Willey 8 Library.
4.4. Statistical Analysis
Data were presented as the mean ± SD. One-way ANOVA was performed using SPSS 23.0 statistical software. Differences were considered significant at p < 0.05. PCA and HCA were used to analyze the interrelationship between the variables and the clustering characteristics in collected samples using MetaboAnalyst 3.0, respectively.
Acknowledgments
All of the authors are grateful to the Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China for providing the necessary facilities for this study. All authors read, revised, and approved the manuscript for publication.
Author Contributions
# G.-L.C. and F.-J.Z. have contributed equally to this work.
This study was financially supported by The China Spark Program (grant go. 2015GA790013), Guangxi Key Technology R&D Program (grant no: GK-AB16380244), Nanning Science and Technology Program of Guangxi (grant no. 20171120-1), Xixiangtang Science and Technology program of Nanning, Guangxi (grant no. 201720103), Guangxi Academy of Agricultural Sciences Basic Research Business Project (grant no. GuiNongKe 2018YT28).
The authors declare no competing financial interest.
References
- Cejudo-Bastante M. J.; Rodríguez Dodero M. C.; Durán Guerrero E.; Castro Mejías R.; Natera Marín R.; García Barroso C. Development and optimisation by means of sensory analysis of new beverages based on different fruit juices and sherry wine vinegar. J. Sci. Food Agric. 2013, 93, 741–748. 10.1002/jsfa.5785. [DOI] [PubMed] [Google Scholar]
- Budak N. H.; Aykin E.; Seydim A. C.; Greene A. K.; Guzel-Seydim Z. B. Functional properties of vinegar. J. Food Sci. 2014, 79, R757–R764. 10.1111/1750-3841.12434. [DOI] [PubMed] [Google Scholar]
- Cejudo-Bastante C.; Castro-Mejías R.; Natera-Marín R.; García-Barroso C.; Durán-Guerrero E. Chemical and sensory characteristics of orange based vinegar. J. Food Sci. Technol. 2016, 53, 3147–3156. 10.1007/s13197-016-2288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Chen T.; Giudici P.; Chen F. Vinegar functions on health: constituents, sources, and formation mechanisms. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1124–1138. 10.1111/1541-4337.12228. [DOI] [PubMed] [Google Scholar]
- Tesfaye W.; Morales M. L.; García-Parrilla M. C.; Troncoso A. M. Wine vinegar: Technology, authenticity and quality evaluation. Trends Food Sci. Technol. 2002, 13, 12–21. 10.1016/s0924-2244(02)00023-7. [DOI] [Google Scholar]
- Perestrelo R.; Silva C.; Silva P.; Câmara J. Establishment of the volatile signature of wine-based aromatic vinegars subjected to maceration. Molecules 2018, 23, 499. 10.3390/molecules23020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.-L.; Zheng F.-J.; Sun J.; Li Z.-C.; Lin B.; Li Y.-R. Production and characteristics of high quality vinegar from sugarcane juice. Sugar Technol. 2015, 17, 89–93. 10.1007/s12355-014-0352-z. [DOI] [Google Scholar]
- Chinnici F.; Durán Guerrero E.; Sonni F.; Natali N.; Natera Marín R.; Riponi C. Gas Chromatography–Mass Spectrometry (GC–MS) Characterization of Volatile Compounds in Quality Vinegars with Protected European Geographical Indication. J. Agric. Food Chem. 2009, 57, 4784–4792. 10.1021/jf804005w. [DOI] [PubMed] [Google Scholar]
- Yu Y.-J.; Lu Z.-M.; Yu N.-H.; Xu W.; Li G.-Q.; Shi J.-S.; Xu Z.-H. HS-SPME/GC-MS and chemometrics for volatile composition of Chinese traditional aromatic vinegar in the Zhenjiang region. J. Inst. Brew. 2012, 118, 133–141. 10.1002/jib.20. [DOI] [Google Scholar]
- Shu X.; Jiang X.-W.; Cheng B. C.-Y.; Ma S.-C.; Chen G.-Y.; Yu Z.-L. Ultra-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry analysis of the impact of processing on toxic components of Kansui Radix. BMC Complementary Altern. Med. 2016, 16, 73. 10.1186/s12906-016-1039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrufo-Curtido A.; Cejudo-Bastante M. J.; Rodríguez-Dodero M. C.; Natera-Marín R.; Castro-Mejías R.; García-Barroso C.; Durán-Guerrero E. Novel vinegar-derived product enriched with dietary fiber: Effect on polyphenolic profile, volatile composition and sensory analysis. J. Food Sci. Technol. 2015, 52, 7608–7624. 10.1007/s13197-015-1908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto R. M.; García N. P.; Hevia A. G.; Valles B. S. Application of purge and trap extraction and gas chromatography for determination of minor esters in cider. J. Chromatogr. A 2005, 1069, 245–251. 10.1016/j.chroma.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Ubeda C.; Callejón R. M.; Hidalgo C.; Torija M. J.; Mas A.; Troncoso A. M.; Morales M. L. Determination of major volatile compounds during the production of fruit vinegars by static headspace gas chromatography-mass spectrometry method. Food Res. Int. 2011, 44, 259–268. 10.1016/j.foodres.2010.10.025. [DOI] [Google Scholar]
- Jo D.; Kim G.-R.; Yeo S.-H.; Jeong Y.-J.; Noh B. S.; Kwon J.-H. Analysis of aroma compounds of commercial cider vinegars with different acidities using SPME/GC-MS, electronic nose, and sensory evaluation. Food Sci. Biotechnol. 2013, 22, 1559–1565. 10.1007/s10068-013-0251-1. [DOI] [Google Scholar]
- Ho C. W.; Lazim A. M.; Fazry S.; Zaki U. K. H. H.; Lim S. J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. 10.1016/j.foodchem.2016.10.128. [DOI] [PubMed] [Google Scholar]
- Yu X.; Yang M.; Dong J.; Shen R. Comparative analysis of the antioxidant capacities and phenolic compounds of oat and buckwheat vinegars during production processes. J. Food Sci. 2018, 83, 844–853. 10.1111/1750-3841.14074. [DOI] [PubMed] [Google Scholar]
- AFRIS (Animal Feed Resources Information System of FAO) . Sugarcane Juice. 2015, http://www.feedipedia.org/node/560 (accessed 25 July, 2020).
- Farah A. G. V.Brazilian Sugarcane Industry. The Brazil Business. 2013, http://thebrazilbusiness.com/article/brazilian-sugarcane-industry (accessed 24 April, 2020).
- Zhang M.; Govindaraju M.. Sugarcane Production in China Sugarcane—Technology and Research. Alexandre Bosco de Oliveira; IntechOpen, 2018. [Google Scholar]
- United States Department of Agriculture Foreign Agricultural Service . Sugar Annual edited by Michael Francom; Gain Global Agricultural Information Network: Beijing, China, 2018. [Google Scholar]
- Kalpana K.; Lal P. R.; Kusuma D. L.; Khanna G. L. The effects of ingestion of sugarcane juice and commercial sports drinks on cycling performance of athletes in comparison to plain water. Asian J. Sports Med. 2013, 4, 181–189. 10.5812/asjsm.34256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira É. R.; Caliari M.; Júnior M. S. S.; Oliveira A. R.; Duarte R. C. M.; Boas E. V. B. V. Assessment of chemical and sensory quality of sugarcane alcoholic fermented beverage. J. Food Sci. Technol. 2018, 55, 72–81. 10.1007/s13197-017-2792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nualsri C.; Reungsang A.; Plangklang P. Biochemical hydrogen and methane potential of sugarcane syrup using a two-stage anaerobic fermentation process. Ind. Crop. Prod. 2016, 82, 88–99. 10.1016/j.indcrop.2015.12.002. [DOI] [Google Scholar]
- James J. B.; Ngarmsak T.. Processing of Fresh-Cut Tropical Fruits and Vegetables: A Technical Guide; Food and Agriculture Organization of the United Nations: Bangkok, 2010. [Google Scholar]
- Kulkarni M. S.; Kininge P. T.; Ghasghase N. V.; Mathapati P. R.; Joshi S. S. Effect of additives on alcohol production and kinetic studies of S. cerevisiae for sugar cane wine production. Int. J. Adv. Biotechnol. Res. 2011, 2, 154–158. [Google Scholar]
- Tzeng D.-I.; Chia Y.-C.; Tai C.-Y.; Ou A. S.-M. Investigation of Chemical Quality of Sugarcane (Saccharum Officinaruml.) Wine During Fermentation Bysaccharomyces Cerevisiae. J. Food Qual. 2010, 33, 248–267. 10.1111/j.1745-4557.2010.00305.x. [DOI] [Google Scholar]
- Le V.-D.; Zheng X.-W.; Chen J.-Y.; Han B.-Z. Characterization of volatile compounds inFen-Daqu- a traditional Chinese liquor fermentation starter. J. Inst. Brew. 2012, 118, 107–113. 10.1002/jib.8. [DOI] [Google Scholar]
- Feng Y.; Liu M.; Ouyang Y.; Zhao X.; Ju Y.; Fang Y. Comparative study of aromatic compounds in fruit wines from raspberry, strawberry, and mulberry in central Shaanxi area. Food Nutr. Res. 2015, 59, 29290. 10.3402/fnr.v59.29290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W.-P.; Zhu B.-Q.; Song R.-R.; Zhang B.; Lan Y.-B.; Zhu X.; Duan C.-Q.; Han S.-Y. Volatile composition and aromatic attributes of wine made with Vitisvinifera L.cv Cabernet Sauvignon grapes in the Xinjiang region of China: effect of different commercial yeasts. Int. J. Food Prop. 2018, 21, 1423–1441. 10.1080/10942912.2018.1479860. [DOI] [Google Scholar]
- Rapp A. Volatile flavour of wine: Correlation between instrumental analysis and sensory perception. Nahrung 1998, 42, 351–363. . [DOI] [PubMed] [Google Scholar]
- Rodríguez-Bencomo J. J.; Conde J. E.; Rodríguez-Delgado M. A.; Garcia-Montelongo F.; Perez-Trujillo J. P. Determination of esters in dry and sweet white wines by headspace solid-phase microextraction and gas chromatography. J. Chromatogr. A 2002, 963, 213. 10.1016/s0021-9673(02)00551-4. [DOI] [PubMed] [Google Scholar]
- Salinas M. R.; Garijo J.; Pardo F.; Zalacain A.; Alonso G. L. Color, polyphenol, and aroma compounds in rose wines after prefermentative maceration and enzymatic treatments. Am. J. Enol. Vitic. 2003, 54, 195–202. [Google Scholar]
- Li H.Wine Tasting; Science Press: Beijing, China, 2006. [Google Scholar]
- Lorenzo C.; Pardo F.; Zalacain A.; Alonso G. L.; Rosario Salinas M. Complementary effect of cabernet sauvignon on monastrell wines. J. Food Compos. Anal. 2008, 21, 54–61. 10.1016/j.jfca.2007.06.003. [DOI] [Google Scholar]
- WHO (World Health Organization) . Strategies for assessing the safety of foods produced by biotechnology: report of a joint FAO/WHO consultation. 1991, http://apps.who.int/iris/bitstream/10665/41465/1/9241561459-eng.pdf (accessed 25 July, 2020).
- OECD (Organization for Economic Co-operation and Development) . Consensus document on compositional considerations for new varieties of sugarcane (Saccharum ssp. hybrids): Key food and feed nutrients, anti-nutrients and toxicants. 2011, http://www.feedipedia.org/node/560 (accessed 25 July, 2020).
- Silva C. C. F.; Caliari M.; Junior M. S. S.; Marques R. C. D.; Beleia A. D. P.; Garcia M. C. Physicochemical and sensory properties of sugar cane candies with roasted peanut and extruded rice bran. J. Food Nutr. Res. 2016, 4, 163–169. 10.12691/jfnr-4-3-6. [DOI] [Google Scholar]
- Oliveira É. R.; Caliari M.; Soares Júnior M. S.; Vilas Boas E. V. d. B. Bioactive composition and sensory evaluation of blended jambolan (Syzygium cumini ) and sugarcane alcoholic fermented beverages. J. Inst. Brew. 2016, 122, 719–728. 10.1002/jib.370. [DOI] [Google Scholar]
- Hamerski F.; da Silva V. R.; Corazza M. L.; Ndiaye P. M.; de Aquino A. D. Assessment of variables effects on sugar cane juice clarification by carbonation process. Int. J. Food Sci. Technol. 2012, 47, 422–428. 10.1111/j.1365-2621.2011.02857.x. [DOI] [Google Scholar]
- Wu L.; Birch R. G. Doubled sugar content in sugarcane plants modified to produce a sucrose isomer. Plant Biotechnol. J. 2007, 5, 109–117. 10.1111/j.1467-7652.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Dellacassa E.; Trenchs O.; Fariña L.; Debernardis F.; Perez G.; Boido E.; Carrau F. Pineapple ( Ananas comosus L. Merr.) wine production in Angola: Characterisation of volatile aroma compounds and yeast native flora. Int. J. Food Microbiol. 2017, 241, 161–167. 10.1016/j.ijfoodmicro.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Masson J.; Cardoso M. d. G.; Zacaroni L. M.; Anjos J. P. d.; Sackz A. A.; Machado A. M. d. R.; Nelson D. L. Determination of acrolein, ethanol, volatile acidity, and copper in different samples of sugarcane spirits. Food Sci. Technol. 2012, 32, 568–572. 10.1590/s0101-20612012005000075. [DOI] [Google Scholar]
- Cabrita M. J.; Torres M.; Palma V.; Alves E.; Patão R.; Costa Freitas A. M. Impact of malolactic fermentation on low molecular weight phenolic compounds. Talanta 2008, 74, 1281–1286. 10.1016/j.talanta.2007.08.045. [DOI] [PubMed] [Google Scholar]
- Naczk M.; Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Tian R.-R.; Pan Q.-H.; Zhan J.-C.; Li J.-M.; Wan S.-B.; Zhang Q.-H.; Huang W.-D. Comparison of phenolic acids and flavan-3-ols during wine fermentation of grapes with different harvest times. Molecules 2009, 14, 827–838. 10.3390/molecules14020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.; Su J.; Liu C.; Zhang H. Y.; Heng Y. W.; Liu Y. B. Analysis of contents of total phenolic compounds and total flavones and DPPH radical scavenging activity during overmature vinegar production. Food Sci. 2009, 17, 038. [Google Scholar]
- Dávalos A.; Bartolomé B.; Gómez-Cordovés C. Antioxidant properties of commercial grape juices and vinegars. Food Chem. 2005, 93, 325–330. 10.1016/j.foodchem.2004.09.030. [DOI] [Google Scholar]
- Li Y.-l.; Hu J.-j.; Li H.-m.; Shan F.; Bian J.-s.; Sun Q.-y. Study on variations of main function ingredients in the Tartary buckwheat vinegar fermentation process with uncooked material. Sci. Technol. Food Ind. 2011, 12, 055. [Google Scholar]
- Wang A.; Zhang J.; Li Z. Correlation of volatile and nonvolatile components with the total antioxidant capacity of Tartary buckwheat vinegar: Influence of the thermal processing. Food Res. Int. 2012, 49, 65–71. 10.1016/j.foodres.2012.07.020. [DOI] [Google Scholar]
- Zhang M.; Pan Q.; Yan G.; Duan C. Using headspace solid phase micro-extraction for analysis of aromatic compounds during alcoholic fermentation of red wine. Food Chem. 2011, 125, 743–749. 10.1016/j.foodchem.2010.09.008. [DOI] [Google Scholar]
- Jewison T.; Knox C.; Neveu V.; Djoumbou Y.; Guo A. C.; Lee J.; Liu P.; Mandal R.; Krishnamurthy R.; Sinelnikov I.; Wilson M.; Wishart D. S. YMDB: the Yeast Metabolome Database. Nucleic Acids Res. 2012, 40, D815–D820. 10.1093/nar/gkr916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.-L.; Zheng F.-J.; Lin B.; Wang T.-S.; Li Y.-R. Preparation and characteristics of sugarcane low alcoholic drink by submerged alcoholic fermentation. Sugar Technol. 2013, 15, 412–416. 10.1007/s12355-013-0248-3. [DOI] [Google Scholar]
- Peña-Neira A.; Hernández T.; García-Vallejo C.; Estrella I.; Suarez J. A. A survey of phenolic compounds in Spanish wines of different geographical origin. Eur. Food Res. Technol. 2000, 210, 445–448. 10.1007/s002170050579. [DOI] [Google Scholar]
- Blanco P.; Mirás-Avalos J. M.; Pereira E.; Orriols I. Fermentative aroma compounds and sensory profiles of Godello and Albariño wines as influenced by Saccharomyces cerevisiae yeast strains. J. Sci. Food Agric. 2013, 93, 2849–2857. 10.1002/jsfa.6122. [DOI] [PubMed] [Google Scholar]