A 71-year-old man came to our pulmonary consultation with 6 weeks of progressive dyspnoea, hoarseness and, more recently, stridor. His past medical history included colorectal cancer treated with surgery and adjuvant chemotherapy, and type 2 diabetes mellitus. Prior to our consultation, his general practitioner had already ordered a chest computed tomography (CT) scan. The patient had undergone this CT scan 3 days before the consultation and it did not reveal any abnormalities.
Short abstract
A flow–volume curve with a flattening of the inspiratory and expiratory limb suggests a proximal obstruction of the upper airways. Plasma cell neoplasms need to be considered in the differential diagnosis of an invasion of the upper respiratory tract. https://bit.ly/2K9lOXj
A 71-year-old man came to our pulmonary consultation with 6 weeks of progressive dyspnoea, hoarseness and, more recently, stridor. His past medical history included colorectal cancer treated with surgery and adjuvant chemotherapy, and type 2 diabetes mellitus. Prior to our consultation, his general practitioner had already ordered a chest computed tomography (CT) scan. The patient had undergone this CT scan 3 days before the consultation and it did not reveal any abnormalities.
Task 1
How do you define a stridor? Which pathologies can trigger a stridor?
Answer 1
Stridor refers to a monophonic sound, typically high pitched, produced as turbulent flow passes through a narrowed segment of the upper respiratory tract. It can be heard without a stethoscope. Stridor can be distinguished from a wheeze because it is predominantly inspiratory and is more prominent over the neck than over the chest. Although stridor is usually inspiratory, it can also be expiratory or biphasic. Causes of stridor in adults include acute epiglottitis, airway oedema after device removal, anaphylaxis, vocal cord dysfunction, inhalation of a foreign body, laryngeal tumours, thyroiditis and tracheal carcinoma [1].
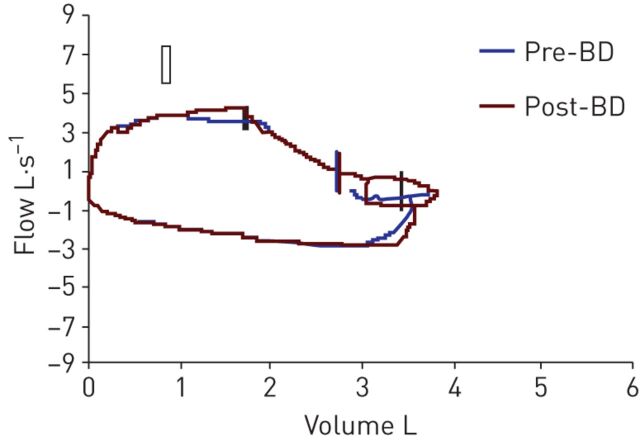
Spirometry was performed and the flow–volume curve is presented in figure 1.
Figure 1.
Flow–volume curve. BD: bronchodilator (salbutamol).
Task 2
What abnormalities do you see on the flow–volume curve? How can they be caused?
Answer 2
The flow–volume curve shows a flattening of the inspiratory and expiratory limbs, suggesting a proximal obstruction of the upper airways.
Task 3
What would be your initial investigations for such a patient?
Answer 3
A CT scan of the neck (along with a CT scan of the chest but in this case, this has already been performed) and a bronchoscopy.
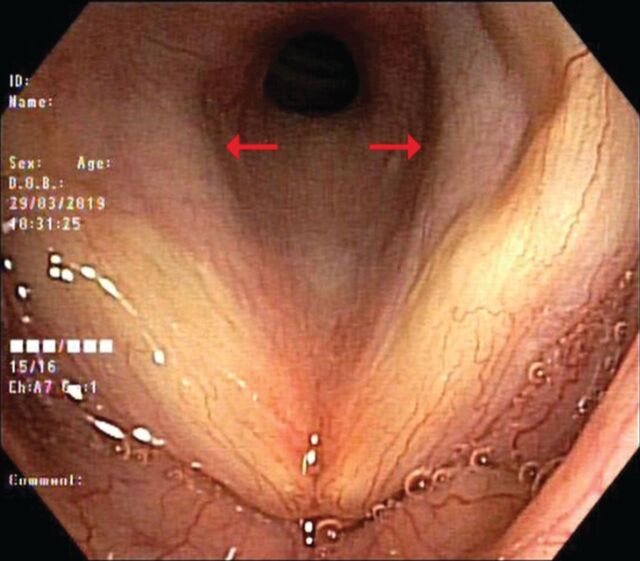
Bronchoscopy was performed before the CT scan of the neck for practical reasons and identified a subglottic stenosis with obstruction of ∼50% of the lumen. There was no impairment in the mobility of the vocal cords. The bronchial exploration below the stenosis was normal (figure 2).
Figure 2.
Bronchoscopy: vocal cords and subglottic stenosis (arrows).
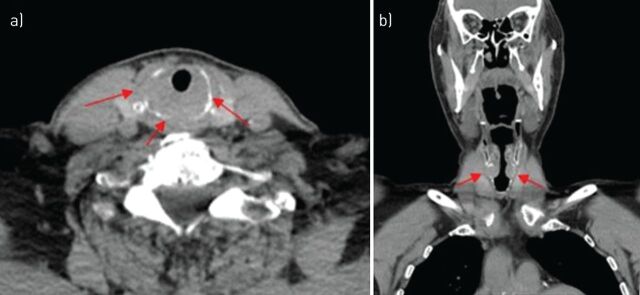
Subsequently, a CT scan of the neck was performed (figure 3).
Figure 3.
CT images of the neck: a) axial view; b) coronal view.
Task 4
Can you describe the singularities indicated by the arrows in figure 3?
Answer 4
This is an infiltrative lesion of the cricoid cartilage leading to subglottic narrowing, with cortical lysis of the external cartilage.
A primary subglottic malignancy invading the cricoid was hypothesised.
Task 5
What are two main diagnoses to rule out?
Answer 5
Squamous cell carcinoma and chondrosarcoma.
Given these hypotheses, a positron emission tomography (PET)-CT was performed. Surprisingly, the PET-CT did not show any hypermetabolism at the cricoid level but identified two hypermetabolic lesions in the left humerus and the left femur (maximum standardised uptake values 3.8 and 4.6, respectively) (figure 4). These two lesions were asymptomatic. Due to the discordance of the PET-CT, a biopsy of the cricoid cartilage was performed by the ear, nose and throat specialist, and revealed a submucosal infiltration of plasma cells (CD138+) with λ-restriction.
Figure 4.
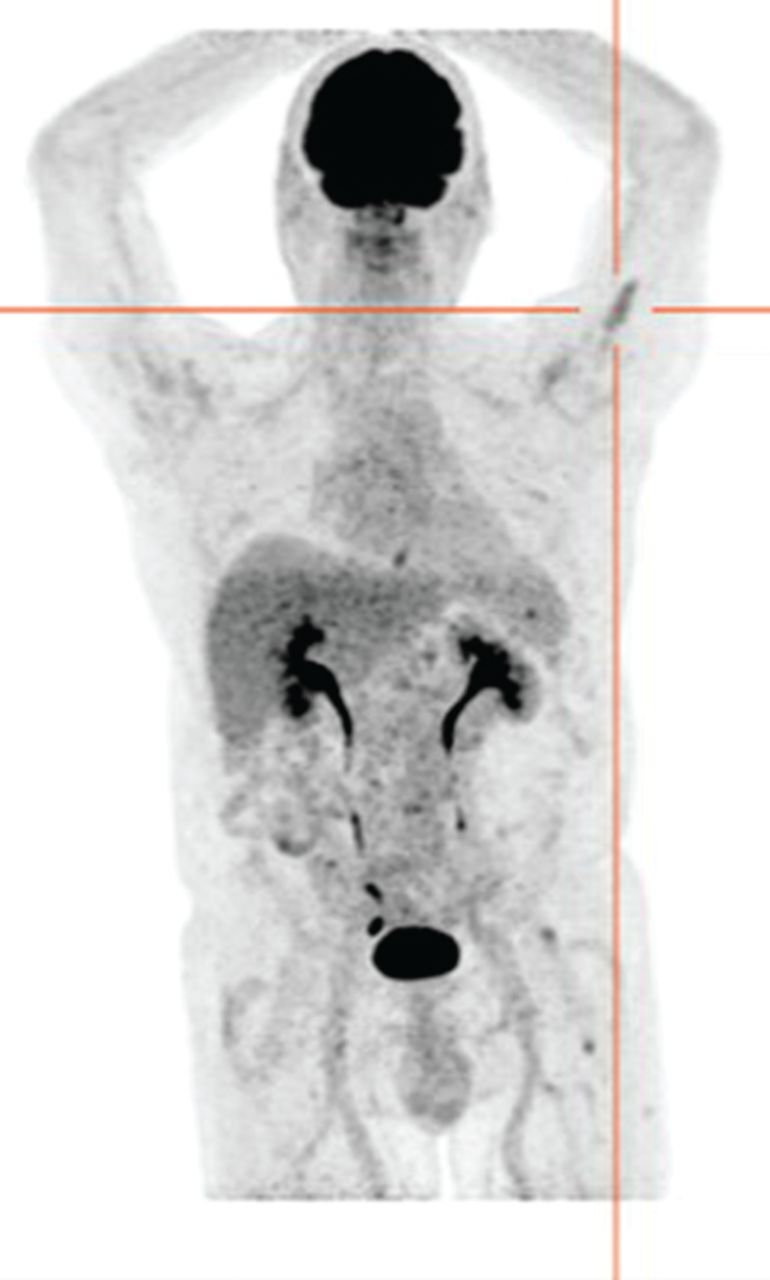
PET-CT: absence of hypermetabolism at the cricoid level but presence of a hypermetabolic lesion in the left humerus.
Task 6
What would be your diagnosis? How could you confirm it?
Answer 6
Owing to the proliferation of plasma cells, multiple myeloma needs to be ruled out by a bone marrow biopsy.
A bone marrow biopsy of the posterior superior iliac crest was performed and revealed the presence of 14.2% plasma cells (CD138+) staining exclusively for λ light chain, confirming the diagnosis of a multiple myeloma. Blood tests did not reveal hypercalcaemia, renal failure or anaemia. Serum immunoglobulin showed a slight IgA increase at 4.6 g·L−1 (normal values 0.7–4.1 g·L−1). The κ/λ ratio was 0.03. Urine immunofixation was normal. The β2-microglobulin level was normal. Two magnetic resonance imaging (MRI) scans confirmed osteolytic lesions compatible with a bone invasion by multiple myeloma in the left humerus and the left femur.
These findings allowed us to confirm an IgA-λ multiple myeloma invading the cricoid cartilage and two long bones. This was a Salmon and Durie stage III and an International Staging System stage 1 myeloma. Given the age of the patient, he received a combination of lenalidomide and dexamethasone.
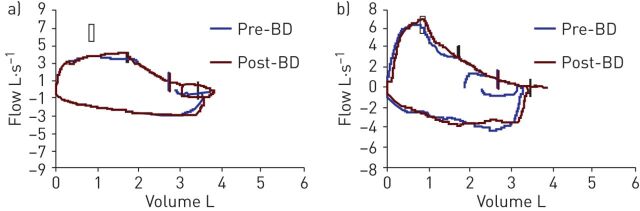
After only one cycle of lenalidomide, the stridor had subsided. Biologically, an 80% drop in the λ-chain peak was observed, and the flow–volume loop no longer showed a flattening of the inspiratory and expiratory curves (figure 5).
Figure 5.
a) The flow–volume curve at the first consultation showed flattening of the inspiratory and expiratory limb, suggesting a proximal obstruction in the airways. b) The flow–volume curve after one cycle of lenalidomide showed a normalisation of the loop. BD: bronchodilator (salbutamol).
Discussion
The exact location of a laryngeal tumour is important for the differential diagnosis. It can be divided into three parts: the supraglottic area (including the epiglottis, false vocal folds and laryngeal ventricles), the glottic area (namely the true vocal cords, usually associated with changes in voice quality) and finally, the subglottic area (10 mm below the free margin of the true vocal fold up to the inferior border of the cricoid cartilage) [2]. Glottic carcinomas represent the majority of laryngeal cancers (50–60%), followed by supraglottic carcinomas (30–40%), while subglottic carcinomas are uncommon (≤5%).
A subglottic mass has a broad differential diagnosis [3], with two main diagnoses to rule out: squamous cell carcinoma (representing >95% of laryngeal tumours) and chondrosarcoma. Other malignancies are rare, and include lymphoma, malignant minor salivary gland tumours and metastasis (especially from melanoma and renal cell carcinoma). Benign tumours of the larynx constitute ≤5% of all laryngeal tumours. Papilloma is the most frequent (85%), and other types include chondroma, haemangioma, fibroma or lipoma [4]. Finally, these masses can be linked to plasma cell neoplasms.
Our case highlights an extremely rare extramedullary involvement of multiple myeloma. To our knowledge, <10 cases of multiple myeloma involvement of cricoid cartilage have been reported [5]. Sometimes, it can be an extramedullary plasmacytoma (EMP), which is defined as an isolated presence of plasma cells outside the bone marrow, such as the cricoid cartilage. It can be distinguished from multiple myeloma by the absence of a plasma cell infiltration in the bone marrow. EMP accounts for ∼3% of plasma cell neoplasms, and <1% of head and neck tumours [6].
It is unclear why the cricoid cartilage did not show an increased uptake whereas the bone lesions did, since both are considered to be part of the same pathology. The bone lesions were not biopsied, but given the MRI findings, these lesions are most likely linked to the multiple myeloma. It is possibile that two different strains of metabolic uptake can exist concomitantly in the same patient. Rasche et al. [7] showed that ≥10% of patients could be misclassified when using fluorodeoxyglucose PET as the only functional imaging technology, compared to with an MRI, as a lower expression of the gene coding for hexokinase-2,which catalyses the first step of glycolysis, can modify PET-CT uptake.
Conclusion
We report a rare case of dyspnoea with stridor and flattening of the flow–volume loop due to an invasion of the cricoid cartilage by plasma cells, in the context of a previously undiagnosed multiple myeloma with an extramedullary localisation. We hope that through this case, plasma cell neoplasms will be considered in the differential diagnosis of upper respiratory tract lesions.
Acknowledgements
We sincerely thank the patient for giving his consent to publish his case and the images.
Footnotes
Conflict of interest: M. Apraxine has nothing to disclose.
Conflict of interest: P. Mineur has nothing to disclose.
Conflict of interest: J. Huet has nothing to disclose.
Conflict of interest: G. Beniuga has nothing to disclose.
Conflict of interest: B. Colinet has nothing to disclose.
References
- 1.Bohadana A, Izbicki G, Kraman S. Fundamentals of lung auscultation. N Engl J Med 2014; 370: 744–751. doi: 10.1056/NEJMra1302901 [DOI] [PubMed] [Google Scholar]
- 2.Mastronikolis NS, Papadas TA, Goumas PD. Head and neck: laryngeal tumors: an overview. Atlas Genet Cytogenet Oncol Haematol 2009; 13: 888–893. doi: 10.4267/2042/44625. [DOI] [Google Scholar]
- 3.Flore B, Hermans R. Multiple myeloma involving the cricoid cartilage. JBR-BTR 2013; 96: 87–88. doi: 10.5334/jbr-btr.217 [DOI] [PubMed] [Google Scholar]
- 4.Kökoğlu K, Canöz O, Doğan S, et al. Laryngeal chondrosarcoma as a rare cause of subglottic stenosis. Case Rep Otolaryngol 2014; 2014: 730643. doi: 10.1155/2014/730643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haser GC, Su HK, Pitman MJ, et al. Extramedullary plasmacytoma of the cricoid cartilage with solitary plasmacytoma of the rib. Am J Otolaryngol 2015; 36: 598–600. doi: 10.1016/j.amjoto.2015.02.010 [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Du J, Zou J, et al. Extramedullary plasmacytoma of the cricoid cartilage progressing to multiple myeloma: a case report. Oncol Lett 2015; 9: 1764–1766. doi: 10.3892/ol.2015.2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasche L, Angtuaco E, McDonald JE, et al. Low expression of hexokinase-2 is associated with false-negative FDG-positron emission tomography in multiple myeloma. Blood 2017; 130: 30–34. doi: 10.1182/blood-2017-03-774422 [DOI] [PMC free article] [PubMed] [Google Scholar]