ABSTRACT
Post-translational modifications (PTMs) are critical drivers and attenuators for proteins that regulate immune signalling cascades in host defence. In this review, we explore functional roles for one such PTM, the small ubiquitin-like modifier (SUMO). Very few of the SUMO conjugation targets identified by proteomic studies have been validated in terms of their roles in host defence. Here, we compare and contrast potential SUMO substrate proteins in immune signalling for flies and mammals, with an emphasis on NFκB pathways. We discuss, using the few mechanistic studies that exist for validated targets, the effect of SUMO conjugation on signalling and also explore current molecular models that explain regulation by SUMO. We also discuss in detail roles of evolutionary conservation of mechanisms, SUMO interaction motifs, crosstalk of SUMO with other PTMs, emerging concepts such as group SUMOylation and finally, the potentially transforming roles for genome-editing technologies in studying the effect of PTMs.
KEYWORDS: Immunity, ptm, signal transduction, sim, ubiquitin, transcriptional Activation/Repression, crosstalk, CRISPR, nfκb
Introduction
Post-translational modifications (PTMs) are regulators of protein function. The PTMs modifying a substrate protein can modulate its structure, folding, stability, dynamics, function or location. On numerous occasions, the effect of the PTM on the substrate protein is context dependent; residues are modified by the same or distinct PTM types in a spatio-temporal manner, determining functional output. PTMs come in all shapes and sizes with a wide variety of chemical groups. PTMs can be small such as phosphate, intermediate in size such as lipids or large such as attachments of sugar polymers [1–4]. Proteins can themselves modify other proteins by conjugating to specific side-chains. The best studied examples of protein modifiers are Ubiquitin-like proteins (UBLs), which include a diverse group of proteins with a Ubiquitin fold, inclusive of Ubiquitin, Nedd8, ISG15, FAU, UBL5, URM5, SUMO, ATG8/12 and many others [5–8]. The protein PTMs conjugate a wide variety of protein substrates, primarily targeting lysine side chains. Considering the large variety of PTMs (>200) [9,10], the number of proteins that they target, and the growing evidence that each substrate protein is a target of multiple PTMs, a functional landscape for each protein can be envisaged, where its native unconjugated state can be tweaked in a multitude of ways depending on the sequence and combination of PTMs[10]. At this point, with the advent of high throughput proteomics, researchers are generating lists of proteins and residues that are post-translationally modified, but functional implications of the effect of a single PTM on protein function, leave alone the combinatorial/sequential effects of multiple PTMs are far from being completely understood.
In this review, we focus on the Small Ubiquitin-like modifier (SUMO)[11], initially christened as Smt3 (Suppressor Of Mif Two 3 Homolog 1) [12–18]. The SUMO family of PTMs is distinct from that of its more famous cousin Ubiquitin, with which it shares a common fold, but <20% sequence identity[5]. SUMO maturation, conjugation and de-conjugation require a distinct set of enzymes that do not appear to overlap with any other UBL [6,11,15,19,20] Figure 1a. Proteomic studies indicate that SUMO modifies 10–30% of the proteome, depending on context [21–24]; a recent proteomics study has identified ~4000 endogenous SUMOylated proteins among the ~12,000 total proteins mapped [25]. We consider the immune signal transduction cascades as an example of a signalling network and have explored roles for SUMO conjugation in regulating these pathways, especially in response to an infection. The evidence for SUMO regulation of proteins involved in immune signalling is growing in the last two decades (reviewed in [26–28]). Proteins with known immune functions are consistently part of published vertebrate and invertebrate SUMO proteomes [21–24], and SUMOylated proteins have also been specifically found during host response to infection [29–32]. In a small subset of cases, evidence for a physiological role for this conjugation is available [33–40], but most of the putative SUMOylated substrates have yet to be investigated. Based on the large number of potential SUMO targets, future studies should uncover major roles for SUMO regulation in host defence.
Figure 1.
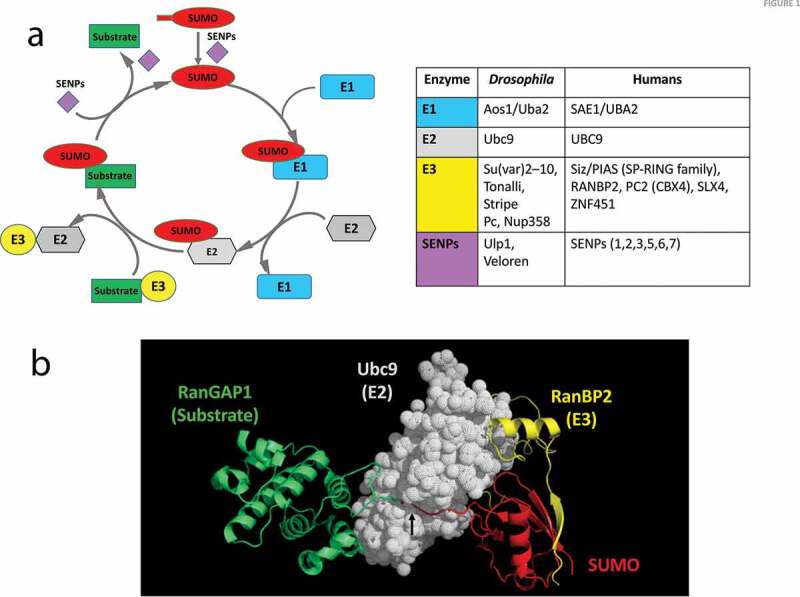
SUMO Conjugation of a substrate protein
(a) The SUMO conjugation/de-conjugation cycle. The addition and removal of SUMO to a target substrate is under enzymatic control. The first step is the maturation of SUMO by an endoprotease, named sentrin-specific protease (SENP) or Ubiquitin-like specific protease (ULP) that exposes the C-terminal di-glycine motif. Next, the E1 heterodimer engages with SUMO via a thioester linkage and subsequently hands it over to the E2 enzyme. The E2 then interacts with the substrate and catalyses the conjugation of the C-terminal COOH of SUMO to a specific lysine side-chain of the substrate. This conjugation step may be either enhanced or directed by an E3 ligase enzyme. The SENPs also serve to de-conjugate SUMO from the target, releasing it for a new cycle. The table lists the enzymes involved in regulating the SUMO cycle. Drosophila enzymes include putative E3 ligases inferred from homology with mammals and also gene ontology analysis. (b) Crystal structure (Protein data bank ID 1Z5S[48]) of SUMO conjugated to RanGAP1 (substrate) by the E2 (Ubc9), with RanBP2 acting as an E3 ligase. The structure shows the interaction between the substrate (RanGAP1) and Ubc9, as well as the cleft/tunnel (black arrow) in Ubc9 that holds the C-terminal GG tail of SUMO for conjugation with the lysine side chain of RanGAP1. The figure was generated using coordinates from the PDB using PyMol (The PyMOLMolecular Graphics System, Version 1.5.0.4 Schrödinger, LLC).
What is SUMO conjugation?
SUMO conjugation or SUMOylation, involves the covalent attachment of the C-terminal carboxyl of the SUMO polypeptide chain to the ε-amino group of a lysine reside on the substrate by an iso-peptide bond. SUMO, synthesized as an inactive precursor, is cleaved to expose a diglycine motif at the SUMO C-terminus, with the aid of a distinct class of proteases termed Sentrin-specific proteases (SENPs) [11,41,42], also called ubiquitin-like specific proteases (ULPs) Figure 1. The hetero-dimeric E1 complex of SUMO activating enzymes (SAE1/SAE2) activates the now mature SUMO, forming a thioester linkage between the E1 cysteine and C-terminus of SUMO, a process dependent on ATP hydrolysis.
SUMO is then transferred to the cysteine of the only known E2 SUMO-conjugating enzyme, Ubc9, which mediates SUMO conjugation to the target lysine. SUMO E3 ligases, a class of enzymes catalysing the transfer of SUMO, can aid the E2 enzyme in transferring SUMO to the substrate, providing specificity in the cell [11,19]. SENPs act as both de-conjugases and maturases, though they exhibit specificity for particular SUMO isoforms[43]. Other SUMO de-conjugating enzymes like DeSUMOylating isopeptidase 1/2 and Ubiquitin specific protease like-1 have recently been discovered, though their functions in SUMO maturation are minimal[44]. A SUMOylated substrate is de-conjugated with the aid of SENPs, making it available for a new conjugation cycle Figure 1a. Genomes of organisms such as S. cerevisiae and Drosophila have a single SUMO gene, while higher vertebrates have five SUMO paralogs [45,46]. A major targeting motif for SUMO is the lysine residue in the core sequence ψ-K-X-α or its inverted variant α-X-K-ψ, with ψ representing a hydrophobic amino acid, usually isoleucine, leucine or valine and α representing a negatively charged side chain, usually glutamic acid or aspartic acid. Other ‘extended’ variants of this canonical conjugation motif have also been defined[47], which allows the prediction of target lysines for experimental testing. Studies over the last decade have however confirmed that prediction accuracy hovers at ~50% suggesting that lysines within non-canonical motifs are also routinely SUMOylated and at this point, we do not have a complete understanding of the molecular basis of motif recognition [8]. The first crystal structure of the E2/E3/Substrate/SUMO1 complex was solved by Christopher Lima’s group [48] Figure 1b, and followed by other structures (PDB: 3UIO, 3UIN) that detailed the molecular interactions between these molecules. These structures confirm that the E2 (Ubc9) brings together the substrate (RanGAP1) with SUMO. The conjugation event occurs in a deep groove in Ubc9 (arrow, Figure 1b). Here, RanBP2 stabilizes the complex and enhances SUMO conjugation, acting as an E3.
What is the effect of SUMO conjugation on the substrate?
The power of SUMO conjugation lies in the versatility that it affords to the biological function of its substrate. For example, SUMO conjugation may change the conformation of a protein, exemplified by the human thymine DNA glycosylase. This SUMO1-modified protein displays altered DNA binding, without altering its enzymatic activity, allowing it to dissociate from DNA[49]. SUMO can also decide the fate of a protein by competing with modifications like ubiquitination, acetylation or methylation at the identical lysine residue. In case of hormone receptors, i.e. the Glucocorticoid receptor (GR) or the Androgen receptor (AR), the SUMO-modified lysine competes with ubiquitin, affecting the rate of turnover by modulating degradation rates [50]. In this way, changes in turnover can affect transcriptional output. In other instances, like the SUMO modification of transcription factors c-Jun and c-Fos, components of the Activator protein 1 (AP-1) dimer, half-life may remain unaltered, but transcriptional activity is reduced [38,39]. Biochemical analysis of non-SUMOylatable c-Fos suggests that accumulation at the promoters may be altered, serving to fine-tune gene regulation, though the exact mechanism remains to be ascertained [51]. These examples demonstrate that SUMO modification of a subset of transcription factors plays a crucial role in their regulation. The heat shock factor HSF1 is SUMO modified upon heat stress and this enhances DNA binding [52]. SUMOylation is also known to alter the subcellular localization of proteins. One such example is that of the transcription factor Medea (Med), the Drosophila ortholog of Smad4. SUMOylation of Med promotes nuclear export and hence negatively regulates Decapentaplegic (Dpp) signalling in the embryo [53].
The identification of a hydrophobic motif, designated SUMO-interacting motif (SIM) or SUMO-binding motif (SBM) that can interact non-covalently with SUMO has helped in understanding SUMO-mediated protein interactions. The trafficking protein RanGAP1 is SUMOylated and associated with the nuclear pore complex protein RanBP2 via the SIM motif of RanBP2 [54]. There are several instances where SUMO has been shown to regulate protein stability, targeting modified proteins to the proteasomal degradation machinery [55–57]. A special case is the ubiquitination of SUMOylated proteins where a dedicated ubiquitin E3 ligase recognizes poly-SUMO chains formed by polymerization of SUMO-2/3 moeities through their internal lysines, and targets them for ubiquitination and subsequent proteasomal degradation [58]. These proteins, designated as STUbLs (SUMO-targeted ubiquitin ligases) represent a specialization and co-evolution of the SUMO and ubiquitin pathways. Key examples are the poly-ubiquitination and subsequent degradation of the SUMOylated promyelocytic leukaemia (PML) nuclear body by the STUbLs RNF4 [59] and Arkadia/Rnf111 [60].
One puzzling aspect of SUMOylation is that only a small proportion of a given protein in the cell is modified by SUMO at a particular time [11]. The rapid conjugation-deconjugation cycles by SUMO-specific proteases bring about this transient state of SUMOylated species. Based on this low proportion, SUMO-conjugation resistant (SCR) variants of the protein should not have a strong effect on function as a large fraction of the protein is in its original ‘non-SUMOylated’ state. However, in most cases, an SCR mutation has a strong effect on function [13,33,36,37,40,51,61]. This indicates that SUMO-conjugation may be a rate-limiting step for an important functional transition. The transition may be related to the folding of the protein, movement between compartments, association/disassociation, all because of a transient SUMO conjugation/de-conjugation step. In the absence of de-SUMOylation, the protein would stall in a non-functional state, reducing the population of the functional state over time, and thus affecting function [11,62,63].
SUMO is required to adapt to cellular stresses, as evidenced by an increase in global SUMO conjugates in response to several abiotic stresses. This global change, termed the SUMO stress response (SSR), involves an increase in SUMO conjugated substrates and is thought to play a pro-survival role [64–67]. Higher molecular weight SUMO2/3 conjugates were found to increase when cells were exposed to detrimental conditions like heat shock, oxidative stress, osmotic stress, etc. The levels of free SUMO rapidly plummet, and SUMOylated proteins increasingly exist as functional clusters. Large-scale mass spectrometric results have corroborated this initial study, providing insight into protein specific and site specific modifications [21,23,25,68–72]. Heat shock and proteasomal inhibition led to an increase in global SUMOylation events by around 50%, with proteins displaying conjugation at multiple sites [25]. Different stresses tended to elicit SUMOylation of certain common proteins, though at different sites[21]. The studies described highlight a possible role for context-specific SUMOylation of distinct sites converging at common stress effector proteins to provide a cyto-protective function.
SUMO in host defence
Stress conditions like DNA damage, ER stress, osmotic stress, etc., can elicit SUMO conjugation in the cell. Similarly, the evolutionarily conserved innate immune pathways are critical to tide over these cellular stresses [73–76]. In the context of SUMO, the immune response can be perceived as a stress response evoked by invading pathogens. Interest in roles for SUMO conjugation regulating the immune response has its origins in the finding that IκBα, the inhibitor of NF-κB is SUMO conjugated[36]. This reversible modification led to a reduction in degradation of IκBα and attenuation of NF-κB signalling. Following this study, the Courey laboratory[33] showed that Drosophila NF-κB, Dorsal was SUMO conjugated and that SUMOylation of Dorsal attenuated the activation of Dorsal target genes and thus the immune response. These seminal studies were followed by a number of studies on other substrate proteins and pathways that confirmed roles for SUMO conjugation and also members of the SUMO cycle in regulating the immune response [61,77–81]. Research in the last decade have further supported the hypothesis that SUMO conjugation of proteins regulates the host immune response. An important landmark for the burgeoning role of SUMO conjugation in host defence was the discovery that pathogens could hijack the SUMO conjugation machinery of the host for increased pathogenicity. Initial studies showed regulation of host E1 activity during adenoviral infection [82,83], followed by similar studies using viruses such as Ebola [84,85] and Influenza[86]. Bacterial pathogens could also regulate the SUMO cycle of the host. Examples include Listeria[87], Shigella[88] and Salmonella[89]. The current era of SUMO proteomics, 2010 onwards, suggests that 10–30% of total proteins in the cell are SUMOylated and that SUMO conjugation is dynamic and context dependent [21–21–25]. The number of studies that have attempted to specifically identify SUMO targets that are modulated in the immune response are limited as are studies that show mechanistic data for SUMO conjugation in immunity. Sloan and co-workers[31] have generated a list of 877 targets for SUMO-2 infection with HSV-1; Our lab has identified 710 Drosophila proteins whose SUMOylation is enriched after an LPS challenge in Schneider cells[29] and Impens and co-workers identified 125 immune-specific SUMO targets for listeriolysin O[90]. Around 5–10% of the total SUMOylated proteins identified belong to immune signalling pathways [29,31,90]. Figure 2 summarizes the major immune signalling pathways in Drosophila Figure 2a and mammals Figure 2b along with identified SUMO conjugation targets.
Figure 2.
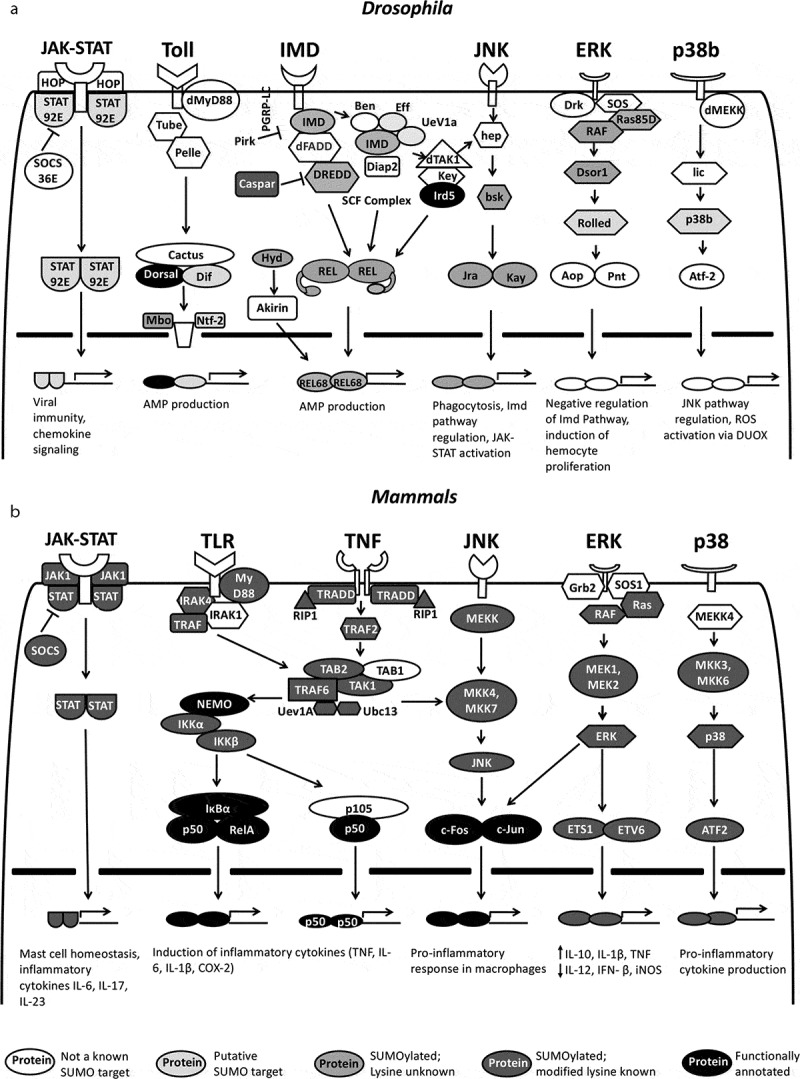
SUMO targets in immune signalling. Schematic for proteins involved in immune cascades in Drosophila (a) and mammals (b). Six orthologous pathways are shown, namely the JAK-STAT, Toll/TLR, IMD/TNF, JNK, ERK and p38. Descriptions of these immune regulatory pathways in Drosophila and their mammalian orthologs can be found in excellent reviews that have been published [91–97]. SUMO targets are marked by varying shades of grey. The darker the shade of grey, the higher the confidence for a role of that protein as a validated SUMOylated substrate, with known target lysine(s), involved in the immune response
Of the proteins displayed in Figure 2, very few (ovals filled with black) have been validated as bona-fide SUMO targets in the context of the host immune response [21]. In Drosophila, Toll/NF-κB and Immune Deficient (IMD)/NF-κB function as the primary signalling pathways for the innate immune response, whereas the Janus kinase (JAK)-signal transducer and activator of transcription (STAT), c-Jun N-terminal kinase (JNK), extracellular-signal-regulated kinase (ERK) and p38 pathways play supporting roles in immune regulation, including roles in cellular immune response [91–98]. Orthologous signalling pathways exist in mammals with signalling playing roles in both innate and adaptive immunity Figure 2. The larger number of SUMO targets in mammals Figure 2b, as emphasized by dark grey shades, are a reflection of the extensive proteomic experiments on mammalian cell lines, with very few examples with actual validation in either cell culture or animal experiments. Suppl. Table 1 lists the predicted SUMO substrates in the major immune signalling pathways in both flies and mammals, along with lysines that are predicted to be targeted by the SUMO machinery.
In the next few sections, we discuss in detail the modulation of the Toll/NF-κB, JAK-STAT and the IMD/NF-κB pathways by SUMO. We also take a few substrates that have been validated in-vivo as SUMO targets as specific examples to bring out the roles for SUMO conjugation from a mechanistic standpoint and to compare and contrast the evolution of SUMO related regulatory mechanisms from flies to mammals.
SUMOylation regulates Toll/TLR signalling
In both flies and mammals, it is evident that SUMO conjugation modulates signalling in both the Toll [33,99] and TLR [36,100] signalling modules. Of the many proteins in the Toll/TLR cascades, in mammals, IκB appears to be a major SUMO target[36] Figure 3. A holistic picture of IκBα regulation has emerged over the years. NF-κB is held in the cytoplasm by IκBα in an inactive state. At this stage, the SUMO-1 modified IκBα is resistant to ubiquitination [36,37]. Upon stimulation with a suitable ligand, a protein kinase cascade is initiated. Phosphorylation of IκBα, aided by the IκB kinase (IKK) complex is thought to recruit SUMO-2/3, possibly through a change in conformation[100]. Though the site of SUMO-2 conjugation remains identical to that of SUMO-1, IκBα now undergoes polyubiquitination, primed by polySUMO. These polySUMO-polyubiquitin hybrid chains target IκBα to the 26S proteasomal machinery, freeing NF-κB for nuclear translocation[100] Figure 3.
Figure 3.
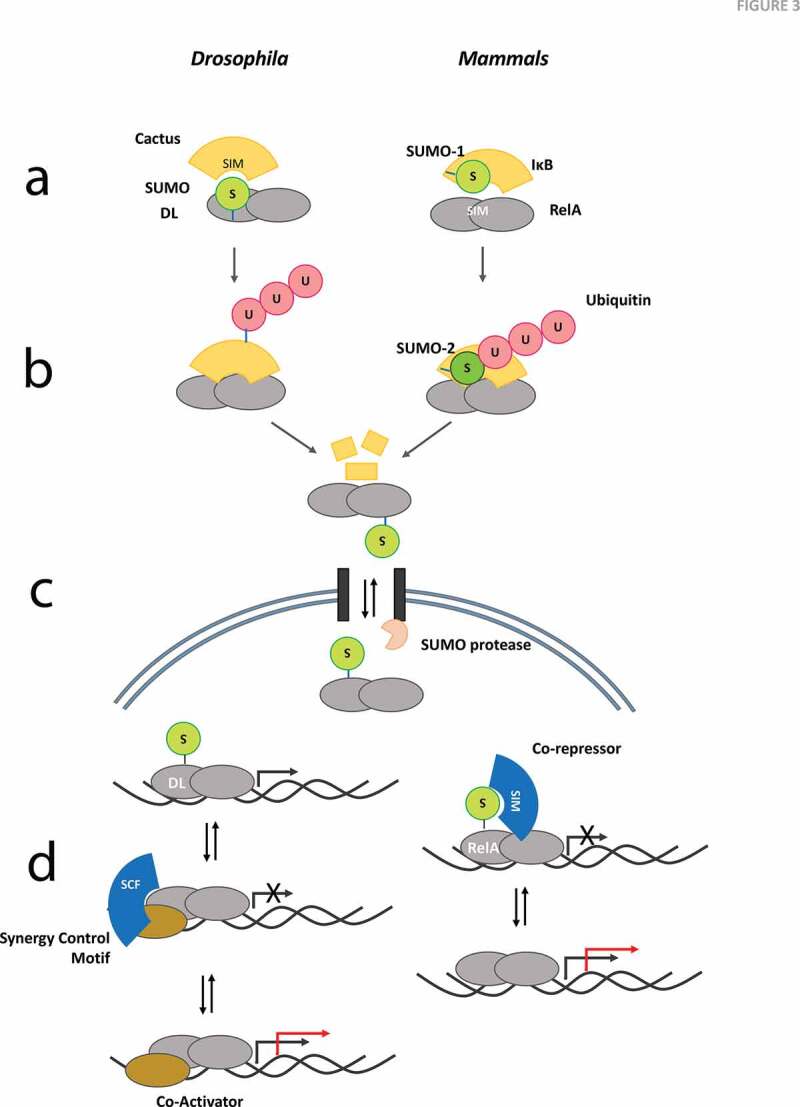
Mechanistic models for SUMO Regulation of Toll/TLR Signalling. Dorsal (DL), SUMOylated at K382 appears to be a key SUMO substrate in the fly Toll pathway in contrast to IκBα SUMOylation at K21 in humans. Possible models/mechanisms for regulation of Toll/TLR Signalling include
(a) SUMO:SIM interactions may play a major role in the evolutionary conserved Dorsal:Cact and NF-κB:IκBα complexes. The stability of the complex, and the release of Dorsal/NF-κB after Cact/IκBα degradation, in response to Toll/TLR signalling is a central feature of the mechanism. SUMO conjugation of Dorsal in flies, and IκBα in mammals, may define the binding dynamics or even enhance poly-ubiquitination and subsequent degradation in a context dependant manner. (b) Poly-ubiquitination of Cact/IκBα is an essential step for release of Dorsal/NF-κB for transcriptional activation and/or nuclear import. In mammals, this step involves a complex exchange of SUMO1 with SUMO2 and subsequent poly-ubiquitination of SUMO2 [36,37,100]. (c) Import of Dorsal into the nucleus may be dependent on a SUMO ‘ticket’ that is cleaved off during transit through the nuclear pore. In mammals, the NF-κB/IκBα complex as a whole can enter the nucleus, allowing the possibility of SUMO dependent nuclear import (or export)[26]. This model highlights the importance of SUMOylation as a rate limiting step for nuclear trafficking and the small fraction of SUMOylated protein, when compared to the non-SUMOylated substrate that can exist and regulate the critical import/export step. (d) A SUMOylation/deSUMOylation cycle may regulate transcriptional activation of defence genes that are activated by Dorsal/NF-κB. SUMO conjugation resistant-Dorsal is a better transcriptional activator, suggesting that SUMO conjugation may restrain Dorsal mediated activation[33]. A synergy control factor (SCF) with a SIM has been hypothesized to bind and regulate SUMOylated Dorsal[33]. Data from mammals suggest that RelA is SUMOylated and SUMO conjugation inhibits RelA transcriptional activity[107]. In the absence of RelA SUMOylation, the co-repressor may not be recruited efficiently. The black arrow at the transcription start site represents normal level of transcription, while the red arrow highlights increased transcriptional output.
In Drosophila, the broad outline of the signalling events is conserved, culminating in phosphorylation of Cact. Cact is then ubiquitinated and degraded, though the biochemical evidence for SUMOylation of the fly ortholog of IκBα, Cact is weak [77]. Also, the fly expresses a single SUMO isoform resembling SUMO2/3 and is unable to form SUMO chains since it lacks the critical lysine [101]. Cact has a single non-consensus site predicted as a SUMO conjugation site (http://www.jassa.fr/ [47]) and researchers in the field have been unable to demonstrate that Cact is conjugated by SUMO. Hence, the scenario for Cact regulation may differ significantly from mammalian IκBα. In mammals, SUMOylation of p100 (NF-κB2) is required for NF-κB inducing kinase (NIK) dependent phosphorylation and processing of inactive p100 [102] while SUMO1 conjugation of RelB has been implicated in converting this NF-κB from an activator to a repressor [103].
In flies, a validated target for SUMO conjugation in Toll signalling is Dorsal, at K382. Dorsal acts redundantly with a related Rel-family protein, Dif to mount an immune response. SUMO-conjugation resistant (SCR), Dorsal (K382R) mutant showed a 5 to 10-fold increase in reporter gene activity, compared to the wild-type protein [33], indicating that SUMO conjugation decreased Toll signalling. The same study, however, had data that suggested that an increase in global SUMO conjugation would lead to increased Toll signalling. Since an increased global SUMO conjugation would lead to an increase in SUMOylated Dorsal, the data were not consistent with the idea of non-SUMOylated Dorsal, mimicked by Dorsal (K382R) being a stronger transcriptional activator. In order to explain the conflicting data, the authors hypothesized that K382 was part of a crucial synergy control motif, facilitating interaction with a transcriptional attenuator, termed synergy control factor (SCF; Figure 3d). A mutation at Dorsal (K382) or SUMO-conjugation at the site would disrupt the interaction with the SCF and hence upregulate Toll signalling, explaining the greater target gene activation in both cases [33].
The idea that SUMO conjugation largely acts as a brake was consistent with experiments in the organism by the Govind lab, which found Ubc9 to be a negative regulator of Toll signalling in the larval immune response [77,78]. Reduction of Ubc9 levels manifests as melanotic masses, caused by the over-proliferation of blood cells. The over-proliferation phenotype was found to be correlated with high levels of nuclear Dorsal in haemocytes. Loss-of-function mutations of Dorsal and Dif in a Ubc9 mutant background suppressed this phenotype, suggesting a genetic interaction of the SUMO machinery with elements of the Toll pathway [77,78]. The authors hypothesized that the physical interaction between Ubc9 and Cact, the fly IκBα ortholog could be important for sequestering Dorsal/Dif in the cytoplasm, which is lost in the ubc9 mutant Figure 3. Another possibility is the SUMOylation of Cact or Dorsal/Dif, preventing the aberrant activation of Cact or the untimely translocation Dorsal/Dif. Further investigation demonstrated that Ubc9 mutant larvae had reduced levels of Cact, re-iterating the significance of SUMO cycle components in regulating the Cact/Dorsal/Dif complex [99]. SUMOylation may also occlude the site for Cact ubiquitination, in agreement with mammalian studies on IκBα [37]. An alternate explanation is the change in Dorsal/Dif stability and/or localization due to altered Cact stability, since the stability of Cact is intimately linked to Dorsal binding [104] largely mediated via its ankyrin repeat domain [105]. Cact also has three hydrophobic core regions – putative SIM motifs nestled in its ankyrin repeat domain (VDVV 243–246, ILLL 284–287, IDIL 375–378)[47], which could also facilitate interaction with SUMOylated Dorsal/Dif, aiding in their cytoplasmic sequestration. Figure 3 lists the possible models that exist that could explain the regulation of Toll/TLR signalling at different levels. At the level of the Dorsal:Cactus or NF-κB:IκB complex, SUMO interactions with SIM my help stabilize (or destabilize) the complex. SUMO may also be important in nuclear import/export with Ulp1 being localized, in Drosophila, to the nuclear side of the nuclear pore complex [106]. At the level of transcriptional activation, as discussed, for both mammals and flies the data strongly suggests that non-SUMOylated Dorsal/NF-κB is a stronger transcriptional activator. In flies this is explained by the loss of binding to a hypothetical SCF, while in mammals the SUMOylated species is postulated to recruit co-repressors to dampen or stop transcriptional activation[107].
SUMOylation regulates TNF/IMD signalling
Immune signalling is a hub of PTM cross-talk. The basic backbone of phosphorylation-dependent relay of the stimulus is further fine-tuned by SUMOylation and ubiquitination. The role of the classical K48-linked ubiquitin-proteasome system and K63-linked-ubiquitin-signalling systems have been investigated in depth. A few instances of SUMO and ubiquitin cross-talk are presented here, with a focus on the IMD/TNF signalling pathways Figure 4. In mammals, binding of a ligand to the tumour necrosis factor (TNF) receptor initiates downstream events, culminating in NF-κB activation. Genotoxic stress also triggers this pathway, deciding the apoptotic fate of the cell. When cells are subjected to DNA-damaging agents, the first step is the recruitment of the adaptor protein RIP1 (receptor-interacting protein 1), to the cytoplasmic tail of the TNF receptor [108]. RIP1 undergoes K63-linked polyubiquitination, an essential step in enlisting TAK1 (Transforming growth factor beta-activated kinase 1) and IκB kinase (IKK) complex proteins through the adaptor, NEMO (NF-κB essential modulator) Figure 4 [109,110]. Interestingly, it was observed that SUMO modification of RIP1 preceded this ubiquitination step [111]. Lysine to arginine mutations of RIP1 at residues 105, 140, 305 and 565 abolished SUMO conjugation. This four lysine to arginine (4KR) mutant failed to undergo ubiquitination, ceasing to activate NF-κB, since the IKK complex remained inactive [111]. Hence, timely action of SUMO dictates further events of ubiquitination and complex formation in this case. Though the cellular inhibitor of apoptosis protein (cIAP) family of proteins have been implicated in the ubiquitination of RIP1 [108], whether their recruitment is contingent upon SUMOylation of RIP1 remains unknown.
Figure 4.
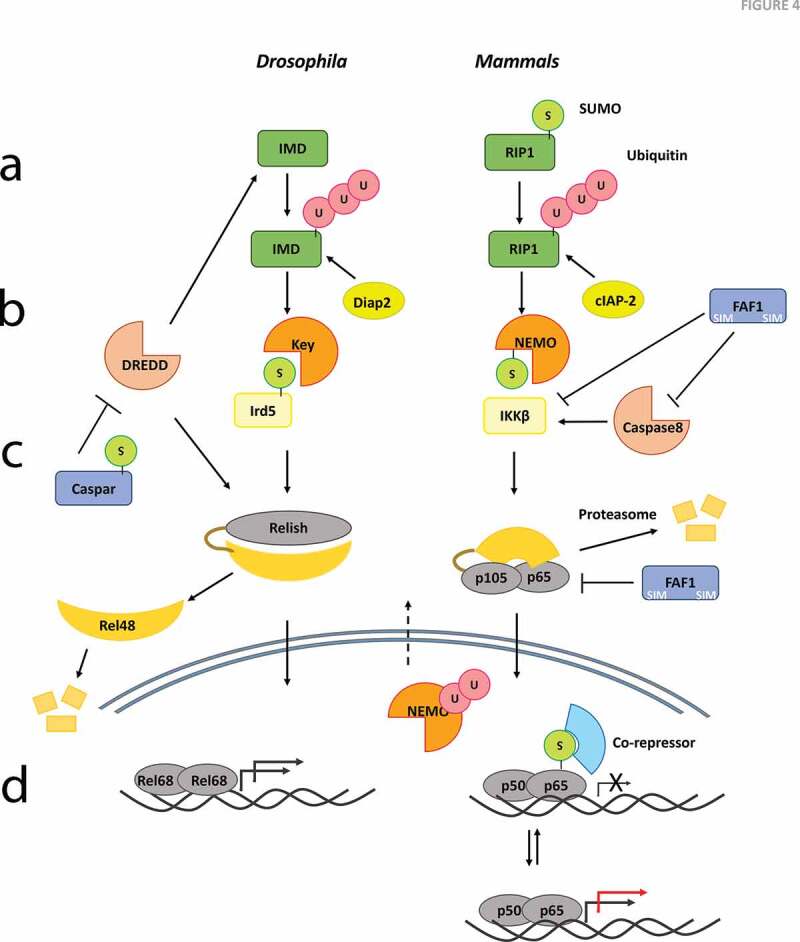
Mechanistic models for SUMO Regulation of IMD/TNF signalling. Group SUMOylation and subsequent protein-interactions may play crucial roles in stabilization of complexes in the IMD/TNF Signalling pathway upon an immune challenge. Possible models/mechanisms include
(a) Ubiquitination of the adaptors IMD/RIP1 appears to be required for signalling to the IKK complex. The ubiquitination may be dependent on SUMOylation of the adaptor. This mechanism has been demonstrated in mammals[111] but not flies. (b) The IKK complex, KEY:IRD5 in flies and IKKα:NEMO:IKKβ may represent another instance of the evolutionary conservation of a functional complex via a SUMO:SIM interaction. SUMO conjugation of IRD5 at 152 may facilitate transduction of signal in the IKK complex, by modulation of its interaction with Kenny, while SUMO modification of NEMO facilitates nuclear import and subsequent ubiquitination, which appears necessary for nuclear export [34,35]. (c) In Drosophila, SUMOylated Caspar may impinge on the DREDD-dependent cleavage of Relish [122], which in turn affects Relish nuclear import and subsequent transcription of defence genes. Interestingly, the mammalian Caspar ortholog, FAF1, is also a negative regulator of NF-κB [123,125]. Its ability to regulate NF-κB signalling appears to depend on its physical interactions with the IKK complex as also p65/p50. FAF1 contains consensus sites for SUMOylation[47] but is not a validated target. (d) There is some evidence for Relish being a direct target of SUMO conjugation [24], but whether this holds true in the immune context needs to be ascertained. Similarly, neither p100 or p105, the Ankyrin domain containing counterparts of Relish in mammals have been shown to be SUMO conjugated upon an immune challenge. The cleaved fragments however can form heterodimers with RelA (p65) or RelB, both SUMO substrates and thus influence transcription. The red arrow at the TSS indicates increased transcriptional output when compared to normal (black arrow).
In Drosophila, the orthologous IMD pathway recognizes gram-negative bacterial cues, deploying Relish (Rel) for the production of anti-microbial peptides (AMPs) [112–115]. The death domain of IMD bears a striking resemblance to that of RIP1 and after processing by the caspase DREDD, IMD is ubiquitinated, by Drosophila inhibitor of apoptosis-2 (Diap2), much like Receptor-interacting serine/threonine-protein kinase 1 (RIP1) [113,116]. Whether this step is evolutionarily conserved with mammals and requires SUMOylation merits further study.
SUMO also forms an integral part of signalling at the nodal IKK complex. Comprised of IKKα, IKKβ and NEMO in mammals, the fly counterpart has two components, immune response-deficient 5 (IRD5/IKKβ) and Kenny (KEY/IKKγ) Figure 4. NEMO is also SUMOylated, at K277/309 [35]. Genotoxic stress, ethanol or hydrogen peroxide are sufficient to move cytoplasmic NEMO to the nucleus, where it is SUMO modified [117]. This facilitates retention in the nucleus and initiation of further signalling events. A cycle of deSUMOylation ensues, leading to phosphorylation and subsequent ubiquitination at the same lysine. Now NEMO is exported from the nucleus where it associates with the IKK complex and activates the NF-κB cascade [117]. In this manner, the sequential modification of NEMO by SUMOylation, phosphorylation and ubiquitination is necessary for altered localization of NEMO and NF-κB activation.
To gain more insight into the Drosophila IMD pathway, Fukuyama and colleagues used a proteomics approach to generate an interactome of 369 proteins in S2 cells challenged by E. coli [40]. In addition to validating previous findings that IMD-Fas associated protein with death domain (FADD)-DREDD and IRD5-KEY exist as complexes, analysis of the IKK complex revealed interaction of IRD5 (IKKβ) and KEY (IKKγ) with SUMO pathway components, hinting at possible SUMO-mediated protein interactions. Furthermore, IRD5 was found to be SUMOylated at K152, and a K152A mutant displayed reduced induction of the AMP Attacin A transcripts in vivo [40]. This is in contrast to the mammalian IKK complex, where IKKγ (NEMO) is SUMOylated. Like in the case of the IκBα/NF-κB complex, the interaction is evolutionarily conserved and might serve a similar purpose, though the SUMOylated entities differ. Coupled with the study demonstrating an enrichment of SUMOylated proteins upon LPS challenge in S2 cells [29], a new paradigm of protein interactions mediated by group SUMOylation in managing the Drosophila immune response is emerging. The phenomenon of group SUMOylation is well documented in case of the double strand break (DSB) repair proteins in the DNA damage response pathway [118–120]. The SUMOylation of target proteins, often at multiple sites, serves to recruit partner proteins via their SIM motifs, culminating in the formation of a protein complex promoting a common function, i.e. DSB repair [120]. In this manner, SUMO is thought to act as a glue, strengthening and reinforcing protein interactions in a SIM-dependent manner [119,120]. Since immune signalling requires the rapid relay of information relying on the interaction of a plethora of signalling molecules, group SUMOylation can be envisaged to hasten and stabilize such SUMO-SIM interactions.
Dampening the immune response after successful resolution of infection and preventing the unrestrained activation of immune effectors in the absence of threats is another facet of signalling [121]. Some of these negative regulators are a target of SUMO modification as well. The Drosophila Fas-associated factor 1 (FAF1) ortholog Caspar was found to be SUMOylated in two independent studies [24,29]. Caspar is required to prevent the untimely processing of Relish via the caspase DREDD [122]. Animals that lack Caspar constitutively express the AMP diptericin and are resistant to bacterial infection [122]. Generating a SUMO-conjugation resistant Caspar mutant will help in deducing the function of SUMO in an immune context. The mammalian ortholog FAF1 also negatively regulates NF-κB by binding to the IKK complex [123] and caspase-8 [124], preventing its activation. Furthermore, FAF1 interacts with RelA, preventing its nuclear localization Figure 4 [125]. Though SUMOylation of FAF1 has not been studied in the immune context, two bona fide SIM motifs are documented in FAF1, though they do not seem to affect NF-κB activation [126], as evidenced by mutational studies in cells. Whether this holds true in an intact organism like the fly in the context of immunity can only be demonstrated unequivocally in a genome-edited mutant for the SUMO/SIM sites.
SUMOylation in anti-viral immunity
Viruses pose a serious threat to the well-being and survival of organisms. To effectively combat them, the innate immune response employs several mechanisms. In mammals, Toll-like receptors (TLRs) and pattern recognition receptors (PRRs) aid in the recognition of viral nucleic acids and viral antigens, inducing autophagy, and triggering the production of interferons (IFNs) and inflammatory cytokines to clear the virus [127,128]. IFNs, a backbone of the antiviral response, also signal to the JAK-STAT pathway to mount a robust antiviral response in the infected cell, while also protecting unaffected neighbouring cells [129,130].
Drosophila has also evolved mechanisms to fight viral pathogens, both natural (Drosophila C virus, Nora virus, etc.) and mammalian (Dengue virus, West Nile virus, etc.) [92,131–133]. The Toll and IMD pathways seem to have a limited role in viral immunity; the Toll-7 receptor mediates aspects of antiviral autophagy [134] while Immune response deficient 5 (IRD5) and Rel in the IMD pathway provide a protective function [135]. The ERK pathway, through p38b signals to JAK-STAT, which is required to mount the antiviral humoral response [98]. At the cellular level, the anti-viral response is multifaceted, engaging autophagy and small interfering RNA (siRNA)-mediated RNA interference (RNAi), among others [92,133,134,136,137].
Experiments done on the Adenovirus protein, Gam1, were one of the earliest studies that revealed a role for host SUMOylation in the mammalian anti-viral response. Gam1, essential for viral replication, adopts a multi-pronged approach to subvert host defences and enhance survival of the virus. It sequesters the SUMO E1, leading to a decrease in global SUMOylation, affecting transcription through factors like Elk-1 and SP3 [82,83]. The decreased SUMOylation further dismantles PML nuclear bodies, whose assembly plays a protective role against viral infections [82,83]. Studies on the Ebola Zaire Virus have found that the deployment of the viral protein VP35 increases SUMOylation of the host transcription factor IRF7, decreasing IFN production. Subsequently, dendritic cells are compromised in their ability to fight off the infection [84]. In the ever evolving arms race between virus and host, the host has also equipped itself to handle the assault on its defences. For instance, a reduction in global host SUMOylation due to the Influenza virus infection alters the SUMOylation status of Tripartite motif-containing 28 (TRIM28), a transcriptional co-repressor, promoting antiviral immunity [138].
The RNA-interference (RNAi) machinery is one of the most robust arms of the insect antiviral defence. Drosophila expresses a repertoire of small interfering RNAs (siRNAs) and micro-RNAs (miRNAs) to bind and degrade the invading viral RNA, halting viral replication [139]. Argonaute (Ago) proteins are crucial components of the RNAi machinery, recruiting siRNAs to the effector RNA-induced silencing complex (RISC) [139,140]. A Drosophila SUMO immune proteome identifies Argonaute-2 (Ago2) as a potential SUMO target, showing an increase in SUMOylation in response to immune challenge [29]. Interestingly, the first report demonstrating a connection between SUMOylation and the RNAi machinery identified the SUMOylation of mammalian Ago2 at K402, albeit in a non-immune context [141]. Loss of Ago2 SUMOylation leads to an increase in Ago2 stability, thereby affecting RNAi efficacy [141].
Autophagy is another essential cellular response against viral infections in insects [136,137]. Activation of JNK leads to the expression of various autophagy-related (ATG) genes, thus regulating stress induced autophagy [142]. Key JNK pathway proteins, Basket, Jra and Kayak are potential SUMO targets (Figure 2; Suppl. Table 1) [29]. Evidence from mammalian studies suggest that SUMOylation plays an important role in the regulation of JNK signalling, through the modification of the transcription factors c-Jun and c-Fos [38]. The JAK/STAT pathway also elicits an anti-viral response in flies [143]. STAT92E, a transcription factor of the JAK/STAT pathway is SUMOylated at K187 and the loss of SUMOylation leads to an increase in STAT92E transcription activity [144]. The SUMOylation of the mammalian ortholog STAT1 indicates an evolutionarily conserved role for SUMO in JAK-STAT signalling [145]. These examples listed above underscore the important roles SUMO conjugation plays in the context of viral infection.
The slow progress of understanding roles for SUMO conjugation in the immune response highlights the complexity of understanding a holistic role for SUMO conjugation in host defence. The complexity increases manifold since it is becoming increasingly apparent that not only are multiple PTMs involved in the regulation of immune signalling, they also cross-talk. The activity of a protein may be modified by not one but multiple PTMs that modify the polypeptide chain, sometimes simultaneously [7]. There is a strong possibility of a PTM code that dictates the functional status of a protein based on the extent of modification [146,147]. Additionally, the incorporation or removal of a PTM may depend on a previous modification by another PTM, highlighted in the case of RIP1 and NEMO. SUMO dependant ubiquitination [58,148,149] where ubiquitin conjugation is dependent on a first step of SUMO conjugation on the same polypeptide, is emerging as a common theme. Simultaneous SUMOylation at multiple sites, polySUMO chains and SUMO-ubiquitin hybrid chains add another layer of complexity to the repertoire of existing PTMs [101,150–153]. They serve as an additional unique motif that can be recognized by specially designated interacting partners. Phosphorylation-dependent SUMOylation and co-modification also dictate the fate of a protein [21]. The different ways these modifications manifest will further help us better understand signalling in general and immunity in particular.
A look to the future
The study of PTMs has proved be challenging due to the low stoichiometry of the modified protein in comparison to the total protein. In the case of SUMO, there are three rate limiting steps that have held back the progress of studies. First is our lack of ability to accurately predict SUMO conjugation sites on substrate proteins. Comparisons of sites predicted with current state-of-the-art programs with actual sites discovered using proteomics suggest that <50% of sites can be predicted based on ‘canonical’ SUMO motifs. The remaining have to be discovered by experimental methods and may be context dependent. The second hurdle is the identification of SUMOylation sites by mass spectrometry. The mammalian SUMO1 has a trypsin cleavage site close to the C-terminal -GG and thus leaves a short tail. Since the total conjugated ‘T-junction’ fragment is small and its mass can be measured accurately by mass spectrometers, efficient identification of the site of SUMOylation is possible. In contrast, the mammalian SUMO2/3 and the fly SUMO ortholog leave behind a large mass remnant after protein digestion, hampering accurate site identification. One way around this problem has been to introduce tagged, cleavable forms of SUMO2/3 [154] but these raise additional concerns. SUMOylation of a fraction of targets could be due to the overexpression and introduction of the tagged SUMO. This means that a fair amount of time and effort have to be expended in identifying target lysines by mutagenesis screens and then validating these as genuine SUMO sites. Recently, researchers have focussed on studying native SUMO modifications in endogenous tissues by utilizing a modified set of proteases that leave behind a digested fragment amenable to mass-spectrometric site-identification [25]. The compatibility of this proteomics pipeline with Drosophila SUMO is a promising indicator of possible SUMO site-identifications in the fly, in the near future.
The third challenge for a SUMO researcher is to create mutant versions of the proteins resistant to SUMO conjugation. This is especially tedious and time-consuming when working with whole organisms, rather than cell lines. The recent advent of genome-editing methods, namely Transcription activator-like effector nucleases (TALEN), Zinc finger nucleases (ZFNs) and Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) [155,156] technologies have revolutionized our ability to make targeted mutations to abolish SUMOylation directly at the genomic locus. Since the SUMOylated lysine residue can be potentially modified by a plethora of PTMs including ubiquitin, the pitfall of a lysine to arginine mutation can be circumvented by modifying the adjacent acidic tract critical for SUMOylation, without affecting other PTMs. CRISPR/Cas9 has especially greatly upgraded the versatile genome-engineering toolbox in Drosophila (Box 1).
Box 1.
Generation of point mutations using CRISPR/Cas9 based Genome editing
| Targeted editing in flies, especially at the level of changing a single amino-acid has been a challenging task. In the past, point mutations were routinely generated by large chemical mutagenesis screens at random sites and mutations of interest subsequently identified, enriched and stabilized 165,166. The second routine method to study mutants was to first generate null flies for the target locus and rescue the null by expression of either wild-type or mutant allele, usually by inserting the transgene at a site distant from the target locus 167,168. In recent years, the utility of the CRISPR/Cas9 toolbox to edit the genomic locus directly and efficiently has revolutionized fly biology. After the initial demonstration of CRISPR/Cas9 genome editing in mammalian cells 169–173, fly biologists developed equivalent methodologies to engineer the fly 158,159,174. Today, a fly biologist can routinely generate site directed mutations, such as replacing a target lysine with an arginine, creating a SUMO resistant site, using the CRISPR/Cas9 toolbox in the fly. |
Targeted editing in flies, especially at the level of changing a single amino-acid has been a challenging task [157–161]. In the past, point mutations were routinely generated by large chemical mutagenesis screens at random sites and mutations of interest subsequently identified, enriched and stabilized [162,163]. The second routine method to study mutants was to first generate null flies for the target locus and rescue the null by expression of either wild-type or mutant allele, usually by inserting the transgene at a site distant from the target locus [164,165]. In recent years, the utility of the CRISPR/Cas9 toolbox to edit the genomic locus directly and efficiently has revolutionized fly biology. After the initial demonstration of CRISPR/Cas9 genome editing in mammalian cells [169–173], fly biologists developed equivalent methodologies to engineer the fly [158,159,174]. Today, a fly biologist can routinely generate site directed mutations, such as replacing a target lysine with an arginine, creating a SUMO resistant site, using the CRISPR/Cas9 toolbox in the fly.
SIMs have also been an understudied aspect of SUMOylation. While in vitro studies exist to demonstrate the importance of SIMs in modulating interaction with a SUMOylated protein, physiological studies elucidating the importance of SIMs are lacking. Advent of genome-editing tools like CRISPR/Cas9 will also help in analysing protein function from both a SIM and a SUMO conjugation perspective, allowing generation of SUMOylation-resistant mutants as well as SIM domain deletions [171,172,174].
Conclusion
A complete understanding of SUMO function in immunity requires data on the effect of SUMO-conjugation of individual immune proteins to the immune response, as also an integrated picture of the total effect of SUMOylation/deSUMOylation on host defence. Dramatic improvements in proteomic and genome editing technology, and a continued interest of researchers in regulation by PTMs should lead to a deeper understanding of the intricacies of PTMs, in the near future.
Supplementary Material
Acknowledgments
The authors have no conflict of interest to declare. We thank Dr Girish Deshpande and two anonymous reviewers for their suggestions and comments that have helped us greatly improve this review.
Funding Statement
Department of Biotechnology, Ministry of Science and Technology, Govt. of India. Genome Engineering Technologies (GET) grant BT/PR26095/GET/119/199/2017 to GR. SH and AS are graduate students supported by a fellowship from the Council for Scientific and Industrial Research (CSIR).
Author contributions
SH, AS, BK and GR wrote the Ms and generated the figures.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Harding JJ, Crabbe MJC.. Post-translational modifications of proteins. Boca Raton (FL): CRC; 1991. [Google Scholar]
- [2].Voet D, Voet JG, Pratt CW.. Fundamentals of biochemistry: life at the molecular level, 5th Edition. USA: Wiley; 2006. [Google Scholar]
- [3].Walsh CT, Garneau-Tsodikova S, Gatto GJ Jr.. Protein posttranslational modifications: The chemistry of proteome diversifications. Angew. Chemie Int. Ed. 2005;44(45):7342–7372. [DOI] [PubMed] [Google Scholar]
- [4].Garcia BA. Post-translational modifications that modulate enzyme activity. Oxford (UK): Elsevier; 2019. (Garcia B, editor). [Google Scholar]
- [5].van der Veen AG, Ploegh HL. Ubiquitin-like proteins. Annu. Rev. Biochem. 2012;81(1):323–357. [DOI] [PubMed] [Google Scholar]
- [6].Vierstra RD. The expanding universe of ubiquitin and ubiquitin-like modifiers. Plant Physiol. 2012;160(1):2 LP– 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eifler K, Vertegaal ACO. Mapping the SUMOylated landscape. Febs J. 2015;282(19):3669–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cappadocia L, Lima CD. Ubiquitin-like protein conjugation: Structures, chemistry, and mechanism. Chem. Rev. 2018;118(3):889–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Consortium TU. The universal protein resource (UniProt) in 2010. Nucleic Acids Res. 2009;38:D142–D148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Minguez P, Parca L, Diella F, et al. Deciphering a global network of functionally associated post-translational modifications. Mol. Syst. Biol. 2012;8(1):599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hay RT. SUMO: A history of modification. Mol Cell. 2005;18(1):1–12. [DOI] [PubMed] [Google Scholar]
- [12].Johnson ES, Schwienhorst I, Dohmen RJ, et al. The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. Embo J. 1997;16(18):5509–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 1996;135(6):1457–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Meluh PB, Koshland D. Evidence that the MIF2 gene of Saccharomyces cerevisiae encodes a centromere protein with homology to the mammalian centromere protein CENP-C. Mol Biol Cell. 1995;6(7):793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Melchior F. SUMO—nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 2000;16(1):591–626. [DOI] [PubMed] [Google Scholar]
- [16].Saitoh H, Pu RT, Dasso M. SUMO-1: Wrestling with a new ubiquitin-related modifier. Trends Biochem. Sci. 1997;22(10):374–376. [DOI] [PubMed] [Google Scholar]
- [17].Wilson VG. Introduction to sumoylation. SUMO Regul. Cell. Processes. 2017;1–12. DOI: 10.1007/978-3-319-50044-7 [DOI] [PubMed] [Google Scholar]
- [18].Seufert W, Futcher B, Jentsch S. Role of a ubiquitin-conjugating enzyme in degradation of S- and M-phase cyclins. Nature. 1995;373(6509):78–81. [DOI] [PubMed] [Google Scholar]
- [19].Geiss-Friedlander R, Melchior F. Concepts in sumoylation: A decade on. Nat. Rev. Mol. Cell Biol. 2007;8(12):947–956. [DOI] [PubMed] [Google Scholar]
- [20].Talamillo A, Sánchez J, Barrio R. Functional analysis of the SUMOylation pathway in. Drosophila. Biochem. Soc. Trans. 2008;36(5):868–873. [DOI] [PubMed] [Google Scholar]
- [21].Hendriks IA, Lyon D, Young C, et al. Site-specific mapping of the human SUMO proteome reveals co-modification with phosphorylation. Nat. Struct. & Mol. Biol. 2017;24(3):325. [DOI] [PubMed] [Google Scholar]
- [22].Golebiowski F, Matic I, Tatham MH, et al. System-wide changes to SUMO modifications in response to heat shock. Sci Signal. 2009;2(72):ra24 LP–ra24. [DOI] [PubMed] [Google Scholar]
- [23].Tammsalu T, Matic I, Jaffray EG, et al. Proteome-wide identification of SUMO2 modification sites. Sci Signal. 2014;7(323):rs2 LP–rs2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pirone L, Xolalpa W, Sigurðsson JO, et al. A comprehensive platform for the analysis of ubiquitin-like protein modifications using in vivo biotinylation. Sci. Rep. 2017;7(1):40756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hendriks IA, Lyon D, Su D, et al. Site-specific characterization of endogenous SUMOylation across species and organs. Nat. Commun. 2018;9(1):2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mabb AM, Miyamoto S. SUMO and NF-κB ties. Cell. Mol. Life Sci. 2007;64(15):1979–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Adorisio S, Fierabracci A, Muscari I, et al. SUMO proteins: Guardians of immune system. J. Autoimmun. 2017;84:21–28. [DOI] [PubMed] [Google Scholar]
- [28].Everett RD, Boutell C, Hale BG. Interplay between viruses and host sumoylation pathways. Nat. Rev. Microbiol. 2013;11(6):400–411. [DOI] [PubMed] [Google Scholar]
- [29].Handu M, Kaduskar B, Ravindranathan R, et al. SUMO-enriched proteome for drosophila innate immune response. G3 (Bethesda). 2015;5(10):2137–2154. DOI: 10.1534/g3.115.020958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Domingues P, Golebiowski F, Tatham MH, et al. Global reprogramming of host SUMOylation during Influenza virus infection. Cell Rep. 2015;13(7):1467–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sloan E, Tatham MH, Groslambert M, et al. Analysis of the SUMO2 Proteome during HSV-1 Infection. PLOS Pathog. 2015;11(7):e1005059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Srikanth CV, Verma S. Sumoylation as an integral mechanism in bacterial infection and disease progression. Adv Exp Med Biol. 2017;963:389–408. DOI: 10.1007/978-3-319-50044-7_22 [DOI] [PubMed] [Google Scholar]
- [33].Bhaskar V, Smith M, Courey AJ. Conjugation of Smt3 to dorsal may potentiate the drosophila immune response. Mol Cell Biol. 2002;22(2):492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wuerzberger-Davis SM, Nakamura Y, Seufzer BJ, et al. NF-κB activation by combinations of NEMO SUMOylation and ATM activation stresses in the absence of DNA damage. Oncogene. 2007;26(5):641–651. [DOI] [PubMed] [Google Scholar]
- [35].Huang TT, Wuerzberger-Davis SM, Wu Z-H, et al. Sequential modification of NEMO/IKKγ by SUMO-1 and ubiquitin mediates NF-κB activation by genotoxic stress. Cell. 2003;115(5):565–576. [DOI] [PubMed] [Google Scholar]
- [36].Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell. 1998;2(2):233–239. [DOI] [PubMed] [Google Scholar]
- [37].Hay RT, Vuillard L, Desterro JMP, et al. Control of NF-κB transcriptional activation by signal induced proteolysis of IκBα. Philos. Trans. R. Soc. B Biol. Sci. 1999;354(1389):1601–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bossis G, Malnou CE, Farras R, et al. Down-Regulation of c-Fos/c-Jun AP-1 Dimer Activity by Sumoylation. Mol Cell Biol. 2005;25(16):6964 LP– 6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Müller S, Berger M, Lehembre F, et al. c-Jun and p53 activity is modulated by SUMO-1 modification. J. Biol. Chem. 2000;275(18):13321–13329. [DOI] [PubMed] [Google Scholar]
- [40].Fukuyama H, Verdier Y, Guan Y, et al. Landscape of protein–protein interactions in Drosophila immune deficiency signaling during bacterial challenge. Proc Natl Acad Sci. 2013;110(26):10717 LP– 10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mukhopadhyay D, Dasso M. The role of SUMO in mitosis. Adv Exp Med Biol. 2017;963:171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hay RT. Decoding the SUMO signal. Biochem. Soc. Trans. 2013;41(2):463–473. [DOI] [PubMed] [Google Scholar]
- [43].Kunz K, Piller T, Müller S. SUMO-specific proteases and isopeptidases of the SENP family at a glance. J Cell Sci. 2018;131(6):jcs211904. [DOI] [PubMed] [Google Scholar]
- [44].Nayak A, Müller S. SUMO-specific proteases/isopeptidases: SENPs and beyond. Genome Biol. 2014;15(7):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Citro S. Sumo paralogs redundancy and divergencies. Frontiers in Bioscience. 2013;S5(2):544–553. [DOI] [PubMed] [Google Scholar]
- [46].Liang Y-C, Lee -C-C, Yao Y-L, et al. SUMO5, a novel poly-SUMO isoform, regulates PML nuclear bodies. Sci. Rep. 2016;6(1):26509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Beauclair G, Bridier-Nahmias A, Zagury J-F, et al. JASSA: A comprehensive tool for prediction of SUMOylation sites and SIMs. Bioinformatics. 2015;31(21):3483–3491. [DOI] [PubMed] [Google Scholar]
- [48].Reverter D, Lima CD. Insights into E3 ligase activity revealed by a SUMO–RanGAP1–Ubc9–Nup358 complex. Nature. 2005;435(7042):687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Steinacher R, Schär P. Functionality of human thymine DNA glycosylase requires SUMO-regulated changes in protein conformation. Curr. Biol. 2005;15(7):616–623. [DOI] [PubMed] [Google Scholar]
- [50].Chymkowitch P, Le May N, Charneau P, et al. The phosphorylation of the androgen receptor by TFIIH directs the ubiquitin/proteasome process. Embo J. 2011;30(3):468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tempé D, Vives E, Brockly F, et al. SUMOylation of the inducible (c-Fos: C-Jun)/AP-1transcription complex occurs on target promoters to limit transcriptional activation. Oncogene. 2014;33(7):921–927. [DOI] [PubMed] [Google Scholar]
- [52].Hong Y, Rogers R, Matunis MJ, et al. Regulation of heat shock transcription factor 1 by stress-induced SUMO-1 modification. J. Biol. Chem. 2001;276(43):40263–40267. [DOI] [PubMed] [Google Scholar]
- [53].Miles WO, Jaffray E, Campbell SG, et al. Medea SUMOylation restricts the signaling range of the Dpp morphogen in the Drosophila embryo. Genes Dev. 2008;22(18):2578–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Song J, Durrin LK, Wilkinson TA, et al. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc Natl Acad Sci U S A. 2004;101(40):14373 LP– 14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ghioni P, D’Alessandra Y, Mansueto G, et al. The protein stability and transcriptional activity of p63α are regulated by SUMO-1 conjugation. Cell Cycle. 2005;4(1):183–190. [DOI] [PubMed] [Google Scholar]
- [56].Gresko E, Möller A, Roscic A, et al. Covalent modification of human homeodomain interacting protein kinase 2 by SUMO-1 at lysine 25 affects its stability. Biochem. Biophys. Res. Commun. 2005;329(4):1293–1299. [DOI] [PubMed] [Google Scholar]
- [57].Klenk C, Humrich J, Quitterer U, et al. SUMO-1 controls the protein stability and the biological function of phosducin. J. Biol. Chem. 2006;281(13):8357–8364. [DOI] [PubMed] [Google Scholar]
- [58].Uzunova K, Gottsche K, Miteva M, et al. Ubiquitin-dependent proteolytic control of SUMO conjugates. J. Biol. Chem. 2007;282(47):34167–34175. [DOI] [PubMed] [Google Scholar]
- [59].Tatham MH, Geoffroy M-C, Shen L, et al. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat. Cell Biol. 2008;10(5):538–546. [DOI] [PubMed] [Google Scholar]
- [60].Erker Y, Neyret-Kahn H, Seeler JS, et al. Arkadia, a novel SUMO-targeted ubiquitin ligase involved in PML degradation. Mol Cell Biol. 2013;33(11):2163 LP– 2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Garaude J, Farrás R, Bossis G, et al. SUMOylation regulates the transcriptional activity of junB in T lymphocytes. J Immunol. 2008;180(9):5983 LP– 5990. [DOI] [PubMed] [Google Scholar]
- [62].Puntambekar SS, Nyayanit D, Saxena P, et al. Identification of unintuitive features of sumoylation through mathematical modeling. J. Biol. Chem. 2016;291(18):9458–9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Celen AB, Sahin U. Sumoylation on its 25th anniversary: Mechanisms, pathology, and emerging concepts. Febs J. 2020;287(15):3110–3140. n/a. [DOI] [PubMed] [Google Scholar]
- [64].Enserink JM. Sumo and the cellular stress response. Cell Div. 2015;10(1). DOI: 10.1186/s13008-015-0010-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kurepa J, Walker JM, Smalle J, et al. The small ubiquitin-like modifier (SUMO) protein modification system in arabidopsis. J. Biol. Chem. 2003;278(9):6862–6872. [DOI] [PubMed] [Google Scholar]
- [66].Jiang Z, Fan Q, Zhang Z, et al. SENP1 deficiency promotes ER stress-induced apoptosis by increasing XBP1 SUMOylation. Cell Cycle. 2012;11(6):1118–1122. [DOI] [PubMed] [Google Scholar]
- [67].Zhou W, Ryan JJ, Zhou H. Global analyses of sumoylated proteins in saccharomyces cerevisiae. J. Biol. Chem. 2004;279(31):32262–32268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hendriks IA, D’Souza RC, Chang J-G, et al. System-wide identification of wild-type SUMO-2 conjugation sites. Nat. Commun. 2015;6(1):7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hendriks IA, Treffers LW, Verlaan-de Vries M, et al. SUMO-2 orchestrates chromatin modifiers in response to DNA damage. Cell Rep. 2015;10(10):1778–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hendriks IA, Vertegaal ACO. A comprehensive compilation of SUMO proteomics. Nat. Rev. Mol. Cell Biol. 2016;17(9):581. [DOI] [PubMed] [Google Scholar]
- [71].Lamoliatte F, McManus FP, Maarifi G, et al. Uncovering the SUMOylation and ubiquitylation crosstalk in human cells using sequential peptide immunopurification. Nat. Commun. 2017;8(1):14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McManus FP, Bourdeau V, Acevedo M, et al. Quantitative SUMO proteomics reveals the modulation of several PML nuclear body associated proteins and an anti-senescence function of UBC9. Sci. Rep. 2018;8(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Karpac J, Younger A, Jasper H. Dynamic coordination of innate immune signaling and insulin signaling regulates systemic responses to localized DNA damage. Dev Cell. 2011;20(6):841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Boltz KA, Carney GE. Loss of p24 function in Drosophila melanogaster causes a stress response and increased levels of NF-κB-regulated gene products. BMC Genomics. 2008;9(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Stergiopoulos K, Cabrero P, Davies S-A, et al. Salty dog, an SLC5 symporter, modulates Drosophila response to salt stress. Physiol Genomics. 2009;37(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cantera R, Barrio R. Do the genes of the innate immune response contribute to neuroprotection in drosophila ? J Innate Immun. 2015;7(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chiu H, Ring BC, Sorrentino RP, et al. dUbc9 negatively regulates the Toll-NF-κB pathways in larval hematopoiesis and drosomycin activation in Drosophila. Dev. Biol. 2005;288(1):60–72. [DOI] [PubMed] [Google Scholar]
- [78].Huang L, Ohsako S, Tanda S. The lesswright mutation activates Rel-related proteins, leading to overproduction of larval hemocytes in Drosophila melanogaster. Dev. Biol. 2005;280(2):407–420. [DOI] [PubMed] [Google Scholar]
- [79].Liu B, Yang Y, Chernishof V, et al. Proinflammatory stimuli induce IKKα-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell. 2007;129(5):903–914. [DOI] [PubMed] [Google Scholar]
- [80].Ozato K, Tailor P, Kubota T. The interferon regulatory factor family in host defense: Mechanism of action. J. Biol. Chem. 2007;282(28):20065–20069. [DOI] [PubMed] [Google Scholar]
- [81].Pascual G, Fong AL, Ogawa S, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature. 2005;437(7059):759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Boggio R, Colombo R, Hay RT, et al. A mechanism for inhibiting the SUMO pathway. Mol Cell. 2004;16(4):549–561. [DOI] [PubMed] [Google Scholar]
- [83].Colombo R, Boggio R, Seiser C, et al. The adenovirus protein Gam1 interferes with sumoylation of histone deacetylase 1. EMBO Rep. 2002;3(11):1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chang T-H, Kubota T, Matsuoka M, et al. Ebola zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLOS Pathog. 2009;5(6):e1000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Vidal S, El Motiam A, Seoane R, et al. Regulation of the ebola virus VP24 protein by SUMO. J. Virol. 2019;94(1):e01687–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pal S, Santos A, Rosas JM, et al. Influenza a virus interacts extensively with the cellular SUMOylation system during infection. Virus Res. 2011;158(1–2):12–27. [DOI] [PubMed] [Google Scholar]
- [87].Ribet D, Hamon M, Gouin E, et al. Listeria monocytogenes impairs SUMOylation for efficient infection. Nature. 2010;464(7292):1192–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Fritah S, Lhocine N, Golebiowski F, et al. Sumoylation controls host anti-bacterial response to the gut invasive pathogen Shigella flexneri. EMBO Rep. 2014;15(9):965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Verma S, Mohapatra G, Ahmad SM, et al. Salmonella engages host MicroRNAs To modulate SUMOylation: A new arsenal for intracellular survival. Mol Cell Biol. 2015;35(17):2932 LP– 2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Impens F, Radoshevich L, Cossart P, et al. Mapping of SUMO sites and analysis of SUMOylation changes induced by external stimuli. Proc. Natl. Acad. Sci. U. S. A. 2014;111(34):12432–12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Govind S, Nehm RH. Innate immunity in fruit flies: A textbook example of genomic recycling. PLOS Biol. 2004;2(8):e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Buchon N, Silverman N, Cherry S. Immunity in Drosophila melanogaster — from microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014;14(12):796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cherry S, Silverman N. Host-pathogen interactions in drosophila: New tricks from an old friend. Nat. Immunol. 2006;7(9):911–917. [DOI] [PubMed] [Google Scholar]
- [94].Lemaitre B, Hoffmann J. The host defense of drosophila melanogaster. Annu. Rev. Immunol. 2007;25(1):697–743. [DOI] [PubMed] [Google Scholar]
- [95].Ferrandon D, Imler J-L, Hetru C, et al. The drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 2007;7(11):862–874. [DOI] [PubMed] [Google Scholar]
- [96].Imler J-L. Overview of Drosophila immunity: A historical perspective. Dev. Comp. Immunol. 2014;42(1):3–15. [DOI] [PubMed] [Google Scholar]
- [97].Ligoxygakis P. Chapter two - genetics of immune recognition and response in drosophila host defense. In: Friedmann T, Dunlap JC, Goodwin SFBT-AG, editors. Advnaces in genetics. Vol. 83. USA: Elsevier; 2013. p. 71–97 [DOI] [PubMed] [Google Scholar]
- [98].West C, Silverman N, Cherry S. p38b and JAK-STAT signaling protect against Invertebrate iridescent virus 6 infection in Drosophila. PLOS Pathog. 2018;14(5):e1007020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Paddibhatla I, Lee MJ, Kalamarz ME, et al. Role for sumoylation in systemic inflammation and immune homeostasis in Drosophila larvae. PLoS Pathog. 2010;6(12):e1001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Aillet F, Lopitz-Otsoa F, Egaña I, et al. Heterologous SUMO-2/3-ubiquitin chains optimize IκBα degradation and NF-κB Activity. PLoS One. 2012;7(12):e51672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ureña E, Pirone L, Chafino S, et al. Evolution of SUMO function and chain formation in insects. Mol. Biol. Evol. 2015;33(2):568–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Vatsyayan J, Qing G, Xiao G, et al. SUMO1 modification of NF-kappaB2/p100 is essential for stimuli-induced p100 phosphorylation and processing. EMBO Rep. 2008;9(9):885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Leidner J, Voogdt C, Niedenthal R, et al. SUMOylation attenuates the transcriptional activity of the NF-κB subunit RelB. J. Cell. Biochem. 2014;115(8):1430–1440. [DOI] [PubMed] [Google Scholar]
- [104].Kubota K, Gay NJ. The dorsal protein enhances the biosynthesis and stability of the Drosophila IkB homologue cactus. Nucleic Acids Res. 1995;23(16):3111–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kidd S. Characterization of the Drosophila cactus locus and analysis of interactions between cactus and dorsal proteins. Cell. 1992;71(4):623–635. [DOI] [PubMed] [Google Scholar]
- [106].Smith M, Bhaskar V, Fernandez J, et al. Drosophila Ulp1, a nuclear pore-associated SUMO protease, prevents accumulation of cytoplasmic SUMO conjugates. J. Biol. Chem. 2004;279(42):43805–43814. [DOI] [PubMed] [Google Scholar]
- [107].Liu Y, Bridges R, Wortham A, et al. NF-κB repression by PIAS3 mediated RelA SUMOylation. PLoS One. 2012;7(5):e37636–e37636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hayden MS, Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014;26(3):253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Devin A, Cook A, Lin Y, et al. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 Recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12(4):419–429. [DOI] [PubMed] [Google Scholar]
- [110].Lee TH, Shank J, Cusson N, et al. The kinase activity of Rip1 is not required for tumor necrosis factor-α-induced IκB Kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J. Biol. Chem. 2004;279(32):33185–33191. [DOI] [PubMed] [Google Scholar]
- [111].Yang Y, Xia F, Hermance N, et al. A cytosolic ATM/NEMO/RIP1 complex recruits TAK1 To mediate the NF-κB and p38 mitogen-activated protein kinase (MAPK)/MAPK-activated protein 2 responses to DNA damage. Mol Cell Biol. 2011;31(14):2774 LP– 2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ganesan S, Aggarwal K, Paquette N, et al. NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr Top Microbiol Immunol. 2011;349:25–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kleino A, Silverman N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Comp. Immunol. 2014;42(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kim C-H, Paik D, Rus F, et al. The caspase-8 homolog dredd cleaves imd and relish but is not inhibited by p35. J. Biol. Chem. 2014;289(29):20092–20101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chen L, Paquette N, Mamoor S, et al. Innate immune signaling in Drosophila is regulated by TGFβ-activated kinase (Tak1)-triggered ubiquitin editing. J Biol Chem. 2017;292(21):8738–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Paquette N, Broemer M, Aggarwal K, et al. Caspase-mediated cleavage, IAP binding, and ubiquitination: Linking three mechanisms crucial for drosophila NF-κB signaling. Mol Cell. 2010;37(2):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Mabb AM, Wuerzberger-Davis SM, Miyamoto S. PIASy mediates NEMO sumoylation and NF-κB activation in response to genotoxic stress. Nat. Cell Biol. 2006;8(9):986–993. [DOI] [PubMed] [Google Scholar]
- [118].Silver HR, Nissley JA, Reed SH, et al. A role for SUMO in nucleotide excision repair. DNA Repair (Amst). 2011;10(12):1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Jentsch S, Psakhye I. Control of nuclear activities by substrate-selective and protein-group SUMOylation. Annu. Rev. Genet. 2013;47(1):167–186. [DOI] [PubMed] [Google Scholar]
- [120].Psakhye I, Jentsch S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell. 2012;151(4):807–820. [DOI] [PubMed] [Google Scholar]
- [121].Wang F, Xia Q. Back to homeostasis: Negative regulation of NF-κB immune signaling in insects. Dev Comp Immunol. 2018;87:216–223. [DOI] [PubMed] [Google Scholar]
- [122].Kim M, Lee JH, Lee SY, et al. Caspar, a suppressor of antibacterial immunity in Drosophila. Proc Natl Acad Sci. 2006;103(44):16358 LP– 16363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Park M-Y, Moon J-H, Lee K-S, et al. FAF1 suppresses IκB kinase (IKK) activation by disrupting the IKK complex assembly. J. Biol. Chem. 2007;282(38):27572–27577. [DOI] [PubMed] [Google Scholar]
- [124].Ryu SW, Lee SJ, Park MY, et al. Fas-associated factor 1, FAF1, Is a member of fas death-inducing signaling complex. J. Biol. Chem. 2003;278(26):24003–24010. [DOI] [PubMed] [Google Scholar]
- [125].Park M-Y, Jang HD, Lee SY, et al. Fas-associated factor-1 inhibits nuclear factor-κB (NF-κB) activity by interfering with nuclear translocation of the RelA (p65) subunit of NF-κB. J. Biol. Chem. 2004;279(4):2544–2549. [DOI] [PubMed] [Google Scholar]
- [126].Wang C-H, Hung P-W, Chiang C-W, et al. Identification of two independent SUMO-interacting motifs in Fas-associated factor 1 (FAF1): Implications for mineralocorticoid receptor (MR)-mediated transcriptional regulation. Biochim. Biophys. Acta - Mol. Cell Res. 2019;1866(8):1282–1297. [DOI] [PubMed] [Google Scholar]
- [127].Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16(2):141–147. [DOI] [PubMed] [Google Scholar]
- [128].Lester SN, Li K. Toll-like receptors in antiviral innate immunity. J. Mol. Biol. 2014;426(6):1246–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Schindler C, Levy DE, Decker T. JAK-STAT signaling: From interferons to cytokines. J. Biol. Chem. 2007;282(28):20059–20063. [DOI] [PubMed] [Google Scholar]
- [130].Majoros A, Platanitis E, Kernbauer-Hölzl E, et al. Canonical and non-canonical aspects of JAK–STAT signaling: Lessons from interferons for cytokine responses. Front. Immunol. 2017;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Xu J, Cherry S. Viruses and antiviral immunity in Drosophila. Dev. Comp. Immunol. 2014;42(1):67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sansone CL, Cohen J, Yasunaga A, et al. Microbiota-dependent priming of antiviral intestinal immunity in drosophila. Cell Host Microbe. 2015;18(5):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Merkling SH, van Rij RP. Beyond RNAi: Antiviral defense strategies in Drosophila and mosquito. J. Insect Physiol. 2013;59(2):159–170. [DOI] [PubMed] [Google Scholar]
- [134].Nakamoto M, Moy R, Xu J, et al. Virus recognition by Toll-7 activates antiviral autophagy in drosophila. Immunity. 2012;36(4):658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Goto A, Okado K, Martins N, et al. The kinase IKKβ regulates a STING- and NF-κB-dependent antiviral response pathway in drosophila. Immunity. 2018;49(2):225–234.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Liu Y, Gordesky-Gold B, Leney-Greene M, et al. Inflammation-induced, STING-dependent autophagy restricts zika virus infection in the drosophila brain. Cell Host Microbe. 2018;24(1):57–68.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Shelly S, Lukinova N, Bambina S, et al. Autophagy Is an essential component of drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30(4):588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Schmidt N, Domingues P, Golebiowski F, et al. An influenza virus-triggered SUMO switch orchestrates co-opted endogenous retroviruses to stimulate host antiviral immunity. Proc Natl Acad Sci. 2019;116(35):17399 LP– 17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wang X-H, Aliyari R, Li W-X, et al. RNA interference directs innate immunity against viruses in adult drosophila. Science. 2006;312(5772):452 LP– 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Liu J, Carmell MA, Rivas FV, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305(5689):1437 LP– 1441. [DOI] [PubMed] [Google Scholar]
- [141].Sahin U, Lapaquette P, Andrieux A, et al. Sumoylation of human argonaute 2 at lysine-402 regulates its stability. PLoS One. 2014;9(7):e102957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Wu H, Wang MC, Bohmann D. JNK protects Drosophila from oxidative stress by trancriptionally activating autophagy. Mech. Dev. 2009;126(8–9):624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Dostert C, Jouanguy E, Irving P, et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat. Immunol. 2005;6(9):946–953. [DOI] [PubMed] [Google Scholar]
- [144].Grönholm J, Ungureanu D, Vanhatupa S, et al. Sumoylation of drosophila transcription factor STAT92E. J. Innate Immun. 2010;2(6):618–624. [DOI] [PubMed] [Google Scholar]
- [145].Grönholm J, Vanhatupa S, Ungureanu D, et al. Structure-function analysis indicates that sumoylation modulates DNA-binding activity of STAT1. BMC Biochem. 2012;13(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Beltrao P, Bork P, Krogan NJ, et al. Evolution and functional cross-talk of protein post-translational modifications. Mol. Syst. Biol. 2013;9(1):714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Venne AS, Kollipara L, Zahedi RP. The next level of complexity: Crosstalk of posttranslational modifications. Proteomics. 2014;14(4–5):513–524. [DOI] [PubMed] [Google Scholar]
- [148].Nie M, Boddy MN. Cooperativity of the SUMO and ubiquitin pathways in genome stability. Biomolecules. 2016;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Nie M, Moser BA, Nakamura TM, et al. SUMO-targeted ubiquitin ligase activity can either suppress or promote genome instability, depending on the nature of the DNA lesion. PLOS Genet. 2017;13(5):e1006776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Spit M, Rieser E, Walczak H. Linear ubiquitination at a glance. J Cell Sci. 2019;132(2):jcs208512. [DOI] [PubMed] [Google Scholar]
- [151].Ikeda F. Linear ubiquitination signals in adaptive immune responses. Immunol. Rev. 2015;266(1):222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Davis ME, Gack MU. Ubiquitination in the antiviral immune response. Virology. 2015;479–480:52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Iwai K. Diverse ubiquitin signaling in NF-κB activation. Trends Cell Biol. 2012;22(7):355–364. [DOI] [PubMed] [Google Scholar]
- [154].Hendriks IA, Vertegaal ACO. A high-yield double-purification proteomics strategy for the identification of SUMO sites. Nat. Protoc. 2016;11(9):1630–1649. [DOI] [PubMed] [Google Scholar]
- [155].Gupta D, Bhattacharjee O, Mandal D, et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci. 2019;232:116636. [DOI] [PubMed] [Google Scholar]
- [156].Harrison PT, Hart S. A beginner’s guide to gene editing. Exp. Physiol. 2018;103(4):439–448. [DOI] [PubMed] [Google Scholar]
- [157].Lumpkin RJ, Gu H, Zhu Y, et al. Site-specific identification and quantitation of endogenous SUMO modifications under native conditions. Nat. Commun. 2017;8(1):1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Hendriks IA, D’Souza RCJ, Yang B, et al. Uncovering global SUMOylation signaling networks in a site-specific manner. Nat. Struct. Mol. Biol. 2014;21(10):927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Nie M, Xie Y, Loo JA, et al. Genetic and proteomic evidence for roles of Drosophila SUMO in cell cycle control, Ras signaling, and early pattern formation. PLoS One. 2009;4(6):e5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Schimmel J, Eifler K, Sigurðsson J, et al. Uncovering SUMOylation dynamics during cell-cycle progression reveals FoxM1 as a key mitotic SUMO target protein. Mol Cell. 2014;53(6):1053–1066. [DOI] [PubMed] [Google Scholar]
- [161].Xiao Z, Chang JG, Hendriks IA, et al. System-wide analysis of SUMOylation dynamics in response to replication stress reveals novel small ubiquitin-like modified target proteins and acceptor lysines relevant for genome stability. Mol. Cell. Proteomics. 2015;14(5):1419 LP– 1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Bökel C. EMS screens: from mutagenesis to screening and mapping. Methods Mol Biol. Totowa (NJ): Humana Press; 2008;420:119–138. DOI: 10.1007/978-1-59745-583-1_7. [DOI] [PubMed] [Google Scholar]
- [163].St Johnston D. The art and design of genetic screens: Drosophila melanogaster. Nat. Rev. Genet. 2002;3(3):176–188. [DOI] [PubMed] [Google Scholar]
- [164].Venken KJT, He Y, Hoskins RA, et al. P[acman]: A BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314(5806):1747 LP– 1751. [DOI] [PubMed] [Google Scholar]
- [165].Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401 LP– 415. [DOI] [PubMed] [Google Scholar]
- [166].Mali P, Yang L, Esvelt KM, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823 LP– 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Yang L, Mali P, Kim-Kiselak C, et al. CRISPR-cas-mediated targeted genome editing in human cells. In: Storici F, editor. Gene Correction. Totowa, NJ:Humana Press; 2014. p. 245–267. DOI: 10.1007/978-1-62703-761-7_16 [DOI] [PubMed] [Google Scholar]
- [168].Jinek M, East A, Cheng A, et al. RNA-programmed genome editing in human cells. Elife. 2013;2:e00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096. [DOI] [PubMed] [Google Scholar]
- [170].Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Kondo S, Ueda R. Highly improved gene targeting by germline-specific Cas9 expression in drosophila. Genetics. 2013;195(3):715 LP– 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Bassett AR, Tibbit C, Ponting CP, et al. Highly efficient targeted mutagenesis of drosophila with the CRISPR/Cas9 system. Cell Rep. 2013;4(1):220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Gratz SJ, Cummings AM, Nguyen JN, et al. Genome engineering of drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194(4):1029 LP– 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Gratz SJ, Cummings AM, Nguyen JN, et al. Genome engineering of drosophila with the CRISPR RNA-guided cas9 nuclease. Genetics. 2013;194(4):1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


