Abstract
Background
To determinate the association relationship of breast cancer bone metastasis and cancer characteristics and molecular subtype. Furthermore, to evaluate the impact of molecular subtype on prevalence and prognosis of bone metastasis from the breast cancer base on a large population real-word program, the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
We collected and analyzed the data obtained from SEER, which showed molecular subtype information for each patient. The prevalence and outcome of bone metastasis in breast cancer were estimated as per the different molecular subtypes.
Results
Occurrence of bone metastasis in conformity with four different molecular subtypes in all 42684 breast cancer patients was 6.2, 9.4, 7.9, and 6.4%, respectively. The most unfavorable subtype was the triple-negative breast cancer (TNBC), followed by the luminal A, luminal B, and HER2 subtypes (hazard ratio [HR] of luminal A compared with TNBC, 0.533, 95% confidence interval, 0.444–0.641; HR of luminal B, 0.482, 95% CI 0.419–0.555; HR of HER2 subtype, 0.542, 95% CI 0.484–0.608). Brain metastasis impacts overall survival (OS) (p < 0.001) fundamentally, and visceral metastases also significantly decreased OS (p < 0.001).
Conclusion
Bone metastasis patients present a more favorable oncological survival consequence than other metastases, and the TNBC subtype with bone metastasis showed the poorest tumor outcome compared with the other three molecular subtypes.
1. Background
With the high incidence, breast cancer has become the most frequent malignant disease of women and the second most common cancer globally [1, 2]. Also, breast cancer had been the frequent main cause of all cancer deaths around the world. As we all know, the bone has been the most common site of distant metastasis and results in unfavorable outcomes [3], including some amount of complications like bone fracture, pain, and hypercalcemia, which can hardly be cured [4]. However, the blooming and implementation of hormone and targeted treatment for the breast cancer subtype have promoted greatly the oncological outcomes even for end-stage patients [5, 6]. In addition, various treatment modalities, including chemotherapy, hormone therapy, targeted therapy, immunotherapy, and radiopharmaceuticals can control cancer growth effectively and improve the quality of life by relieving symptoms like pain and constipation [7]. Therefore, predicting prognosis precisely of bone metastasis has become gradually urgent in choosing the most suitable therapy strategy.
Breast cancer molecular subtypes categorized as HER2 (HR−/HER2+), triple-negative breast cancer (TNBC, HR−/HER2−), luminal A (hormone receptor (HR+)/human epidermal growth factor receptor 2 (HER2−), and luminal B (HR+/HER2+), have been displayed empirically in clinical value in conducting therapeutic plans [8]. The choice of whether to execute HER2-targeted or hormone therapies is mainly dependent on the breast cancer molecular subtype. Previous researches have noted that the molecular classifications of breast cancer are meaningfully linked with risks of early tumor progression such as recurrence and metastasis [9], therapeutic response [10], and overall survival (OS) [11].
Our main objective of this study is to determine the association relationship of breast cancer bone metastasis and cancer characteristics, molecular subtype, and all other metastasis statuses and to value the effects of molecular types on prevalence and end results of bone metastatic breast cancer based on a large-scale population real-word database, the Surveillance, Epidemiology, and End Results (SEER) program.
2. Materials and Methods
2.1. Patient Selection
This study collected and analyzed the data obtained from the SEER latest release covering about 30% of the U.S. population in 2016. The details of each molecular subtypes were provided in the SEER-Medicare database. Moreover, the information was also obtainable regarding different metastasis containing bone, brain, lung, and liver at the first diagnosis of breast cancer.
Selection criteria of our study were shown as follows: (1) female breast cancer patients; (2) diagnosed year from 2011 to 2015; (3) age at diagnosis no less than 20 years old; (4) the information of breast cancer molecular subtype was available, and metastasis status existing in distant organs was known; and (5) breast cancer was first diagnosed or only cancer. Exclusive criteria were patients with untraced follow-up outcome information and diagnosed via autopsy or death certificate.
2.2. Statistical Analyses
Chi-square test was used to compare the incidence of bone metastasis among different subtypes. To predict the probability of bone metastasis more visibly, a nomogram was calculated and visualized. We separate randomly and evenly all patients into one training cohort and one validation cohort. Univariate and multivariate logistic regression analyses were implemented.
In subgroup analyses, including patients with stage IV cancer, bone metastasis, and each different molecular type, median overall survival (OS) of every group was evaluated by applying Kaplan-Meier estimates with log-rank tests. Of course, we also operate univariate and multivariate analyses to explore the risk factors of OS in these subgroups. We applied “Hmisc” and “rms” R packages to construct and visualize the nomogram of the prognosis prediction model with R software. In this study, we consider the two-sided p value less than 0.05 as statistically significant. All data were calculated using SPSS software (version 24.0, SPSS Inc., Chicago, IL, USA) and R studio platform under R 3.5.0 (http://www.r-project.org/).
3. Results
3.1. Bone Metastasis Rates Differ from Molecular Subtypes
Each breast cancer molecular subtype group in whole breast cancer patients were the following: luminal A, luminal B, HER2, and TNBC were 28931 (67.8%), 4502 (10.5%), 2244 (5.3%), and 7007 (16.4%), respectively. The incidence of bone metastasis in molecular subtype cohorts was 6.2, 9.4, 7.9, and 6.4% (Table 1). The luminal B and HER2 group have higher rates of bone metastasis. In younger patients with age less than 40 years old, the TNBC subtype presents even lower incidence of bone metastasis (4.9%).
Table 1.
Incidence of bone metastasis in different breast cancer molecular subtypes.
| Characteristics | Luminal A | Luminal B | HER2 | TNBC | p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (HR+/HER2-) | (HR+/HER2+) | (HR-/HER2+) | (HR-/HER2-) | ||||||||||
| Bone | All | (%) | Bone | All | (%) | Bone | All | (%) | Bone | All | (%) | ||
| Total | 1800 | 28931 | 6.2 | 422 | 4502 | 9.4 | 178 | 2244 | 7.9 | 447 | 7007 | 6.4 | <0.001 |
| Age (yrs) | |||||||||||||
| <40 | 104 | 1229 | 8.5 | 34 | 410 | 8.3 | 15 | 178 | 8.4 | 32 | 653 | 4.9 | 0.035 |
| 40-59 | 687 | 11834 | 5.8 | 193 | 2184 | 8.8 | 83 | 1071 | 7.7 | 189 | 3253 | 5.8 | <0.001 |
| ≥60 | 1009 | 15868 | 6.4 | 195 | 1908 | 10.2 | 80 | 995 | 8 | 226 | 3101 | 7.3 | <0.001 |
| T stage | |||||||||||||
| ≤1 | 215 | 16607 | 1.3 | 49 | 1930 | 2.5 | 20 | 789 | 2.5 | 45 | 2217 | 2 | <0.001 |
| 2 | 583 | 8515 | 6.8 | 127 | 1622 | 7.8 | 38 | 811 | 4.7 | 121 | 2870 | 4.2 | <0.001 |
| 3 | 336 | 2059 | 16.3 | 79 | 470 | 16.8 | 29 | 263 | 11 | 85 | 912 | 9.3 | <0.001 |
| 4 | 666 | 1750 | 38.1 | 167 | 480 | 34.8 | 91 | 381 | 23.9 | 196 | 1008 | 19.4 | <0.001 |
| N stage | |||||||||||||
| 0 | 427 | 18074 | 2.4 | 94 | 2337 | 4 | 35 | 973 | 3.6 | 82 | 3512 | 2.3 | <0.001 |
| 1 | 831 | 7329 | 11.3 | 203 | 1394 | 14.6 | 82 | 759 | 10.8 | 217 | 2084 | 10.4 | 0.001 |
| 2 | 254 | 2064 | 12.3 | 55 | 443 | 12.4 | 25 | 275 | 9.1 | 60 | 706 | 8.5 | 0.023 |
| 3 | 288 | 1464 | 19.7 | 70 | 328 | 21.3 | 36 | 237 | 15.2 | 88 | 705 | 12.5 | <0.001 |
| Other metastases | |||||||||||||
| Brain(+)lung(-)liver(-) | 60 | 83 | 72.3 | 14 | 26 | 53.8 | 8 | 16 | 50 | 13 | 49 | 26.5 | <0.001 |
| Brain(-)lung(+)liver(-) | 316 | 479 | 66 | 47 | 105 | 44.8 | 20 | 75 | 26.7 | 68 | 254 | 26.8 | <0.001 |
| Brain(-)lung(-)liver(+) | 206 | 344 | 59.9 | 94 | 156 | 60.3 | 37 | 92 | 40.2 | 77 | 170 | 45.3 | <0.001 |
| Brain(+)lung(+)liver(-) | 30 | 43 | 69.8 | 12 | 16 | 75 | 4 | 13 | 30.8 | 15 | 40 | 37.5 | 0.002 |
| Brain(+)lung(-)liver(+) | 16 | 21 | 76.2 | 8 | 9 | 88.9 | 5 | 10 | 50 | 9 | 12 | 75 | 0.264 |
| Brain(-)lung(+)liver(+) | 142 | 190 | 74.7 | 69 | 98 | 70.4 | 40 | 62 | 64.5 | 54 | 68 | 50 | <0.001 |
| Brain(+)lung(+)liver(+) | 31 | 37 | 83.8 | 14 | 18 | 77.8 | 14 | 17 | 82.4 | 20 | 24 | 83.3 | 0.958 |
| Brain(-)lung(-)liver(-) | 999 | 27734 | 3.6 | 164 | 4074 | 4 | 50 | 1959 | 2.6 | 191 | 6350 | 3 | 0.003 |
HR: hormone receptor; HER2: human epidermal growth factor receptor 2; TNBC: triple-negative breast cancer.
Our data also showed that high clinical T and N stages were associated with a high prevalence of bone metastasis, while compared to other molecular subtypes, TNBC had a lower rate of bone metastasis relatively. In the brain only without the liver or lung metastasis group, the occurrence of bone metastasis in TNBC subtypes was lowest (13/49, 26.5%, p < 0.001). For the patients accompanied by liver or lung metastasis without brain metastasis, the HER2 and TNBC suggested lower frequencies of bone metastasis than the other two types. However, the percentage of bone metastasis had no significant difference in these four molecular subtypes (p = 0.958) in the group who had both brain and visceral metastases. If patients had none of the brain, liver, and lung metastases, the number of bone metastasis in both TNBC (191/6350, 3%) and HER2 (50/1959, 2.6%) patients was lower than that in luminal A (999/27734, 3.6%) and B (164/4074, 4%) subtypes (p = 0.003).
3.2. Nomogram Predictive Model
Our multivariate regression found that the status of other metastases including brain, lung, and liver, older age at diagnosis, white and black race, high pathological grade, high clinical T and N stages, and luminal A was attributed to a high rate of bone metastasis (Table 2). To evaluate the trend of bone metastasis more precisely, we generated a nomogram base on the above logistic analysis (Figure 1). Based on the nomogram, if there is a breast cancer patient with 60 years of age, grade 3, T4N2, and luminal A, the tendency of bone metastasis is over 40% with a final total score of 148. Conversely, the occurrence of bone metastasis was nearly 10% at a lower level for patients aged 70 years, grade 3, T3, N2, and TNBC.
Table 2.
Multivariate logistic regression analysis predicting bone metastasis from breast cancer.
| Characteristic | OR | 95% CI | p value |
|---|---|---|---|
| Age | 1.004 | 1.001-1.008 | 0.01 |
| Race | |||
| White | Reference | ||
| Black | 1.024 | 0.902-1.162 | 0.719 |
| Other | 0.785 | 0.655-0.942 | 0.009 |
| Unknown | 0.237 | 0.033-1.708 | 0.153 |
| Grade | |||
| 1 | Reference | ||
| 2 | 1.521 | 1.283-1.803 | <0.001 |
| 3 | 1.101 | 0.921-1.318 | 0.291 |
| 4 | 1.486 | 0.856-2.577 | 0.159 |
| T stage | |||
| ≤1 | Reference | ||
| 2 | 3.02 | 2.62-3.482 | <0.001 |
| 3 | 5.576 | 4.722-6.585 | <0.001 |
| 4 | 10.355 | 8.816-12.163 | <0.001 |
| N stage | |||
| 0 | Reference | ||
| 1 | 2.157 | 1.917-2.426 | <0.001 |
| 2 | 1.833 | 1.562-2.150 | <0.001 |
| 3 | 2.519 | 2.151-2.951 | <0.001 |
| Subtype | |||
| Luminal A | Reference | ||
| Luminal B | 0.849 | 0.737-0.979 | <0.024 |
| Her2+ | 0.377 | 0.304-0.469 | <0.001 |
| TNBC | 0.431 | 0.373-0.496 | <0.001 |
| Brain metastasis | |||
| No | Reference | ||
| Yes | 6.436 | 4.987-8.306 | <0.001 |
| Liver metastasis | |||
| No | Reference | ||
| Yes | 10.59 | 9.173-12.226 | <0.001 |
| Lung metastasis | |||
| No | Reference | ||
| Yes | 6.31 | 5.508-7.229 | <0.001 |
OR: odds ratio; CI: confidence interval.
Figure 1.
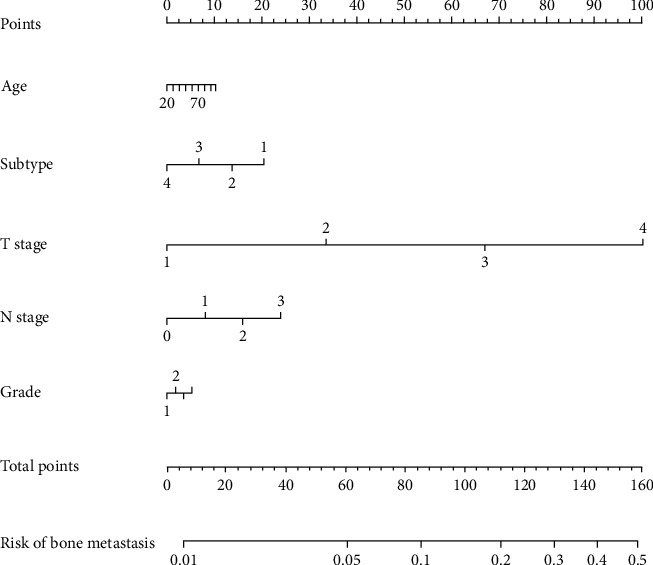
Nomogram predicting the probability of brain metastasis at the time of breast cancer diagnosis. Subtype: 1: luminal A; 2: luminal B; 3: HER2+; 4: TNBC. T stage: 1: ≤1.
3.3. Prognosis of Bone Metastasis Patients in Subgroup Analysis
The overall survival time for stage IV cases was assessed depending on different molecular subtypes (Table 3). The median overall survival period was 18 months, 18 months, 11 months, and 9 months in the luminal A, luminal B, HER2, and TNBC group, respectively, revealing that the TNBC and HER2 cohorts featured the most disadvantageous survival outcome. For patients who had brain and lung metastases with or without liver metastasis, the prevalence of bone metastasis had no significant difference among varied molecular subtypes (p = 0.182 and p = 0.591, respectively). The HER2 and TNBC have shown a similar survival outcome, whether bone metastasis existed or not. With bone metastasis, however, unlike the HER2 subtype, the prognosis of luminal B patients was obviously better.
Table 3.
Median survival of stage IV patients by molecular subtype and bone metastasis.
| Characteristics | All | Luminal A | Luminal B | HER2+ | TNBC | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (HR+/Her2-) | (HR+/Her2+) | (HR-/Her2+) | (HR-/Her2-) | ||||||||
| Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | ||
| Stage IV | |||||||||||
| No bone metastasis | 12 | 10.883-13.117 | 14 | 11.969-16.031 | 15 | 10.293-19.707 | 11 | 8.419-13.581 | 9 | 7.598-10.402 | <0.001 |
| Bone metastasis | 16 | 15.126-16.874 | 19 | 17.724-20.276 | 20 | 16.779-23.221 | 10 | 6.887-13.113 | 9 | 7.828-10.172 | <0.001 |
| Multiple metastases | |||||||||||
| Brain(+)lung(-)liver(-) | 8 | 5.127-10.873 | 9 | 4.942-13.058 | 13 | 0-31.739 | 10 | 6.080-13.92 | 6 | 4.05-7.95 | 0.028 |
| Brain(-)lung(+)liver(-) | 15 | 13.618-16.382 | 16 | 13.924-18.076 | 23 | 18.698-27.302 | 11 | 7.608-14.392 | 12 | 10.219-13.781 | <0.001 |
| Brain(-)lung(-)liver(+) | 13 | 11.445-14.555 | 15 | 13.035-16.965 | 11 | 5.202-16.798 | 19 | 13.024-24.976 | 8 | 6.108-9.892 | <0.001 |
| Brain(+)lung(+)liver(-) | 7 | 4.934-9.066 | 7 | 3.337-10.663 | 10 | 4.120-15.880 | 6 | 1.890-10.110 | 6 | 4.458-7.542 | 0.182 |
| Brain(+)lung(-)liver(+) | 7 | 4.352-9.648 | 7 | 4.009-9.991 | 17 | 2.391-31.609 | 7 | 2.445-11.555 | 3 | 0-6.395 | 0.011 |
| Brain(-)lung(+)liver(+) | 8 | 6.626-9.374 | 10 | 7.753-12.247 | 9 | 5.132-12.868 | 5 | 1.915-8.085 | 6 | 3.605-8.395 | 0.001 |
| Brain(+)lung(+)liver(+) | 4 | 2.876-5.124 | 4 | 2.537-5.463 | 7 | 4.934-9.066 | 2 | 0.674-3.326 | 4 | 0.173-7.827 | 0.591 |
In the HER2 and TNBC group, patients in all age groups showed lower survival rates (Table 4), compared with the luminal A and B patients, and youth was related to a favorable prognosis. Tumor grade and T stage were not notable prognostic elements for bone metastasis except for the luminal A molecular subtype. Higher N stage was relevant to better prognosis in the luminal A and HER2 subtypes of bone metastatic breast cancer patients. In all four molecular subtypes, other site metastases were obviously related to the decreased median survival time of bone metastasis patients, especially in the brain metastasis subgroup. Meanwhile, brain and visceral metastases also correlated with survival time in TNBC and HER2 subtype patients.
Table 4.
Median survival time of bone metastasis cancer patients according to molecular subtype and patient characteristics.
| Characteristics | Luminal A | Luminal B | HER2+ | TNBC | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| (HR+/Her2-) | (HR+/Her2+) | (HR-/Her2+) | (HR-/Her2-) | ||||||
| Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | ||
| All | 19 | 17.724-20.276 | 20 | 16.779-23.221 | 10 | 6.887-13.113 | 9 | 7.828-10.172 | <0.001 |
| Age | |||||||||
| <40 | 27 | 21.289-32.711 | 25 | 20.429-29.571 | 19 | 9.532-28.468 | 13 | 9.832-16.168 | <0.001 |
| 40-59 | 24 | 22.144-25.856 | 24 | 19.463-28.537 | 12 | 6.792-17.208 | 10 | 8.263-11.737 | <0.001 |
| ≥60 | 15 | 13.553-16.447 | 14 | 8.137-19.863 | 6 | 2.017-9.983 | 7 | 5.530-8.470 | <0.001 |
| p value | <0.001 | <0.001 | 0.177 | 0.003 | |||||
| Race | |||||||||
| White | 19 | 17.446-20.554 | 19 | 14.887-23.113 | 11 | 6.617-15.383 | 9 | 7.714-10.286 | <0.001 |
| Black | 16 | 13.831-18.169 | 22 | 17.254-26.746 | 8 | 3.118-12.882 | 10 | 8.013-11.987 | <0.001 |
| Other | 24 | 20.056-27.944 | 7 | 0-22.245 | 12 | 0-24.834 | 7 | 2.340-11.660 | 0.006 |
| p value | <0.001 | 0.594 | 0.422 | 0.971 | |||||
| Grade | |||||||||
| 1-2 | 22 | 20.406-23.594 | 19 | 12.275-25.725 | 11 | 4.575-17.425 | 8 | 5.613-10.387 | <0.001 |
| 3-4 | 16 | 14.645-17.355 | 20 | 16.340-23.660 | 10 | 6.405-13.595 | 9 | 7.754-10.246 | <0.001 |
| p value | <0.001 | 0.996 | 0.739 | 0.736 | |||||
| T stage | |||||||||
| 0-2 | 22 | 20.445-23.555 | 23 | 19.581-26.419 | 10 | 3.607-16.393 | 10 | 7.712-12.288 | <0.001 |
| 3-4 | 17 | 15.409-18.591 | 17 | 11.878-22.122 | 10 | 6.933-13.067 | 9 | 7.671-10.329 | <0.001 |
| p value | <0.001 | 0.117 | 0.341 | 0.114 | |||||
| N stage | |||||||||
| 0-1 | 18 | 16.455-19.545 | 17 | 12.033-21.967 | 9 | 5.656-12.344 | 8 | 6.697-9.303 | <0.001 |
| 2-3 | 21 | 18.792-23.208 | 22 | 18.577-25.423 | 16 | 8.355-23.645 | 10 | 8.013-11.987 | <0.001 |
| p value | 0.042 | 0.577 | 0.038 | 0.487 | |||||
| Brain metastasis | |||||||||
| No | 21 | 19.732-22.268 | 21 | 18.027-23.973 | 12 | 7.380-16.620 | 9 | 7.731-10.269 | <0.001 |
| Yes | 7 | 4.822-9.178 | 9 | 4.756-13.244 | 6 | 1.637-10.363 | 6 | 4.522-7.478 | 0.002 |
| p value | <0.001 | 0.003 | 0.002 | 0.001 | |||||
| Liver metastasis | |||||||||
| No | 22 | 20.623-23.377 | 25 | 22.414-27.586 | 11 | 6.905-15.095 | 11 | 9.673-12.327 | <0.001 |
| Yes | 11 | 9.013-12.987 | 9 | 4.945-13.055 | 9 | 3.404-14.596 | 6 | 4.623-7.377 | <0.001 |
| p value | <0.001 | <0.001 | 0.008 | <0.001 | |||||
| Lung metastasis | |||||||||
| No | 22 | 20.661-23.339 | 21 | 17.898-24.102 | 18 | 12.664-23.336 | 10 | 8.388-11.612 | <0.001 |
| Yes | 12 | 10.108-13.892 | 14 | 7.818-20.182 | 5 | 2.403-7.597 | 7 | 4.663-9.337 | <0.001 |
| p value | <0.001 | 0.081 | <0.001 | 0.002 | |||||
3.4. Univariate and Multivariate Analyses
In univariate analysis, variables including age, tumor grade, T stage, molecular subtypes, brain metastasis, liver metastasis, and lung metastasis were statistically significant (p < 0.05) for OS of bone metastatic breast cancer patients. So, we put all these variables into multivariate analysis. The molecular classification was considerably associated with OS (Table 5). Patients with the most unfavorable prognosis were the TNBC, poorer than luminal A, luminal B, and HER2 subtypes (hazard ratio [HR] of luminal A compared with TNBC, 0.533, 95% confidence interval, 0.444–0.641, p < 0.001; HR of luminal B, 0.482, 95% CI 0.419–0.555, p < 0.001; HR of HER2 subtype, 0.542, 95% CI 0.484–0.608, p < 0.001). Brain metastasis impacts OS (p < 0.001) fundamentally, and liver and lung metastases also significantly decreased OS (p < 0.001 and p < 0.001, respectively). Multivariate Cox regression analysis of OS in bone metastasis which had no brain metastases was also calculated. This analysis is taken in age at diagnosis, molecular classification, and liver or lung metastases. Similar results have been found in that the most inauspicious result was also the TNBC subtype, followed by the HER2, luminal A, and luminal B subtypes (HR of HER2 subtype, 0.484, 95% CI 0.395–0.593, p < 0.001; HR of luminal A compared with TNBC, 0.484, 95% CI 0.395–0.593, p < 0.001; HR of luminal B, 0.481, 95% CI 0.414–0.559, p < 0.001) (Table 6; Figure 2). High T stage and tumor grade and liver or lung metastases also related to the poor OS of bone metastatic breast cancer without brain metastasis (HR of T stage, 1.216, 95% CI 1.118-1.322; HR of tumor grade, 1.183, 95% CI 1.084-1.291; HR of liver metastasis, 1.688, 95% CI 1.536-1.855; and HR of lung metastasis, 1.228, 95% CI, 1.122-1.344).
Table 5.
Cox multivariate analysis of overall survival in bone metastasis from breast cancer.
| Characteristics | HR | 95% CI | p value |
|---|---|---|---|
| Age at diagnosis (continuous) | 1.018 | 1.015-1.021 | <0.001 |
| Subtype | |||
| TNBC | Reference | ||
| Luminal A | 0.533 | 0.444-0.641 | <0.001 |
| Luminal B | 0.482 | 0.419-0.555 | <0.001 |
| HER2+ | 0.542 | 0.484-0.608 | <0.001 |
| Brain metastasis | |||
| No | Reference | ||
| Yes | 1.686 | 1.479-1.922 | <0.001 |
| Liver metastasis | |||
| No | Reference | ||
| Yes | 1.673 | 1.531-1.828 | <0.001 |
| Lung metastasis | |||
| No | Reference | ||
| Yes | 1.236 | 1.135-1.345 | <0.001 |
| Grade | |||
| 1-2 | Reference | ||
| 3-4 | 1.193 | 1.098-1.297 | <0.001 |
| T stage | |||
| 0-2 | Reference | ||
| 3-4 | 1.169 | 1.079-1.266 | <0.001 |
Table 6.
Cox multivariate analysis of overall survival in bone metastasis from breast cancer without brain metastasis.
| Characteristics | HR | 95% CI | p value |
|---|---|---|---|
| Age at diagnosis (continuous) | 1.018 | 1.015-021 | <0.001 |
| Subtype | |||
| TNBC | Reference | ||
| Luminal A | 0.484 | 0.395-0.593 | <0.001 |
| Luminal B | 0.481 | 0.414-0.559 | <0.001 |
| HER2+ | 0.484 | 0.395-0.593 | <0.001 |
| Liver metastasis | |||
| No | Reference | ||
| Yes | 1.688 | 1.536-1.855 | <0.001 |
| Lung metastasis | |||
| No | Reference | ||
| Yes | 1.228 | 1.122-1.344 | <0.001 |
| Grade | |||
| 1-2 | Reference | ||
| 3-4 | 1.183 | 1.084-1.291 | <0.001 |
| T stage | |||
| 0-2 | Reference | ||
| 3-4 | 1.216 | 1.118-1.322 | <0.001 |
Figure 2.
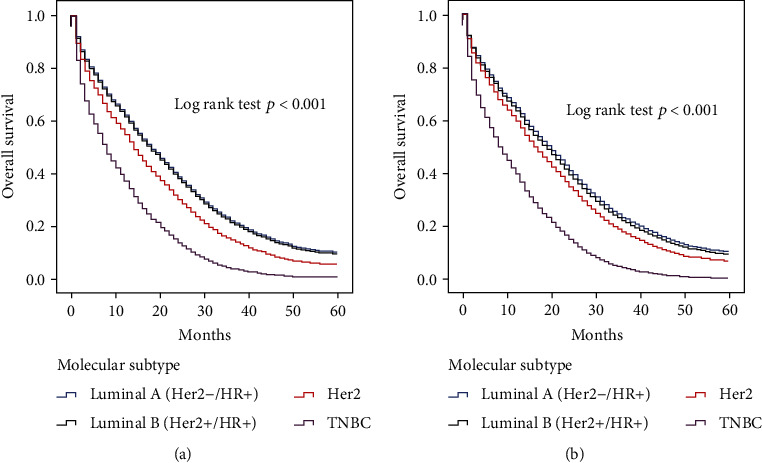
Kaplan-Meier survival curves according to molecular subtype: (a) in all patients with brain metastasis from breast cancer; (b) in patients with brain metastasis from breast cancer without brain metastasis.
4. Discussion
In our study of cancer patients from 2011 to 2015 (N = 42684), luminal B breast cancer displayed the highest occurrence of bone metastasis (9.4%, 422/4502), and even in elderly patients with luminal B molecular subtype, the rate of bone metastasis was over 10%. As the most common metastasis, bone metastasis could notably increase the risk of other metastases, especially brain metastasis [12–14]. The predictive nomogram allows us to assess bone metastasis's probability by molecular features and some clinical characteristics. This nomogram may be pretty useful due to the high occurrence of bone metastasis in breast cancer patients. The nomogram in our study estimates the prevalence of accompanying bone metastasis at the diagnostic time of breast cancer. Because of the nature of observed research, our nomogram could be more appropriate to make the decision to add a bone scan when the probability of bone metastasis is really high according to our nomogram.
Our results indicated that the incidence of bone metastasis in luminal A and B were apparently higher than that in HER2 and TNBC patients, which was consistent with previous research conducted by Xiao et al. [9]. Interestingly, with the age growth, the rate of bone metastasis in TNBC was also increased, while the incidence in other molecular subtypes was not changed visibly. Also, we noted that the rate of bone metastasis was seemingly increased in brain and visceral metastasis breast cancer patients compared with brain metastasis lacking visceral metastasis. While lung metastasis existed, the brain metastasis was associated with a low rate of bone metastasis. A previous study demonstrated a difference in time duration to distant recurrence [15, 16] largely. ER-negative tumor related to early recurrence, while ER-positive tumor is associated with a sustained low risk of more than five years. In agreement with our study, Xiao et al. [9]. found that TNBC had a higher rate of brain, liver, and lung metastases but a significantly lower rate of bone metastases than luminal A tumors.
A recent study conducted by Kono et al. showed that patients with bone-only first metastasis tend to have longer OS than patients with others-only first metastasis [17]. Similarly, in our research, we also demonstrated that the median survival of stage IV breast cancer was longer in the bone metastasis cohort than for the patient without bone metastasis, especially in luminal A and B subtypes. Patients with initial bone metastasis had a more favorable 5-year survival proportion than those with other metastases. Luminal A and luminal B patients had a propensity of bone metastasis and were related to better tumor outcomes of patients with initial bone metastasis.
Breast cancer cell transmission and final metastasis organs that grow into the distance—mainly bones, lung, and the brain—represent a significant clinical problem. The disease is incurable and is the main cause of death in the general population of most patients with TNBC. Metastatic spread of tumor cells is a highly complex but difficult to understand process, with many complicated biological processes, such as invasive, angiogenic, genetic, and epigenetic changes, tumor-interstitial interaction, basal permeation membrane, and some extravasation of cancer cells to the distal tissue [18]. However, disseminated cells are often in a new environment, and proapoptotic signals stay quiescent in long-term secondary organ latency, also known as dormancy [19, 20]. At this stage, breast cancer cells could hardly be detected and show resistance to chemotherapy [19]. This is still an important clinical issue, because patients are usually viewed as “survivors” and can progress to metastatic disease many years later. Disseminated tumor cells (DTCs) can enter into the secondary organs' sleep state by existing for an indefinite period of the proliferative cycle or by balancing the proliferation and apoptosis. The success of dormancy emergence results from further development of surviving DTCs by assembling molecular genetic changes allowing interaction with the tumor microenvironment [19]. By illuminating these features, patients with metastatic sites could well adapt to the host microenvironment and start the colonization. Although critical issues have been made, some specific problems need to be highlighted. Recent efforts focus on clarifying the role of key genes, potential molecular mechanisms, and effects of the signal pathway involved in fatal metastasis propagation. These studies are crucial for research progress on new effective treatment methods for antitumor metastasis in TNBC.
This study presents some vital significations for clinical practice. At first, the nomogram including breast cancer molecular subtypes can help clinicians to identify and predict patients at increased risk for bone metastasis at diagnosis. Second, the risk evaluation of molecular feature-based bone metastasis may play a crucial role in the age of precision medicine. Meanwhile, undeniably, our study has several limitations. First of all, our study is retrospective, so there is inevitably selection bias. Details of systemic therapy including targeted, hormone, chemical, and radiation therapy information is not available in our study. In the second place, the data of the sequence of different metastases during the follow-up time are not quite available yet due to the SEER program not supporting its related data. Last but not least, the specific location or number of bone and other metastases could not be acquired, which results in making it difficult to evaluate prognostic value.
5. Conclusions
The predictive model we constructed in our study could calculate the probability of bone metastasis of breast cancer at an initial diagnosis based on different molecular subtypes and other critical clinical characteristics. Patients with bone metastasis had a more favorable oncological survival than other metastases, and TNBC with bone metastasis patients showed the poorest tumor outcome compared with the other three molecular subtypes.
Abbreviations
- SEER:
Surveillance, Epidemiology, and End Results
- HR:
Hormone receptor
- TNBC:
Triple-negative breast cancer
- OS:
Overall survival
- HR:
Hazard ratio
- HER2:
Human epidermal growth factor receptor 2
- DTC:
Disseminated tumor cells.
Data Availability
The datasets generated and analyzed during the current study are available in the SEER database repository, https://seer.cancer.gov/.
Ethical Approval
Our study was approved by the Linyi Central Hospital ethics committee.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
SZ collected the data from the SEER program and wrote the main body of the manuscript, and LL analyzed the data by using SPSS. YZ contributed to constructing the nomogram of bone metastasis. FW and GL designed the whole study. All authors read and approved the final manuscript. Dongning Shi, Junwen Bai and Yibo Chen contributed equally to this work.
References
- 1.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2016. CA: a Cancer Journal for Clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Leong S. P. L., Tseng W. W. Micrometastatic cancer cells in lymph nodes, bone marrow, and blood: clinical significance and biologic implications. CA: a Cancer Journal for Clinicians. 2014;64(3):195–206. doi: 10.3322/caac.21217. [DOI] [PubMed] [Google Scholar]
- 4.Makhoul I., Montgomery C. O., Gaddy D., Suva L. J. The best of both worlds - managing the cancer, saving the bone. Nature Reviews Endocrinology. 2016;12(1):29–42. doi: 10.1038/nrendo.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn R. S., Crown J. P., Lang I., et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. The Lancet Oncology. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 6.Swain S. M., Baselga J., Kim S. B., et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. The New England Journal of Medicine. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenlee H., DuPont-Reyes M. J., Balneaves L. G., et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA: a Cancer Journal for Clinicians. 2017;67(3):194–232. doi: 10.3322/caac.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperduto P. W., Kased N., Roberge D., et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. International Journal of Radiation Oncology • Biology • Physics. 2012;82(5):2111–2117. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao W., Zheng S., Yang A., et al. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: a population-based study. Cancer Management and Research. 2018;Volume 10:5329–5338. doi: 10.2147/CMAR.S176763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Y. J., Kim J. S., Kim I. A. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. Journal of Cancer Research and Clinical Oncology. 2018;144(9):1803–1816. doi: 10.1007/s00432-018-2697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawood S., Broglio K., Buzdar A. U., Hortobagyi G. N., Giordano S. H. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2010;28(1):92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckhardt B. L., Francis P. A., Parker B. S., Anderson R. L. Strategies for the discovery and development of therapies for metastatic breast cancer. Nature Reviews Drug Discovery. 2012;11(6):479–497. doi: 10.1038/nrd2372. [DOI] [PubMed] [Google Scholar]
- 13.Futakuchi M., Fukamachi K., Suzui M. Heterogeneity of tumor cells in the bone microenvironment: mechanisms and therapeutic targets for bone metastasis of prostate or breast cancer. Advanced Drug Delivery Reviews. 2016;99(Part B):206–211. doi: 10.1016/j.addr.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Harbeck N., Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 15.Kennecke H., Yerushalmi R., Woods R., et al. Metastatic behavior of breast cancer subtypes. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2010;28(20):3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 16.Cortesi L., Toss A., Cirilli C., et al. Twenty-years experience with de novo metastatic breast cancer. International Journal of Cancer. 2015;137(6):1417–1426. doi: 10.1002/ijc.29503. [DOI] [PubMed] [Google Scholar]
- 17.Kono M., Fujii T., Matsuda N., et al. Somatic mutations, clinicopathologic characteristics, and survival in patients with untreated breast cancer with bone-only and non-bone sites of first metastasis. Journal of Cancer. 2018;9(19):3640–3646. doi: 10.7150/jca.26825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen D. X., Massague J. Genetic determinants of cancer metastasis. Nature Reviews Genetics. 2007;8(5):341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 19.Giancotti F. G. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155(4):750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valastyan S., Weinberg R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available in the SEER database repository, https://seer.cancer.gov/.


