Abstract
To detect potentially curable hepatocellular carcinoma (HCC), clinical practice guidelines recommend semiannual surveillance US of the liver in adult patients at risk for developing this malignancy, such as those with cirrhosis and some patients with chronic hepatitis B infection. However, cirrhosis and a large body habitus, both of which are increasingly prevalent in the United States and the rest of the world, may impair US visualization of liver lesions and reduce the sensitivity of surveillance with this modality. The low sensitivity of US for detection of early-stage HCC contributes to delayed diagnosis and increased mortality. Abbreviated MRI, a shortened MRI protocol tailored for early-stage detection of HCC, has been proposed as an alternative surveillance option that provides high sensitivity and specificity. Abbreviated MRI protocols include fewer sequences than a complete multiphase MRI examination and are specifically designed to identify small potentially curable HCCs that may be missed at US. Three abbreviated MRI strategies have been studied: (a) nonenhanced, (b) dynamic contrast material–enhanced, and (c) hepatobiliary phase contrast-enhanced abbreviated MRI. Retrospective studies have shown that simulated abbreviated MRI provides high sensitivity and specificity for early-stage HCC, mostly in nonsurveillance cohorts. If it is supported by scientific evidence in surveillance populations, adoption of abbreviated MRI could advance clinical practice by increasing early detection of HCC, allowing effective treatment and potentially prolonging life in the growing number of individuals with this cancer.
Online supplemental material is available for this article.
©RSNA, 2020
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Describe the at-risk patient groups and guideline recommendations for HCC surveillance.
■ List the key differences among the three strategies for abbreviated MRI for HCC surveillance: nonenhanced, dynamic contrast-enhanced, and hepatobiliary phase contrast-enhanced abbreviated MRI.
■ Discuss the abbreviated MRI protocols that are tailored for detection of HCC and involve the use of fewer sequences than a complete multiphase MRI examination.
Introduction
Imaging surveillance has been shown to reduce mortality for patients at risk for hepatocellular carcinoma (HCC) (1–4). Detection of HCC at early stages allows application of curative therapies such as ablation, resection, or a transplant. Imaging surveillance for HCC primarily and historically relies on US performed every 6 months, with or without serum α-fetoprotein testing. For surveillance, high sensitivity is essential, especially to detect small lesions (ie, early-stage potentially curable disease). Likewise, high specificity is desired to reduce false-positive results and consequent patient harm. Unfortunately, the diagnostic accuracy of US is reduced in patients with obesity, fatty liver disease, and cirrhosis (5,6). Reduced sensitivity can result in delayed diagnosis and missed opportunities to treat and potentially cure HCC. With the rise in obesity in the United States, there is interest in identifying alternative more effective strategies for HCC surveillance.
In this article, we provide an overview of current guidelines and recommendations for HCC surveillance, challenges associated with the current strategy, and emerging options, with a focus on abbreviated or shortened MRI protocols. Abbreviated MRI could replace US in some components of the current surveillance paradigm.
Epidemiology
In the last 2 decades, the incidence of HCC has doubled in the United States and increased by 75% worldwide (7). In fact, HCC has become the fastest-growing cause of cancer mortality in the United States (8). In the United States and other Western nations, the most important risk factor for HCC is cirrhosis. Presently, approximately 600 000 Americans are living with cirrhosis, most commonly due to hepatitis C virus (HCV) infection, alcohol consumption, and, increasingly, from obesity-related nonalcoholic steatohepatitis (NASH) (9). In the past 2 decades, the number of cases of compensated cirrhosis more than doubled because of NASH (10). With the increasing prevalence of NASH, the incidence of HCC is expected to increase also, and the reported annual incidence is approximately 5.3 per 1000 person-years (95% CI: 0.75, 37.56) in patients with NASH (11). From 2002 to 2016, NASH-related HCC increased 11.8-fold in the United States, becoming the fastest-growing cause for liver transplant (12). The future looks even bleaker, because the estimated the number of Americans with cirrhosis due to NASH may increase to more than 3 million by 2030 (13).
With the growing use of novel antiviral treatment of HCV, decreasing HCV incidence, and aggressive vaccination programs for hepatitis B (HBV), we will likely see an even more pronounced epidemiologic shift toward NASH-associated HCC cases. According to Surveillance, Epidemiology, and End Results (SEER) program data of Medicare patients in the United States, obese patients with NASH could be contributing to 36.6% of new HCC diagnoses (14).
Guidelines and Recommendations for HCC Surveillance
Rationale for HCC Surveillance
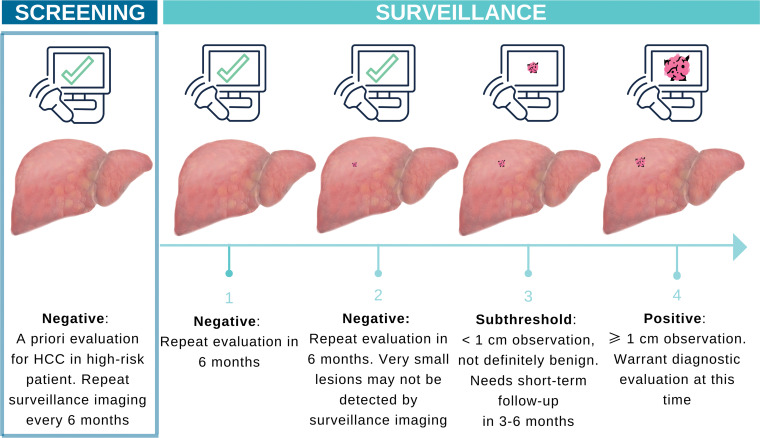
Screening refers to a one-time use of a test to detect cancer prevalence in a population, while surveillance refers to the repetition of the test at scheduled intervals to identify incidental cancers (15) (Fig 1). The rationale for surveillance is to identify patients with asymptomatic HCC at an early stage to facilitate curative therapy. The best outcomes of curative treatment options such as radiofrequency ablation, surgical resection, and liver transplant are achieved in patients who are discovered to have early-stage disease (3,16). Patients whose cancer has progressed to late stages have a poor prognosis and invariably die of cancer (17,18). Randomized and retrospective studies support a 31%–38% reduction in HCC-associated mortality for patients who undergo surveillance US (1,2,19). Surveillance leads to detection of earlier-stage tumors (20) and allows curative treatment options (21) that may extend life and reduce mortality.
Figure 1.
Illustration shows the differences between HCC screening and surveillance. Cancer surveillance involves screening examinations that are repeated at regular intervals in patients at high risk for HCC. HCC surveillance with US is repeated every 6 months because US lacks high sensitivity, especially for small early-stage cancers. If adherence to the surveillance schedule is maintained, US may allow detection of a previously missed lesion when the lesion reaches a more conspicuous size at follow-up.
Who Should Undergo Surveillance
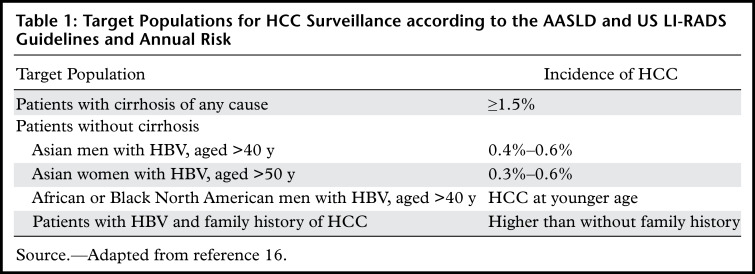
Major international societies including the American Association for the Study of Liver Diseases (AASLD), the Asian Pacific Association for the Study of the Liver (APASL), and the European Association for the Study of the Liver (EASL) recommend surveillance in patients with increased risk for HCC who would benefit from early-stage detection and curative treatment (3,4,22). The decision to begin a surveillance program is determined according to the patient’s level of risk for HCC and also takes into account the patient’s age, overall health, functional status, and willingness and ability to comply with surveillance and potential treatment requirements (16). The 2018 AASLD guidelines recommend surveillance imaging every 6 months for adults with Child-Pugh classification A or B cirrhosis (ie, if the cause of cirrhosis is associated with an estimated ≥1.5% annual risk of incidental HCC) and certain adults with chronic HBV infection but without cirrhosis (ie, those who have demographic characteristics that are associated with an estimated ≥0.2% annual risk) (16). This applies to patients with Child-Pugh A or B cirrhosis of virtually any cause, and patients without cirrhosis but with chronic HBV infection who meet specific clinical criteria (3). Patients with Child-Pugh class C cirrhosis should undergo surveillance only if they are awaiting liver transplant; if they are not on the liver transplant list, surveillance is not recommended because there are no effective treatment options and little if any benefit to detecting early-stage HCC.
Surveillance has not been proven to be cost effective in adults without cirrhosis but with chronic liver disease from causes other than HBV infection (eg, HCV, alcohol, or NASH). Thus, surveillance is not recommended by the AASLD in such patients. Some international societies, including the European Association for the Study of the Liver–European Organization for Research and Treatment of Cancer (EASL-EORTC), the Korean Liver Cancer Study Group, and the National Cancer Center recommend surveillance in adults with stage 3 fibrosis due to HCV infection (4,23,24). A summary of HCC surveillance eligibility criteria according to the AASLD and the American College of Radiology (ACR) Liver Imaging Reporting and Data System (LI-RADS) is listed in Table 1.
Table 1:
Target Populations for HCC Surveillance according to the AASLD and US LI-RADS Guidelines and Annual Risk
Recommended Modality for Surveillance
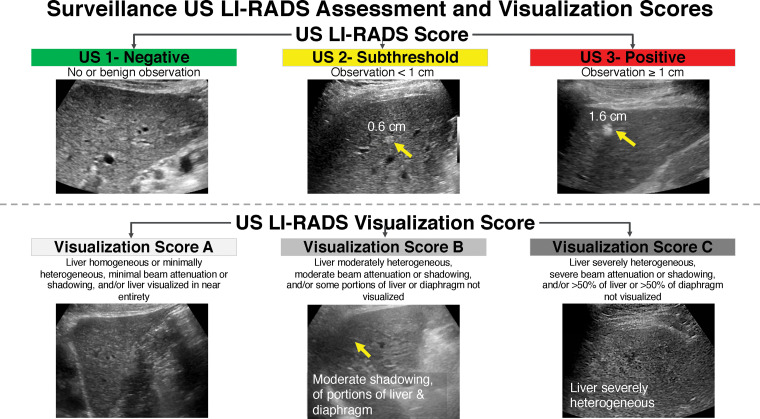
All international guidelines currently recommend US as the standard imaging modality for HCC screening and surveillance, given its low cost, its high tolerability and availability, and evidence that US-based surveillance programs prolong life. Screening with US is also endorsed by the ACR in the US LI-RADS, a standardized system for interpretation and reporting (25). Guidelines for technical imaging acquisition, reporting, and management are provided with the US LI-RADS Core and are available free of charge on the ACR website (25). Figure 2 demonstrates the US LI-RADS algorithm. The AASLD also recommends the optional use of α- fetoprotein testing. Patients on the liver transplant list are an exception. In these patients, surveillance is usually performed with multiphase CT or MRI, rather than US. A detailed discussion of surveillance in liver transplant candidates is beyond the scope of this review.
Figure 2.
US LI-RADS assessment and visualization scores for surveillance. Positive screening US results (suspected malignant liver lesion ≥1 cm) prompt diagnostic imaging with multiphase contrast-enhanced CT, MRI, or contrast-enhanced US for further characterization. Diagnostic follow-up examinations are interpreted by using the LI-RADS CT, MRI, or contrast-enhanced US diagnostic algorithms. The visualization score indicates whether the study is limited by liver steatosis, a poor acoustic window, or other factors. Although the AASLD does not recommend additional imaging in patients with limited US findings, many physicians opt for alternative surveillance strategies in these instances, such as MRI or CT.
Challenges of US Surveillance
Although US remains the recommended HCC surveillance strategy according to many societal guidelines, it has notable limitations. In patients with cirrhosis, US does not allow detection of more than one-half of early-stage HCCs (26). This is partly explained by the poor acoustic window because of cirrhosis, body habitus, or liver steatosis (6). Prospective evaluation has shown reduced sensitivity and specificity and higher likelihood of US inadequacy in male patients and in those with a larger body habitus, an echogenic or nodular liver due to high fat content or fibrosis, and ascites (6,27,28). Studies have shown that the per-patient sensitivity of US for small HCCs (<2 cm), defined as Barcelona Clinic Liver Cancer stage 0 or A, is as low as 47%–63% (5,26,29). A 13-year meta-analysis (30) in which US was compared with alternative surveillance strategies found US to have the lowest overall sensitivity and positive predictive value (59.3% and 77.4%, respectively), compared with contrast material–enhanced CT (73.6% and 85.8%, respectively) and contrast-enhanced MRI (77.5% and 83.6%, respectively).
US with intravascular contrast material improves liver lesion conspicuity compared with that at standard US (31). However, there is a paucity of evidence for the use of intravascular contrast–enhanced US in primary surveillance of liver cancers. Growing evidence supports the use of perfluorobutane microbubble enhancement with US. This contrast agent is taken up by the Kupffer cells, allowing for Kupffer phase imaging, which is similar to hepatobiliary phase (HBP) imaging at MRI with hepatobiliary agents. In a randomized controlled study (32) comparing perfluorobutane-enhanced US to gray-scale US for surveillance of HCC, Kupffer phase imaging allowed detection of significantly smaller HCCs. Unfortunately, at this time, Kupffer cell contrast-enhanced US agents are not available for use in the United States.
Considering the low sensitivity of US and the rising prevalence of obesity and the incidence of HCC in the United States and other Western nations, there is growing interest in developing an improved surveillance strategy. Alternatives to US surveillance have been suggested, including the use of CT and MRI. Both CT and MRI may be more sensitive than US for detection of HCC, especially early-stage HCC (30,33). However, these alternatives come with some disadvantages.
Alternative Imaging Options for HCC Surveillance
Complete CT and MRI
Dynamic multiphase contrast-enhanced CT and MRI have higher sensitivity and specificity for the diagnosis of HCC than does US. Annual CT surveillance with a multiphase protocol has reported sensitivity of 68%–73% (5) and has been shown in a prospective randomized trial (34) to perform similarly to semiannual US, with a trend toward detection of HCC at an earlier stage than that with US. MRI has even greater diagnostic yields than US. In a surveillance population, multiphase contrast-enhanced MRI performed with gadoxetate disodium contrast material provided better detection of HCC (86% vs 28%, P < .001) and a lower false-positive results rate (3% vs 6%, P = .004) compared with standard US in patients with HCC risk primarily due to HBV infection (35).
Despite the diagnostic advantages of multiphase contrast-enhanced CT and MRI, there are challenges and drawbacks that prevent their widespread use for surveillance. CT is associated with the risks of ionizing radiation exposure and adverse events related to iodinated contrast agents (36). Due to the repetitive nature of surveillance imaging, the use of CT results in an unacceptably high cumulative radiation risk, especially in patients with HBV infection and well-compensated cirrhosis, who have longer disease courses. Because of the large number of imaging sequences and the length and complexity of complete diagnostic MRI protocols (37), they are not cost- or time-effective for HCC surveillance. In recent years, with improvements in MRI technology and a focus on value in radiology, several investigators (38–43) have suggested abbreviated MRI strategies in an effort to make MRI a more feasible option for clinical HCC surveillance.
Abbreviated MRI
The concept of abbreviated MRI is not new. The greatest body of work and adoption of abbreviated MRI has been in the area of breast cancer screening, which shows benefits in diagnosis and resource use (44,45). Abbreviated MRI strategies have also been suggested for evaluation of hepatic steatosis and fibrosis and follow-up of pancreatic cysts, adrenal adenomas, renal masses, and gynecologic lesions (46). These abbreviated MRI protocols are simplified shorter protocols comprising a small number of sequences that are tailored to evaluate a particular disease, and therefore, are less time intensive to perform and less laborious to interpret. In the context of HCC surveillance, multiphase abdominal MRI may take approximately 40 minutes to complete and US may take approximately 30 minutes; whereas, an abbreviated MRI protocol is typically performed in 15 minutes or less and includes only the sequences necessary for detection of HCC.
Abbreviated MRI Protocols for HCC Surveillance
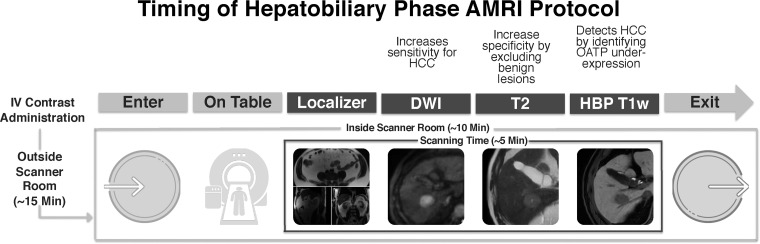
Several abbreviated MRI strategies have been proposed for HCC surveillance. These are summarized and compared in Table E1 and can be categorized as (a) nonenhanced imaging, (b) dynamic contrast-enhanced imaging, and (c) HBP imaging with a hepatobiliary contrast agent. From the vantage point of resource optimization, any of the three abbreviated MRI strategies can be completed in less than or equal to 15 minutes (including set-up time). Figure 3 shows the approximate imaging time for hepatobiliary contrast-enhanced abbreviated MRI that has been adopted in clinical practice at the University of California, San Diego, since 2013. Several multireader retrospective simulated studies (38–40,42,43) have been performed in which abbreviated image sets were extracted from complete, multiphase contrast-enhanced MRI, providing preliminary evidence that supports the efficacy and feasibility of abbreviated MRI for detection of HCC.
Figure 3.
Illustration shows timing of the HBP abbreviated MRI (AMRI) protocol. DWI = diffusion-weighted imaging, IV = intravenous,T1w = T1-weighted.
LI-RADS provides major features (eg, arterial phase hyperenhancement, washout, and capsule appearance) and ancillary features (eg, restricted diffusion, mild to moderate T2 hyperintensity, and fat or iron sparing) that can be used to determine the probability that an observation is HCC. Abbreviated MRI strategies target detection of either major or ancillary features to identify possible HCC and exclude benign lesions such as cysts or hemangiomas (Table 2). For a more in-depth discussion of imaging features and LI-RADS categories, please refer to the recent articles in RadioGraphics by Elsayes et al (52) and Cerny et al (53). Similar to US, an abbreviated MRI study that is positive for suspected HCC may trigger a callback for a full diagnostic evaluation to allow LI-RADS categorization and staging (37).
Table 2:
CT and MRI LI-RADS Major and Ancillary Features as They Relate to Abbreviated MRI for a Surveillance Population
Dynamic Contrast-enhanced Abbreviated MRI
Dynamic abbreviated MRI protocols include dynamic contrast-enhanced multiphase imaging (ie, nonenhanced, arterial phase, portal venous phase, and delayed phase imaging) with a standard extracellular (ie, rather than hepatobiliary) contrast agent, with or without T2-weighted MRI acquisition, diffusion-weighted imaging (DWI), or HBP imaging (Table E1, Fig 4). Although dynamic abbreviated MRI protocols do not meet the minimum sequence requirements for CT or MRI according to LI-RADS 2018 (37), LI-RADS major features (but not most ancillary features) can be applied to dynamic abbreviated MRI.
Figure 4.
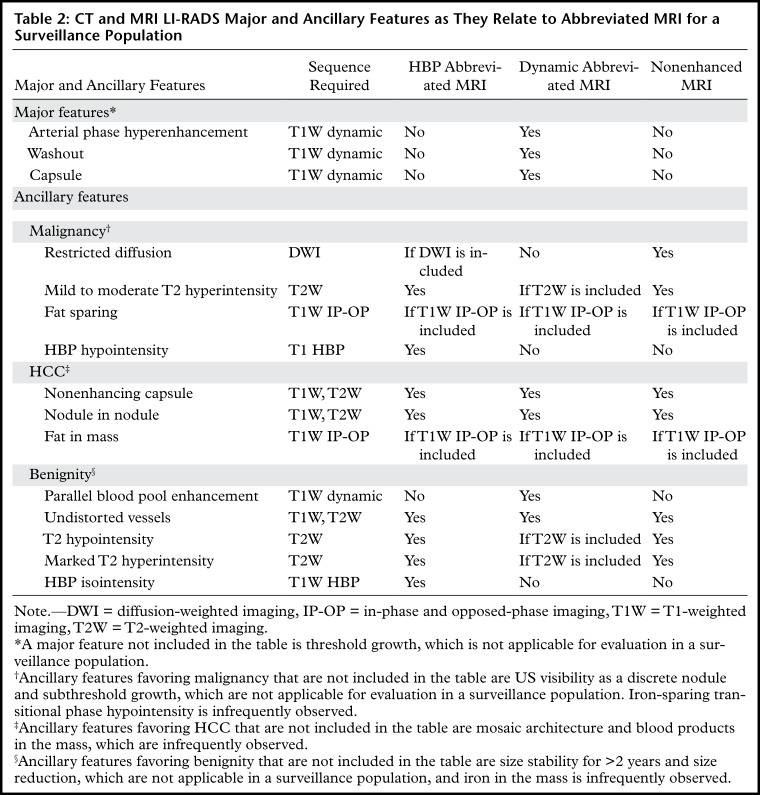
Abbreviated MRI (AMRI) protocol for HCC screening and surveillance. Axial dynamic extracellular contrast-enhanced T1-weighted (T1w) MR images in a 62-year-old man with HCV cirrhosis show a 2.0-cm observation (arrows) in segment 7 or 8 that appears hypointense and shows hyperenhancement on the arterial phase MR image, washout on the portal venous phase MR image, and a capsule appearance on the delayed phase MR image. This finding meets the criteria for LR-5, and this study was considered positive for suspected HCC. GRE = gradient-recalled echo.
Dynamic abbreviated MRI allows characterization of focal liver observations in the context of LI-RADS major features—arterial phase hyperenhancement, washout appearance, and capsule appearance (40). During carcinogenesis, hepatocellular nodules may lose their normal portal triads and develop unpaired arteries (50). This process, in part, explains arterial phase hyperenhancement and washout kinetics that have been described in progressed HCC. Pathologically, capsule appearance may represent a true fibrous capsule or compressed parenchyma around a growing tumor (54); either is characteristic of HCC. The presence of these major features in combination allows a definite diagnosis of HCC with imaging (ie, LR-5).
Although no studies have been performed, to our knowledge, with dynamic abbreviated MRI for surveillance, emerging literature suggests it may be suitable for HCC detection, with per-patient sensitivity of 85%–92% for HCC and specificity of 89%–100% (Table E1) (39,47). In a simulated abbreviated MRI study, Lee et al (40) observed only a small difference in LI-RADS categorization of liver observations between dynamic abbreviated MRI and full diagnostic MRI. Of the 5% of observations that were affected, most were LR-3 observations upgraded to LR-4 by the presence of ancillary features. Further research is needed to determine how dynamic abbreviated MRI should be reported and how indeterminate lesions detected at dynamic abbreviated MRI, such as LR-3 observations, should be followed.
HBP Abbreviated MRI with Gadoxetic Acid
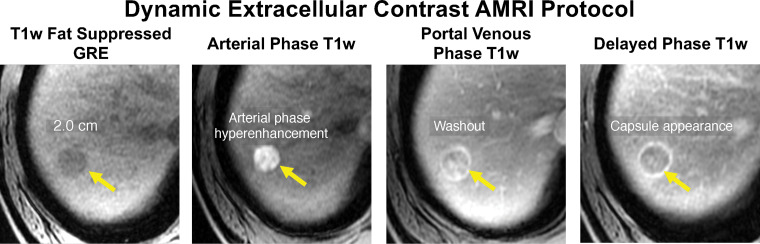
HBP abbreviated MRI is performed after intravenous administration of the hepatobiliary contrast agent gadoxetate disodium (Eovist; Bayer, Whippany, NJ; Primovist outside of the United States.) (Table E1, Fig 5) (39,42,43). HBP abbreviated MRI comprises T1-weighted HBP MRI, and T2-weighted MRI with and without DWI. The primary mechanism for detection of HCC is evaluation for ancillary features of HBP hypointensity and restricted diffusion. The T2-weighted MR images help to preserve specificity by allowing cysts and hemangiomas, which otherwise might resemble and be mistaken for HCC with the other sequences, to be ruled out (Fig 6).
Figure 5.
Example of an HBP abbreviated MRI (AMRI) protocol for HCC screening and surveillance. Axial MR images in a 78-year-old woman with HCV cirrhosis show a new 1.9-cm hypointense observation in segment 5 (arrows) with mild hyperintensity on the T2-weighted (T2W) and DWI MR images, and hypointensity on the HBP T1-weighted (T1w) fat-suppressed MR image. This study was considered positive for suspected HCC, and the patient was referred for diagnostic imaging. SSFSE = single-shot fast spin-echo.
Figure 6.
Standardized reporting of abbreviated MRI (AMRI) for HCC surveillance with hepatobiliary contrast agent. Abbreviated MR images show various findings (arrows).
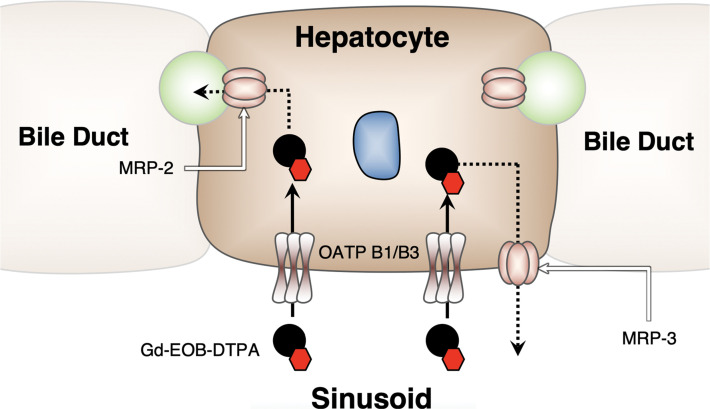
Unlike extracellular contrast agents, which distribute in the intravascular and interstitial spaces, gadoxetate disodium is taken up by hepatocytes by means of hepatocyte-specific organic anion- transporter proteins (OATPs) that are present on the cellular membrane, yielding accumulation in the liver parenchyma and peak enhancement at approximately 20 minutes after contrast agent injection, at which point, the liver enhances relative to the vessels during the HBP (Fig 7). Lesions that lack normal functioning hepatocytes are nonenhancing relative to the liver parenchyma. The hepatocarcinogenesis and the associated imaging features of HCC at HBP and arterial phase MRI are described in detail in Figure 8.
Figure 7.
Illustration shows gadoxetate disodium taken up by hepatocytes by means of specific OATP transporters on the cellular membrane, yielding normal hepatocytes to display peak enhancement at approximately 20 minutes after contrast material injection (ie, during the HBP). (Reprinted, with permission from the ACR, reference 37.)
Figure 8.
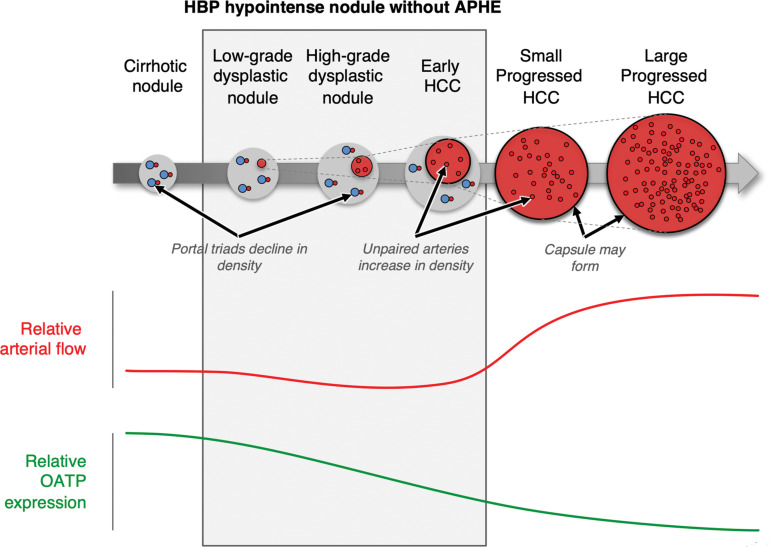
Hepatocarcinogenesis and timing of associated imaging features at HBP and arterial phase MRI. Apparent hypointensity in the HBP occurs because early progression to HCC results in decreased OATP transporter function before development of vascular alterations. A minority of HCCs (approximately 5%) show increased OATP expression and may appear hyperintense on HBP images. HBP imaging may allow detection of additional lesions, including small HCCs and early HCCs that are not visible on images from any other sequence. Because OATP expression decreases during carcinogenesis before complete neoarterialization, some HCCs may be first visible on HBP images as hypointense nodules, before they develop vascular alterations. (Reprinted, with permission from the ACR, reference 37.)
OATP expression progressively declines in most HCCs during hepatocarcinogenesis, resulting in HBP hypointensity (Fig 7). Because OATP expression decreases during carcinogenesis before complete neoarterialization, some HCCs may be first visible on HBP MR images as hypointense nodules, before arterial phase hyperenhancement is detectable (Fig 8). A minority of HCCs (ie, approximately 5%) show increased OATP expression and may appear hyperintense on HBP MR images (37). A possible pitfall of HBP abbreviated MRI is the fact that in patients with severe cirrhosis or cholestasis, HBP uptake may be reduced, which can decrease lesion conspicuity and negatively affect sensitivity for HCC detection. To address this pitfall and other potential artifacts (eg, respiratory motion artifacts) that may reduce sensitivity for detection of HCC, at the University of California, San Diego, we assign a visualization score that is analogous to the LI-RADS US visibility score. A score of B refers to minor technical limitations that may limit complete evaluation of lesions that are smaller than 1 cm, while a visualization score of C refers to considerable technical limitations that limit adequate evaluation for any size HCC. At our institution, nondiagnostic HBP MRI occurs for a minority of patients (approximately 4% with a visualization score of C). Approximately 10% of our institutional abbreviated MRI examinations have a visualization score of B, which indicates a mild reduction in uptake. Our combined rate of visualization scores B and C is similar to another published rate of suboptimal HBP MRI (ie, 15%) (55), which is objectively lower than the published rate of inadequate US examinations for HCC surveillance (ie, 20.3%) (6).
A head-to-head comparison in sensitivity between HBP abbreviated MRI and dynamic abbreviated MRI with extracellular contrast material has not been reported in the literature, to our knowledge. HBP abbreviated MRI has been shown to have sensitivity of 81%–92% and a negative predictive value of 90%–96% (38,42,43,47,48) in simulated retrospective studies.
Nonenhanced Abbreviated MRI
Nonenhanced abbreviated MRI comprises T1- and T2-weighted MRI with DWI. Detection of HCC with these sequences relies on the presence of LI-RADS ancillary features. Ancillary features that are evaluable with nonenhanced abbreviated MRI include restricted diffusion, mild-to-moderate T2 hyperintensity, iron or fat sparing (if in- and opposed-phase images are included), fat in the mass, and potential blood products in the mass (Table E1, Fig 9) (38,56,57). In- and opposed-phase MRI can be included without additional acquisition time with any abbreviated MRI protocol that includes T1-weighted gradient-recalled-echo sequences (Fig 10). Similar to HBP abbreviated MRI, the presence of marked T2-hyperintensity can help to exclude cysts or hemangiomas. DWI may help to improve sensitivity for detection of focal lesions, especially malignant lesions, on the basis of their higher cellularity and microstructural heterogeneity (58).
Figure 9.
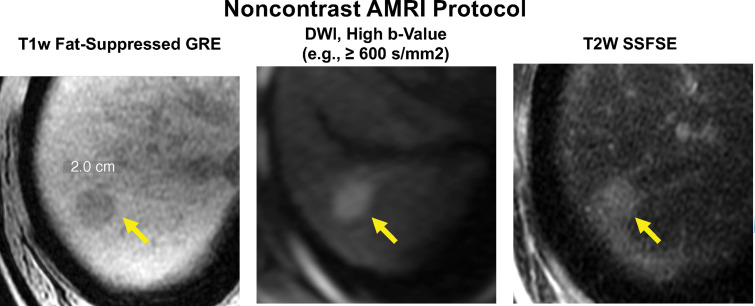
Abbreviated MRI protocol for HCC screening and surveillance. Nonenhanced abbreviated MR images in a 62-year-old man with HCV cirrhosis. A 2.0-cm hypointense observation (arrow) is seen in segment 7-8 on the T1-weighted (T1w) fat-suppressed gradient-recalled-echo (GRE) MR image, with restricted diffusion on the high b-value DWI MR image and moderate hyperintensity on the T2-weighted (T2w) steady-state fast spin-echo (SSFSE) MR image. This study was considered positive for suspected HCC.
Figure 10.
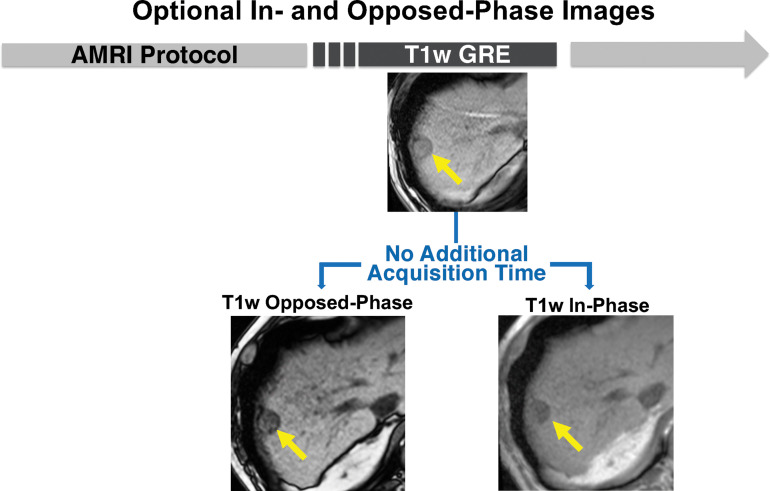
In-phase and opposed-phase chemical shift MRI can be performed simultaneously with Dixon T1-weighted (T1w) gradient-recalled-echo (GRE) MRI. The in- and opposed-phase sequences are performed to identify pathologic (intravoxel) fat content of tissues by showing a decrease in signal intensity on the opposed- phase images compared with the in-phase images. T1-weighted fat-suppressed GRE MR images show decreased signal intensity of the 2.5-cm mass (arrows) in segment 7-8. This sequence pair can be performed as part of any of the abbreviated MRI (AMRI) strategies, without additional acquisition time.
Similar to the literature for other abbreviated MRI strategies, to our knowledge, there are few studies that evaluate nonenhanced abbreviated MRI in true surveillance cohorts. In retrospective nonsurveillance cohorts, nonenhanced MRI achieved per-patient sensitivity of 79.1%–91.7% and specificity of 76%–98% (41,49,51,59), demonstrating significant superiority over historically reported standard US (per-patient sensitivity, 47%–63%) (29,56). In a single retrospective surveillance cohort (47) in which nonenhanced abbreviated MRI was compared to HBP and dynamic abbreviated MRI, the per-patient sensitivity was lower (62% vs 81%–85%) for nonenhanced abbreviated MRI.
Evidence for Abbreviated MRI in HCC Screening and Surveillance
To our knowledge, no prospective studies have been performed to compare abbreviated MRI strategies to US in surveillance cohorts. Furthermore, it is challenging to synthesize the existing retrospective data on abbreviated MRI, given that authors have used different protocols and in most studies have not examined surveillance cohorts. The incidence of HCC in surveillance cohorts is low, approximately 3%–8% per year, hence the true diagnostic performance (ie, negative and positive predictive values) of abbreviated MRI and US is challenging to assess in cohorts with a high prevalence of HCC and a relatively low number of benign lesions. In a recent retrospective study, Vietti Violi et al (47) compared the three simulated abbreviated MRI strategies (dynamic, HBP, and nonenhanced abbreviated MRI) used in 237 patients under surveillance for HCC who underwent gadoxetic acid–enhanced complete MRI. Although both dynamic and HBP abbreviated MRI performed well, there was no independent reference standard.
Despite the limitations of the current evidence, the per-patient sensitivity and specificity of abbreviated MRI for early-stage HCC is substantially higher than those reported historically for US. Abbreviated MRI also provides high reliability, with moderate to high interreader agreement (per-patient κ = 0.51–0.8, per-lesion κ = 0.67–0.88) (Table E1), which suggests that consistent interpretation can be achieved (43). The reproducibility of US in the detection of HCC is unknown and likely varies by institution and sonographer experience.
Rates of false-positive results were not available for most studies of abbreviated MRI that we evaluated, because the majority were conducted with simulated image sets. However, our unpublished single-center data for 417 patients who underwent 1160 prospective abbreviated MRI examinations with hepatobiliary contrast material yielded a per-study false-positive results rate of 2%. Our rate of false-positive examinations is similar to that of complete dynamic MRI with hepatobiliary contrast material (3%) (35) and is significantly lower than that of US in a contemporary Western cohort.
A final but important point is that the rate of inadequate examination with US may be as high as 20% (6). The rate of inadequate examination with abbreviated MRI is likely lower. In our single-center experience with clinically applied HBP abbreviated MRI, the rate of nondiagnostic HBP examinations was approximately 4%. The inadequacy rates were not reported in the literature because studies often excluded patients with inadequate examinations. Technical adequacy ultimately contributes to the overall effectiveness of a surveillance modality, and comparison of US with abbreviated MRI should be included in future prospective studies.
Implementation Considerations
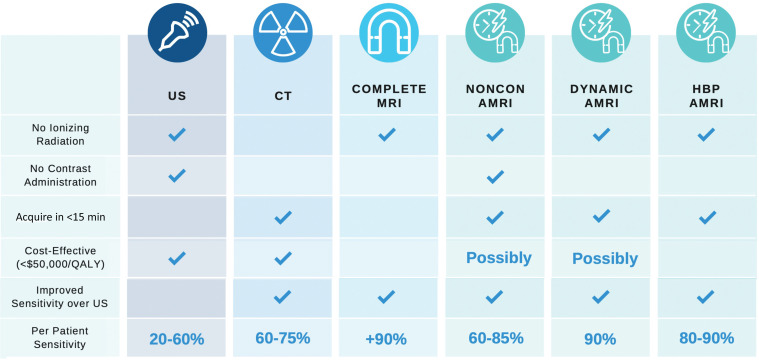
Deciding which imaging strategy to use for HCC surveillance is complex. There are advantages and disadvantages to all strategies for HCC surveillance (Fig 11).
Figure 11.
Chart shows a comparison of HCC surveillance strategies. AMRI = abbreviated MRI, noncon = non–contrast-enhanced, QALY = quality-adjusted life years.
Standardized Template Reporting
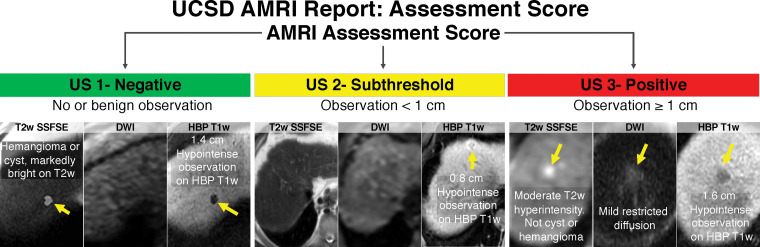
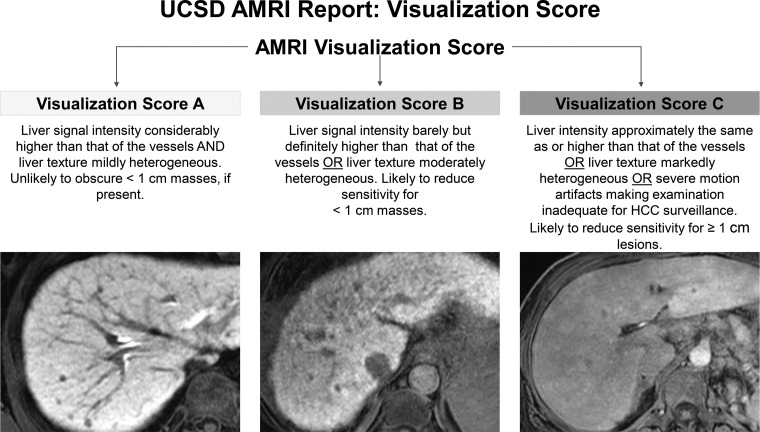
Standardized reporting is recognized as a quality metric for HCC imaging and is the impetus for the LI-RADS. Unlike surveillance US, the LI-RADS does not currently provide guidance on reporting abbreviated MRI. At our institution, we have devised a template that mirrors that used in US surveillance reports. The primary assessment of each study is reported as negative, subthreshold, or positive for suspected HCC, similar to that for the US LI-RADS (Fig 6). We also categorize and report the study quality and the presence or absence of limiting artifacts (Fig 12). This template approach provides consistent recommendations to referring clinicians (Fig 13). However, further data are needed to validate these follow-up study and time-interval recommendations. To our knowledge, no authors have suggested or provided templated options for nonenhanced or dynamic abbreviated MRI. The presence of indeterminate observations (such as LR-3 or LR-4) may complicate the reporting of and the recommended time intervals for dynamic abbreviated MRI surveillance.
Figure 12.
Standardized reporting of abbreviated MRI (AMRI) for HCC surveillance with HBP contrast agent. The optimal visualization score shows the liver parenchyma with considerably higher signal intensity than that of the vessels. An image quality score of A is unlikely to obscure masses smaller than 1 cm, if present.
Figure 13.
Standardized reporting template for abbreviated MRI (AMRI) for HCC surveillance with an HBP contrast agent. At our institution, we have devised and refined a template that mirrors that used in US surveillance reports and includes clinical recommendations for follow-up imaging based on the assessment score of each surveillance abbreviated MRI examination. UCSD = University of California, San Diego.
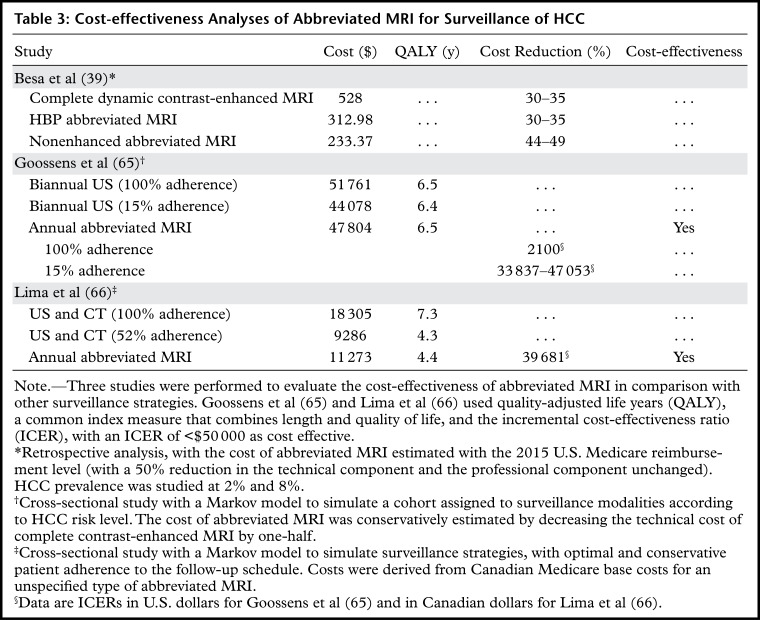
Cost Analysis of HCC Surveillance Strategies
Although a detailed discussion of cost-effectiveness is beyond the scope of this article, there are a few points that readers should consider. Surveillance for HCC improves life expectancy and is cost-effective in patients who may be eligible for effective therapies (ie, those with early-stage HCC) (61). Although MRI is superior to both US and CT for characterization and detection of HCC (5,35,62–64), the relatively high cost of full diagnostic MRI and limited geographic availability has impeded its acceptance for surveillance. To narrow the cost gap, abbreviated MRI techniques have been developed as alternatives to the current surveillance strategy. Recent cost-effectiveness analyses (39,65,66) have shown that semiannual abbreviated MRI may be cost-effective for high- and intermediate-risk patients with cirrhosis and may outperform currently recommended nonstratified semiannual US in patients with cirrhosis. The results of these studies are summarized in Table 3.
Table 3:
Cost-effectiveness Analyses of Abbreviated MRI for Surveillance of HCC
Future Directions
Clinical Adoption of Abbreviated MRI
In addition to abbreviated protocols, MRI has several benefits over US that may improve its cost-effectiveness. These include likely lower false-positive rates and potentially fewer follow-up imaging studies and/or biopsies. MRI may be appropriate in specific patients who are either at high risk for HCC or who are not ideal candidates for US surveillance. This is especially true in the United States and other Western nations where obesity and nonalcoholic fatty liver disease, which can reduce the sensitivity of US, are common. With more evidence, there may also be an opportunity to decrease MRI screening frequency from semiannual to annual, given the improved sensitivity for detection of small lesions. This would lead to further reduction in cost if it were proven to be noninferior to semiannual screening.
Determining the Optimal Abbreviated MRI Surveillance Strategy
There is currently insufficient evidence to suggest one abbreviated MRI strategy over another. With growing interest in abbreviated protocols and a growing need for HCC surveillance, prospective trials (67,68) evaluating surveillance every 6 months (the MIRACLE-HCC trial) or every 12 months (the MAGNUS-HCC trial) with nonenhanced abbreviated MRI versus US are currently underway in Korea and will provide prospective data to validate preliminary data in a true surveillance setting. In addition, an Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN) funded prospective multicenter evaluation, Liver Imaging For Early Cancer Detection (LIFE) study will soon be performed to evaluate HBP abbreviated MRI versus US for HCC surveillance (IUMICA18928–01).
Maintaining Focus and Brevity
Abbreviated protocols not only shorten acquisition time for the patient but also shorten interpretation and reporting time for the radiologist. In our experience, evaluation of fewer images allows the interpreting radiologist to maintain focus and efficiency. For example, although most imaging units today can obtain in- and-opposed phase images with the use of the Dixon technique (Fig 10), inclusion of these “free” images may prolong the reading time for the radiologist, with minimal diagnostic yield for HCC. In a surveillance population, the presence of fat is seen in approximately 1.3% of HCCs (51). Hence, adding sequences may have minimal positive effect on detection but could result in longer interpretation time and greater complexity. To our knowledge, no literature exists on radiologists’ perception of complexity or interpretation times related to diagnostic performance or burnout. Future studies may be performed to evaluate these aspects in addition to diagnostic accuracy.
Improving Patient Adherence to HCC Surveillance
Despite the proven efficacy of surveillance related to survival, many at-risk patients do not undergo surveillance at appropriate intervals or at all. In the United States, fewer than 20% of eligible patients undergo regular surveillance before HCC diagnosis (69,70). Retrospective evaluations of patients who were eligible for surveillance identified that 3%–17% of Americans receive consistent surveillance every 6 months, and 66% receive inconsistent annual surveillance (69–71). Lack of adherence is likely multifactorial but may be worse because of the semiannual frequency of US examinations. In an evaluation of more than 2000 screening or surveillance examinations in more than 600 surveillance-appropriate patients, only 34.5% of patients underwent two or more screening or surveillance encounters per year (72). Hence, only one-third of patients underwent adequate semiannual screening as recommended by the AASLD. In the same study, 76% of patients underwent more than one surveillance encounter, which suggests that a larger number of patients maintain an annual screening regimen. Although there is no evidence to suggest that abbreviated MRI would have higher compliance rates than US surveillance, if a longer surveillance interval can be adopted for a more sensitive test, perhaps patient adherence to the HCC surveillance schedule may also be improved.
Other factors may contribute to the lack of adherence, such as patient preference. Currently there are no data on patient satisfaction with US versus that with MRI. This could be a focus of future research that may help inform reasons for nonadherence and whether abbreviated MRI is a preferred alternative.
Circulating Biomarkers
In addition to imaging options, there is growing interest in genetic and cellular serum biomarkers for early detection of HCC, and radiologists should be aware of this emerging and potentially synergistic diagnostic technology. Although α-fetoprotein is the most commonly used circulating biomarker for HCC, its role in surveillance for detection of early-stage cancer is limited by its poor sensitivity and specificity (5,73). An emerging technology known as liquid biopsy allows detection of circulating tumor cells, genetic byproducts, or specific tumor-associated proteins. Liquid biopsies are a promising approach for noninvasive blood-based diagnosis. Although there are currently not enough data to recommend the use of liquid biopsy in clinical practice (20), a recent study confirmed high sensitivity and specificity (100% and 94%, respectively) in a prospective surveillance population (74). With further refinement and validation, liquid biopsy may be important to the early diagnosis and initial management of patients who are undergoing surveillance for HCC (74) and could be combined with abbreviated MRI strategies.
Conclusion
For detection of potentially curable early-stage HCC, clinical practice guidelines recommend semiannual surveillance with liver US. However, cirrhosis and body habitus can impair US visualization of liver lesions in patients who are at risk for HCC. The low sensitivity of US may mean that small potentially curable HCCs are not identified. Abbreviated MRI protocols are specifically tailored for detection of HCC and use fewer sequences than a complete multiphase MRI examination. Retrospective studies have shown that simulated abbreviated MRI provides high sensitivity and specificity for early-stage HCC but mostly in nonsurveillance cohorts. Data are needed to further define the diagnostic accuracy of abbreviated MRI in the surveillance population. Prospective studies, ideally randomized controlled trials that compare US and abbreviated MRI strategies, may provide sufficient evidence to allow adoption of abbreviated MRI in societal guidelines. The optimal protocol and interval and cost-related issues still must be resolved for widespread clinical implementation.
SUPPLEMENTAL TABLE
Disclosures of Conflicts of Interest.—: B.T. Activities related to the present article: grant and consultancy from Bayer. Activities not related to the present article: consultancy for Alexion and grant/grants pending from Takeda. Other activities: disclosed no relevant relationships. C.B.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: grants from Bayer, GE, Gilead, Philips, and Siemens; consultancy for Blade, Boehringer, and Epigenomics; consulting under the auspices of the University of California for AMRA Medical, Bristol-Myers Squibb, Exact Sciences, GE digital, and IBM-Watson; laboratory services from Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva, and Takeda; and royalties from Wolters Kluwer. Other activities: disclosed no relevant relationships. K.J.F. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultancy for Epigenomics; grants/grants pending from Bayer and the U.S. Department of Defense; lectures for the Los Angeles Radiological Society, Medscape, and the University of California, San Diego; and support for travel expenses from Bayer. Other activities: disclosed no relevant relationships.
J.Y.A. supported by the NIH T32EB005970 institutional training grant through the Clinician-Scientist Radiology Residency Program.
For this journal-based SA-CME activity, the authors B.T., C.B.S., and K.J.F. have reported disclosures; the other authors, the editor, and the reviewers have disclosed no relevant relationships.
J.Y.A. and M.A.P. contributed equally to this work.
Abbreviations:
- AASLD
- American Association for the Study of Liver Diseases
- ACR
- American College of Radiology
- DWI
- diffusion-weighted imaging
- HBP
- hepatobiliary phase
- HBV
- hepatitis B
- HCC
- hepatocellular carcinoma
- HCV
- hepatitis C
- LI-RADS
- Liver Imaging Reporting and Data System
- NASH
- nonalcoholic steatohepatitis
- OATP
- organic anion-transporting polypeptides
References
- 1.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130(7):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh YP, Hu TH, Cho PY, et al. Evaluation of abdominal ultrasonography mass screening for hepatocellular carcinoma in Taiwan. Hepatology 2014;59(5):1840–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67(1):358–380. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69(1):182–236 [Published correction appears in J Hepatol 2019;70(4):817.]. [DOI] [PubMed] [Google Scholar]
- 5.Colli A, Fraquelli M, Casazza G, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol 2006;101(3):513–523. [DOI] [PubMed] [Google Scholar]
- 6.Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45(1):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HS, El-Serag HB. The Epidemiology of Hepatocellular Carcinoma in the USA. Curr Gastroenterol Rep 2019;21(4):17. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 9.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sepanlou SG, Safiri S, Bisignano C, et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5(3):245–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 12.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol 2019;17(4):748–755.e3. [DOI] [PubMed] [Google Scholar]
- 13.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67(1):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016;122(11):1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cucchetti A, Cescon M, Erroi V, Pinna AD. Cost-effectiveness of liver cancer screening. Best Pract Res Clin Gastroenterol 2013;27(6):961–972. [DOI] [PubMed] [Google Scholar]
- 16.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68(2):723–750. [DOI] [PubMed] [Google Scholar]
- 17.Si MS, Amersi F, Golish SR, et al. Prevalence of metastases in hepatocellular carcinoma: risk factors and impact on survival. Am Surg 2003;69(10):879–885. [PubMed] [Google Scholar]
- 18.Yeung CSY, Chiang CL, Wong NSM, et al. Palliative Liver Radiotherapy (RT) for Symptomatic Hepatocellular Carcinoma (HCC). Sci Rep 2020;10(1):1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittal S, Kanwal F, Ying J, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: A United States cohort. J Hepatol 2016;65(6):1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular Carcinoma Screening Associated with Early Tumor Detection and Improved Survival Among Patients with Cirrhosis in the US. Am J Med 2017;130(9):1099–1106.e1. [DOI] [PubMed] [Google Scholar]
- 21.Choi DT, Kum HC, Park S, et al. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2019;17(5):976–987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4(2):439–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY) 2018;43(1):13–25. [DOI] [PubMed] [Google Scholar]
- 24.Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC) . 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol 2015;16(3):465–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US LI-RADS v2017 Core . US LI-RADS v2017 Working Group. American College of Radiology. https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-US-Algorithm-Portrait-2017.pdf. Published 2017. Accessed June 20, 2020. [Google Scholar]
- 26.Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018;154(6):1706–1718.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fetzer DT, Rodgers SK, Seow JH, et al. Ultrasound Evaluation in Patients at Risk for Hepatocellular Carcinoma. Radiol Clin North Am 2019;57(3):563–583. [DOI] [PubMed] [Google Scholar]
- 28.Wong LL, Reyes RJ, Kwee SA, Hernandez BY, Kalathil SC, Tsai NC. Pitfalls in surveillance for hepatocellular carcinoma: How successful is it in the real world? Clin Mol Hepatol 2017;23(3):239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanna RF, Miloushev VZ, Tang A, et al. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY) 2016;41(1):71–90. [DOI] [PubMed] [Google Scholar]
- 31.Gui J, Kelly W, Wilson SR. Contrast-enhanced US in Local Ablative Therapy and Secondary Surveillance for Hepatocellular Carcinoma. 2016;(5):1302-1322. [DOI] [PubMed] [Google Scholar]
- 32.Kudo M, Ueshima K, Osaki Y, et al. B-Mode Ultrasonography versus Contrast-Enhanced Ultrasonography for Surveillance of Hepatocellular Carcinoma: A Prospective Multicenter Randomized Controlled Trial. Liver Cancer 2019;8(4):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008;6(12):1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography: a randomised study. Aliment Pharmacol Ther 2013;38(3):303–312. [DOI] [PubMed] [Google Scholar]
- 35.Kim SY, An J, Lim YS, et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol 2017;3(4):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morcos SK, Thomsen HS. Adverse reactions to iodinated contrast media. Eur Radiol 2001;11(7):1267–1275. [DOI] [PubMed] [Google Scholar]
- 37.CT/MR LI-RADS v2018 Core . American College of Radiology. https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf. Published 2018. June 20, 2020.
- 38.Besa C, Lewis S, Pandharipande PV, et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol (NY) 2017;42(1):179–190 [Published correction appears in Abdom Radiol (NY) 2018;43(3):760.]. [DOI] [PubMed] [Google Scholar]
- 39.Khatri G, Pedrosa I, Ananthakrishnan L, et al. Abbreviated-protocol screening MRI vs. complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS v2018. J Magn Reson Imaging 2020;51(2):415–425. [DOI] [PubMed] [Google Scholar]
- 40.Lee JY, Huo EJ, Weinstein S, et al. Evaluation of an abbreviated screening MRI protocol for patients at risk for hepatocellular carcinoma. Abdom Radiol (NY) 2018;43(7):1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan MV, McDonald SJ, Ong YY, et al. HCC screening: assessment of an abbreviated non-contrast MRI protocol. Eur Radiol Exp 2019;3(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marks RM, Ryan A, Heba ER, et al. Diagnostic per-patient accuracy of an abbreviated hepatobiliary phase gadoxetic acid-enhanced MRI for hepatocellular carcinoma surveillance. AJR Am J Roentgenol 2015;204(3):527–535. [DOI] [PubMed] [Google Scholar]
- 43.Tillman BG, Gorman JD, Hru JM, et al. Diagnostic per-lesion performance of a simulated gadoxetate disodium-enhanced abbreviated MRI protocol for hepatocellular carcinoma screening. Clin Radiol 2018;73(5):485–493. [DOI] [PubMed] [Google Scholar]
- 44.Greenwood HI. Abbreviated protocol breast MRI: The past, present, and future. Clin Imaging 2019;53:169–173. [DOI] [PubMed] [Google Scholar]
- 45.Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L. An Abbreviated Protocol for High-Risk Screening Breast MRI Saves Time and Resources. J Am Coll Radiol 2016;13(11S):R74–R80. [DOI] [PubMed] [Google Scholar]
- 46.Canellas R, Rosenkrantz AB, Taouli B, et al. Abbreviated MRI protocols for the abdomen. RadioGraphics 2019;39(3):744–758. [DOI] [PubMed] [Google Scholar]
- 47.Vietti Violi N, Lewis S, Liao J, et al. Gadoxetate-enhanced abbreviated MRI is highly accurate for hepatocellular carcinoma screening. Eur Radiol 2020. 10.1007/s00330-020-07014-1. Published online June 25, 2020. [DOI] [PubMed] [Google Scholar]
- 48.Brunsing RL, Chen DH, Schlein A, et al. Gadoxetate-enhanced Abbreviated MRI for Hepatocellular Carcinoma Surveillance: Preliminary Experience. Radiol Imaging Cancer 2019;1(2):e190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whang S, Choi MH, Choi J-I, Youn SY, Kim DH, Rha SE. Comparison of diagnostic performance of non-contrast MRI and abbreviated MRI using gadoxetic acid in initially diagnosed hepatocellular carcinoma patients: a simulation study of surveillance for hepatocellular carcinomas. Eur Radiol 2020;30(8):4150–4163. [DOI] [PubMed] [Google Scholar]
- 50.Park HJ, Jang HY, Kim SY, et al. Non-enhanced magnetic resonance imaging as a surveillance tool for hepatocellular carcinoma: Comparison with ultrasound. J Hepatol 2020;72(4):718–724. [DOI] [PubMed] [Google Scholar]
- 51.Han S, Choi JI, Park MY, Choi MH, Rha SE, Lee YJ. The Diagnostic Performance of Liver MRI without Intravenous Contrast for Detecting Hepatocellular Carcinoma: A Case-Controlled Feasibility Study. Korean J Radiol 2018;19(4):568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elsayes KM, Hooker JC, Agrons MM, et al. 2017 version of LI-RADS for CT and MR imaging: An update. RadioGraphics 2017;37(7):1994–2017. [DOI] [PubMed] [Google Scholar]
- 53.Cerny M, Chernyak V, Olivié D, et al. LI-RADS version 2018 ancillary features at MRI. RadioGraphics 2018;38(7):1973–2001. [DOI] [PubMed] [Google Scholar]
- 54.Ishigami K, Yoshimitsu K, Nishihara Y, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology 2009;250(2):435–443. [DOI] [PubMed] [Google Scholar]
- 55.Kobi M, Paroder V, Flusberg M, Rozenblit AM, Chernyak V. Limitations of GD-EOB-DTPA-enhanced MRI: can clinical parameters predict suboptimal hepatobiliary phase? Clin Radiol 2017;72(1):55–62. [DOI] [PubMed] [Google Scholar]
- 56.Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med 2000;124(7):1061–1065. [DOI] [PubMed] [Google Scholar]
- 57.Sutherland T, Watts J, Ryan M, et al. Diffusion-weighted MRI for hepatocellular carcinoma screening in chronic liver disease: Direct comparison with ultrasound screening. J Med Imaging Radiat Oncol 2017;61(1):34–39. [DOI] [PubMed] [Google Scholar]
- 58.Tang L, Zhou XJ. Diffusion MRI of cancer: From low to high b-values. J Magn Reson Imaging 2019;49(1):23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim YK, Kim YK, Park HJ, Park MJ, Lee WJ, Choi D. Noncontrast MRI with diffusion-weighted imaging as the sole imaging modality for detecting liver malignancy in patients with high risk for hepatocellular carcinoma. Magn Reson Imaging 2014;32(6):610–618. [DOI] [PubMed] [Google Scholar]
- 60.Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65(4):1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruggeri M. Hepatocellular carcinoma: cost-effectiveness of screening. A systematic review. Risk Manag Healthc Policy 2012;5:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee YJ, Lee JM, Lee JS, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015;275(1):97–109. [DOI] [PubMed] [Google Scholar]
- 63.Yu NC, Chaudhari V, Raman SS, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9(2):161–167. [DOI] [PubMed] [Google Scholar]
- 64.Di Martino M, De Filippis G, De Santis A, et al. Hepatocellular carcinoma in cirrhotic patients: prospective comparison of US, CT and MR imaging. Eur Radiol 2013;23(4):887–896. [DOI] [PubMed] [Google Scholar]
- 65.Goossens N, Singal AG, King LY, et al. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin Transl Gastroenterol 2017;8(6):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lima PH, Fan B, Bérubé J, et al. Cost-utility analysis of imaging for surveillance and diagnosis of hepatocellular carcinoma. AJR Am J Roentgenol 2019;213(1):17–25. [DOI] [PubMed] [Google Scholar]
- 67.An C, Kim DY, Choi JY, Han KH, Roh YH, Kim MJ. Noncontrast magnetic resonance imaging versus ultrasonography for hepatocellular carcinoma surveillance (MIRACLE-HCC): study protocol for a prospective randomized trial. BMC Cancer 2018;18(1):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HA, Kim KA, Choi JI, et al. Comparison of biannual ultrasonography and annual non-contrast liver magnetic resonance imaging as surveillance tools for hepatocellular carcinoma in patients with liver cirrhosis (MAGNUS-HCC): a study protocol. BMC Cancer 2017;17(1):877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology 2010;52(1):132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singal AG, Yopp AC, Gupta S, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila) 2012;5(9):1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farvardin S, Patel J, Khambaty M, et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology 2017;65(3):875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Majeed A, Roberts SK, Kemp W. RE: No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology 2019;156(4):1217. [DOI] [PubMed] [Google Scholar]
- 73.Ahn JC, Teng PC, Chen PJ, et al. Detection of circulating tumor cells and their implications as a novel biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology 2020. 10.1002/hep.31165. Published online February 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qu C, Wang Y, Wang P, et al. Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAg-seropositive individuals by liquid biopsy. Proc Natl Acad Sci U S A 2019;116(13):6308–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.