ABSTRACT
Preclinical data suggest that a “prime-boost” vaccine regimen using a target-expressing lentiviral vector for priming, followed by a recombinant protein boost, may be effective against cancer; however, this strategy has not been evaluated in a clinical setting. CMB305 is a prime-boost vaccine designed to induce a broad anti-NY-ESO-1 immune response. It is composed of LV305, which is an NY-ESO-1 expressing lentiviral vector, and G305, a recombinant adjuvanted NY-ESO-1 protein. This multicenter phase 1b, first-in-human trial evaluated CMB305 in patients with NY-ESO-1 expressing solid tumors. Safety was examined in a 3 + 3 dose-escalation design, followed by an expansion with CMB305 alone or in a combination with either oral metronomic cyclophosphamide or intratumoral injections of a toll-like receptor agonist (glucopyranosyl lipid A). Of the 79 patients who enrolled, 81.0% had sarcomas, 86.1% had metastatic disease, and 57.0% had progressive disease at study entry. The most common adverse events were fatigue (34.2%), nausea (26.6%), and injection-site pain (24.1%). In patients with soft tissue sarcomas, a disease control rate of 61.9% and an overall survival of 26.2 months (95% CI, 22.1–NA) were observed. CMB305 induced anti-NY-ESO-1 antibody and T-cell responses in 62.9% and 47.4% of patients, respectively. This is the first trial to test a prime-boost vaccine regimen in patients with advanced cancer. This approach is feasible, can be delivered safely, and with evidence of immune response as well as suggestion of clinical benefit.
KEYWORDS: NY-ESO-1, vaccine, immunotherapy, LV305, G305, synovial sarcoma, myxoid liposarcoma, prime-boost, lentivirus
Introduction
Based on preclinical studies, therapeutic cancer vaccines designed to induce an immune response against tumor cells are a promising treatment option for cancer.1–3 However, clinical cancer vaccine studies have resulted in only marginal efficacy to date, particularly in the advanced and metastatic settings, and identifying the optimal vaccine platform, patient (sub)population, and tumor antigen target(s) remains a challenge.4–6 New York esophageal squamous cell carcinoma-1 (NY-ESO-1) is a cancer-testis antigen expressed only in the spermatogonia of the testis, the placenta, and in certain malignancies, and serves as an immunotherapeutic target for a wide variety of solid tumors, including melanoma, lung, and ovarian cancers.7–10 Multiple trials targeting NY-ESO-1 in these cancers and others using both vaccine and adoptive T-cell therapy approaches have demonstrated clear clinical benefit.11–13 In this regard, two soft tissue sarcoma (STS) subtypes, synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCL), have been of particular interest because of the very high consistency and homogeneity of their NY-ESO-1 expression.8,14
CMB305 was developed as a clinical prime-boost vaccine regimen Figure 1. Heterologous prime-boost regimens that use two different vaccines to first prime the immune system and then boost its response have been shown to improve the efficacy of cancer vaccines in numerous preclinical animal models, and lentiviral vectors as the priming component have emerged as a promising new vaccine modality.15–19
Figure 1.
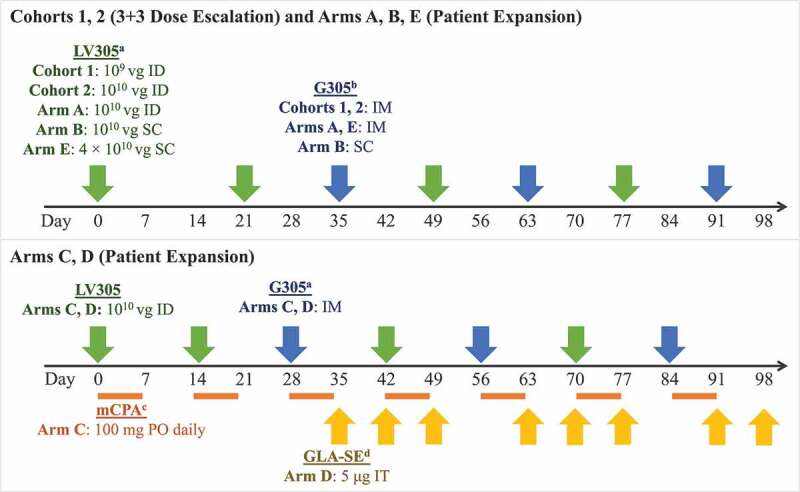
Dose, route, and timing of treatment administration by study arm. a LV305 is a NY-ESO-1 expressing, dendritic-cell tropic lentiviral vector. b G305 is recombinant NY-ESO-1 protein formulated in an oil-in-water stable emulsion with the synthetic TLR4 GLA. G305 dose for all study arms consisted of 250 μg NY-ESO-1 protein mixed with 5-μg GLA-SE. Patients were also given a boosting dose of G305 at each follow-up visit during the first year. c mCPA was only administered to patients in Arm C. It was dosed at 100 mg PO once daily for 7 days, then was not given for the next 7 days, in cycles that repeated until day 97. Patients were given a 1-week supply at each visit.d IT GLA-SE (5 µg/dose) was only administered to patients in Arm D and could have been injected into accessible primary tumors or distant metastases. If no accessible tumor was present at weeks 10, 11, 13, or 14, GLA-SE was not administered. Abbreviations: GLA-SE = glucopyranosyl lipid A-stable emulsion; ID = intradermal; IM = intramuscular; mCPA = metronomic cyclophosphamide; PO = oral; SC = subcutaneous; IT = intratumoral; μg = microgram; vg = viral genomes
The priming component of CMB305 is LV305, which is a replication-incompetent, integration-deficient, improved third-generation lentiviral vector that contains RNA encoding for the full-length NY-ESO-1 protein.20 Further, LV305 is based on the ZVex® platform, which has been shown to transduce dendritic cells through pseudotyping with an engineered Sindbis virus glycoprotein called SINVar1 that binds the C-type lectin receptor DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin) expressed on immature dendritic cells.21,22 As a result, the vector induces direct major histocompatibility complex class I presentation of cluster of differentiation 8 (CD8) epitopes and robust CD8 T-cell immune responses. A phase I clinical trial demonstrated that LV305 is safe with evidence of inducing an anti-NY-ESO-1 CD4 and CD8 T-cell immune response, but no anti-NY-ESO-1 antibodies.23 Dosing of LV305 led to a partial remission in one SS patient refractory to multiple lines of prior therapy.24
The boost component of CMB305 is G305, which is composed of full-length recombinant E. coli-produced NY-ESO-1 protein co-formulated with glucopyranosyl lipid A (GLA), a potent toll-like receptor 4 (TLR4) agonist as an adjuvant, in a stable squalene oil-in-water emulsion (SE). G305 can induce anti-NY-ESO-1 specific CD4 T-cell and antibody responses as a single agent and has been shown to be safe at doses ranging from 2 to 10 µg.25 The rationale of combining LV305 and with G305 was to induce stronger T-cell responses and integrated immune responses (CD4 and CD8 T-cells, and antibodies), which preclinically resulted in improved tumor control.15
This phase 1b, first-in-human study of CMB305 evaluated the safety, efficacy, and immunogenicity LV305 and G305 administered in a prime-boost vaccine regimen in patients with advanced solid tumors. The CMB305 regimen was also tested in a cohort receiving metronomic cyclophosphamide (mCPA) in order to eliminate regulatory T-cell populations.26,27 In addition, CMB305 was tested in an intratumoral “prime-pull” strategy that was designed to first stimulate (prime) the systemic innate immune response and then recruit (pull) NY-ESO-1-specific CD8 T-cells to the tumor by adding GLA dosed locally. This approach was shown in preclinical models to increase the T-cell inflammation of tumors and greatly enhance clinical efficacy.28
Materials and methods
Patient population
Patients aged 18 years or older with Eastern Cooperative Oncology Group (ECOG) Performance Status score of 0 or 1 who had locally advanced, relapsed, and/or metastatic solid tumors positive for NY-ESO-1 expression by immunohistochemistry staining were eligible to participate. Table 1 displays the tumor types eligible for each study arm. Key exclusion criteria were the receipt of cancer therapies ≤3 weeks prior to CMB305 dosing; prior administration of LV305, G305, or NY-ESO-1 targeting immunotherapy; and concurrent or recent immunosuppression from systemic corticosteroids or other immunosuppressive medications (the use of physiologic doses of corticosteroids may have been approved after consultation with the Sponsor).
Table 1.
Eligible tumor types and rationale for each study arm
| Arm | Eligible Tumor Types | Rationale |
|---|---|---|
| Part 1a | ||
| Cohort 1 | NSCLC, ovarian, melanoma, sarcoma (any subtype) | Dose finding |
| Cohort 2 | NSCLC, ovarian, melanoma, sarcoma (any subtype) | Dose finding |
| Part 2b | ||
| Arm A | NSCLC, ovarian, SS, MRCL | Monotherapy of ID route |
| Arm B | SS, MRCL | Monotherapy of SC route |
| Arm C | SS, MRCL | Evaluate mCPA effect |
| Arm D | SS, MRCL | Evaluate IT GLA-SE effect |
| Arm E | Any soft tissue sarcoma | Dose finding of increased dose via SC route |
Abbreviations: GLA-SE = glucopyranosyl lipid A-stable emulsion; ID = intradermal; IT = intratumoral; mCPA = metronomic cyclophosphamide; MRCL = myxoid/round cell liposarcoma; NSCLC = non-small cell lung carcinoma; SC = subcutaneous; SS = synovial sarcoma
a3 + 3 design.
bPatients in Arms C, D, and E must have had tumors accessible for biopsy and must have provided consent for biopsies.
Study design
This phase 1b, multi-center, open-label study conducted in the United States occurred from January 29, 2015 to August 3, 2019. The study (ClinicalTrials.gov identifier NCT02387125) was conducted according to the principles outlined in the Declaration of Helsinki and Good Clinical Practice guidelines. Patients were not involved in the design of the study. Informed consent was obtained from all patients prior to participation, and the Institutional Review Boards and Institutional Biosafety Committees at the participating study sites approved the study protocol and the use of the lentiviral vector LV305 (biosafety level 2).
In Part 1, dose escalation, a standard 3 + 3 design was used to study the safety of intradermal (ID) administration of 2 dose levels (109 and 1010 viral genomes [vg]) of the LV305 component of CMB305. A fixed dose of G305 (250 μg NY-ESO-1 recombinant protein mixed with 5 μg GLA-SE) was used in Part 1 and all arms in Part 2. Dosing was to be suspended at any dose level if dose-limiting toxicity (DLT) was observed in 2 or more patients. In Part 2, there were 5 separate study arms: A, B, C, D, and E. Study treatment doses, routes, and schedules for each arm are presented in Figure 1. The CMB305 vaccine regimen was administered over 91 days for the dose-escalation cohorts and all arms except Arms C and D, for which administration occurred over 84 days. Arm A included a 1010 vg ID dose of LV305 and intramuscular (IM) administration of G305. Arm B examined subcutaneous (SC) administration of both 1010 vg of LV305 and G305. Arms C and D were added in August 2016; patients in these arms received 100 mg of oral mCPA or intratumoral injections of 5 μg GLA-SE, respectively, in addition to ID administration of 1010 vg of LV305 and IM G305. Finally, Arm E was added in October 2017 and used a 3 + 3 design to evaluate the safety of a higher 4 × 1010 vg SC dose of LV305 with the standard dose of IM G305; dosing was to be suspended if DLTs were observed in one-third or more of subjects. The sample sizes in Part 2 were designed to provide adequate preliminary data to inform subsequent trials and to reject an indication should no clinical benefit have occurred.
The primary objective was to evaluate the safety and tolerability of CMB305 in Cohorts 1 and 2 and in Arms A, B, and E, and then CMB305 in combination with oral mCPA or intratumoral GLA in Arms C and D, respectively. Adverse events (AEs) and serious adverse events (SAEs) were reported up to 30 days after the last dose. The potential for DLTs was assessed for 42 days, based on AE severity using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03.29 An LV305 persistence assay to evaluate for replication competent lentivirus was run using peripheral blood mononuclear cell (PBMC) pellets collected at different time points post-treatment (Day 168, Month 12, Month 24, and beyond) using a polymerase chain reaction-based assay (Molecular MD, Cambridge, MA).
The secondary objectives included evaluation of clinical responses, overall survival (OS), and progression-free survival (PFS). Tumor imaging was performed at baseline and every 8 weeks (12 weeks in Arms C and D) until confirmed disease progression per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 modified to use the immune-related response criteria (irRC).30,31 Survival visits were completed every 3 months until the end of the study. Additional secondary objectives included evaluation of time to next treatment, time to progression, cellular and humoral immune responses to NY-ESO-1, and evaluation of pre- and post-regimen blood samples for potential biomarkers of immunogenicity and clinical tumor response. Tumor biopsies were obtained from all patients at baseline to evaluate NY-ESO-1 expression, which was done by immunohistochemistry staining at Mosaic laboratory (Lake Forest, CA).
Systemic NY-ESO-1 immune response assessment was performed on all patients with biomarker samples using methods that have been published previously.24 Pre- and post PBMC and plasma collection occurred at baseline and pre-specified timepoints throughout the study. Assays for antibody response to NY-ESO-1 tumor antigen were evaluated by enzyme-linked immunosorbent assay using recombinant NY-ESO-1 protein and peptide pools. The induction of antibodies was defined as ≥4-fold increase in antibody titer as compared to baseline or seroconversion from negative (titer <100) to positive (titer ≥100). Cellular (T-cell) immune response to NY-ESO-1 was evaluated by interferon gamma (IFNγ) enzyme-linked immune absorbent spot (ELISpot). After bead-guided selection, CD4 and CD8 T-cells were independently cultured with peptide pulsed, irradiated T-cell depleted PBMC (serving as antigen-presenting cells) in RPMI + 10% serum type AB (to avoid potential reactivity) supplemented with interleukin-2 (10 U/mL) and interleukin-7 (20 ng/mL) twice a week. Cells were assessed for specificity at days 10 and 20 of culture, respectively for CD8 and CD4, using autologous antigen-presenting cells pulsed with NY-ESO-1 peptides or controls (influenza nucleoprotein peptide pool or dimethyl sulfoxide). A pool of overlapping 20-mer peptides covering the entire sequence of NY-ESO-1 was used as antigen, which ensured that any naturally processed Class I and Class II-restricted epitopes were detected rather than requiring up-front selection of minimal peptides. The assay was repeated for confirmation at day 14 and day 25 in most patients. The induction of CD4 or CD8 T-cells was defined as ≥2-fold increase as compared to baseline in spots per well in ELISpot.
Statistical analysis
Safety and efficacy analyses were performed with the safety population, which included all patients who received at least one injection/dose of study drug. All statistical tests were exploratory, two-sided and tested at alpha = 0.05. The nominal P values were presented without multiplicity adjustment. All statistical analyses were performed using SAS® version 9.4. Throughout the study, key safety analyses were performed quarterly for the purposes of safety monitoring.
Overall survival and PFS were analyzed using the Kaplan-Meier methodology. Stepwise Cox regression analysis was used to investigate prognostic baseline factors associated with OS and PFS. Tumor response was assessed by RECIST v1.1 criteria modified to use the unidimensional measurements approach of the irRC.30 At each tumor assessment, the response in index and new measurable lesions was defined based on the change in the sum of the longest diameters. Best overall response was defined as the best overall tumor response assessment assigned to a patient at any time-point during the study. Overall response rate was defined as percent of patients with immune-related complete response (irCR) or partial response (irPR) and the confidence interval (CI) was estimated using Clopper-Pearson exact method. Disease control rate was defined as the number of patients whose best overall response was irCR, irPR, or immune-related stable disease (irSD) divided by the number of evaluable patients. The minimum amount of time to establish irSD was 42 days (6 weeks). Median duration of response (DOR), time to next treatment, and time to progression with the corresponding 95% CIs were estimated using the Kaplan-Meier method in each treatment arm and disease type.
Results
Patient characteristics
A total of 90 patients were screened and 79 patients were enrolled at 8 sites (Appendix Figure 1). The median age of patients was 50 years (range: 20–80), and 40 (50.6%) patients were female Table 2. At study entry, 64 (81.0%) patients had sarcomas, 68 (86.1%) had metastatic disease, and 45 (57.0%) had progressive disease (PD). Twenty-eight (35.4%) patients had received ≥3 prior therapies. The highest level of NY-ESO-1 expression (>75% of tumor cells positive) was observed in 46 (58.2%) patients, while 9 (11.4%) patients had moderate (>25–75% of cells positive) and 24 (30.4%) patients had low (≤25% of cells positive) NY-ESO-1 expression levels, respectively (Appendix Figure 2). The majority of patients with non-small cell lung carcinoma (NSCLC) and ovarian cancer had ≤25% NY-ESO-1 expression (75.0% and 72.7%, respectively), whereas most patients with STS (69.8%) had >75% expression of NY-ESO-1 (Appendix Table 1). Clinical development of CMB305 ended in early 2019 and patients participating in this trial were taken off study drug treatment and completed end of study visits regardless of their status in the protocol visit schedule.
Table 2.
Patient demographics and baseline characteristics
|
Number (%) of Patients |
||||||||
|---|---|---|---|---|---|---|---|---|
|
Part 1: 3 + 3 Dose Escalation |
Part 2: Patient Expansion |
Total (N = 79) | ||||||
|
Cohort 1 109 vg dose of LV305 (N = 3) |
Cohort 2 1010 vg dose of LV305 (N = 3) |
Arm A CMB305 expansion (N = 35) |
Arm B SC admin of CMB305 (N = 9) |
Arm C CMB305 + mCPA (N = 10) |
Arm D CMB305 + GLA-SE (N = 10) |
Arm E CMB305 escalation (N = 9) |
||
| Age (years), mean (SD) | 62.3 (19.5) | 34.0 (7.0) | 53.8 (15.3) | 41.8 (13.7) | 48.0 (14.2) | 51.6 (14.9) | 48.4 (14.8) | 50.4 (15.3) |
| Female | 2 (66.7) | 1 (33.3) | 18 (51.4) | 5 (55.6) | 4 (40.0) | 6 (60.0) | 4 (44.4) | 40 (50.6) |
| Race | ||||||||
| White | 3 (100) | 1 (33.3) | 30 (85.7) | 7 (77.8) | 6 (60.0) | 9 (90.0) | 6 (66.7) | 62 (78.5) |
| Asian | 0 | 1 (33.3) | 1 (2.9) | 0 | 1 (10.0) | 0 | 2 (22.2) | 5 (6.3) |
| Black or African American | 0 | 1 (33.3) | 2 (5.7) | 0 | 1 (10.0) | 1 (10.0) | 0 | 5 (6.3) |
| Not Reported/Other | 0 | 0 | 2 (5.7) | 2 (22.2) | 2 (20.0) | 0 | 1 (11.1) | 7 (8.9) |
| Ethnicity | ||||||||
| Not Hispanic or Latino | 2 (66.7) | 3 (100) | 32 (91.4) | 6 (66.7) | 7 (70.0) | 9 (90.0) | 6 (66.7) | 65 (82.3) |
| Hispanic or Latino | 1 (33.3) | 0 | 2 (5.7) | 1 (11.1) | 2 (20.0) | 1 (10.0) | 2 (22.2) | 9 (11.4) |
| Not reported | 0 | 0 | 1 (2.9) | 2 (22.2) | 1 (10.0) | 0 | 1 (11.1) | 5 (6.3) |
| ECOG Performance Status | ||||||||
| 0 | 1 (33.3) | 0 | 20 (57.1) | 3 (33.3) | 0 | 7 (70.0) | 5 (55.6) | 36 (45.6) |
| 1 | 2 (66.7) | 3 (100) | 15 (42.9) | 6 (66.7) | 10 (100) | 3 (30.0) | 4 (44.4) | 43 (54.4) |
| Disease typea | ||||||||
| NSCLC | 0 | 0 | 4 (11.4) | 0 | 0 | 0 | 0 | 4 (5.1) |
| Ovarian | 0 | 0 | 11 (31.4) | 0 | 0 | 0 | 0 | 11 (13.9) |
| Sarcoma | 3 (100) | 3 (100) | 20 (57.1) | 9 (100) | 10 (100) | 10 (100) | 9 (100) | 64 (81.0) |
| MRCL | 0 | 0 | 9 (25.7) | 2 (22.2) | 4 (40.0) | 8 (80.0) | 4 (44.4) | 27 (34.2) |
| SS | 2 (66.7) | 1 (33.3) | 11 (31.4) | 7 (77.8) | 6 (60.0) | 2 (20.0) | 4 (44.4) | 33 (41.8) |
| Other sarcoma | 1 (33.3) | 2 (66.7) | 0 | 0 | 0 | 0 | 1 (11.1) | 4 (5.1) |
| Disease status | ||||||||
| Metastatic | 2 (66.7) | 3 (100) | 27 (77.1) | 9 (100) | 10 (100) | 9 (90.0) | 8 (88.9) | 68 (86.1) |
| Locally advanced | 1 (33.3) | 0 | 8 (22.9) | 0 | 0 | 1 (10.0) | 1 (11.1) | 11 (13.9) |
| Progression status based on physician assessment | ||||||||
| Stable disease | 0 | 0 | 14 (40.0) | 2 (22.2) | 4 (40.0) | 2 (20.0) | 5 (55.6) | 27 (34.2) |
| Any tumor growth/PD | 1 (33.3) | 3 (100) | 17 (48.6) | 6 (66.7) | 6 (60.0) | 8 (80.0) | 4 (44.4) | 45 (57.0) |
| No evidence of disease | 2 (66.7) | 0 | 4 (11.4) | 1 (11.1) | 0 | 0 | 0 | 7 (8.9) |
| Current TNM stageb | ||||||||
| Stage IIIA | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 1 (11.1) | 2 (2.5) |
| Stage IIIC | 0 | 0 | 6 (17.1) | 0 | 0 | 0 | 0 | 6 (7.6) |
| Stage IV | 2 (66.7) | 3 (100) | 23 (65.7) | 9 (100) | 10 (100) | 9 (90.0) | 8 (88.9) | 64 (81.0) |
| Not staged/Missing | 0 | 0 | 6 (17.1) | 0 | 0 | 1 (10.0) | 0 | 7 (8.9) |
| NY-ESO-1 expression (% tumor cells positive) | ||||||||
| 1–25% | 1 (33.3) | 0 | 15 (42.9) | 1 (11.1) | 2 (20.0) | 2 (20.0) | 3 (33.3) | 24 (30.4) |
| >25–50% | 0 | 0 | 3 (8.6) | 0 | 0 | 0 | 1 (11.1) | 4 (5.1) |
| >50–75% | 0 | 2 (66.7) | 2 (5.7) | 1 (11.1) | 0 | 0 | 0 | 5 (6.3) |
| >75–100% | 2 (66.7) | 1 (33.3) | 15 (42.9) | 7 (77.8) | 8 (80.0) | 8 (80.0) | 5 (55.6) | 46 (58.2) |
| Number of lines of prior therapy (any type) | ||||||||
| 1 | 1 (33.3) | 1 (33.3) | 11 (31.4) | 6 (66.7) | 4 (40.0) | 2 (20.0) | 0 | 25 (31.6) |
| 2 | 0 | 0 | 8 (22.9) | 2 (22.2) | 3 (30.0) | 5 (50.0) | 4 (44.4) | 22 (27.8) |
| ≥3 | 2 (66.7) | 2 (66.7) | 14 (40.0) | 1 (11.1) | 3 (30.0) | 2 (20.0) | 4 (44.4) | 28 (35.4) |
| Missing | 0 | 0 | 2 (5.7) | 0 | 0 | 1 (10.0) | 1 (11.1) | 4 (5.1) |
| Type of prior therapy | ||||||||
| Radiotherapy | 3 (100) | 2 (66.7) | 19 (54.3) | 4 (44.4) | 8 (80.0) | 9 (90.0) | 8 (88.9) | 53 (67.1) |
| Immunotherapy | 1 (33.3) | 0 | 5 (14.3) | 0 | 1 (10.0) | 0 | 2 (22.2) | 9 (11.4) |
| Chemotherapy | 3 (100) | 3 (100) | 33 (94.3) | 9 (100) | 10 (100) | 9 (90.0) | 8 (88.9) | 75 (94.9) |
| Other therapy | 1 (33.3) | 1 (33.3) | 4 (11.4) | 1 (11.1) | 1 (10.0) | 1 (10.0) | 2 (22.2) | 11 (13.9) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; GLA-SE = glucopyranosyl lipid A-stable emulsion; mCPA = metronomic cyclophosphamide; MRCL = myxoid/round cell liposarcoma; NSCLC = non-small cell lung carcinoma; PD = progressive disease; SC = subcutaneous; SD = standard deviation; SS = synovial sarcoma; TNM = tumor node metastasis
aPatients with melanoma were eligible to participate, but no patients with melanoma enrolled in the study.
bNo patients had a current TNM stage of 0, I, II, IIB, or IIIB.
Safety
In total, 72 (91.1%) patients who received CMB305 experienced at least 1 AE. The frequency of AEs was similar across study arms, with 3 (100%) patients experiencing AEs in Cohort 1, 2 (66.7%) in Cohort 2, and 32 (91.4%), 9 (100%), 10 (100%), 9 (90.0%), and 7 (77.8%) in Arms A, B, C, D, and E, respectively Figure 2. The most common AEs overall were fatigue (27; 34.2%), nausea (21; 26.6%), injection-site pain (19; 24.1%), decreased appetite (17; 21.5%), and dyspnea (13; 16.5%) (Appendix Table 2).
Figure 2.
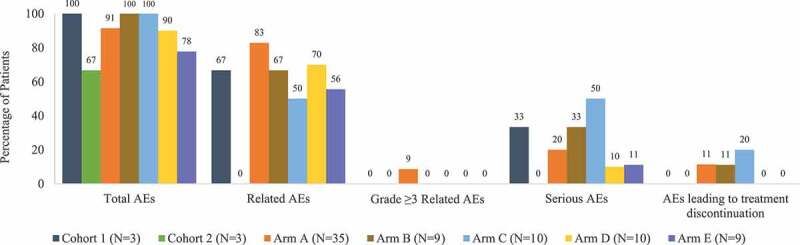
Summary of adverse events by study arm. Three patients experienced dose-limiting toxicities, but there were no AEs or safety concerns reported with these dose-limiting toxicities. Two patients experienced treatment-related serious AEs in Arm A (prostatic pain in a patient with metastatic synovial sarcoma, and pneumonitis in a patient with non-small cell lung carcinoma who had a previous history of pneumonitis); no other patients experienced serious AEs considered related to treatment. One patient in Arm A experienced an AE of acute respiratory failure not considered related to treatment that led to death. Abbreviation: AE = adverse event
Fifty-four (68.4%) patients experienced AEs considered related to study treatment; among these, the most common AEs were fatigue (19; 24.1%), injection-site pain (18; 22.8%), influenza-like illness (11; 13.9%), myalgia (10; 12.7%), and injection-site reaction (9; 11.4%). Among patients who received CMB305 monotherapy (Cohorts 1 and 2 and Arms A, B, and E), AEs considered related to treatment occurred in 66.7%, 0%, 82.9%, 66.7%, and 55.6% of patients, respectively. In Arm C (CMB305 plus mCPA), 5 (50.0%) patients experienced AEs related to CMB305 and 5 (50.0%) related to mCPA. In Arm D (CMB305 plus GLA-SE), 7 (70.0%) patients experienced AEs related to CMB305 and 4 (40.0%) related to GLA-SE.
The majority of patients had AEs of maximum severity grade 1 (22; 27.8%) or grade 2 (27; 34.2%). Grade 3 AEs occurred in 21 (26.6%) patients; of these, 3 (3.8%) were considered related to treatment. One patient experienced two grade 4 AEs (sepsis and platelet count decreased) and one patient experienced a grade 5 AE of acute respiratory failure that resulted in death, but these events were considered not related to CMB305 treatment. There were no clinically relevant changes in laboratory parameters related to CMB305.
A total of 18 (22.8%) patients experienced SAEs. Of the SAEs reported, 2 (2.5%) were grade 3 events that were considered related to treatment: prostatic pain in a patient with metastatic SS, and pneumonitis in a patient with NSCLC who had a previous history of pneumonitis.
Adverse events that led to study treatment discontinuation occurred in 7 (8.9%) patients; 1 (1.3%; pneumonitis) was considered possibly related to treatment. Protocol-defined DLTs were reported for 3 patients (1 in Arm A and 2 in Arm B), but none prevented a patient from receiving further injections and there were no associated AEs or safety concerns reported with these DLTs. Four patients had medical events of interest: 1 patient had grade 3 vomiting considered unrelated to the study drug and 3 patients (2 in Arm A and 1 in Arm D) had non-serious events of overdose of study drug stemming from dispensing errors that did not result in any sequelae or change to dosing.
Depending on the availability of PBMC, LV305 persistence assay was performed in 51 (64.6%) patients, who all tested negative at 1 (25.3%), 2 (21.5%), or more (17.7%) timepoints tested. Twenty-eight (35.4%) patients had no LV305 persistence test performed due to death, withdrawal of consent, study termination, or unknown reasons.
Efficacy
In Part 1 of the study, the median OS was 19.2 (95% CI, 7.1–not available [NA]) and 23.7 (95% CI, 7.5–NA) months in Cohorts 1 and 2, respectively. In Part 2, the median OS was 28.9 months (95% CI, 13.5–33.8) for the 35 patients in Arm A and 18.4 months (95% CI, 6.9–NA) for the 9 patients in Arm B (Figure 3; Appendix Table 3). The median OS for Arms C, D, and E was not reached, with a 30-month OS rate of 50.0%, 100%, and 88.9% and a median duration of observation of 11.99, 20.42, and 9.23 months, respectively. Among patients with SS, MRCL, ovarian cancer, and NSCLC, the median OS was 26.2 (95% CI, 13.0–NA), 29.5 (95% CI, 22.1–NA), 30.3 (95% CI, 8.4–33.8), and 7.7 (95% CI, 1.2–13.5) months, respectively (Appendix Table 4; Appendix Figure 3). The median PFS in Part 1 was 14.0 months in Cohort 1 and 3.1 months in Cohort 2, and ranged from 2.0 (Arm C) to 3.7 months (Arm A) in Part 2 (Figure 3; Appendix Table 3). Patients with SS, MRCL, ovarian cancer, and NSCLC had a median PFS of 2.4 (95% CI, 2.1–5.6), 5.1 (95% CI, 2.6–7.2), 3.3 (95% CI, 1.8–3.9), and 2.3 (95% CI, 1.2–2.5), respectively. Among patients with STS, 6 patients with SS (2 in Cohort 1, 1 in Arm A, 1 in Arm B, and 2 in Arm C) remained progression-free for 12.0 to 30.4 months, and 2 patients with MRCL in Arm A remained progression-free for 23.0 and 35.1 months, respectively. In a subgroup analysis, patients with STS who had PD at screening but achieved stable disease during the study had a median PFS of 6.0 months (95% CI, 3.1–9.2).
Figure 3.
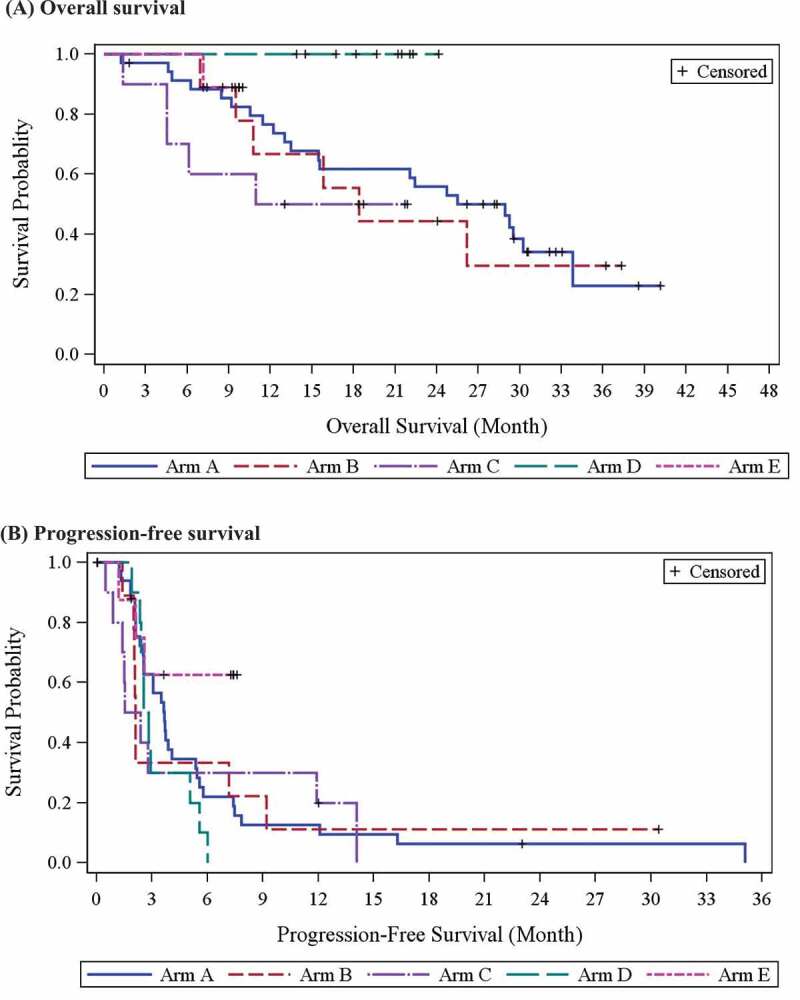
(a) Overall survival and (b) Progression-free survival by study arm
In Part 2, disease control rates were 68.6% in Arm A, 33.3% in Arm B, 40.0% in Arm C, 90.0% in Arm D, and 66.7% in Arm E, with a total of 50 (63.3%) of patients on the study achieving irSD based on irRC. The disease control rate for patients with STS was 61.9% with a median DOR of 4.6 months (95% CI, 2.0–7.1), and 81.8% of patients with ovarian cancer and 50.0% of patients with NSCLC had irSD, with a median DOR of 1.4 (95% CI, 0.5–3.7) and 0.4 (95% CI, NA–NA) months, respectively (Appendix Table 4). No objective responses were observed. Time to next treatment and time to progression results are available in Appendix Tables 5 and 6.
Immune response
At baseline, evidence of preexisting NY-ESO-1 specific antibodies (sarcomas 28.3%, ovarian 45.5%, and NSCLC 33.3%) and T-cells (sarcomas 38.0%, ovarian 37.5%, and NSCLC 33.3%) were comparable across disease types (Appendix Figure 4). There was a weak positive correlation between NY-ESO-1 expression level (0–100%) and preexisting T-cells (r = 0.3107; p = .0148), but not preexisting NY-ESO-1 antibodies (r = 0.1000; p = .3965) (Appendix Figure 5). CMB305 induced antibody responses to NY-ESO-1 in 62.9% of patients and T-cell responses in 47.4%; a total of 22.8% of patients had both Figure 4. Appendix Figure 6 displays the time course of CD4 and CD8 T-cell and NY-ESO-1 antibody responses in a patient with an induced integrated response. No difference was observed by changing administration routes (ID LV305 and IM G305 in Arm A vs SC for both LV305 and G305 in Arm B), addition of oral mCPA (in Arm C), or addition of intratumoral GLA-SE injection (in Arm D).
Figure 4.
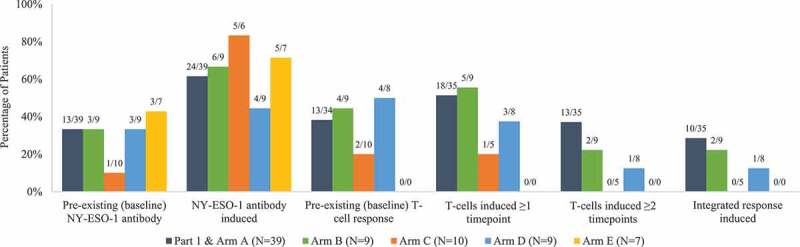
Immune response frequencies by study arm. Complete biomarker data were not available for all patients. The Ns for each study arm denote the total number of patients with biomarker data in that arm. Numerators and denominators are shown above each bar. Integrated response was defined as positive if both NY-ESO-1 antibody and T-cells (CD4 and CD8) were positive. T-cell analysis was not performed for patients in Arm E due to early study termination
This preliminary study was not powered to evaluate correlations between efficacy and immune outcomes. However, a signal indicating a potentially higher 1-year OS rate was observed in patients with SS treated with CMB305 alone when they also had preexisting NY-ESO-1 antibody (100% vs 69.2%, with a difference of 30.8%; 95% CI, 5.7–55.9; p = .0162), as well as those for whom T-cells were induced on ≥2 time points (100% vs 75.0%, with a difference of 25.0%; 95% CI, 0.5–49.5; p = .0455) or had an integrated response post-study treatment (100% vs 76.9%, with a difference of 23.1%; 95% CI, 0.2–46.0; p = .0483) (Appendix Figure 7).
Discussion
While prime-boost vaccines built around a lentiviral vector as the priming component have been evaluated in the context of infectious diseases such as HIV (human immunodeficiency virus),32,33 to our knowledge, this phase 1b trial is the first report of a clinical study using this vaccination strategy in cancer. CMB305 treatment either alone or in combination with oral mCPA or intratumoral GLA-SE was well-tolerated in the dose-escalation phase and across tumor types in this trial, with the most common AEs being fatigue, nausea, injection-site pain, decreased appetite, and dyspnea. While 54 (68.4%) patients had AEs considered related to study treatment, most of these patients (51; 94.4%) had AEs that were only grade 1 or 2 in severity and transient. Three patients experienced protocol-defined DLTs, but they were not associated with other AEs or safety concerns and did not prevent resumption of study treatment. Overall, the safety profile of CMB305 appeared to be similar across treatment arms, with most patients in each study arm experiencing at least one mild to moderate adverse event. The CMB305 vaccine regimen was generally well tolerated in each arm, with expected toxicity profiles observed.
CMB305 demonstrated an ability to induce anti-NY-ESO-1 antibody and T-cell responses across treatment arms and disease types. Eighteen percent of patients experienced the induction of an integrated immune response, which has previously been linked to enhanced tumor control in melanoma patients treated with ipilimumab.34 A signal indicating a potentially higher 1-year survival rate was observed in patients with SS treated with CMB305 alone who had preexisting NY-ESO-1 antibodies, T-cells induced at ≥2 time points, or an integrated response post-study treatment. Overall, there was no significant difference in OS between patients with and without induced NY-ESO-1 antibodies, T-cells, or an integrated immune response. In approximately half of the patients who had an induced T-cell response at the first evaluated timepoint, a response was not present at the second evaluated timepoint. These results may indicate that the induction of an immune response is not sufficient to produce durable tumor control in this population with advanced oncologic disease. The ability to interpret the efficacy data is limited by the small sample size and heterogeneity within the treatment arms and the lack of a controlled comparator group.
Previous cancer vaccines studies have had inconsistent outcomes regarding immune responses and have not led to tumor regressions, but prolonged survival has been noted.35–39 In this study, the median OS of 26.2 and 29.5 months in patients with SS and MRCL, respectively, compares favorably with published data (OS of 11.7 to 13.5 months) for patients with advanced or metastatic STS in second-line and beyond.40–43 In addition, a total of 51.5% of patients with SS and 74.1% of patients with MRCL experienced irSD on the study. The observed median PFS ranged from 2.0 to 3.7 months in Part 2, which is consistent with other published trials in this patient population (PFS of 1.5 to 4.6 months).41–43 It is important to consider that evaluation of PFS in this study included clinical progression/symptomatic deterioration, which leads to shorter median PFS compared to later phase studies that include only radiological PD. Patients receiving the higher dose of LV305 in Arm E (4 × 1010 vg SC) had an OS rate of 88.9% and a PFS rate of 62.5% at the time the study was terminated, with the median OS and PFS not yet reached. The study termination and small number of patients prevent interpretation about the long-term benefit of the higher SC LV305 dose.
Several confounding factors must be considered when interpreting the clinical outcomes in this study. Patients had a relatively high level of disease burden overall, with 86.1% having metastatic disease at the start of the study. However, there was considerable heterogeneity both across and between treatment arms, with each arm having multiple tumor types, NY-ESO-1 expression levels, and types and lines of prior therapy. Combined with the small sample size and lack of a control arm, these factors limit the interpretation and generalizability of the clinical outcome findings for any specific disease type.
To enhance the clinical activity of vaccine-based approaches, strategies that combine the vaccine with checkpoint inhibitors or other immunomodulatory therapies to alleviate immunosuppression in the tumor microenvironment have been discussed.36,39,44–46 In this study, administering the CMB305 vaccine in combination with intratumoral GLA-SE, a synthetic TLR4 agonist (Arm D), resulted in positive activity in patients with SS or MRCL. With a median follow-up of 20.4 months, 100% of the patients in Arm D were still alive at the time of study termination. Additionally, patients in Arm D achieved a disease control rate of 90.0% (95% CI, 56%–100%), even though 9 (90.0%) patients in Arm D patients had metastatic disease, 8 (80.0%) had PD at study entry, and 9 (90.0%) had prior chemotherapy, including 7 (70.0%) with ≥2 prior lines of chemotherapy. Further research is necessary to evaluate the clinical benefit of a “prime-pull” strategy combining treatments such as CMB305 with intratumoral GLA-SE in patients with advanced or metastatic SS or MRCL. While CMB305 administration with mCPA resulted in a less robust clinical response (disease control rate of 40.0%), the patients in this arm also had the lowest percentage of preexisting NY-ESO-1 antibodies and T-cells. Given that patients with preexisting NY-ESO-1 antibodies exhibited better 1-year survival rates, this may explain the reduced clinical activity demonstrated with the CMB305 and mCPA combination.
With limited treatment options and continued poor outcomes for patients with SS and MRCL, there has been growing interest in vaccine strategies to induce an immune response directed against these cancers.36,45,47 Given that effective therapies for patients with SS or MRCL remain inadequate despite ongoing research,48–50 this study argues that novel vaccination strategies could potentially benefit patients with SS and MRCL and that further exploration is warranted.
Conclusions
In summary, administering a lentiviral vector as the priming component in a prime-boost vaccine regimen was feasible, safe, and well-tolerated in this Phase 1b trial of 79 patients with locally advanced, relapsed, or metastatic cancer expressing NY-ESO-1. The prime-boost regimen exhibited both clinical and immunogenic activity across study arms and disease types. This study will serve as a benchmark for future studies of vaccine trials using prime-boost regimens, as well as those using dendritic cell-targeted lentiviral agents.
Supplementary Material
Acknowledgments
Colleen Brown of Mark Consulting, Inc. provided writing and formatting assistance for this manuscript. This study was presented as an oral presentation at the ASCO Annual Meeting 2017 and a poster at the ESMO 2018 Congress.
Funding Statement
Immune Design Corp., a wholly-owned subsidiary of Merck & Co., Inc., Kenilworth, NJ USA, provided funding for this study and participated in the design of the study; collection, analysis, and interpretation of the data; writing the manuscript; and the decision to submit the manuscript for publication.
Disclosure of interest
NS reports support from Immune Design during the conduct of the study and a grant from Merck & Co. outside the submitted work. SC reports grants from Amgen, Roche, Threshold Pharmaceuticals, Immune Design, and Karyopharm Therapeutics outside the submitted work. MB reports institutional support from Immune Design and Merck & Co. during the conduct of the study, and grants from Bristol Myers Squibb, Genentech, Pharmacyclics, Marker Therapeutics, and Transgene SA outside the submitted work. JK reports support from Immune Design during the conduct of the study. MD reports receipt of consultation fees from Adaptimmune Therapeutics, Daiichi Sankyo, Epizyme, Blueprint Medicines, and Deciphera Pharmaceuticals outside of the submitted work. PH reports personal fees from Immatics, Dragonfly Therapeutics, GSK, and Sanofi outside of the submitted work. RJ reports grants/research support from MSD and GSK and receipt of consultation fees from Adaptimmune Therapeutics, Athenex, Blueprint Medicines, Clinigen, Eisai, Epizyme, Daichii Sankyo, Deciphera Pharmaceuticals, Immune Design, Lilly, Merck & Co., Pharma Mar S.A., and UpToDate. SG reports research funding from Immune Design during the conduct of the study; consultancy/advisory roles from Merck & Co. and OncoMed and research funding from Regeneron Pharmaceuticals, Genentech, Pfizer, and Takeda Pharmaceutical Company outside of the submitted work; and a patent for NY-ESO-1 peptides issued to GSK. The Human Immune Monitoring Center received support from Cancer Center P30 grant CA196521. HL was a full-time employee of Immune Design with stock options during the conduct of the study. AY was an employee and shareholder of Immune Design during the conduct of the study. JtM was a full-time employee and shareholder of Immune Design during the conduct of the study, and has patents US20160058852 (Immunotherapy of cancer through combination of local and systemic immune stimulation) and US20170196954 (Prime-boost regimens with a TLR4 agonist adjuvant and a lentiviral vector) pending with Merck & Co. MC was an employee of Immune Design during the conduct of the study. RK was an employee of Immune Design and subsequently employed by ClinReg Biologics, LLC as a consultant to Immune Design during the conduct of the study. CB was an employee of Immune Design during the conduct of the study and reports prior employment and current stock with Amgen and current employment and stock with Macrogenics, Inc. SP reports grants from Immune Design and Merck & Co. during the conduct of the study; grants from EMD Serono, Presage Biosciences, Janssen Pharmaceuticals, OncoSec, and Juno Therapeutics outside the submitted work; and personal fees from Daiichi Sankyo, GSK, and Blueprint Medicines outside the submitted work. The remaining authors (JM, KD, KS, SKS, KT, and MY) have nothing to disclose.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY.. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–12. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlom J, Hodge JW, Palena C, Tsang K-Y, Jochems C, Greiner JW, Farsaci B, Madan RA, Heery CR, Gulley JL. Therapeutic cancer vaccines. Adv Cancer Res. 2014;121:67–124. doi: 10.1016/B978-0-12-800249-0.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butterfield LH. Cancer vaccines. BMJ. 2015;350:h988. doi: 10.1136/bmj.h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatti-Mays ME, Redman JM, Collins JM, Bilusic M. Cancer vaccines: enhanced immunogenic modulation through therapeutic combinations. Hum Vaccin Immunother. 2017;13(11):2561–2574. doi: 10.1080/21645515.2017.1364322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solinas C, Aiello M, Migliori E, Willard-Gallo K, Emens LA. Breast cancer vaccines: heeding the lessons of the past to guide a path forward. Cancer Treat Rev. 2020;84:101947. doi: 10.1016/j.ctrv.2019.101947. [DOI] [PubMed] [Google Scholar]
- 6.van der Burg SH. Correlates of immune and clinical activity of novel cancer vaccines. Semin Immunol. 2018;39:119–136. doi: 10.1016/j.smim.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Endo M, de Graaff MA, Ingram DR, Lim S, Lev DC, Briaire-de Bruijn IH, Somaiah N, Bovée JVMG, Lazar AJ, Nielsen TO. NY-ESO-1 (CTAG1B) expression in mesenchymal tumors. Mod Pathol. 2015;28(4):587–595. doi: 10.1038/modpathol.2014.155. [DOI] [PubMed] [Google Scholar]
- 8.Jungbluth AA, Antonescu CR, Busam KJ, Iversen K, Kolb D, Coplan K, Chen YT, Stockert E, Ladanyi M, Old LJ. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer. 2001;94(2):252–256. doi: 10.1002/ijc.1451. [DOI] [PubMed] [Google Scholar]
- 9.Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, Intengan M, Beck A, Keitz B, Santiago D,et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63(18):6076–6083. [PubMed] [Google Scholar]
- 10.Raza A, Merhi M, Inchakalody VP, Krishnankutty R, Relecom A, Uddin S, Dermime S. Unleashing the immune response to NY-ESO-1 cancer testis antigen as a potential target for cancer immunotherapy. J Transl Med. 2020;18(1):140. doi: 10.1186/s12967-020-02306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maraskovsky E, Sjölander S, Drane DP, Schnurr M, Le TTT, Mateo L, Luft T, Masterman K-A, Tai T-Y, Chen Q,et al. NY-ESO-1 protein formulated in ISCOMATRIX adjuvant is a potent anticancer vaccine inducing both humoral and CD8+ t-cell-mediated immunity and protection against NY-ESO-1+ tumors. Clin Cancer Res. 2004;10(8):2879–2890. doi: 10.1158/1078-0432.ccr-03-0245. [DOI] [PubMed] [Google Scholar]
- 13.Thomas R, Al-Khadairi G, Roelands J, Hendrickx W, Dermime S, Bedognetti D, Decock J. NY-ESO-1 based immunotherapy of cancer: current perspectives. Front Immunol. 2018;9:947. doi: 10.3389/fimmu.2018.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollack SM, Jungbluth AA, Hoch BL, Farrar EA, Bleakley M, Schneider DJ, Loggers ET, Rodler E, Eary JF, Conrad III EU, et al. NY-ESO-1 is a ubiquitous immunotherapeutic target antigen for patients with myxoid/round cell liposarcoma. Cancer. 2012;118(18):4564–4570. doi: 10.1002/cncr.27446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albershardt TC, Parsons AJ, Reeves RS, Flynn PA, Campbell DJ, Ter Meulen J, Berglund P. Therapeutic efficacy of PD1/PDL1 blockade in B16 melanoma is greatly enhanced by immunization with dendritic cell-targeting lentiviral vector and protein vaccine. Vaccine. 2020;38(17):3369–3377. doi: 10.1016/j.vaccine.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Alpizar YA, Karwacz K, Arce F, Rivera AY, Fernández LE, Collins MK, Ramírez BS. Lentiviral vector followed by protein immunisation breaks tolerance against the self-antigen Her1 and results in lung cancer immunotherapy. J Gene Med. 2012;14(3):151–157. doi: 10.1002/jgm.2606. [DOI] [PubMed] [Google Scholar]
- 17.Otten GR, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J, Kazzaz J, Lian Y, Singh M, Ugozzoli M, et al. Enhanced potency of plasmid DNA microparticle human immunodeficiency virus vaccines in rhesus macaques by using a priming-boosting regimen with recombinant proteins. J Virol. 2005;79(13):8189–8200. doi: 10.1128/JVI.79.13.8189-8200.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richmond JF, Lu S, Santoro JC, Weng J, Hu S-L, Montefiori DC, Robinson HL. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J Virol. 1998;72(11):9092–9100. doi: 10.1128/JVI.72.11.9092-9100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Parker C, Taaffe J, Solórzano A, García-Sastre A, Lu S. Heterologous HA DNA vaccine prime–inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine. 2008;26(29–30):3626–3633. doi: 10.1016/j.vaccine.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albershardt TC, Campbell DJ, Parsons AJ, Slough MM, Ter Meulen J, Berglund P. LV305, a dendritic cell-targeting integration-deficient ZVex TM -based lentiviral vector encoding NY-ESO-1, induces potent anti-tumor immune response. Mol Ther Oncolytics. 2016;3:16010. doi: 10.1038/mto.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odegard JM, Kelley-Clarke B, Tareen SU, Campbell DJ, Flynn PA, Nicolai CJ, Slough MM, Vin CD, McGowan PJ, Nelson LT, et al. Virological and preclinical characterization of a dendritic cell targeting, integration-deficient lentiviral vector for cancer immunotherapy. J Immunother. 2015;38(2):41–53. doi: 10.1097/CJI.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tareen SU, Kelley-Clarke B, Nicolai CJ, Cassiano LA, Nelson LT, Slough MM, Vin CD, Odegard JM, Sloan DD, Van Hoeven N, et al. Design of a novel integration-deficient lentivector technology that incorporates genetic and posttranslational elements to target human dendritic cells. Mol Ther. 2014;22(3):575–587. doi: 10.1038/mt.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somaiah N, Block MS, Kim JW, Shapiro GI, Do KT, Hwu P, Eder JP, Jones RL, Lu H, Ter Meulen JH, et al. First-in-class, first-in-human study evaluating LV305, a dendritic-cell tropic lentiviral vector, in sarcoma and other solid tumors expressing NY-ESO-1. Clin Cancer Res. 2019;25(19):5808–5817. doi: 10.1158/1078-0432.CCR-19-1025. [DOI] [PubMed] [Google Scholar]
- 24.Pollack SM, Lu H, Gnjatic S, Somaiah N, O’Malley RB, Jones RL, Hsu FJ, Ter Meulen J. First-in-human treatment with a dendritic cell-targeting lentiviral vector-expressing NY-ESO-1, LV305, induces deep, durable response in refractory metastatic synovial sarcoma patient. J Immunother. 2017;40(8):302–306. doi: 10.1097/CJI.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahipal A, Ejadi S, Gnjatic S, Kim-Schulze S, Lu H, Ter Meulen JH, Kenney R, Odunsi K. First-in-human phase 1 dose-escalating trial of G305 in patients with advanced solid tumors expressing NY-ESO-1. Cancer Immunol Immunother. 2019;68(7):1211–1222. doi: 10.1007/s00262-019-02331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72(14):3439–3444. doi: 10.1158/0008-5472.CAN-11-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003;63(23):8408–8413. [PubMed] [Google Scholar]
- 28.Albershardt TC, Leleux J, Parsons AJ, Krull JE, Berglund P, Ter Meulen J. Intratumoral immune activation with TLR4 agonist synergizes with effector T-cells to eradicate established murine tumors. NPJ Vaccines. 2020;5:50. doi: 10.1038/s41541-020-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Cancer Institute . Common terminology criteria for adverse events (CTCAE), version 4.03. Bethesda (MD): U.S. Department of Health and Human Services - National Institutes of Health; 2010. [Google Scholar]
- 30.Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19(14):3936–3943. doi: 10.1158/1078-0432.CCR-13-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebb éC, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 32.Norton TD, Miller EA. Recent advances in lentiviral vaccines for HIV-1 infection. Front Immunol. 2016;7:243. doi: 10.3389/fimmu.2016.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu B, Tai A, Wang P. Immunization delivered by lentiviral vectors for cancer and infectious diseases. Immunol Rev. 2011;239(1):45–61. doi: 10.1111/j.1600-065X.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108(40):16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melief CJM, van Hall T, Arens R, Ossendorp F, van der Burg SH. Therapeutic cancer vaccines. J Clin Invest. 2015;125(9):3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, Chuang E, Sanborn RE, Lutzky J, Powderly J, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med. 2014;6(232):232ra51. doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawaguchi S, Tsukahara T, Ida K, Kimura S, Murase M, Kano M, Emori M, Nagoya S, Kaya M, Torigoe T, et al. SYT-SSX breakpoint peptide vaccines in patients with synovial sarcoma: a study from the Japanese musculoskeletal oncology group. Cancer Sci. 2012;103(9):1625–1630. doi: 10.1111/j.1349-7006.2012.02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carvajal RD, Agulnik M, Ryan CW, Milhem MM, George S, Jones RL, Chmielowski B, Van Tine BA, Tawbi HAH, Elias AD, et al. Trivalent ganglioside vaccine and immunologic adjuvant versus adjuvant alone in metastatic sarcoma patients rendered disease-free by surgery: A randomized phase 2 trial [abstract]. J Clin Oncol. 2014;32(15_Suppl):10520. doi: 10.1200/jco.2014.32.15_suppl.10520. [DOI] [Google Scholar]
- 39.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savina M, Le Cesne A, Blay J-Y, Ray-Coquard I, Mir O, Toulmonde M, Cousin S, Terrier P, Ranchere-Vince D, Meeus P, et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: the METASARC observational study. BMC Med. 2017;15(1):78. doi: 10.1186/s12916-017-0831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Graaf WTA, Blay J-Y, Chawla SP, Kim D-W, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP, Beppu Y, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 42.Schöffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, Grignani G, Camargo V, Bauer S, Rha SY, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387(10028):1629–1637. doi: 10.1016/S0140-6736(15)01283-0. [DOI] [PubMed] [Google Scholar]
- 43.Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, Milhem M, Elias A, Ganjoo K, Tawbi H, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34(8):786–793. doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollack SM. The potential of the CMB305 vaccine regimen to target NY-ESO-1 and improve outcomes for synovial sarcoma and myxoid/round cell liposarcoma patients. Expert Rev Vaccines. 2018;17(2):107–114. doi: 10.1080/14760584.2018.1419068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pender A, Jones RL, Pollack S. Optimising cancer vaccine design in sarcoma. Cancers (Basel). 2018;11(1):1. doi: 10.3390/cancers11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang S, Kohli K, Black RG, Yao L, Spadinger SM, He Q, Pillarisetty VG, Cranmer LD, Van Tine BA, Yee C, et al. Systemic interferon-gamma increases MHC Class I expression and T-cell infiltration in cold tumors: results of a phase 0 clinical trial. Cancer Immunol Res. 2019;7(8):1237–1243. doi: 10.1158/2326-6066.CIR-18-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Angelo SP, Tap WD, Schwartz GK, Carvajal RD. Sarcoma immunotherapy: past approaches and future directions. Sarcoma. 2014;2014:391967. doi: 10.1155/2014/391967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tap WD, Wagner AJ, Schöffski P, Martin-Broto J, Krarup-Hansen A, Ganjoo KN, Yen C-C, Abdul Razak AR, Spira A, Kawai A, et al. Effect of doxorubicin plus olaratumab vs doxorubicin plus placebo on survival in patients with advanced soft tissue sarcomas: the ANNOUNCE randomized clinical trial. JAMA. 2020;323(13):1266–1276. doi: 10.1001/jama.2020.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riedel RF, Jones RL, Italiano A, Bohac C, Thompson JC, Mueller K, Khan Z, Pollack SM, Van Tine BA. Systemic anti-cancer therapy in synovial sarcoma: a systematic review. Cancers (Basel). 2018;10(11):417. doi: 10.3390/cancers10110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollack SM, Ingham M, Spraker MB, Schwartz GK. Emerging targeted and immune-based therapies in sarcoma. J Clin Oncol. 2018;36(2):125–135. doi: 10.1200/JCO.2017.75.1610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


