Abstract
Impairment in glutamate neurotransmission mediates the development of dependence upon nicotine (NIC) and ethanol (EtOH). Previous work indicates that continuous access to EtOH or phasic exposure to NIC reduces expression of the glutamate transporter-1 (GLT-1) and cystine/glutamate antiporter (xCT) but not the glutamate/aspartate transporter (GLAST). Additionally, metabotropic glutamate receptors (mGluRs) expression was affected following exposure to EtOH or NIC. However, little is known about the effects of EtOH and NIC co-consumption on GLT-1, xCT, GLAST, and mGluR1 expression. In this study, peri-adolescent female alcohol preferring (P) rats were given binge-like access to water, sucrose (SUC), SUC-NIC, EtOH, or EtOH-NIC for four weeks. The present study determined the effects of these reinforcers on GLT-1, xCT, GLAST, and mGluR1 expression in the nucleus accumbens (NAc), hippocampus (HIP) and prefrontal cortex (PFC). GLT-1 and xCT expression were decreased in the NAc following both SUC-NIC and EtOH-NIC. In addition, only xCT expression was downregulated in the HIP in both of these latter groups. Also, glutathione peroxidase (GPx) activity in the HIP was reduced following SUC, SUC-NIC, EtOH, and EtOH-NIC consumption. Similar to previous work, GLAST expression was not altered in any brain region by any of the reinforcers. However, mGluR1 expression was increased in the NAc in the SUC-NIC, EtOH, and EtOH-NIC groups. These results indicate that peri-adolescent binge-like drinking of EtOH or SUC with or without NIC may exert differential effects on astroglial glutamate transporters and receptors. Our data further parallel some of the previous findings observed in adult rats.
Keywords: Co-abuse, Ethanol, Nicotine, GLT-1, xCT, mGluR1
1. Introduction
Recent evidence indicates a significant increase in ethanol (EtOH) and tobacco consumption in adolescents worldwide (Primack et al., 2015; Xi et al., 2016; Esser, 2017; Miech et al., 2017; Wang et al., 2017). Additionally, EtOH consumption has been shown to increase pleasure from cigarettes smoking in young adults (Gubner et al., 2017). Moreover, several clinical studies indicate that EtOH abusers are more likely to abuse tobacco (Bierut et al., 2000; Falk et al., 2008). Similarly, studies have reported that EtOH drinking increases tobacco use, and conversely tobacco use increases EtOH intake (Bobo and Husten, 2000; Grant et al., 2004; Falk et al., 2006; Falk et al., 2008). Thus, it appears that EtOH and nicotine (NIC) modulate, at least some, common reinforcing/rewarding neurocircuits, which result in increased intake when both compounds are available concurrently (Griffiths et al., 1976; Bito-Onon et al., 2011). Delineating the pharmacological mechanisms involved in the development of EtOH and NIC co-dependence could lead to the discovery of molecular targets for pharmacotherapeutic intervention, in particular targets within the central glutamatergic reward neurocircuitry.
Within the mesocorticolimbic reward pathway, the nucleus accumbens (NAc) receives glutamatergic inputs from different brain regions, including the prefrontal cortex (PFC) and hippocampus (HIP) (Floresco et al., 2001; Kalivas and Volkow, 2005). These glutamatergic projections to the NAc have been implicated in the development of drug-seeking and drug-taking behavior (LaLumiere and Kalivas, 2008). The HIP also receives input from, and sends glutamatergic projections to, the PFC, which appears to mediate the development of drug dependence as well [for review see (Feduccia et al., 2012)]. Thus, it is important to investigate the effects of binge-like drinking of EtOH or sucrose (SUC) with or without NIC on the glutamatergic system in these three brain areas. Importantly, exposure to EtOH or NIC has been associated with significant increases in total extracellular glutamate concentrations in these critical reward-related brain regions (Floresco et al., 2001; Lambe et al., 2003; Saellstroem Baum et al., 2006; Bancila et al., 2009). For instance, a recent study from Deehan et al. (2015) reported that female P rats exposed to EtOH-NIC showed significant increases in extracellular glutamate concentrations compared with EtOH, saccharin-NIC or saccharin self-administered groups. However, the effects of co-exposure of NIC and EtOH on astroglial glutamate transporter and metabotropic glutamate receptor-1 (mGluR1) expression in the mesocorticolimbic neurocircuit have not been studied yet.
Glutamate uptake is primarily regulated by the glutamate transporter-1 (GLT-1), of which the excitatory amino acid transporter 2 (EAAT2) is the human homolog (Danbolt, 2001). Previous work indicates that EtOH drinking for five weeks or intravenous NIC self-administration for 21 days reduced the expression of GLT-1 in the NAc (Knackstedt et al., 2009; Goodwani et al., 2015). In addition, repeated oral gavage of high dose of EtOH reduced GLT-1 expression in the HIP (Alshehri et al., 2017). This suggests that long-term exposure to EtOH or NIC may induce a marked increase in the total extracellular glutamate concentrations in the mesocorticolimbic brain areas, including the NAc, PFC and HIP. However, less is known about the effects of EtOH and NIC co-consumption on GLT-1 expression in these mesocorticolimbic nuclei. Additionally, cystine/glutamate antiporter (xCT) has an important role in regulating glutamate homeostasis [for review see (Bridges et al., 2012)], where extracellular glutamate is exchanged for intracellular cystine (Baker et al., 2002; Shih et al., 2006). The glutamate/aspartate transporter (GLAST) is another glutamate transporter co-localized with GLT-1 and xCT in astroglia. Regarding Group I metabotropic glutamate receptors (mGluRs), mGluR1 is upregulated in animals exposed to EtOH or NIC (Kane et al., 2005; Obara et al., 2009). Moreover, it has been shown that mGluR1 antagonists significantly attenuate both EtOH- and NIC-seeking behavior in animals (Dravolina et al., 2007; Besheer et al., 2008; Lum et al., 2014; Goodwani et al., 2017) suggesting a potential role for mGluR1 in regulating the reinforcing effects of EtOH and NIC. However, the effect of EtOH-NIC co-exposure on the expression of GLAST or mGluR1 has not been directly investigated.
Interestingly, xCT has shown neuroprotective and antioxidant effects in part by stimulating the biosynthesis of glutathione (Shih et al., 2006; Albrecht et al., 2010). Intracellular cystine is converted to cysteine, which is involved in glutathione synthesis and inhibition of oxidative stress (Shih et al., 2003; Amin et al., 2014). Importantly, oxidized glutathione is formed by the oxidation of glutathione via glutathione peroxidase (GPx), which is widely used as a biomarker for neuroprotection (Deponte, 2013). However, the association between GPx activity and xCT expression has not been yet evaluated in the context of co-consumption of NIC and EtOH.
In the present study, we investigated changes in the expression of glutamate transporters (GLT-1, xCT and GLAST) and mGluR1 in the NAc, HIP, and PFC of female P rats exposed to a free-choice, binge-like drinking protocol with SUC, SUC-NIC, EtOH, or EtOH-NIC as the reinforcers. Since NIC has a bitter taste, SUC and EtOH were mixed with NIC to stimulate NIC drinking in P rats. Thus, the effects of SUC or EtOH, SUC-NIC and EtOH-NIC on astroglial glutamate transporter and mGluR1 expression were investigated to determine any significant interactions between these reinforcers on the glutamatergic system. Additionally, GPx activity in the HIP was assessed. The rationale for using female, rather than male, P rats in this study is based on reported literature indicating heightened EtOH and NIC seeking behavior in female, compared with male, animals and humans (Donny et al., 2000; Torres et al., 2014; Frydenberg et al., 2015). A positive correlation has been associated between levels of estrogen and EtOH consumption in female humans (Frydenberg et al., 2015). In addition, female rats have been shown to have greater motivation to consume NIC or EtOH compared to female ovariectomized or male rats (Torres et al., 2014; Flores et al., 2016). The reason for using P rat model in this study is that these rats have several alcoholic phenotypes such as physiological, neurochemical and behavioral characteristics (Murphy et al., 2002; Bell et al., 2011; Bell et al., 2016; Bell et al., 2017). Additionally, female P rats were previously used in a study from our laboratory that found upregulating GLT-1 levels and/or function by ceftriaxone attenuated drinking behavior of EtOH, SUC and NIC (Sari et al., 2016). Thus, these animals have an avidity to consume EtOH, SUC and NIC.
A prior study reported that chronic NIC exposure reduced glutamate content in the NAc of female adult rats exposed to EtOH, but not similarly treated adolescents (Lallemand et al., 2009). Given most studies investigated the effects of chronic exposure to EtOH or NIC on glutamatergic in adult animals, it is imperative that this research question also be investigated during the adolescent stage of development. Owing to the clinically and physiologically relevance to human exposure, a multiple scheduled binge-like drinking of EtOH or SUC with or without NIC procedure was used in this study to simulate binge-like drinking (Bell et al., 2011; Bell et al., 2014). Overall, this study was designed to provide evidence regarding the differential or synergistic effects of binge-like EtOH with or without NIC consumption on the glutamatergic system relative to neutral-(water) and positive-control (Ryu et al.) conditions.
2. Materials and methods
2.1. Animals and drinking protocol
Female P rats used in this study were maintained in a facility fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All research protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Indiana University School of Medicine (IUSM, Indianapolis, IN) and were in accordance with the guidelines of the IACUC of the National Institutes of Health (NIH) and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council 1996). Animals were weaned at 21 days old [post-natal day (PND) 21] and group housed by sex. At PND 25, animals were transferred to a vivarium room maintained on a 12/12 h reverse dark/light cycle with light offset at 10:00 a.m.
At PND 30 ± 2, animals were transferred to hanging stainless steel wire-mesh cages and given ad lib access to food and water until tissue harvesting. On the third day that the rats were housed in hanging stainless steel wire-mesh cages, each animal was randomly assigned to one of the five different treatment groups. All groups experienced a binge-like drinking-in-the-dark—multiple-scheduled-access procedure (Bell et al., 2011) of two 1-hour (h) sessions separated by 2 h, with the first session occurring during the first h of the dark cycle. Access to the respective solutions occurred five days a week (Monday through Friday) for four weeks. Rats were grouped into five groups and were given free-choice access to three bottles [three bottle-choice (3BC)] as follows: a water bottle and concurrent access to (1) two bottles of water, (2) a bottle of 15% as well as a bottle of 30% EtOH, (3) a bottle of 15% EtOH and 0.07 mg/mL NIC as well as a bottle of 30% EtOH and 0.14 mg/mL NIC, (4) a bottle of 10% SUC and 0.07 mg/mL NIC as well as a bottle of 10% SUC and 0.14 mg/ml NIC, and finally (5) a bottle of 10% SUC as well as a bottle of 10% SUC. Each treatment group had nine animals and the treatment groups are depicted in Fig. 1. NIC was mixed with either EtOH or SUC to stimulate NIC drinking in P rats and avoid unpleasant taste of NIC. The NIC concentrations (0.07 mg/mL and 0.14 mg/mL) were chosen based on studies showing that the intake of NIC (mg/kg/day) using these concentrations results in blood NIC levels that are comparable to non-dependent chronic smokers (Benowitz, 1984; Hauser et al., 2012).
Fig. 1.
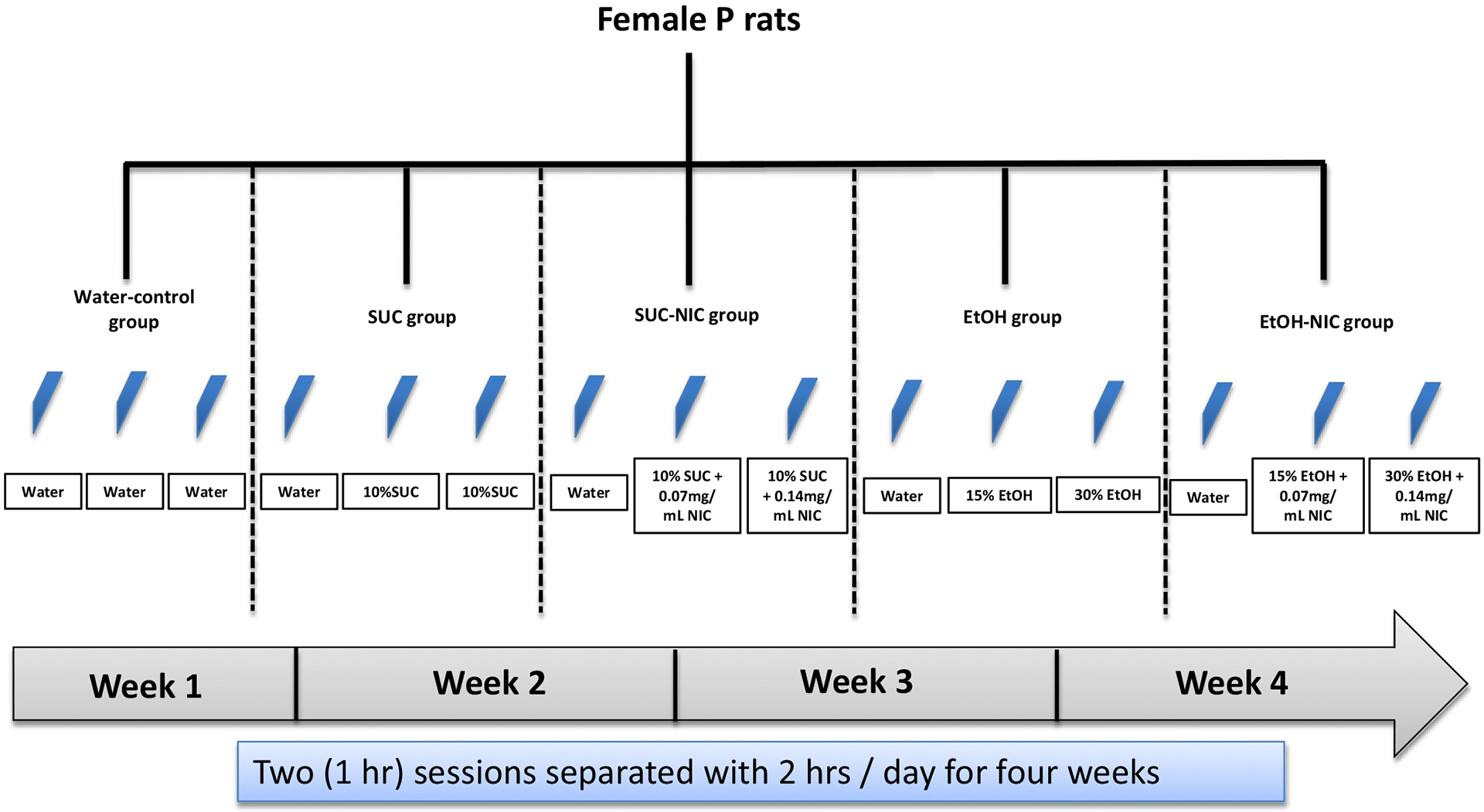
Time-line and group conditions for binge-like drinking of SUC or EtOH with or without NIC in female P rats.
2.2. Brain harvesting
Approximately 2 h after the last binge-like access period, rats were euthanized and brains were immediately extracted and immediately stored at −80 °C for immunoblotting assay. Brain regions (NAc, PFC, and HIP) were dissected using a Cryostat machine maintained at −20 °C. The Paxinos and Watson Atlas was used to determine the boundaries of these brain regions (Paxinos and Watson, 2007). In this study, we dissected (freehand) these brain regions such as NAc (~1 mm of thickness), HIP (~2 mm of thickness) and PFC (~2 mm of thickness). Random samples were chosen from each group (n = 6, for each group) for Western Blotting.
2.3. Western Blot analyses
Western Blot assay was performed to determine changes in expression of GLT-1, xCT, GLAST, mGluR1, and GAPDH in the NAc, HIP, and PFC as described previously (Alasmari et al., 2016b; Hammad et al., 2017). Briefly, brain tissue samples were homogenized gently using lysis buffer-containing protease inhibitor. For protein quantification, Bio-Rad quantification reagents (Bio-Rad, Hercules, CA, USA) were used to determine the total protein in each tissue sample. Specific volume from each sample of tissue was mixed with laemmli dye and then loaded on polyacrylamide gels (10–20%). Subsequently, a transfer apparatus system was used to transfer proteins from each gel onto a PVDF membrane. Subsequently, membranes were blocked using 3% free fat milk in Tris-buffered saline Tween-20 (TBST) for 30 min to 1 h at room temperature. The PVDF membranes were incubated with the desired primary antibodies (overnight) at 4 °C. The primary antibodies used in this study included guinea pig anti-GLT-1 (1:5000, Millipore), rabbit anti-xCT antibody (1: 1000; Abcam), rabbit anti-GLAST antibody (1: 5000; Abcam), and rabbit anti-mGluR1 (1: 3000; Millipore Bioscience Research Reagents). These antibodies have been used in our previous studies (Hammad et al., 2017). The control loading protein used in this study was mouse anti-GAPDH (1:5000, Millipore). On the second day, all membranes were washed five times with TBST followed by 30-minute blocking in 3% free fat milk. Subsequently, membranes were incubated with the appropriate secondary antibody [anti-rat IgG, anti-guinea pig IgG, anti-rabbit IgG or anti-mouse IgG, respectively] at a dilution of 1:3000 for 90 min. This was followed by five washes with TBST and drying. To detect proteins, the dried membranes were incubated with the developing Chemiluminescent kit (Super Signal West Pico, Pierce Inc.). Membranes exposed to HyBlot CL Film (Thermo Fisher Scientific) were then developed using SRX-101A processor. An MCID machine was used to quantify and analyze the expression of GLT-1, xCT, GLAST, mGluR1, and GAPDH on digitized blot images. In each run of the gels, we loaded the gels as follows: 1) Water, SUC, and SUC-NIC; and 2) Water, EtOH, and EtOH-NIC. Thus, each group was compared for changes in the expression of GLT-1, xCT, GLAST, and mGluR1 in the NAc, PFC, and HIP. The data for the water-control group were expressed as 100% to determine significant changes in expression of these proteins (relative to water control group) in these mesocorticolimbic brain regions of animals exposed to binge-like access to different drinking solutions as performed previously (Li et al., 2003; Raval et al., 2003; Miller et al., 2008; Zhang and Tan, 2011; Devoto et al., 2013; Alasmari et al., 2015). Thus, we normalized all the expression of proteins of interest in each treatment groups to the matched water control group that was run in the same gel set.
2.4. Glutathione peroxidase activity (GPx)
Glutathione peroxidase (GPx) activity in the HIP region of the treated rats was determined using the commercially available glutathione peroxidase kit (Cayman Chemical, Ann Arbor, USA). To examine GPx activity, the frozen brain tissue samples were retrieved from −80° C and homogenized in ice-cold PBS (pH 7.4) supplemented with 1 mM EDTA. The homogenate was centrifuged at 22,000g for 30 min at 4 °C and the supernatant was assessed immediately for GPx activity using the kit protocol. Briefly, the rate of change in absorbance at 340 nm (A340) over 1 to 6 min was determined for the background wells, positive control and the collected brain tissue samples in duplicate. The GPx activity was then determined using the rate change of A340 and the NADPH extinction coefficient. Protein concentration of samples was determined using a Pierce BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, USA).
2.5. Statistical analyses
2.5.1. Drinking-solution data
The average intake of SUC, EtOH, and NIC for the last five sessions of the four-week binge-like scheduled drinking procedure was calculated and analyzed using unpaired t-test. We used in this experiment, unpaired t-test, to compare between SUC (g/kg) or EtOH (g/kg) drinking with or without addition of NIC. In addition, we used unpaired t-test to determine the effects of EtOH and SUC on NIC intake (mg/kg). It is important to consider that SUC or EtOH and NIC were used with different concentrations and the drinking was measured with different units of amount (e.g., g/kg/time for EtOH).
2.5.2. Western Blot and glutathione peroxidase (GPx) data
Data obtained from the Western Blot assays, for the proteins of interest, were analyzed as a percentage ratio, of water-control group, relative to the control-loading protein (GAPDH) using one-way ANOVA followed by Newman Keuls post-hoc multiple comparison tests. The percentage of GPx activity was also analyzed using a one-way ANOVA followed by Newman Keuls multiple comparisons. The densities of immunoblot bands and GPx activity obtained from the water control group served as the 100% bench-mark. All data are shown for a p < 0.05 level of significance. The sample sizes differed between the behavioral studies (n = 9) and Western Blot studies (n = 6) due to the greater effect sizes observed in Western Blot studies.
3. Results
3.1. Average intake of SUC, SUC-NIC, EtOH, or EtOH-NIC
Independent t-test analysis revealed that addition of NIC significantly reduced SUC intake [p < 0.0001, (Fig. 2A)]. An unpaired t-test showed that the average intake of NIC (mg/kg) was significantly lower in animals exposed to the binge-like schedule of EtOH-NIC drinking compared with the SUC-NIC group [p < 0.0001, (Fig. 2B)]. However, no significant differences were observed for the average intake of EtOH relative to EtOH-NIC [p > 0.05, (Fig. 2C)].
Fig. 2.
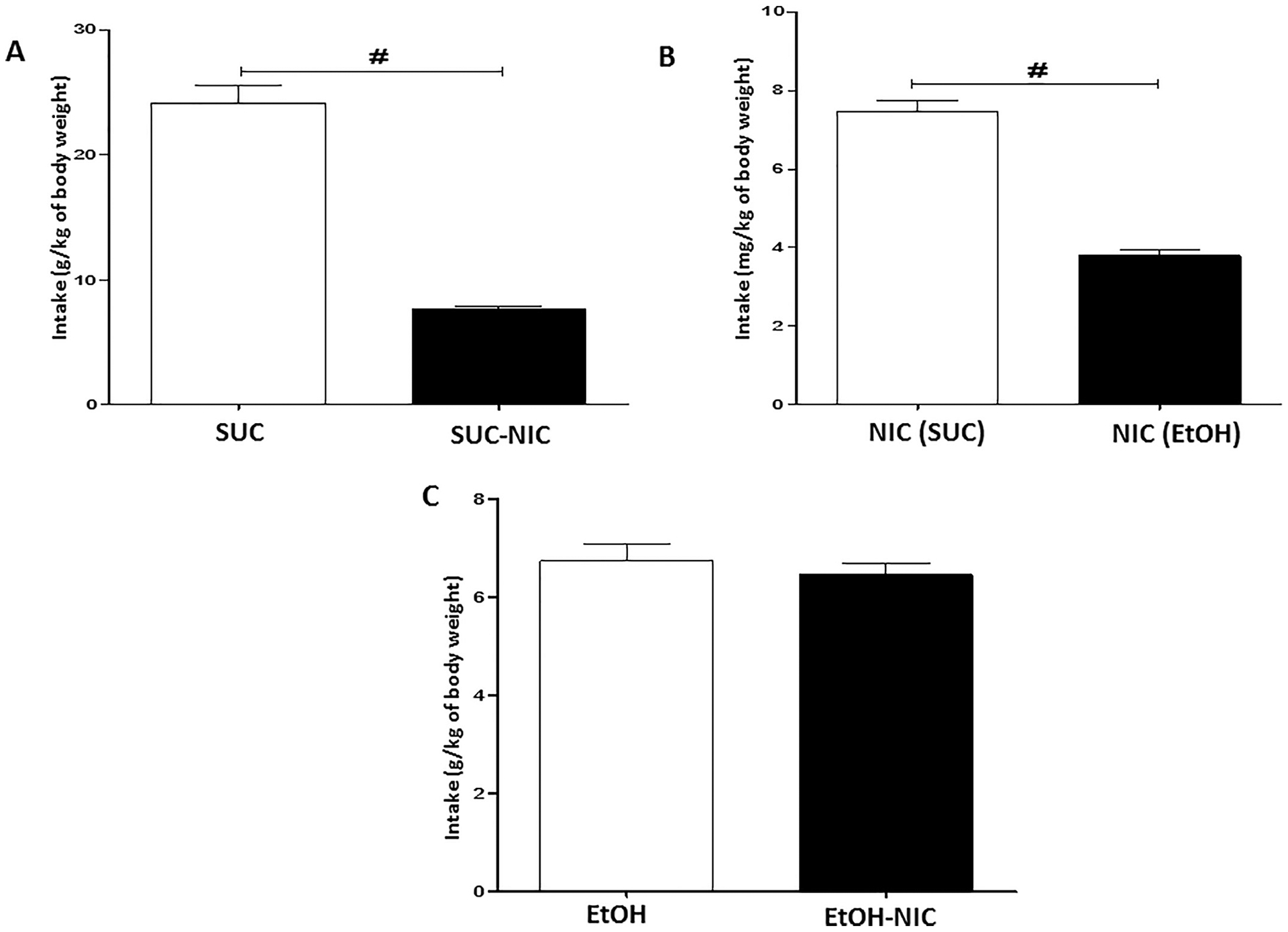
Average last five sessions binge-like drinking of; (A) SUC (g/kg of average body weight) and SUC-NIC (g/kg of body weight), (B) NIC (Ryu et al.) (mg/kg of body weight) and NIC (EtOH) (mg/kg of body weight) NIC (mg/kg of body weight) in female P rats, and (C) EtOH (g/kg of body weight) and EtOH-NIC (g/kg of body weight). Unpaired t-test revealed that addition of NIC reduced SUC consumption, however, the analysis did not show any change in EtOH consumption following addition of NIC. Unpaired t-test revealed that NIC consumption was higher in SUC-NIC group compared to EtOH-NIC group. Data are represented as mean ± SEM (n = 9 for each group), (# p < 0.0001).
3.2. Effects of binge-like drinking of SUC or SUC-NIC on GLT-1, xCT, GLAST and mGluR1 expression in the NAc
One-way ANOVA showed a significant difference among water control, SUC, and SUC-NIC groups in GLT-1 expression [F (2, 15) = 10.99, p = 0.0011, (Fig. 3A)], xCT expression [F (2, 15) = 6.91, p = 0.0075, (Fig. 3B)], and mGluR1 expression [F (2, 15) = 4.53, p = 0.028, (Fig. 3D)], but not in GLAST expression [F (2, 15) = 0.63, p = NS, (Fig. 3C)] in the NAc. Newman-Keuls multiple comparison post-hoc tests revealed that while there was no significant changes in GLT-1 expression between SUC and water groups in the NAc, SUC-NIC drinking reduced GLT-1 and xCT expression and increased mGluR1 expression in the NAc compared to water and SUC groups, neutral- and positive-control groups respectively.
Fig. 3.
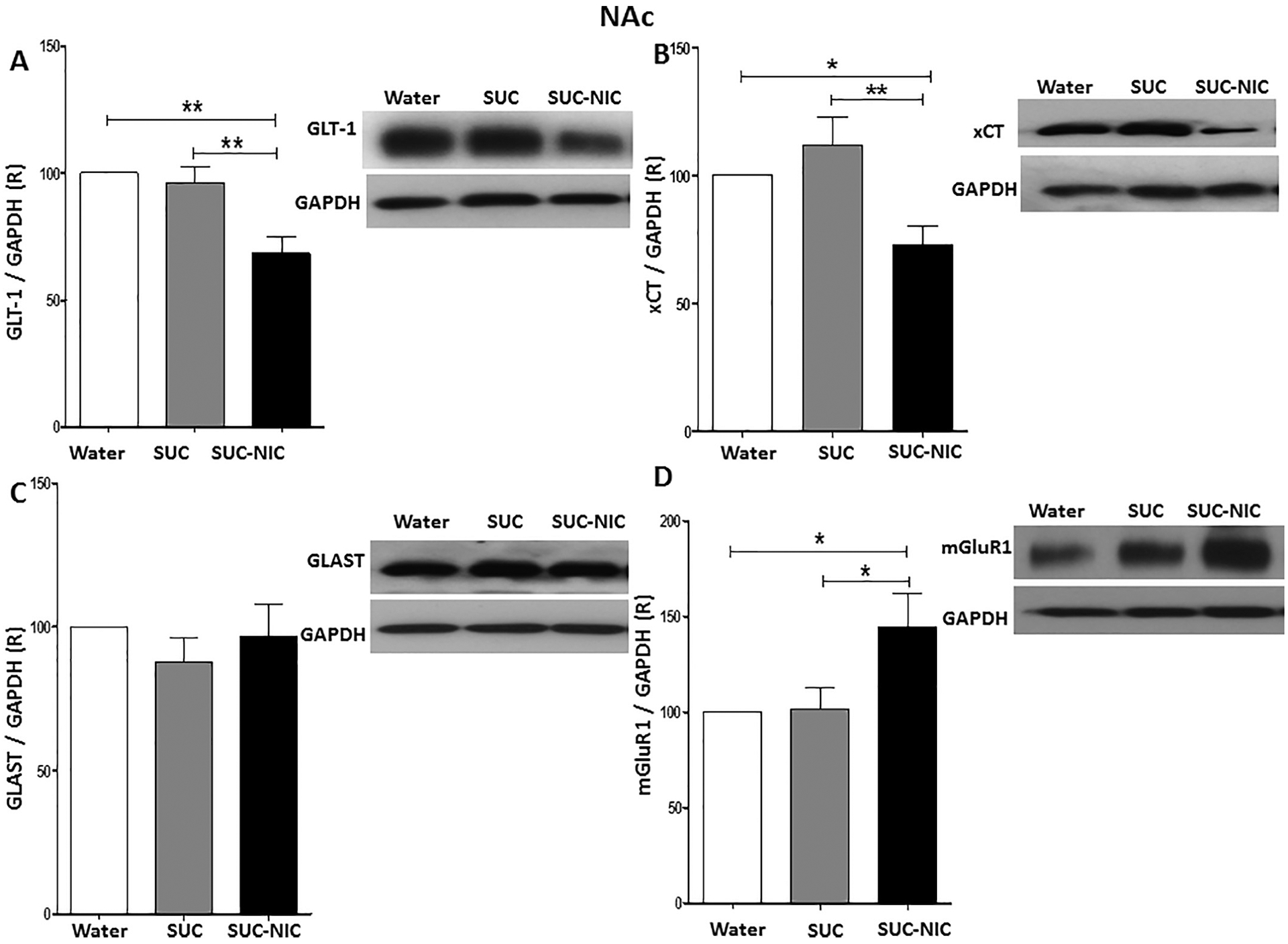
Effects of binge-like drinking of SUC and SUC-NIC on relative expression (R) of (A) GLT-1, (B) xCT, (C) GLAST, and (D) mGluR1 in the NAc. One way ANOVA followed by Newman-Keuls analysis revealed that SUC-NIC but not SUC exposure reduced GLT-1/GAPDH and xCT/GAPDH ratios in the NAc. The analysis did not show any significant reduction in GLAST/GAPDH ratio in the NAc. One way ANOVA followed by Newman-Keuls analysis revealed that SUC-NIC but not SUC exposure increased mGluR1/ratio in the NAc. Data are represented as mean ± SEM (n = 6 for each group), (* p < 0.05 and ** p < 0.01).
3.3. Effects of binge-like drinking of EtOH or EtOH-NIC on GLT-1, xCT, GLAST, and mGluR1 expression in the NAc
We further determined the effects of four-week binge-like access drinking of EtOH and EtOH-NIC on the expression of GLT-1, xCT, GLAST, and mGluR1 in the NAc. One-way ANOVA showed a significant difference among these groups in the NAc in GLT-1 expression [F (2,15) = 5.28, p = 0.018, (Fig. 4A)], xCT expression [F (2, 15) = 7.26, p = 0.0062, (Fig. 4B)], and mGluR1 expression [F (2,15) = 4.58, p = 0.028, (Fig. 4D)], but not in GLAST expression [F (2, 15) = 0.55, p = NS, (Fig. 4C)]. Newman-Keuls multiple comparisons showed that while there were no significant changes in GLT-1 and xCT expression between water control and EtOH groups in the NAc, EtOH-NIC, exposure reduced GLT-1 and xCT expression compared to water control and EtOH groups in the NAc. In addition, EtOH and EtOH-NIC exposure increased mGluR1 expression in the NAc compared to the water-control group and there were significant changes in mGluR1 expression in the NAc between EtOH and EtOH-NIC groups.
Fig. 4.
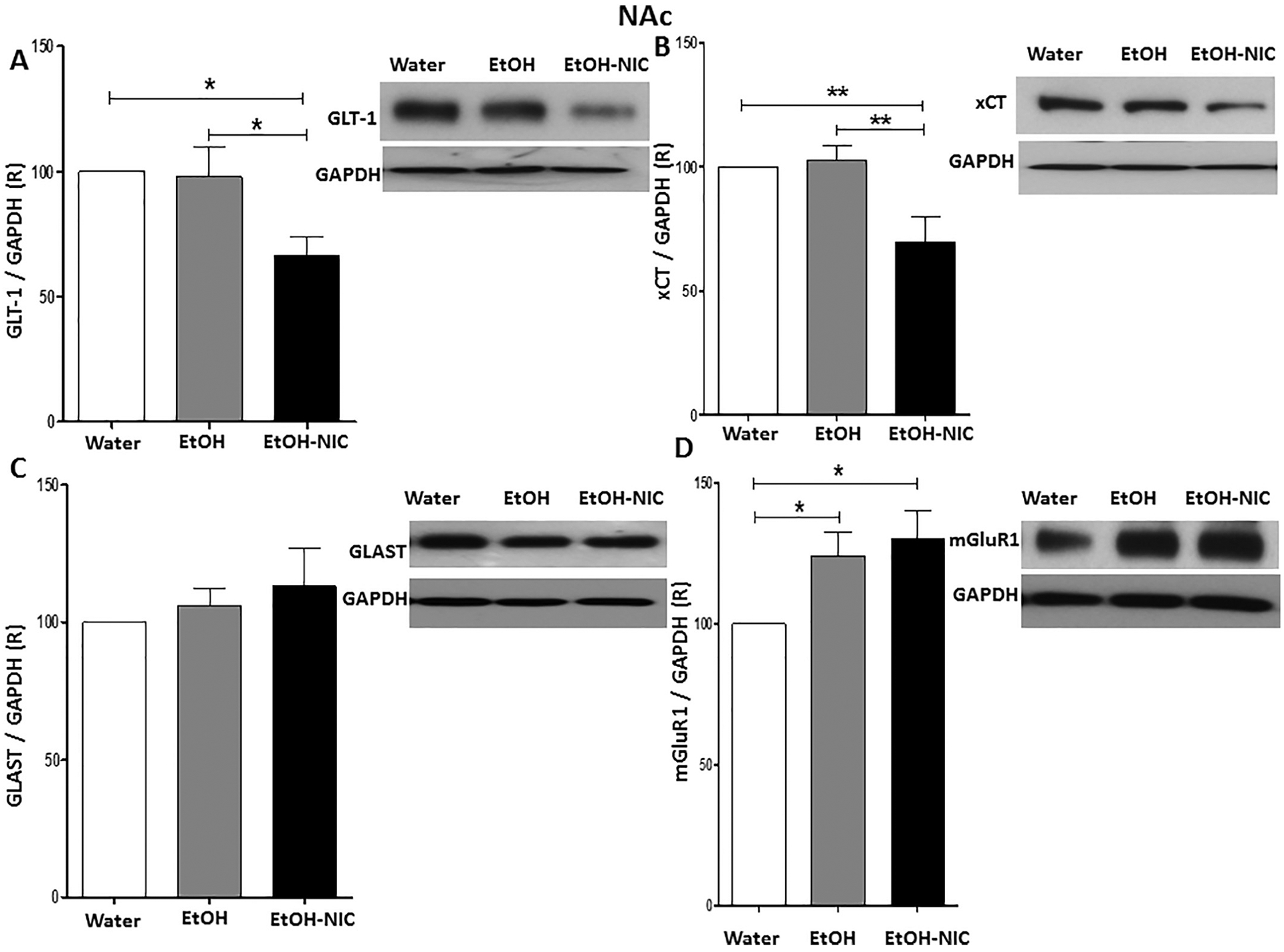
Effects of binge-like drinking of EtOH and EtOH-NIC on relative expression (R) of (A) GLT-1, (B) xCT, (C) GLAST, and (D) mGluR1 in the NAc. One way ANOVA followed by Newman-Keuls analysis revealed that EtOH-NIC but not EtOH exposure reduced GLT-1/GAPDH and xCT/GAPDH ratios in the NAc. The analysis did not show any significant reduction in GLAST/GAPDH ratio between the groups in the NAc. One way ANOVA followed by Newman-Keuls analysis revealed that EtOH-NIC and EtOH exposure increased mGluR1/GAPDH ratio in the NAc. Data are represented as mean ± SEM (n = 6 for each group), (* p < 0.05 and ** p < 0.01).
3.4. Effects of binge-like drinking of SUC or SUC-NIC on GLT-1, xCT, GLAST and mGluR1 expression in the HIP
One-way ANOVA demonstrated a significant difference in xCT expression among water control, SUC, and SUC-NIC groups in the HIP [F (2, 15) = 9.05, p = 0.0026, (Fig. 5B)]. The statistical analysis did not show any significant differences among water control, SUC, and SUC-NIC groups in GLT-1 expression [F (2, 15) = 0.70, p = NS, (Fig. 5A)], GLAST expression [F (2, 15) = 0.98, p = NS, (Fig. 5C)], and mGluR1 expression [F (2, 15) = 0.65, p = NS, (Fig. 5D)]. Newman-Keuls analysis revealed that SUC-NIC drinking reduced xCT expression significantly in the HIP compared to the SUC and water control groups, with no difference between the SUC and water control groups.
Fig. 5.
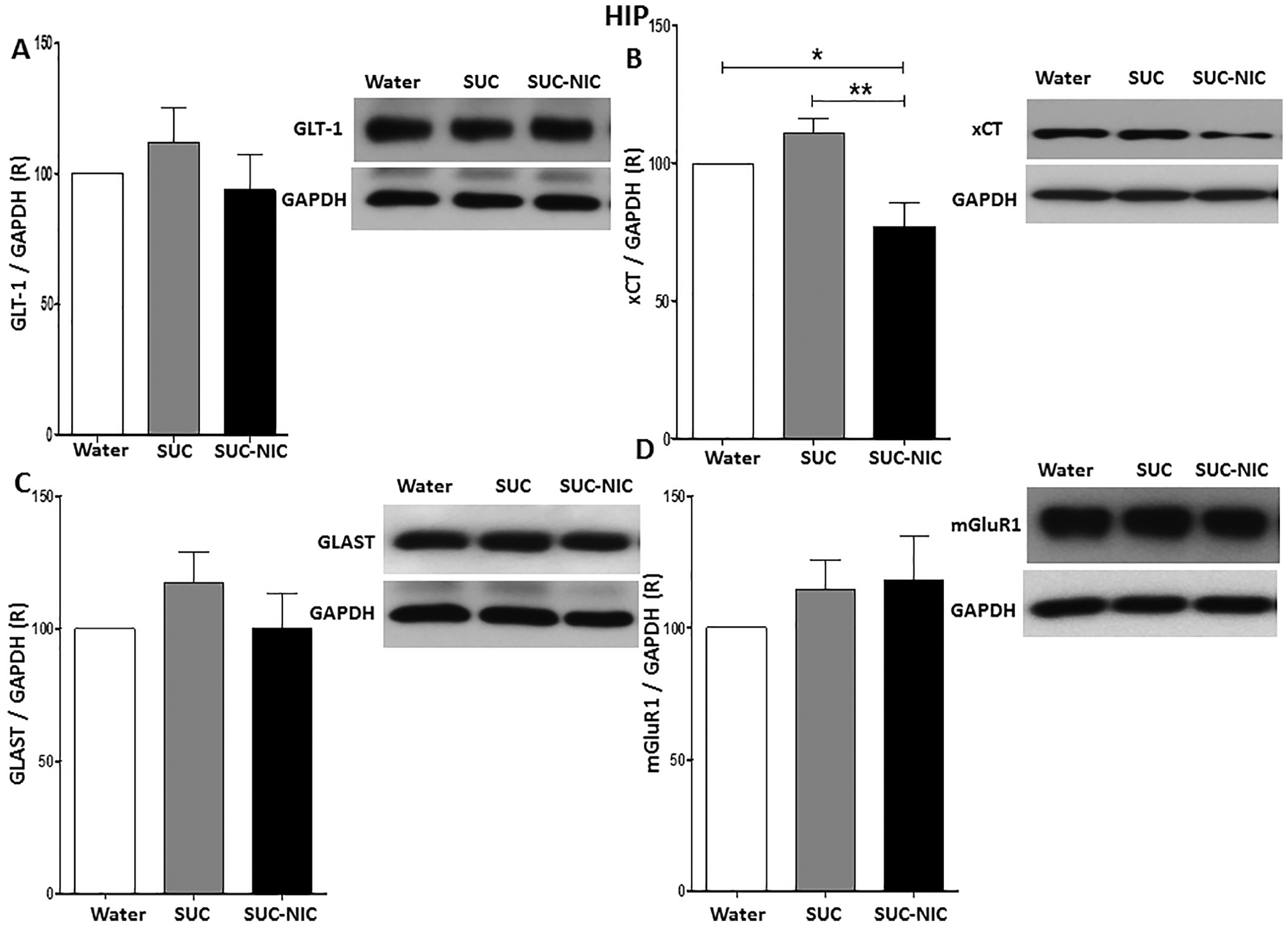
Effects of binge-like drinking of SUC and SUC-NIC on relative expression (R) of (A) GLT-1, (B) xCT, (C) GLAST, and (D) mGluR1 in the HIP. One way ANOVA followed by Newman-Keuls analysis revealed that SUC-NIC but not SUC exposure reduced xCT/GAPDH ratio in the HIP. The analysis did not show any significant reduction in GLT-1/GAPDH, GLAST/GAPDH and mGluR1/GAPDH ratios between all groups in the HIP. Data are represented as mean ± SEM (n = 6 for each group), (* p < 0.05 and ** p < 0.01).
3.5. Effects of binge-like drinking of EtOH or EtOH-NIC on GLT-1, xCT, GLAST, and mGluR1 expression in the HIP
We further investigated expression of GLT-1, xCT, GLAST, and mGluR1 between water, EtOH, and EtOH–NIC groups. One-way ANOVA revealed a significant change in xCT expression among these groups in the HIP [F (2, 15) = 5.08, p = 0.021, (Fig. 6B)], but not in GLT-1 expression [F (2, 15) = 0.37, p = NS, (Fig. 6A)], GLAST expression [F (2, 15) = 0.11, p = NS, (Fig. 6C)], or mGluR1 expression [F (2, 15) = 0.25, p = NS, (Fig. 6D)]. Newman-Keuls multiple comparisons revealed that there was no significant changes in xCT expression in the HIP following binge-like EtOH drinking as compared to the water control group. However, EtOH–NIC drinking decreased xCT expression significantly in the HIP compared to EtOH and water control groups.
Fig. 6.
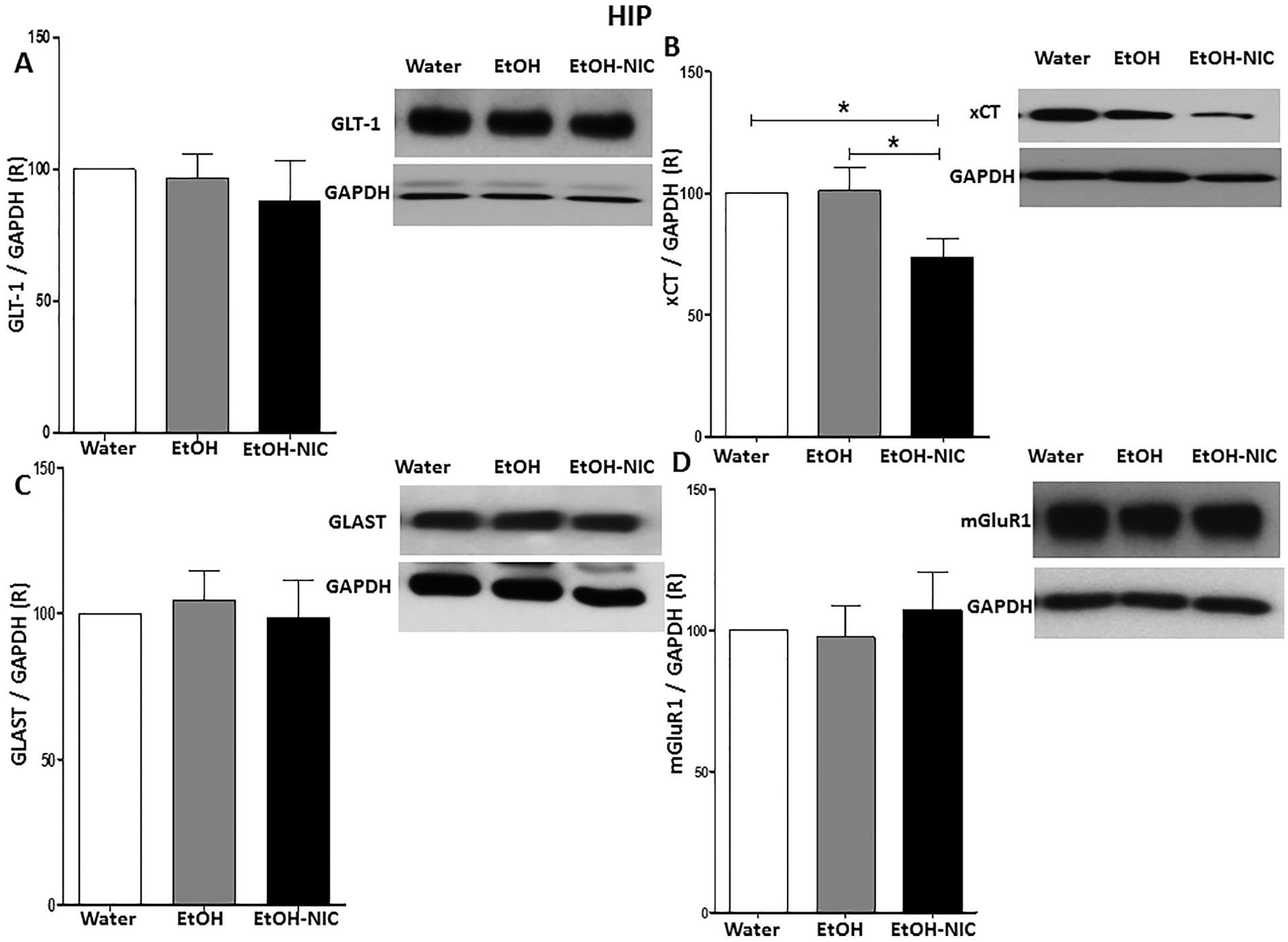
Effects of binge-like drinking of EtOH and EtOH-NIC on relative expression (R) of (A) GLT-1, (B) xCT, (C) GLAST and (D) mGluR1 in the HIP. One way ANOVA followed by Newman-Keuls analysis revealed that EtOH-NIC but not EtOH exposure reduced xCT/GAPDH ratio in the HIP. The analysis did not show any significant reduction in GLT-1/GAPDH, GLAST/GAPDH and mGluR1/GAPDH ratios between the groups in the HIP. Data are represented as mean ± SEM (n = 6 for each group), (* p < 0.05).
3.6. Effects of binge-like drinking of SUC or SUC-NIC on GLT-1, xCT, GLAST and mGluR1 expression in the PFC
One-way ANOVA did not revealed any significant differences in GLT-1 expression [F (2, 15) = 0.14, p = NS, (Fig. 7A)], xCT expression [F (2, 15) = 0.48, p = NS, (Fig. 7B)], GLAST expression [F (2, 15) = 0.20, p = NS, (Fig. 7C)] and mGluR1 expression [F (2, 15) = 0.37, p = NS, (Fig. 7D)] among water control, SUC and SUC-NIC groups expression in the PFC.
Fig. 7.
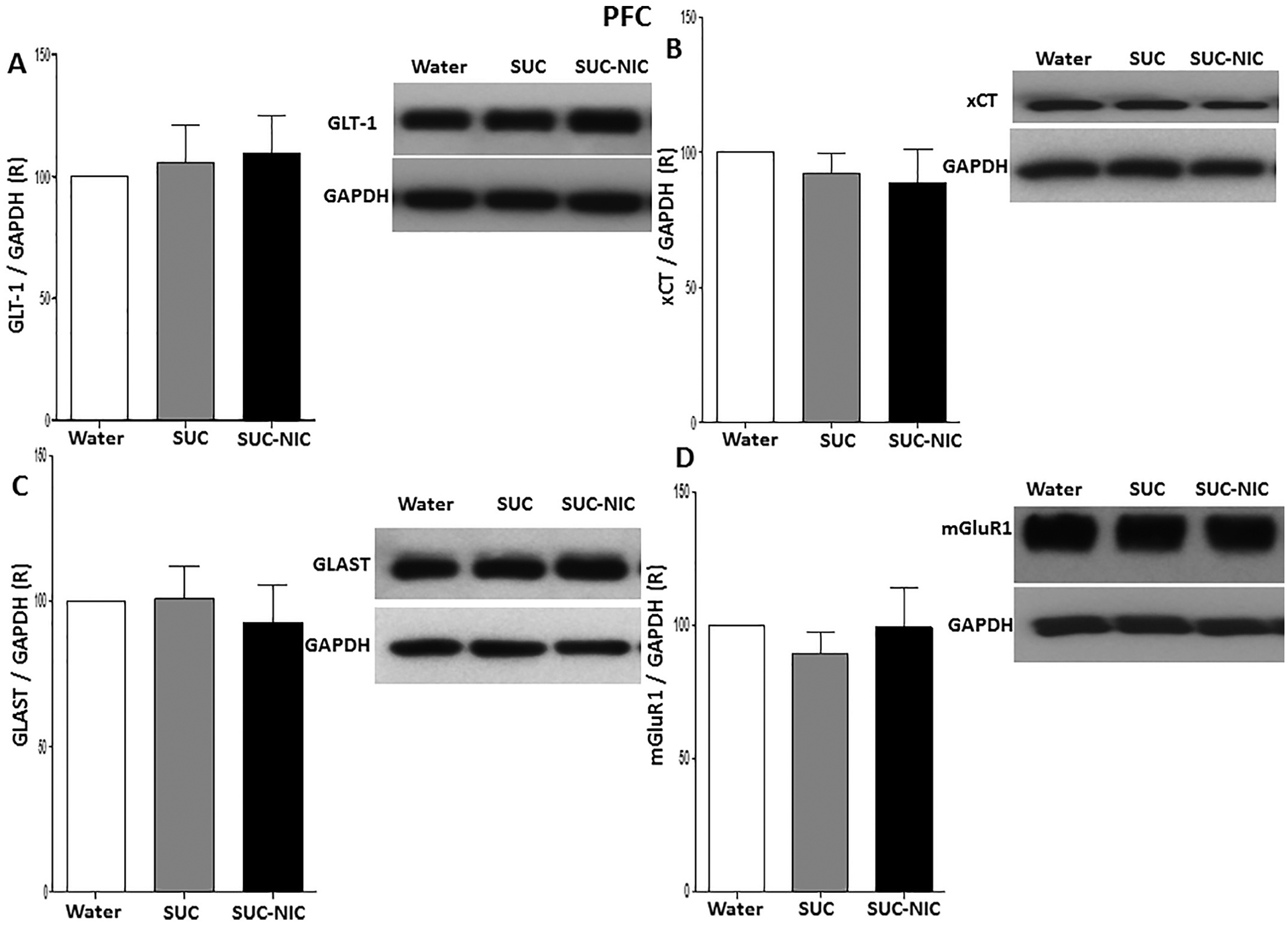
Effects of binge-like drinking of SUC and SUC-NIC on relative expression (R) of (A) GLT-1, (B) xCT, (C) GLAST, and (D) mGluR1 in the PFC. One way ANOVA followed by Newman-Keuls analysis did not show any significant reduction in GLT-1/GAPDH, xCT/GAPDH GLAST/GAPDH and mGluR1/GAPDH ratios between all groups in the PFC. Data are represented as mean ± SEM (n = 6 for each group).
3.7. Effects of binge-like drinking of EtOH, or EtOH-NIC on GLT-1, xCT, GLAST and mGluR1 expression in the PFC
We further determined the expression of GLT-1, xCT, GLAST and mGluR1 between water control, EtOH and EtOH–NIC groups in all studied brain regions. One-way ANOVA did not demonstrate any significant differences in GLT-1 expression [F (2, 15) = 0.41, p = NS, (Fig. 8A)], xCT expression [F (2, 15) = 0.35, p = NS, (Fig. 8B)], GLAST expression [F (2, 15) = 0.15, p = NS, (Fig. 8C)] and mGluR1 expression [F (2, 15) = 0.26, p = NS, (Fig. 8D)] among these groups in the PFC.
Fig. 8.
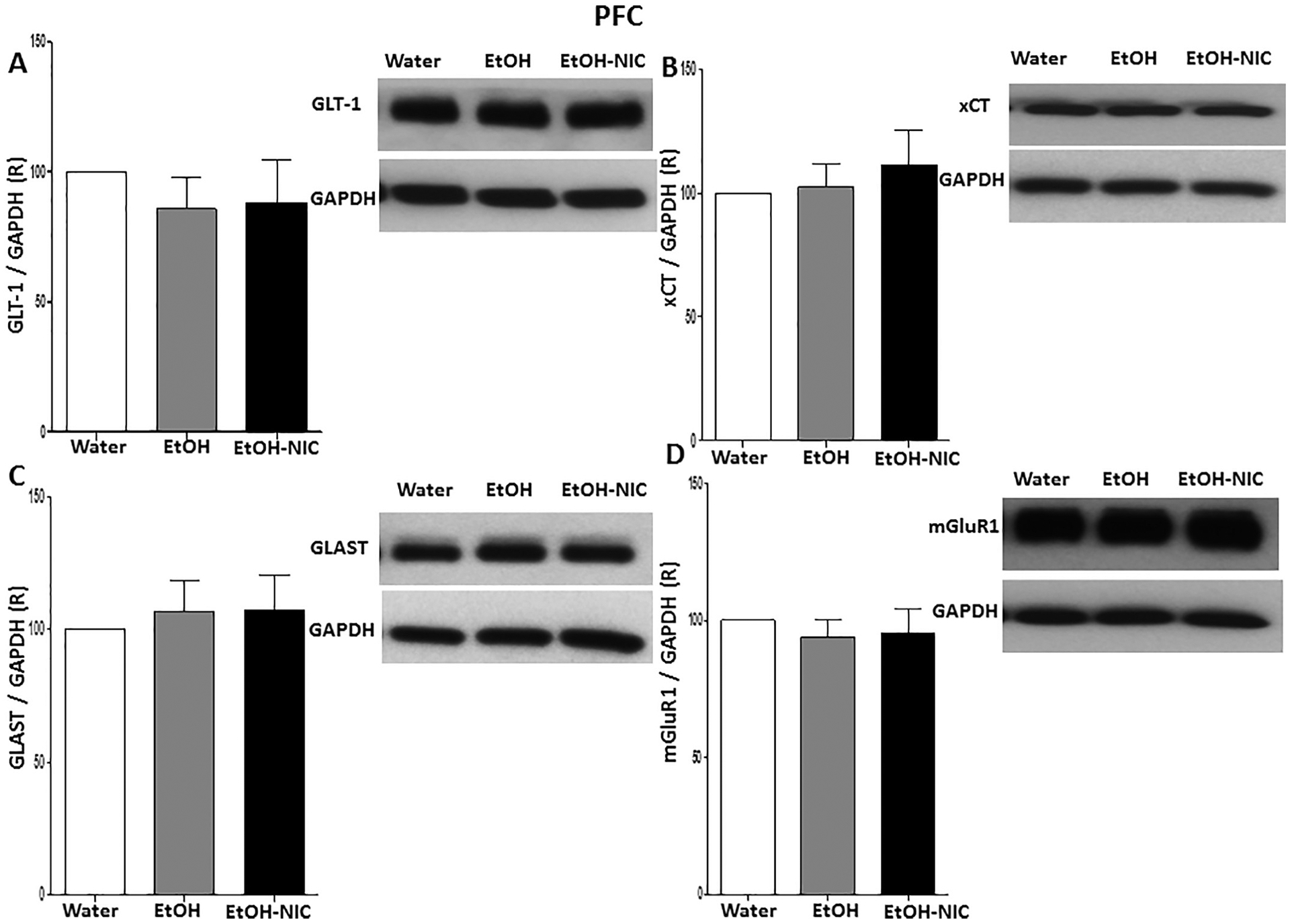
Effects of binge-like drinking of EtOH and EtOH-NIC on relative expression (R) of (A) GLT-1, (B) xCT, (C) GLAST, and (D) mGluR1 in the PFC. One way ANOVA followed by Newman-Keuls analysis did not show any significant reduction in GLT-1/GAPDH, xCT/GAPDH GLAST/GAPDH and mGluR1/GAPDH ratios between all groups in the PFC. Data are represented as mean ± SEM (n = 6 for each group).
3.8. Effects of SUC, SUC-NIC, EtOH, or EtOH-NIC drinking on GPx activity in the HIP
One-way ANOVA followed by Newman-Keuls analysis indicated a significant reduction in GPx activity in the HIP of SUC and SUC-NIC groups as compared to the water control group [F (2,15) = 5.06, p = 0.021, (Fig. 9A)]. In addition, one-way ANOVA followed by Newman-Keuls multiple comparisons showed that % of GPx activity was also significantly reduced in EtOH and EtOH-NIC groups compared to the water control group in the HIP [F (2,15) = 4.19, p = 0.035, (Fig. 9B)].
Fig. 9.
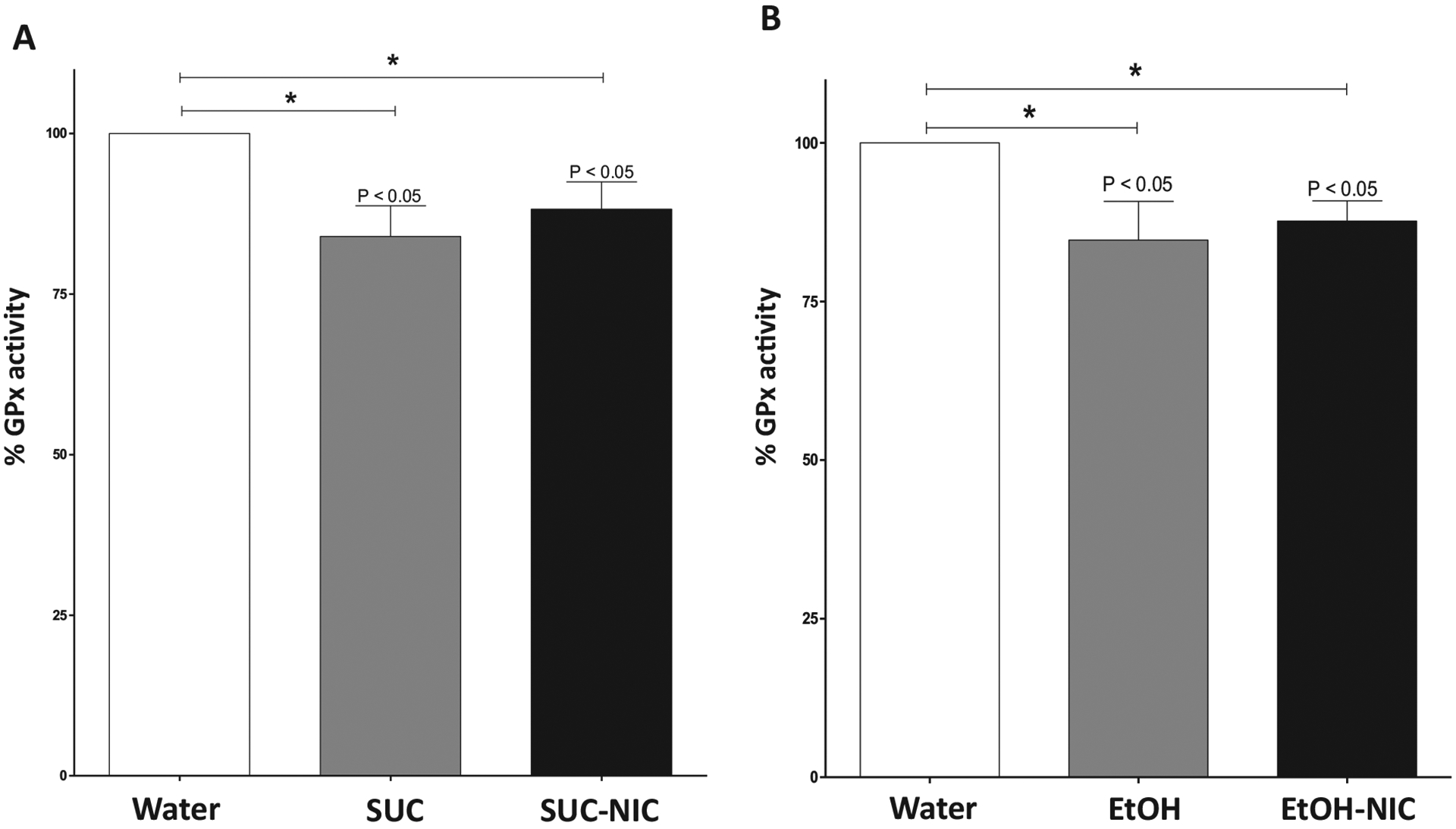
Effects of binge-like drinking of SUC, SUC-NIC EtOH, or EtOH-NIC on the activity of GPx in the HIP. A) One way ANOVA followed by Newman-Keuls analysis revealed that SUC and SUC-NIC exposure reduced GPx activity the HIP. B) One way ANOVA followed by Newman-Keuls analysis revealed that EtOH and EtOH-NIC exposure reduced GPx activity in the HIP. Data are represented as mean ± SEM (n = 6 for each group), (* p < 0.05).
4. Discussion
The present study examined the effects of binge-like drinking of EtOH with or without NIC on astroglial glutamate transporters and mGluR1 expression in three brain regions of female P rats. In addition, the study investigated the effects of SUC on GLT-1, xCT, GLAST and mGluR1 expression to determine whether there is an interaction between SUC and NIC on the glutamatergic transporters and receptor (i.e., SUC as the positive-control). The findings indicate that binge-like SUC-NIC drinking decreased GLT-1 and increased mGluR1 expression in the NAc, while xCT expression was decreased in both the NAc and the HIP compared to the water and SUC control groups. In addition, binge-like EtOH drinking did not affect astroglial glutamate transporter expression in the brain regions examined but did increase mGluR1 expression in the NAc compared to the water control group. Moreover, binge-like EtOH-NIC drinking reduced GLT-1 in the NAc and xCT in both the NAc and HIP, while increasing mGluR1 expression in the NAc compared to the water control group.
In the present study, the addition of NIC in the SUC solution resulted in a significant reduction in SUC consumption. However, EtOH intake was not significantly changed by the addition of NIC, although it did decrease slightly. This indicates that the bitter taste of NIC may reduce SUC consumption but not necessarily EtOH intake. It important to note that the present study used a binge-like drinking procedure in peri-adolescent P rats, whereas previous work has generally been done in adult rats given continuous access to EtOH and/or NIC. In addition, the present findings indicate that NIC intake was significantly higher in the group given binge-like scheduled access to SUC-NIC compared to those given access to EtOH-NIC. This effect was probably due to the significantly higher consumption of SUC relative to EtOH (Fig. 2). Previous work has revealed conflicting results for the effects of NIC exposure on SUC and EtOH consumption (Bito-Onon et al., 2011; Grimm et al., 2012; Sari et al., 2016). One reason for these inconsistent findings may be the route of NIC administration. For instance, oral NIC self-administration reduced SUC and EtOH intake (Sari et al., 2016) in one study, while others have reported that EtOH and SUC consumption are increased following i.p. injections of NIC (Bito-Onon et al., 2011; Grimm et al., 2012).
A prior study from our group reported that upregulating GLT-1 in the NAc and the PFC by ceftriaxone treatment led to an attenuation of EtOH and/or NIC drinking behavior in female P rats (Sari et al., 2016). This indicates that astroglial glutamate transporters, mainly GLT-1, plays a critical role in regulating EtOH or NIC seeking [for review see (Alasmari et al., 2016a, Goodwani et al., 2017)]. In the present work, for the first time, we found that peri-adolescent binge-like scheduled access to SUC-NIC downregulated GLT-1 in the NAc compared to water and SUC control groups, while peri-adolescent EtOH-NIC binge-like drinking reduced GLT-1 expression in the NAc compared to the water control and EtOH groups. However, neither SUC nor EtOH binge-like scheduled drinking altered the expression of GLT-1 in the NAc. Previously, continuous exposure to EtOH for five weeks and phasic exposure to NIC for 21 days reduced GLT-1 expression in the NAc of adult rats (Knackstedt et al., 2009; Alhaddad et al., 2014b; Goodwani et al., 2015). However, others have shown that chronic limited access to EtOH did not induce any changes in GLT-1 or xCT expression in adult animals (Griffin et al., 2015; Pati et al., 2016; Stennett et al., 2017). Together these findings indicate that continuous (i.e., not-scheduled) exposure to EtOH reduces the expression of GLT-1 in the NAc. Our data also suggest that multiple scheduled NIC self-administrations are able to decrease the expression of GLT-1 in the NAc. Although continuous five-week EtOH consumption reduced GLT-1 expression in the HIP (Aal-Aaboda et al., 2015), we did not observe any changes in GLT-1 expression in the HIP following binge-like drinking of EtOH with or without NIC using a limited access procedure. A previous study reported that phasic exposure to electronic cigarettes (i.e., vapors-containing NIC) for six months did not reduce GLT-1 in the HIP (Alasmari et al., 2017). Our present data are in agreement with previous findings using adult rats wherein EtOH or NIC exposure did not cause any changes in GLT-1 expression in the PFC (Knackstedt et al., 2009; Goodwani et al., 2015). In addition, a recent study found that blocking GLT-1 in the NAc did not attenuate reinstatement of SUC seeking (Bobadilla et al., 2017). It is noteworthy that the present study provided some corroboration for the latter finding, such that chronic exposure to SUC did not affect GLT-1 expression in the NAc, HIP, or PFC.
Several studies, using adult rats, have reported that chronic exposure to EtOH or NIC led to a reduction in xCT expression in specific brain regions, including the NAc, HIP, and ventral tegmental area (Knackstedt et al., 2009; Alhaddad et al., 2014b; Aal-Aaboda et al., 2015). In this study, for the first time, we investigated the effects of peri-adolescent binge-like drinking of EtOH, SUC, SUC-NIC, or EtOH-NIC on xCT expression in the NAc, PFC, and HIP. We found that SUC-NIC or EtOH-NIC intake induced a significant downregulation of xCT expression in the NAc and HIP. This effect was not observed in the group exposed to EtOH drinking. Previous work showed that xCT expression was reduced after scheduled phasic exposure to NIC but not EtOH in the NAc, dorsal striatum, and HIP (Knackstedt et al., 2009; Griffin III et al., 2014; Alasmari et al., 2017; Stennett et al., 2017). Additionally, previously reported studies and our data have not demonstrated any significant reductions in the expression of xCT in the PFC following chronic exposure to NIC (Knackstedt et al., 2009; Alasmari et al., 2017). The present data indicated that scheduled binge-intake of EtOH did not affect the expression of xCT in the PFC, although continuous consumption of EtOH has been shown to reduce xCT expression in the PFC (Alhaddad et al., 2014a). Thus, adult and peri-adolescent EtOH, with or without NIC, binge-like exposure may have differential effects, relative to continuous exposure, on the expression of astroglial glutamate transporters.
In this study, we did not observe any changes in GLAST expression following binge-like scheduled drinking of EtOH, SUC-NIC, or EtOH-NIC for four weeks. Although GLAST is localized throughout the brain, GLAST is highly expressed in the cerebellum rather than the forebrain (Lehre and Danbolt, 1998; Danbolt, 2001). Moreover, GLAST was found highly expressed in the retina (Lehre and Danbolt, 1998). Continuous EtOH drinking for five weeks or chronic NIC inhalation did not alter the expression of GLAST in the NAc, dorsal striatum, or PFC (Hakami et al., 2016; Alasmari et al., 2017).
The expression of mGluR1 was also determined in the NAc, HIP, and PFC following peri-adolescent multiple scheduled binge-like intakes of EtOH with or without NIC. We report that limited access to EtOH and/or NIC upregulated mGluR1 expression in the NAc. This is in agreement with a previous study that revealed that exposure to EtOH for six months increased mGluR1 expression in the NAc core of P rats (Obara et al., 2009). This suggests that mGluR1 activity in the NAc of P rats is affected by EtOH and/or NIC consumption. In addition, mGluR1 expression in the amygdala was found to be increased in animals exposed to binge-like drinking of EtOH (Cozzoli et al., 2014). Other reports have indicated that selectively bred High Drinking-in-the-Dark mice showed a significant increase in mGluR1 expression of the NAc (Cozzoli et al., 2009; Cozzoli et al., 2012). However, less is known about the effects of EtOH and NIC co-exposure on the expression of mGluR1 in central reward brain areas. The present study indicated that co-exposure of EtOH and NIC increased mGlurR1 expression in the NAc. Studies have found that phasic exposure to EtOH or NIC resulted in a marked increase in the total extracellular concentrations of glutamate, which might indicate increased firing of medium spiny neurons (Griffin III et al., 2014; Griffin et al., 2015; Ryu et al., 2017). We suggest that this effect might increase the expression of post-synaptic glutamate receptors such as mGluR1 as a compensatory mechanism. However, we did not observe any changes in the expression of mGluR1 in the HIP or PFC regions. Since little is known about the effects of intermittent exposure to EtOH or NIC on the mGluR1 expression and synaptic glutamate concentrations in the HIP and PFC, further studies are required to delineate the relationship between extracellular glutamate concentrations and the expression of post-synaptic glutamate receptors, including mGluR1 in these brain regions.
Several drugs of abuse, including EtOH, are well-known inducers of oxidative stress in the HIP (Pant et al., 2017). Since the activity of GPx has a direct impact on the ability of cells to defend against oxidative stress, recent studies have focused on examining the impact of drug abuse on GPx activity (Biala et al., 2017; Gong et al., 2017). Exposure to water pipe smoke, for instance, was found to reduce the activity of GPx in the HIP (Alzoubi et al., 2015). This study also found a significant impairment in the memory and learning of these male Wistar rats. Intuitively, an increase in GPx activity would be associated with protective effects against oxidative stress in animals (Miyamoto et al., 2003). A prior study reported that ceftriaxone, a GLT-1 and xCT upregulator, was able to normalize glutathione content (Amin et al., 2014) suggesting a strong association between glutathione system and astroglial glutamate transporter activity. In this study, we found that SUC-NIC and EtOH-NIC drinking decreased both xCT and GPx activity in the HIP compared to the water control group suggesting a possible correlation between reduction of xCT expression and attenuation of GPx activity in NIC treated rats. Interestingly, exposure to SUC or EtOH reduced GPx activity but had no effect on xCT expression compared to the water control group. This indicates that SUC and EtOH might affect the activity of GPx through other mechanisms, including the generation of free radicals (Oh et al., 1998; Rosas-Villegas et al., 2017). Thus, our data do not provide conclusive evidence suggesting a direct link between expression of xCT and the activity of GPx in the HIP following exposure to NIC and/or EtOH.
In summary, our work indicates that peri-adolescent binge-like co-access to EtOH and NIC induces significant changes in the expression of astroglial glutamate transporters and mGluR1 in the NAc and the HIP. Future studies are needed to investigate the effects of these drugs of abuse on the glutamatergic system in NAc subregions such as NAc shell and core. These data further confirmed previous findings observed in studies using adult animals. In the future, the differential and/or similar effects of these drugs of abuse on glutamatergic transporters and receptors in adolescent vs adult mesocorticolimbic brain regions are warranted further investigation. It is important to note that adolescents and young adults have high rates of EtOH-NIC co-abuse, which highlights the need for more knowledge on the long-term biological and physiological effects of these drugs. In addition, further studies are needed to investigate the effects of EtOH and/or NIC on oxidative stress parameters in these mesocorticolimbic nuclei.
Acknowledgements
The presented research was supported in part by AA019458 (Y. Sari) and AA13522 (R.L. Bell) from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Aal-Aaboda M, Alhaddad H, Osowik F, Nauli SM, Sari Y, 2015. Effects of (R)-(−)-5-methyl-1-nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. J. Neurosci. Res 93, 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Abuhamdah S, Sari Y, 2015. Effects of ampicillin on cystine/glutamate antiporter and glutamate transporter 1 isoforms as well as ethanol drinking in male P rats. Neurosci. Lett 600, 148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Al-Rejaie SS, AlSharari SD, Sari Y, 2016a. Targeting glutamate homeostasis for potential treatment of nicotine dependence. Brain Res. Bull 121, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Rao PS, Sari Y, 2016b. Effects of cefazolin and cefoperazone on glutamate transporter 1 isoforms and cystine/glutamate exchanger as well as alcohol drinking behavior in male alcohol-preferring rats. Brain Res. 1634, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Alexander LEC, Nelson JA, Schiefer IT, Breen E, Drummond CA, Sari Y, 2017. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α−7 nicotinic acetylcholine receptor in female CD-1 mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 77, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht P, Lewerenz J, Dittmer S, Noack R, Maher P, Methner A, 2010. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc as a neuroprotective drug target. CNS Neurol. Disord. Drug Targets 9, 373–382. [DOI] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y, 2014a. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology 231, 4049–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, Wei Y, Sari Y, 2014b. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front. Behav. Neurosci 8, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri FS, Althobaiti YS, Sari Y, 2017. Effects of administered ethanol and methamphetamine on glial glutamate transporters in rat striatum and hippocampus. J. Mol. Neurosci 61, 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzoubi KH, Khabour OF, Alharahshah EA, Alhashimi FH, Shihadeh A, Eissenberg T, 2015. The effect of waterpipe tobacco smoke exposure on learning and memory functions in the rat model. J. Mol. Neurosci 57, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin B, Hajhashemi V, Abnous K, Hosseinzadeh H, 2014. Ceftriaxone, a beta-lactam antibiotic, modulates apoptosis pathways and oxidative stress in a rat model of neuropathic pain. Biomed. Res. Int 2014, 937568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW, 2002. The origin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci 22, 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancila V, Cordeiro JM, Bloc A, Dunant Y, 2009. Nicotine-induced and depolarisation-induced glutamate release from hippocampus mossy fibre synaptosomes: two distinct mechanisms. J. Neurochem 110, 570–580. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, McBride WJ, 2011. Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol. Biochem. Behav 100, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Engleman EA, Toalston JE, McBride WJ, 2014. Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: modeling adolescent and adult binge-like drinking. Alcohol 48, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Hauser SR, McClintick J, Rahman S, Edenberg HJ, Szumlinski KK, McBride WJ, 2016. Ethanol-associated changes in glutamate reward neurocircuitry: a minireview of clinical and preclinical genetic findings In: Progress in Molecular Biology and Translational Science. 137 Elsevier, pp. 41–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Hauser SR, Liang T, Sari Y, Maldonado-Devincci A, Rodd ZA, 2017. Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology 122, 201–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, 1984. Daily intake of nicotine during cigarette smoking. Clin. Pharmacol. Ther 35, 499–504. [DOI] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin J, Hodge C, 2008. Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol 42, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Pekala K, Boguszewska-Czubara A, Michalak A, Kruk-Slomka M, Budzynska B, 2017. Behavioral and biochemical interaction between nicotine and chronic unpredictable mild stress in mice. Mol. Neurobiol 54, 904–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Schuckit MA, Hesselbrock V, Reich T, 2000. Co-occurring risk factors for alcohol dependence and habitual smoking. Alcohol Res. Health 24, 233–241. [PMC free article] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE, 2011. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict. Biol 16, 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobadilla AC, Garcia-Keller C, Heinsbroek JA, Scofield MD, Chareunsouk V, Monforton C, Kalivas PW, 2017. Accumbens mechanisms for cued sucrose seeking. Neuropsychopharmacology 42, 2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo JK, Husten C, 2000. Sociocultural influences on smoking and drinking. Alcohol Res. Health 24, 225–232. [PMC free article] [PubMed] [Google Scholar]
- Bridges R, Lutgen V, Lobner D, Baker DA, 2012. Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacol. Rev 64, 780–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu J-H, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, 2009. Binge drinking upregulates accumbens mGluR5–Homer2–PI3K signaling: functional implications for alcoholism. J. Neurosci 29, 8655–8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, Wroten MG, Zhang PW, Xiao B, Hu JH, 2012. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol. Clin. Exp. Res 36, 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Maliniak D, Worley PF, Jonquieres G, 2014. Binge alcohol drinking by mice requires intact group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdale. Neuropsychopharmacology 39, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC, 2001. Glutamate uptake. Prog. Neurobiol 65, 1–105. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Hauser SR, Waeiss RA, Knight CP, Toalston JE, Truitt WA, … Rodd ZA, 2015. Co-administration of ethanol and nicotine: the enduring alterations in the rewarding properties of nicotine and glutamate activity within the mesocorticolimbic system of female alcohol-preferring (P) rats. Psychopharmacology 232 (23), 4293–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deponte M, 2013. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta Gen. Subj 1830, 3217–3266. [DOI] [PubMed] [Google Scholar]
- Devoto VP, Bogetti ME, de Plazas SF, 2013. Developmental and hypoxia-induced cell death share common ultrastructural and biochemical apoptotic features in the central nervous system. Neuroscience 252, 190–200. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S, 2000. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology 151, 392–405. [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY, 2007. mGlu1 receptor blockade attenuates cue-and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology 52, 263–269. [DOI] [PubMed] [Google Scholar]
- Esser MB, 2017. Current and binge drinking among high school students—United States, 1991–2015. MMWR Morb. Mortal. Wkly Rep 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Yi H, Hiller-Sturmhofel S, 2006. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders. Alcohol Res. Health 29, 162–171. [PMC free article] [PubMed] [Google Scholar]
- Falk D, Yi HY, Hiller-Sturmhofel S, 2008. An epidemiologic analysis of co-occurring alcohol and drug use and disorders: findings from the National Epidemiologic Survey of Alcohol and Related Conditions (NESARC). Alcohol Res. Health 31, 100–110. [PMC free article] [PubMed] [Google Scholar]
- Feduccia AA, Chatterjee S, Bartlett SE, 2012. Neuronal nicotinic acetylcholine receptors: neuroplastic changes underlying alcohol and nicotine addictions. Front. Mol. Neurosci 5, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores RJ, Pipkin JA, Uribe KP, Perez A, O’Dell LE, 2016. Estradiol promotes the rewarding effects of nicotine in female rats. Behav. Brain Res 307, 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA, 2001. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J. Neurosci 21, 4915–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydenberg H, Flote VG, Larsson IM, Barrett ES, Furberg AS, Ursin G, Wilsgaard T, Ellison PT, McTiernan A, Hjartaker A, Jasienska G, Thune I, 2015. Alcohol consumption, endogenous estrogen and mammographic density among premenopausal women. Breast Cancer Res. 17, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y-S, Hu K, Yang L-Q, Guo J, Gao Y-Q, Song F-L, Hou F-L, Liang C-Y, 2017. Comparative effects of EtOH consumption and thiamine deficiency on cognitive impairment, oxidative damage, and β-amyloid peptide overproduction in the brain. Free Radic. Biol. Med 108, 163–173. [DOI] [PubMed] [Google Scholar]
- Goodwani S, Rao PS, Bell RL, Sari Y, 2015. Amoxicillin and amoxicillin/clavulanate reduce ethanol intake and increase GLT-1 expression as well as AKT phosphorylation in mesocorticolimbic regions. Brain Res. 1622, 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwani S, Saternos H, Alasmari F, Sari Y, 2017. Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder. Neurosci. Biobehav. Rev 77, 14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA, 2004. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and relatedconditions. Arch. Gen. Psychiatry 61, 1107–1115. [DOI] [PubMed] [Google Scholar]
- Griffin WC III, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC, 2014. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 39, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Ramachandra VS, Knackstedt LA, Becker HC, 2015. Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Front. Pharmacol 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I, 1976. Facilitation of human tobacco self-administration by ethanol: a behavioral analysis. J. Exp. Anal. Behav 25, 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Ratliff C, North K, Barnes J, Collins S, 2012. Nicotine increases sucrose self-administration and seeking in rats. Addict. Biol 17, 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubner NR, Thrul J, Kelly OA, Ramo DE, 2017. Young adults report increased pleasure from smoking cigarettes when drinking alcohol but not when using marijuana. Addict. Res. Theory 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami AY, Hammad AM, Sari Y, 2016. Effects of amoxicillin and augmentin on cystine-glutamate exchanger and glutamate transporter 1 isoforms as well as ethanol intake in alcohol-preferring rats. Front. Neurosci 10, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad AM, Alasmari F, Althobaiti YS, Sari Y, 2017. Modulatory effects of ampicillin/sulbactam on glial glutamate transporters and metabotropic glutamate receptor 1 as well as reinstatement to cocaine-seeking behavior. Behav. Brain Res 332, 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Katner SN, Deehan GA, Ding ZM, Toalston JE, Scott BJ, Bell RL, McBride WJ, Rodd ZA, 2012. Development of an oral operant nicotine/ethanol co-use model in alcohol-preferring (P) rats. Alcohol. Clin. Exp. Res 36, 1963–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND, 2005. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatr 162, 1403–1413. [DOI] [PubMed] [Google Scholar]
- Kane J, Hwang Y, Konu O, Loughlin S, Leslie F, Li M, 2005. Regulation of Homer and group I metabotropic glutamate receptors by nicotine. Eur. J. Neurosci 21, 1145–1154. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW, 2009. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol. Psychiatry 65, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand F, Ward RJ, De Witte P, 2009. The influence of chronic nicotine administration on behavioural and neurochemical parameters in male and female rats after repeated binge drinking exposure. Alcohol Alcohol. 44, 535–546. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW, 2008. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J. Neurosci 28, 3170–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK, 2003. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology 28, 216–225. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC, 1998. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J. Neurosci 18, 8751–8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Olinger A, Dassow M, Abel M, 2003. Up-regulation of GABA B receptor mRNA and protein in the hippocampus of cocaine-and lidocaine-kindled rats. Neuroscience 118, 451–462. [DOI] [PubMed] [Google Scholar]
- Lum EN, Campbell RR, Rostock C, Szumlinski KK, 2014. mGluR1 within the nucleus accumbens regulates alcohol intake in mice under limited-access conditions. Neuropharmacology 79, 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R, Patrick ME, O’malley PM, Johnston LD, 2017. What are kids vaping? Results from a national survey of US adolescents. Tob. Control 26, 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV, 2008. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience 153, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Koh YH, Park YS, Fujiwara N, Sakiyama H, Misonou Y, Ookawara T, Suzuki K, Honke K, Taniguchi N, 2003. Oxidative stress caused by inactivation of glutathione peroxidase and adaptive responses. Biol. Chem 384, 567–574. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li T-K, 2002. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav. Genet 32, 363–388. [DOI] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK, 2009. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol. Clin. Exp. Res 33, 1924–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SI, Kim C-I, Chun HJ, Park SC, 1998. Chronic ethanol consumption affects glutathione status in rat liver. J. Nutr 128, 758–763. [DOI] [PubMed] [Google Scholar]
- Pant R, Jangra A, Kwatra M, Singh T, Kushwah P, Bezbaruah BK, Gurjar SS, Phukan S, 2017. Cognitive deficits induced by combined exposure of stress and alcohol mediated through oxidative stress-PARP pathway in the hippocampus. Neurosci. Lett 653, 208–214. [DOI] [PubMed] [Google Scholar]
- Pati D, Kelly K, Stennett B, Frazier CJ, Knackstedt LA, 2016. Alcohol consumption increases basal extracellular glutamate in the nucleus accumbens core of Sprague–Dawley rats without increasing spontaneous glutamate release. Eur. J. Neurosci 44, 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2007. The Rat Brain in Stereotaxic Coordinates in Stereotaxic Coordinates. Elsevier. [DOI] [PubMed] [Google Scholar]
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD, 2015. Progression to traditional cigarette smoking after electronic cigarette use among US adolescents and young adults. JAMA Pediatr. 169, 1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Pérez-Pinzón MA, 2003. εPKC is required for the induction of tolerance by ischemic and NMDA-mediated pre-conditioning in the organotypic hippocampal slice. J. Neurosci 23, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Villegas A, Sanchez-Tapia M, Avila-Nava A, Ramirez V, Tovar AR, Torres N, 2017. Differential effect of sucrose and fructose in combination with a high fat diet on intestinal microbiota and kidney oxidative stress. Nutrients 9, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu IS, Kim J, Seo SY, Yang JH, Oh JH, Lee DK, Cho H-W, Yoon SS, Seo J-W, Chang S, 2017. Behavioral changes after nicotine challenge are associated with α7 nicotinic acetylcholine receptor-stimulated glutamate release in the rat dorsal striatum. Sci. Rep 7, 15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saellstroem Baum S, Huebner A, Krimphove M, Morgenstern R, Badawy AA, Spies CD, 2006. Nicotine stimulation on extracellular glutamate levels in the nucleus accumbens of ethanol-withdrawn rats in vivo. Alcohol. Clin. Exp. Res 30, 1414–1421. [DOI] [PubMed] [Google Scholar]
- Sari Y, Toalston JE, Rao PS, Bell RL, 2016. Effects of ceftriaxone on ethanol, nicotine or sucrose intake by alcohol-preferring (P) rats and its association with GLT-1 expression. Neuroscience 326, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH, 2003. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci 23, 3394–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH, 2006. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J. Neurosci 26, 10514–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennett BA, Frankowski JC, Peris J, Knackstedt LA, 2017. Ceftriaxone reduces alcohol intake in outbred rats while upregulating xCT in the nucleus accumbens core. Pharmacol. Biochem. Behav 159, 18–23. [DOI] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O’dell LE, 2014. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcohol. Clin. Exp. Res 38, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mamudu HM, Collins C, Wang YF, 2017. High prevalence of tobacco use and exposure to secondhand tobacco smoke among adolescents in low- and middle-income countries. Ann. Transl. Med 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi B, Liang Y, Liu Y, Yan Y, Zhao M, Ma C, Bovet P, 2016. Tobacco use and second-hand smoke exposure in young adolescents aged 12–15 years: data from 68 low-income and middle-income countries. Lancet Glob. Health 4, e795–e805. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Tan Y, 2011. Nerve growth factor augments neuronal responsiveness to noradrenaline in cultured dorsal root ganglion neurons of rats. Neuroscience 193, 72–79. [DOI] [PubMed] [Google Scholar]


