ABSTRACT
Chromatin accessibility is generally perceived as a common property of active regulatory elements where transcription factors are recruited via DNA-specific interactions and other physico-chemical properties to regulate gene transcription. Recent work in the context of mitosis provides less trivial and potentially more interesting relationships than previously anticipated.
KEWORDS: Transcription factor, chromatin accessibility, mitosis, mitotic bookmarking, epigenetics
Introduction
Transcription factors (TFs) play an essential role in gene regulation, they modulate transcriptional activity by binding specific regions of DNA [1]. The capacity of TFs to bind DNA depends on functional interactions with nucleosomes, which are the structural unit of chromatin and can inhibit TF binding by occluding their cognate motifs [2]. These interactions are often mediated by chromatin remodelers, which assist TFs by displacing or evicting nucleosomes [3]. As a consequence, TF binding sites are associated with chromatin regions that can be experimentally identified by their accessibility to enzymes that will digest or cut exposed DNA (for example, DNAseI, MNase, restriction enzymes, Tn5 transposase, for review see [4]). This property has recently become the focus of a large number of deep sequencing studies that aim to establish the repertoires of active gene regulatory elements. However, does chromatin accessibility necessarily imply that TFs are actively engaged at a certain region? Conversely, and putting aside TFs with pioneering activity, does TF binding imply chromatin accessibility as measured by current methodologies? More conceptually, does chromatin accessibility in and of itself represent a mechanism of gene regulatory inheritance, and if so, how? In this point-of-view we will briefly address these questions from the perspective of studies performed in mitotic cells, when chromatin undergoes major rearrangements that are associated with a loss of TF activity [5,6]. Studying chromatin accessibility, nucleosome positioning and TF behaviors in the context of mitosis therefore provides a unique perspective on how TFs interact with the chromatin. These studies call for cautious interpretation of chromatin accessibility results while providing rich hypotheses to its function in transcriptional control and its potential role in conveying gene regulatory information through mitosis.
Mitosis – does chromosome condensation necessarily imply loss of accessibility?
The equal distribution of replicated DNA between the two daughter cells is achieved by mitosis, the process during which the chromatin condenses into macroscopic filaments (from Greek “mitos”, thread); first placed on the metaphasic plate, then broken into two identical chromatids that are pulled to the opposite poles of the dividing cell. Given its macroscopic, condensed nature, it has been thought for decades that mitotic chromosomes would behave as inert objects, refractory to most – if not all – DNA transactions [7]. Indeed, early reports showed that TFs are evicted and transcription globally silenced [5,8]. Nevertheless, pioneering work in the 90’s identified that the Hsp70 promoter remains accessible to in vivo chemical footprinting in mitotically-arrested cells [9]. Yet, it was reported that the TFs binding this promoter in interphase are evicted during mitosis [5]. Twenty-five years later this paradoxical observation has been largely generalized: in several models, including mouse Embryonic Stem (ES) cells (Figure 1), drosophila embryos and multiple human cell lines, genome-wide assessment of chromatin accessibility using DNase-seq or ATAC-seq has shown that the vast majority of active promoters remain accessible in mitotic cells [10–14]. In contrast, enhancers display more variable results, both within and between cell lines [10,14]. One important conclusion can be drawn from these observations: mitotic chromosomes preserve significant, although variable levels of chromatin accessibility. Since many TFs are evicted from mitotic DNA, it is unlikely that all regions accessible in mitosis are occupied by subsets of the TFs that normally target them in interphase. This suggests that, at least during mitosis, chromatin accessibility is not necessarily an immediate proxy for TF binding.
Figure 1.
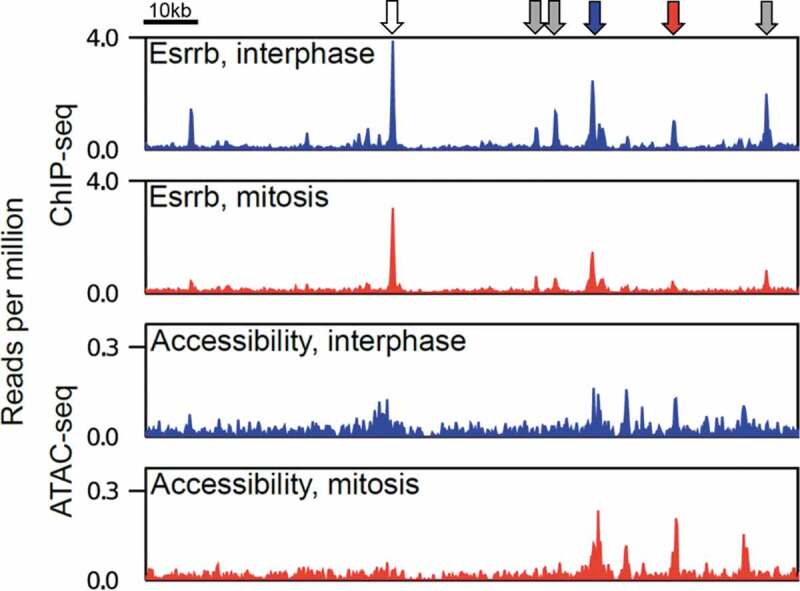
Chromatin accessibility and binding of the TF Esrrb in interphase and mitosis. Genome snapshot of Esrrb binding highlighting non-trivial relationships with chromatin accessibility in interphase and mitosis. The arrows illustrate binding of Esrrb in the absence of detectable chromatin accessibility (gray), binding of Esrrb in interphase and mitosis (blue) or only in interphase (red) with persistent chromatin accessibility in mitosis, and binding of Esrrb in interphase and mitosis with a mitotic loss of accessibility (white)
Mitotic bookmarking TFs, chromatin accessibility and nucleosome positioning
The effective binding of a TF to its specific binding sites during mitosis, a process known as mitotic bookmarking, was thought to locally prevent the collapse of chromatin accessibility such that the bookmarked regions would me more effectively targeted by functional regulatory complexes in the daughter cells [15]. While this may well be the case for active promoters, which retain binding of the TATA-binding protein and possibly other General TFs during mitosis [16–20], the status of more complex regulatory elements, such as enhancers, appears less simple. For example, the level of chromatin accessibility between regions mitotically bound by the pluripotency-associated mitotic bookmarking TF, Esrrb, and sites bound exclusively in interphase, is relatively similar: regions retaining Esrrb binding are not significantly more accessible during mitosis than those losing binding [14] (Figure 1). Nevertheless, Esrrb-responding genes in early interphase are statistically enriched in the vicinity of Esrrb-bookmarked enhancers [21]. How then does Esrrb mitotic retention provide a regulatory advantage at its targets over regions it only binds in interphase? The elucidation of this required going beyond measurement of accessibility to the assessment of the precise organization of the nucleosomes at Esrrb bound regions. Several nucleosomal array configurations can theoretically allow equivalent access to the enzymes used to establish accessibility maps; but statistically most are not compatible with precise, DNA-sequence-specific recognition of a TF. It was observed that regions that are bound by Esrrb both in interphase and mitosis maintain ordered nucleosomal arrays centered on a nucleosome-depleted region at the exact position of Esrrb binding motifs [14]. In contrast, nucleosomes are repositioned in mitosis at regions losing Esrrb binding. These observations were consolidated by functional assays focusing on another mitotic bookmarking factor in ES cells, CTCF: upon CTCF depletion, the nucleosomes at CTCF binding sites are readily disorganized, in interphase and in mitosis [22]. More generally, while enhancers appeared, on average, to preserve very high levels of chromatin accessibility in mitotic ES cells, presumably more than in other cell types analyzed so far, large repositioning of nucleosomes was observed. Hence, whereas chromatin accessibility does not appear to drastically change during mitosis, particularly in ES cells, a primary consequence of the relatively general loss of TF binding seems to be loss of nucleosome order/positioning.
An epigenetic mechanism preserving chromatin accessibility?
If chromatin accessibility is maintained at some sites in mitosis by mechanisms that seem different from those required to establish and continuously maintain it in interphase, what can we learn? This is a far-reaching question, potentially calling into play epigenetic mechanisms that render the maintenance of gene regulation independent from the initial instructors required for its initiation – in the same way X–inactivation is maintained across cellular generations independently of its trigger, the Xist lncRNA [23]. If a TF establishes a given region as accessible during interphase, and is required to maintain the region accessible, but chromatin accessibility remains when the TF is evicted during mitosis, what does this mean? Can this qualify as an epigenetic process? At first glance this could well be the case and specific experimental strategies are required to test this possibility and unravel the underlying mechanisms. Briefly, two main options appear possible. First, local chromatin accessibility remains detectable because, in absence of de novo loading of nucleosomes the passive movements of those that preexist are simply not sufficient to abrogate the capacity of the enzymes used to measure accessibility to access and cleave DNA. Such nucleosome diffusion in 1D would imply a scaling law that would specify the accessibility in mitosis given that in interphase, and in principle, this hypothesis could be computationally examined with existing data [24]. Second, specific activities that are yet to be identified could take over the regulation of chromatin accessibility at regulatory elements. Obvious candidates are ATP-dependent chromatin remodelers as they can open chromatin and organize nucleosomes at TF binding sites in interphase [25–27]. A plethora of proteins that play important functions during mitosis, such as Condensins, APC/C, Topoisomerases and the CPC are also to be considered. Notably, the activity of both Condensins and Topoisomerases is associated with DNA torsional stress, known to alter nucleosome stability and already hypothesized to play a role in mitotic bookmarking [9,15]. Hence, as recently proposed for the mitotic control of promoters by the APC/C complex [28], we may well be here facing an integral, previously unappreciated and nearly systemic mechanism maintaining chromatin accessibility in mitotic cells. In turn, this would increase the efficacy of TF rebinding in the following interphase without the involvement of a site-specific mitotic bookmarking TF.
Conclusions
Over the last years assays such as ATAC-seq and DNase-seq have become instrumental to dissect how gene regulatory networks are wired. However, these studies start from the premise that local accessibility reflects TF binding. Is this necessarily the case? As TF binding in interphase does not necessarily imply measurable accessibility (Figure 1), the study of discrepancies between chromatin accessibility, nucleosome positioning and detectable TF binding in mitotic cells, suggest a more delicate and, in fact, interesting relationship. Acknowledging that the aforementioned techniques measure the operational accessibility of relatively small enzymes (17kDa for MNase, 31kDa for DNAse [29] and 53kDa for Tn5 [30]) that act alone or as dimers (Tn5) without DNA specificity, while TFs often bind as larger molecular complexes engaging in base-specific contacts with cognate motifs, arises as an important reminder. Nevertheless, scrutinizing the divergent information obtained from these three parameters (TF binding, chromatin accessibility and nucleosome positioning), may take us today to the gates of a new understanding in how gene regulatory processes are conveyed from mother to daughter cells. On the one hand, several examples have already demonstrated how robust epigenetic marks can be in conveying repressive gene information throughout cellular generations; on the other, bona-fide bookmarking TFs [15] are being increasingly and convincingly reported as means to jumpstart active transcription in the daughter cells. Identifying and dissecting a mechanism that would link the strict concept of epigenetic regulation with the mitotic preservation of chromatin accessibility at regulatory elements may be a new frontier with unexpected implications in our understanding of developmental processes and other normal and pathological phenomena characterized by cell proliferation.
Acknowledgments
We thank our laboratories for critical discussions. RX.C is supported by a Postdoctoral fellowship from the Revive Labex (Investissement d’Avenir; ANR-10-LABX-73). N.O acknowledges Research England’s Expanding Excellence in England (E3) award for funding. Work in P.N laboratory is supported by recurrent funding from the Institut Pasteur, the CNRS and Revive, as well as by funding obtained from the Agence Nationale de la Recherche (ANR 16 CE120004 01 MITMAT), the Ligue contre le Cancer (LNCC EL2018 NAVARRO), and the European Research Council (ERC-CoG-2017 BIND).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Spitz F, Furlong EEM.. Transcription factors: from enhancer binding to developmental control. Nat Rev Genet. 2012;13:613–626. [DOI] [PubMed] [Google Scholar]
- [2].Zhu F, Farnung L, Kaasinen E, et al. The interaction landscape between transcription factors and the nucleosome. Nature. 2018;562:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brahma S, Henikoff S. Epigenome regulation by dynamic nucleosome unwrapping. Trends Biochem Sci. 2020;45:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Klemm SL, Shipony Z, Greenleaf WJ. Chromatin accessibility and the regulatory epigenome. Nat Rev Genet. 2019;20:207–220. [DOI] [PubMed] [Google Scholar]
- [5].Martínez-Balbás MA, Dey A, Rabindran SK, et al. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell Com. 1995;83:29–38. [DOI] [PubMed] [Google Scholar]
- [6].Rizkallah R, Alexander KE, Hurt MM. Global mitotic phosphorylation of C2H2 zinc finger protein linker peptides. Cell Cycle. 2011;10:3327–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ma Y, Kanakousaki K, Buttitta L. How the cell cycle impacts chromatin architecture and influences cell fate. Front Genet. 2015;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Prescott DM, Bender MA. Synthesis of RNA and protein during mitosis in mammalian tissue culture cells. Exp Cell Res. 1962;26:260–268. [DOI] [PubMed] [Google Scholar]
- [9].Michelotti EF, Sanford S, Levens D. Marking of active genes on mitotic chromosomes. Nature. 1997;388:895–899. [DOI] [PubMed] [Google Scholar]
- [10].Hsiung CC-S, Morrissey CS, Udugama M, et al. Genome accessibility is widely preserved and locally modulated during mitosis. Genome Res. 2015;25:213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blythe SA, Wieschaus EF. Establishment and maintenance of heritable chromatin structure during early Drosophila embryogenesis. Elife. 2016;5:e1003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Teves SS, An L, Hansen AS, et al. A dynamic mode of mitotic bookmarking by transcription factors. Elife. 2016;5:e22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xu J, Carter AC, Gendrel A-V, et al. Landscape of monoallelic DNA accessibility in mouse embryonic stem cells and neural progenitor cells. Nat Genet. 2017;49:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Festuccia N, Owens N, Papadopoulou T, et al. Transcription factor activity and nucleosome organization in mitosis. Genome Res. 2019;29:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Festuccia N, Gonzalez I, Owens N, et al. Mitotic bookmarking in development and stem cells. Development. 2017;144:3633–3645. [DOI] [PubMed] [Google Scholar]
- [16].Segil N, Guermah M, Hoffmann A, et al. Mitotic regulation of TFIID: inhibition of activator-dependent transcription and changes in subcellular localization. Genes Dev. 1996;10:2389–2400. [DOI] [PubMed] [Google Scholar]
- [17].Chen D, Hinkley CS, Henry RW, et al. TBP dynamics in living human cells: constitutive association of TBP with mitotic chromosomes. Mol Biol Cell. 2002;13:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Christova R, Oelgeschläger T. Association of human TFIID-promoter complexes with silenced mitotic chromatin in vivo. Nat Cell Biol. 2002;4:79–82. [DOI] [PubMed] [Google Scholar]
- [19].Xing H, Vanderford NL, Sarge KD. The TBP-PP2A mitotic complex bookmarks genes by preventing condensin action. Nat Cell Biol. 2008;10:1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Teves SS, An L, Bhargava-Shah A, et al. A stable mode of bookmarking by TBP recruits RNA Polymerase II to mitotic chromosomes. Elife. 2018;7:e35621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Festuccia N, Dubois A, Vandormael-Pournin S, et al. Mitotic binding of Esrrb marks key regulatory regions of the pluripotency network. Nat Cell Biol. 2016;18(11):1139–1148. [DOI] [PubMed] [Google Scholar]
- [22].Owens N, Papadopoulou T, Festuccia N, et al. CTCF confers local nucleosome resiliency after DNA replication and during mitosis. Elife. 2019;8:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Galupa R, Heard E. X-chromosome inactivation: a crossroads between chromosome architecture and gene regulation. Annu Rev Genet. 2018;52:535–566. [DOI] [PubMed] [Google Scholar]
- [24].Rudnizky S, Khamis H, Malik O, et al. The base pair-scale diffusion of nucleosomes modulates binding of transcription factors. Proc Natl Acad Sci USA. 2019;116:12161–12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wiechens N, Singh V, Gkikopoulos T, et al. The chromatin remodelling enzymes SNF2H and SNF2L position nucleosomes adjacent to CTCF and other transcription factors. PLoS Genet. 2016;12:e1005940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].King HW, Klose RJ. The pioneer factor OCT4 requires the chromatin remodeller BRG1 to support gene regulatory element function in mouse embryonic stem cells. Elife. 2017;6:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Barisic D, Stadler MB, Iurlaro M, et al. Mammalian ISWI and SWI/SNF selectively mediate binding of distinct transcription factors. Nature. 2019;569:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Oh E, Mark KG, Mocciaro A, et al. Gene expression and cell identity controlled by anaphase-promoting complex. Nature. 2020;579:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nicieza RG, Huergo J, Connolly BA, et al. Purification, characterization, and role of nucleases and serine proteases in Streptomyces differentiation. Analogies with the biochemical processes described in late steps of eukaryotic apoptosis. J Biol Chem. 1999;274:20366–20375. [DOI] [PubMed] [Google Scholar]
- [30].Picelli S, Björklund AK, Reinius B, et al. Tn5 transposase and tagmentation procedures for massively scaled sequencing projects. Genome Res. 2014;24:2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]


