Abstract
Background
Nebulized heparin has been effectively used in the management of many pulmonary diseases. However, its effect on mechanically ventilated patients with acute exacerbation chronic obstructive pulmonary disease (AECOPD) has never been studied. This study aimed to assess the efficacy of nebulized heparin and salbutamol to increase ventilator-free days (VFD) in mechanically ventilated AECOPD patients and the effect of nebulized heparin on respiratory and coagulation functions.
Methods
In this double-blind controlled study, 60 mechanically ventilated adult patients with AECOPD were randomly allocated into two groups; heparin and salbutamol (HS) group and salbutamol only (S) group. In the Group HS, patients received nebulized heparin (25,000 IU) and salbutamol (5 mg) every 6 hours. Patients in the Group S received nebulized salbutamol only (5 mg). The treatment was continued while patients remained ventilated for a maximum of 14 days. The primary outcome was VFDs at day 14. PaCO2, PaO2/FiO2 ratio, number of nebulizations withheld, C-reactive protein (CRP) titer and activated partial thromboplastin time (APTT) were secondary outcomes.
Results
Patients in the Group HS had significantly more VFDs (4.7 ± 3.3) compared with those in the Group S (2.4 ± 2.6), P = 0.007. PaCO2 levels, PaO2/FiO2, the decrease in the CRP level and the increase in the APTT from the baseline showed no evidence of difference in both groups.
Conclusions
The co-administration of nebulized heparin and salbutamol, compared with salbutamol alone, significantly increased (VFDs) among mechanically ventilated AECOPD patients without increasing bleeding risks.
Keywords: Albuterol, Artificial respiration, Asthma chronic obstructive pulmonary disease overlap syndrome, C-reactive protein; Heparin, Nebulizers
Introduction
Chronic obstructive pulmonary disease (COPD) is considered the fifth leading cause of mortality in the world and is subjected to be the fourth by 2030 [1]. The prevalence of COPD in Egypt is almost 10% [2]. COPD is a progressive disease and is associated with acute exacerbation (AE) periods [1]. Despite the widespread use of non-invasive positive pressure ventilation (NIPPV) in the management of AECOPD, it is not suitable for all patients and may be associated with a 60% risk of intubation [1,3,4]. The leading pathophysiologic causes of AECOPD are peribronchial inflammation and bronco-constriction. Accordingly, short-acting β2 agonists (e.g., salbutamol), antibiotics, and corticosteroids are considered cornerstones in the management of COPD [1,5].
Besides its anticoagulant effect, heparin decreases the adherence of bacteria and viruses to the bronchial surface and has an anti-inflammatory effect [6,7]. Recently nebulized heparin has been added for the management of many pulmonary conditions including the exacerbation of bronchial asthma, smoke inhalational injuries and critically ill mechanically ventilated patients [8-10]. However, its effect on mechanically ventilated AECOPD patients is unknown and has not yet been studied. In theory, adding nebulised heparin to ventilated AECOPD treatment may shorten the duration of mechanical ventilation (MV).
This study aimed to assess the efficacy of nebulized heparin and salbutamol (albuterol) to increase ventilator-free days (VFDs) among mechanically ventilated AECOPD patients (primary outcome) and its effect on respiratory, coagulation functions and C-reactive protein (CRP) (secondary outcomes).
Materials and Methods
This double-blind randomized controlled study was approved by the ethics committee of the Faculty of Medicine, Ain Shams University, Cairo, Egypt (FMASU R 26/2017) and registered at ClinicalTrials.gov (NCT03333395). The study was conducted between the 1st of February 2017 to the 30th of September 2017 at the Internal Medicine Intensive Care Unit of Ain-Shams University Hospital. The study was conducted in accordance with the Declaration of Helskinki and Good Clinical Practice.
Sixty adult patients with COPD and primary respiratory failure that were not responding to NIPPV were included in this study [11]. The randomization was done using a computer-generated table of random numbers. Allocation concealment was achieved by drawing a sequential numbered, opaque, and sealed envelope to randomize the patients into two groups: heparin and salbutamol (HS) group and salbutamol (S) group. There were 30 patients allocated to each group. The randomization day was considered to be day 0. Written informed consent was taken from all the patients, and their parents or guardians if applicable before the study were commenced. Patients were aged between 18 and 70 years old, had a body mass index of ≤40 kg/m2 with stage II to IV COPD, according to the Global Initiative for Chronic Obstructive Lung Disease spirometric classification [12]. The research team excluded patients who had already been mechanically ventilated for more than 24 h, were expected to be extubated within 48 h, pregnant, or had a history of ischemic heart disease, pulmonary bleeding (within the previous three months), bleeding diathesis, allergy to heparin, or history of heparin induced- thrombocytopenia (HIT).
According to our intensive care unit (ICU) policy and guidelines, on patient admission, an arterial cannula (in the radial artery of the non-dominant hand) and a central line were inserted and daily blood samples were taken. Patients received standard medications, including analgesics, sedatives, fluid management, antibiotics according to a sputum culture, steroids and thrombo-prophylaxis (enoxaparin 40 IU SC once/day) [13].
All patients received nebulization of a 5 ml solution; 2.5 ml (5 mg) of nebulized salbutamol solution (preservative-free Ventolin, GlaxoSmithKline, UK) added to 2.5 ml normal saline (NS). This was followed by either nebulization of heparin (25,000 IU, heparin sodium; Nile Company for Pharmaceuticals and Chemical Industries, Egypt) in the Group HS or 5 ml of NS in the Group S. The medication for the second nebulization was prepared by the ICU pharmacist and then handed to the nurse in charge who was blinded to the nature of the medication and did not further take part in the study. All data were recorded by ICU resident doctors who followed-up the patients and were unaware of the contents used in the second nebulization medication. Additionally, they were not involved in any other part of the study.
The nebulization session lasted for at least 10 min for each medication. This regimen was repeated every 6 h and continued while patients remained ventilated for a maximum of 14 days.
The nebulization medication was added to a nebulization chamber (Ameco Technology; particle size: 0.5–10 μm, nebulization rate: > 0.3 ml/min) connected to the inspiratory limb of the breathing circuit 15 cm from the Y of the circuit. The heat and moisture exchanger was removed during nebulization. All patients were mechanically ventilated using the Synchronized Intermittent (SIMV) mode with or without pressure support (PS), with a targeted tidal volume: 6–8 ml/kg, rate: 12–14 breaths/min inspiratory : expiratory ratio (I : E ratio) = 1 : 3, positive end-expiratory pressure (PEEP): 5–10 cmH2O, fraction of inspired oxygen (FiO2) (40–60%) and upper pressure levels were maintained at or below 35 cmH2O to target arterial oxygen saturation (SpO2) of 88–92%. PS ventilation was used to wean the patient.
The weaning process was started by optimizing the mechanical and biochemical respiratory parameters; i) treatment of the cause of exacerbation, ii) spontaneous respiratory volume (Vt) > 0.005 L/kg of body weight, iii) maximal spontaneous inspiratory effort (PImax) ≤ 25 cmH2O, iv) heart rate <140/min, v) body temperature <37.5°C, vi) hemoglobin >100 g/L, vii) partial arterial oxygen pressure (PaO2) > 60 mmHg with inspired oxygen fraction (FiO2) ≤ 0.4, extrinsic PEEP < 5 cmH2O, viii) no need for vasoactive and/or inotropic support, PaO2/FiO2 ratio > 200, and RR/Vt ratio < 100. Patients who fulfilled those criteria were given a two-hours spontaneous breathing trial (SBT) using PSV with an initial positive pressure of 15 cmH2O. Patients who withstood the SBT were extubated, while those who had a spontaneous respiratory rate > 25/min, SatO2< 90%, FiO2 ≤ 0.4, heart rate > 140/min (or more than 20% change from the initial heart rate), PaO2 ≤ 60 mmHg, pH ≤ 7.30, and restlessness were tagged as failing to wean and (MV) with SIMV was continued.
The duration of VFDs (the primary outcome) was evaluated at day 14. The number of patients successfully weaned from MV, changes in PaCO2 level (during MV), the average daily ratio of PaO2/FiO2 (during MV), the CRP quantitative titer (measured daily), activated partial thromboplastin time (APTT), and any complications were recorded (secondary outcomes). The PaO2/FiO2 and PaCO2 were measured each day at 7 AM. No changes in the ventilator settings or the patient’s position were permitted for the 10 min before these measurements were taken. In cases of suction or lavage of blood-tinged sputum, the next nebulization cession was withheld, and the number of those cessions were evaluated and were added to the secondary outcomes. In cases of HIT occurrence, increased APTT more than double the normal, evident pulmonary bleeding, or death the patient was dropped out of the study.
To facilitate blinding, the study medications were prepared by a pharmacist and given by the nurse in charge. Both of those staff members were not involved in any other part of the study.
The PaCO2, the PaO2/FiO2 and CRP titer were assessed daily. They were presented every other day to avoid redundancy of data.
Statistical analysis
Based on a similar previous study [14], 26 patients were required in each group, assuming a power of 0.80 and a 5% alpha error (2-tailed): [15]. To compensate for dropouts, 30 patients in each group were recruited. Coded data were tabulated and statistically analyzed using the Statistical Package for Social Sciences version 22.0 (IBM Corp., USA). Data are presented as mean ± SD, numbers, frequencies and percentages. Data were analyzed using the independent t-test, repeated measures analysis of variance (RMANOVA), chi-square test, or the log-rank test as appropriate. The level of significance was set to a P value of < 0.05.
Results
A total of 55 patients completed the study, of which 28 were in the Group HS and 27 in the Group S (Fig. 1). Patients' characteristics, associated co-morbidities, basal respiratory variables, known risk factors, and laboratory results showed no evidence of difference (Table 1).
Fig. 1.
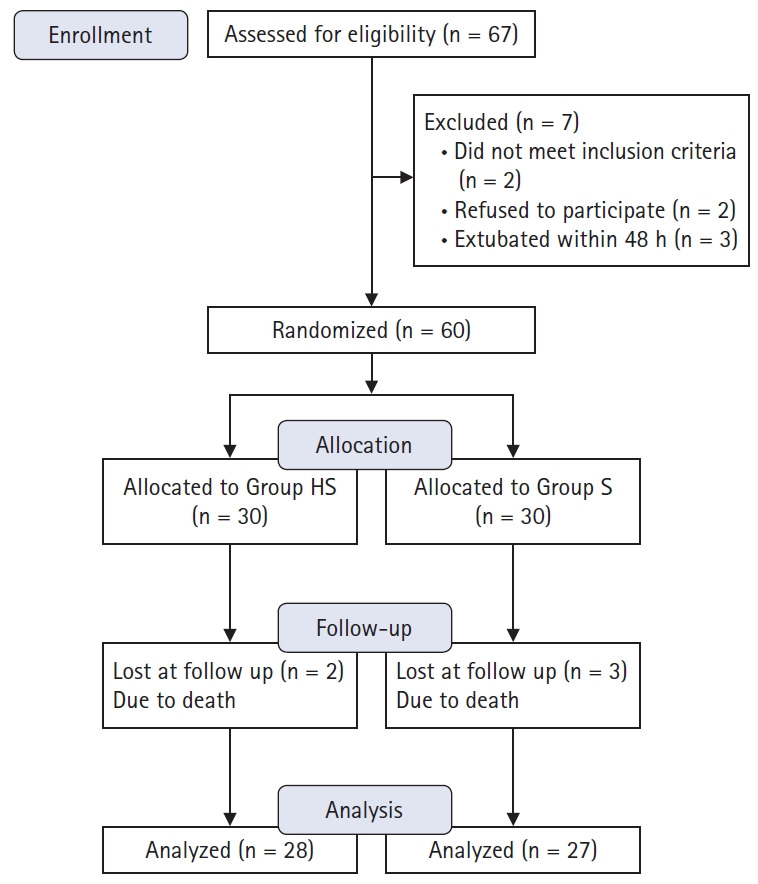
CONSORT flow diagram. Group HS: heparin and salbutamol group, Group S: salbutamol only group.
Table 1.
Demographic and Clinical Characteristics of the Study Participants
| Group HS (n = 28) | Group S (n = 27) | P value | |
|---|---|---|---|
| Age (yr) | 45.8 ± 5.5 | 45.2 ± 5.7 | 0.673 |
| BMI (kg/m2) | 27.9 ± 2.0 | 27.5 ± 2.2 | 0.556 |
| Sex (M/F) | 23/5 | 21/6 | 0.686 |
| Current smoker | 23 | 25 | 0.422 |
| Vasopressors | 6 | 7 | 0.695 |
| Associated co-morbidities | |||
| Diabetes Mellitus | 16 | 20 | 0.187 |
| Previous Stroke | 9 | 7 | 0.612 |
| Hypertension | 25 | 23 | 0.705 |
| Chronic liver disease | 4 | 6 | 0.503 |
| Renal impairment | 3 | 5 | 0.469 |
| Etiology of exacerbation | |||
| H. influenza | 10 | 11 | 0.901 |
| Streptococcus pneumoniae | 8 | 5 | |
| Common cold (viral) | 3 | 4 | |
| Exposure to dust | 4 | 5 | |
| Unidentifiable cause | 3 | 2 | |
| Base line values | |||
| Hb (g/dl) | 12.7 ±1.0 | 12.8 ± 0.8 | 0.668 |
| PLT (103/μl) | 260.1 ± 48.1 | 273.7 ± 35.0 | 0.235 |
| APTT (s) | 38.6 ± 1.6 | 39.0 ± 1.3 | 0.421 |
| FEV1 % predicted | 40.4 ± 2.3 | 39.2 ± 2.6 | 0.074 |
| FEV1/FVC (%) | 61.7 ± 2.4 | 61.7 ± 2.7 | 0.945 |
| SO2 (%) | 88.0 ± 2.4 | 87.2 ± 2.2 | 0.219 |
| PaO2 (mmHg) | 72.7 ± 2.6 | 71.8 ± 2.5 | 0.248 |
| pH | 7.3 ± 0.1 | 7.3 ± 0.1 | 0.274 |
| Risk factors of worsening COPD | |||
| Duration of COPD (yr) | 18.7 ± 1.5 | 18.1 ± 1.6 | 0.604 |
| COPD related hospitalization in preceding year (number) | 5 | 6 | 0.686 |
| Exacerbations in previous year | 16 | 14 | 0.694 |
| Antibiotic therapy | 20 | 19 | 0.931 |
| Inhaled steroid therapy | 22 | 23 | 0.729 |
| Theophylline therapy | 13 | 10 | 0.480 |
| Mucous hypersecretion | 21 | 22 | 0.561 |
Values are presented as mean ± SD or a frequency as appropriate. Group HS: heparin and salbutamol group, Group S: salbutamol only group.
BMI: body mass index, Hb: hemoglobin, PLT: platelet count, APTT: activated partial thromboplastin time, FEV1: forced expiratory volume in first second % of predicted, FEV1/FVC: ratio between forced expiratory volume in first second and forced vital capacity, SO2: arterial oxygen saturation, PaCO2: partial pressure of CO2 in arterial blood, PaO2: partial pressure of O2 in arterial blood, pH: decimal logarithm of the reciprocal of the hydrogen ion activity, COPD: chronic obstructive pulmonary disease. All spirometry values were taken when the patient first presented in the emergency room.
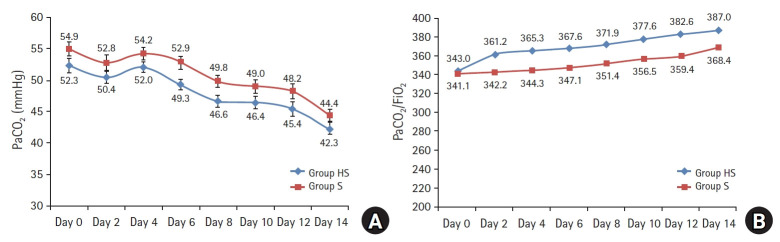
The patients in the Group HS had significantly longer VFDs (4.7 ± 3.3 vs. 2.4 ± 2.6, P = 0.007; Table 2). The survival curve showed that the percentage of ventilated patients in the Group HS was lower than the Group S during the 14 days (P = 0.009, Fig. 2). The other respiratory variables; PaCO2 levels (P = 0.075, Fig. 3), PaO2/FiO2 (P = 0.069, Fig. 3) and the rate of decrease in CRP (P = 0.185, Fig. 4) showed no evidence of difference in both groups.
Table 2.
Outcomes, Tolerability, and Safety
| Group HS (n = 28) | Group S (n = 27) | P value | |
|---|---|---|---|
| Ventilator free days | 4.7 ± 3.3 | 2.4 ± 2.6 | 0.007* |
| Number of doses of nebulization withheld/patient | 5.8 ± 2.2 | 4.8 ± 1.4 | 0.064 |
| Blood transfusion | 9 | 7 | 0.612 |
| APTT Max (s) | 39.5 ± 2.5 | 39.1 ± 1.0 | 0.407 |
| APTT elevation (s) | 0.9 ± 1.8 | 0.2 ± 0.9 | 0.053 |
| APTT Max ≥ 40.0 (s) | 13 | 9 | 0.322 |
| Double APTT | 0 | 0 | -- |
| HIT | 0 | 0 | -- |
| Death | 2 | 3 | 0.669 |
Values are presented as mean ± SD or a frequency as appropriate. Group HS: heparin and salbutamol group, Group S: salbutamol only group. APTT: activated partial thromboplastin time, HIT: heparin-induced thrombocytopenia.
Statistically significant with P < 0.05.
Fig. 2.
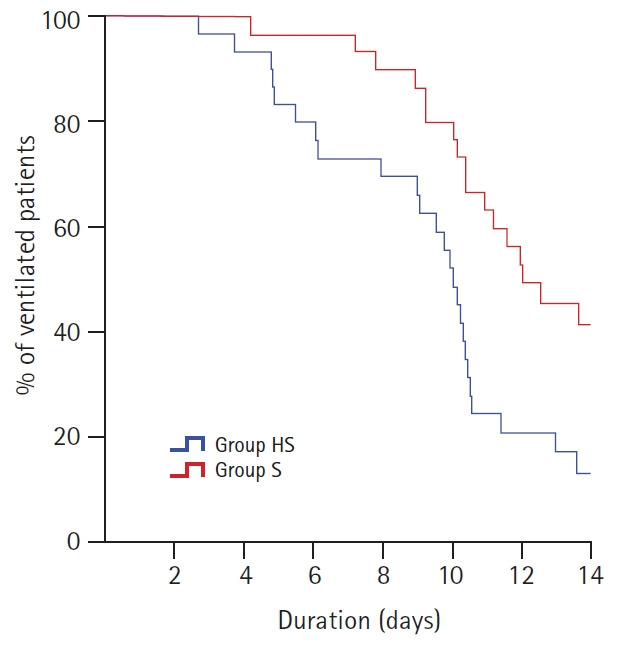
The survival curve showed that the percentage of ventilated patients in the Group HS was lower than the Group S during the 14 days (P = 0.009). Group HS: heparin and salbutamol group, Group S: salbutamol only group.
Fig. 3.
(A) PaCO2 levels showed no evidence of difference in both groups (P = 0.075). (B) PaO2/FiO2 ratios (P = 0.069) showed no evidence of difference between groups. Group HS: heparin and salbutamol group, Group S: salbutamol only group.
Fig. 4.
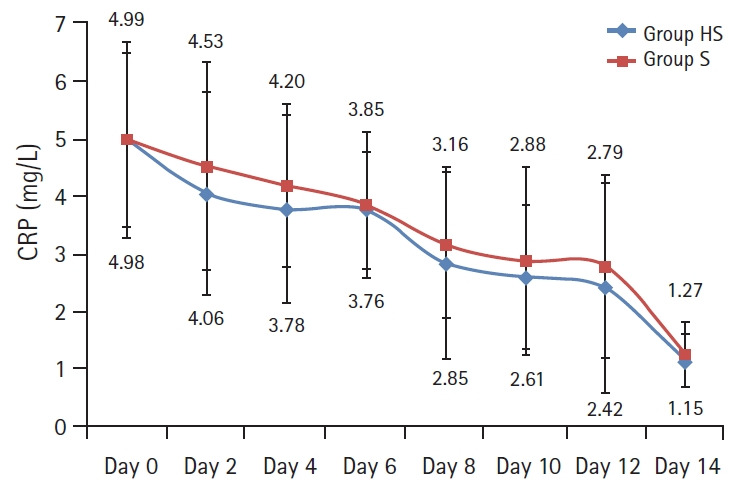
Rate of decrease of the CRP levels in both groups showed no evidence of difference and is presented by a line graph with error bars. An RMANOVA was used for the analysis; Group effect (P = 0.185). Group HS: heparin and salbutamol group, Group S: salbutamol only group. CRP: C-reactive protein, RMANOVA: repeated-measures analysis of variance.
Generally, the study medications were well tolerated by patients in both groups. The number of withheld of nebulisations/patient in the Group HS showed no evidence of difference to that of the Group S (5.8 ± 2.2 vs. 4.8 ± 1.4, P = 0.064; Table 2). Similarly, blood product usage did not show any significant differences between the groups, with only nine patients requiring blood transfusions in the Group HS and seven in the Group S. None of the patients in both groups had suspected HIT (a decrease in platelet count). The maximum increase in the APTT from baseline over the study period was higher in the Group HS. However, this was not statistically significant (39.5 ± 2.5 vs. 39.1 ± 1.0, P = 0.407; Table 2).
Discussion
Invasive or noninvasive MV is a form of life support until the cause of underlying acute respiratory failure is reversed with medical therapy. This study demonstrated that the Group HS was associated with higher VFDs as compared to the Group S. This effect may be due to an improvement of oxygenation, ventilation (Fig. 3), and/or inflammation (Fig. 4). Despite not reaching statistical significance an improving trend in the Group HS was observed.
Many studies have confirmed the anti-inflammatory and immune-modulatory effects of heparin [16,17] (Fig. 5). A variety of clinical trials studying patients with inflammatory processes e.g. inflammatory bowel disease and cardiopulmonary bypass have confirmed this [18,19]. It was even found to reduce the histological and clinical evidence of pulmonary microvascular thrombosis in patients with acute pulmonary inflammation following cardiac surgery [20]. Moreover, inhalational heparin has anti-asthmatic properties as confirmed by different clinical models of bronchial asthma [8,21] and inpatients with smoke inhalational injuries [8,9,21,22]. Heparin nebulization also improved oxygenation and increased the VFDs in critically ill mechanically ventilated patients and was found to be comparable to a nebulized corticosteroid (budesonide) in decreasing the risk of ventilator-induced lung injury [14,23], highlighting its anti-inflammatory effect. Subcutaneous low-molecular-weight heparin also improved the pulmonary functions and decreased the days of MV among AECOPD ventilated patients [24,25].
Fig. 5.
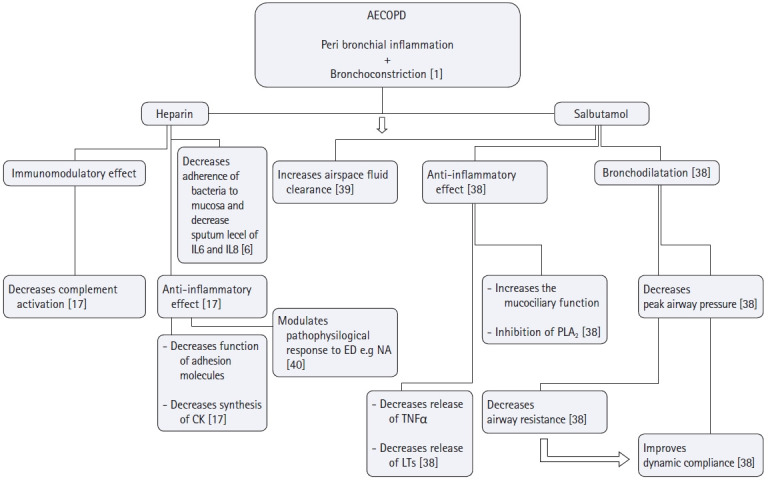
Possible roles of heparin and salbutamol nebulization in targeting the pathophysiology of AECOPD. Main targets of heparin and salbutamol in the management of AECOPD are to hit its two pathological components; peribronchial inflammation and bronchoconstriction. As for heparin, it prevents the adhesion of Pseudomonas aeruginosa, Burkholderia cepacia, Burkholderia pseudomalleior Legionella pneumophilato bronchial mucosa and decrease sputum levels of IL-6 and IL-8 confirming its anti-inflammatory action. Heparin's anti-inflammatory effect is exerted also by other mechanisms; i) Heparin preparations have been shown to inhibit chemokine synthesis, as well as chemokine function (a cytokine that regulates the extravasations of cells from blood stream to tissues), ii) Heparin inhibits adhesion molecules (which with cytokines are essential for the extravasations of neutrophils to tissues), iii) Animal studies highlighted the effect of low molecular weight heparin on the modulation of pathophysiologic response to endotoxins by decreasing neutrophils adhesion, solubilization of TNF-α receptors and regulation of thromoboxane A2 biosynthesis. Salbutamol on the other hand through its bronchodilator effect improves dynamic compliance secondary to the decrease of peak airway pressure, it also possesses a unique anti-inflammatory effect by inhibiting the mast cell release to histamine and its inhibitory effect on phospholipase A2 subsequently decreasing the microvascular permeability and enhancing the air space fluid clearance. AECOPD: acute exacerbation chronic obstructive pulmonary disease, IL-6: Interleukin 6, IL-8: Interleukin 8, CK: cytokines, ED: endotoxins, NA: neutrophil aggregation, TNFα: tissue necrosis factor α, LTs: Leukotrienes, PLA2: Phospholipase A2.
In contrast to our study, a retrospective review designed by Kashefi et al. [26] concluded that alternating treatments of heparin and N-acetylcysteine/albuterol nebulization every 4 h on adult inhalation injury patients did not reduce mortality or duration of MV. This finding may be explained by the low dose of (5,000 IU/ per dose) used.
In a trial studying the effects of different doses of nebulized heparin on the coagulation activation, nebulized heparin was found to increase APTT in a dose-dependent manner. However at a dose of 100,000 IU/day this increase was modest and was not associated with any adverse events [27].
Also, Shute et al. [28], proved that nebulized heparin of 75,000 or 150,000 IU/day days in moderate to very severe COPD patients significantly increased FVC following 7 days of treatment. These results might be explained by an earlier study on intrapulmonary administered heparin. This study proved that it was absorbed rapidly by the alveolar membrane and released gradually into the blood. A study done by Bendstrup et al. concluded that nebulized heparin slowly dissipated form the lungs, and that 39% of it was still present in the lungs 24 h after nebulization [29,30]. Based on such results, the research team decided to adjust the treatment protocol to add enoxaparin SC as a prophylaxis against thromboembolic complications and to withhold heparin nebulisation if APTT increased more than double the normal or serious bleeding occurred. Our results correlated well with all the studies done on the nebulized heparin regarding the absence of any bleeding hazards in response to its usage [8,10,14,23–25,27,28].
Heparin nebulization on the other hand, did not prove to be effective in reversing histamine-induced bronchospasm, suggesting its effects are mediated by mechanisms not involving smooth muscles [8,16]. This finding mandated the addition of bronchodilators to the management of histamine-induced bronchspasm. In this context, short-acting β2 agonists alone or in combination are recommended [5].
The dose of 5.0 mg of nebulized salbutamol was based on a review study on the management of COPD exacerbations by Rodriguez-Roisin [5]. He found that inspiratory capacity increased significantly at 30 and 90 min after the administration of 5.0 mg of nebulized salbutamol to acute-on-chronic air trapping and lung hyperinflation patients.
Dixon et al. [14] showed that the pulmonary lavage markers of coagulation activation did not decrease in the heparin group. However, later, he contradicted this in a letter to the editor when he studied higher doses (up to 400,000 IU/day) and was allowed to do further coagulation markers [31].
In this study, the team chose CRP as a biomarker of inflammation. CRP is not only an easy and important marker in COPD but is also an early marker of exacerbation [32]. Its increase indicates bacterial infection in AECOPD and the need for antibiotics in the treatment [32–35]. IIts serial measurements were beneficial in assessing the efficacy of treatment [33,34]. Despite the absence of a statistical significance between the two groups regarding CRP, the research team observed a decreasing trend of CRP in the heparin treated patients (Fig. 4). It could be explained that heparin decreases the adherence of bacteria and viruses to the bronchial surface and it has an anti-inflammatory effect [6,7].
This study had several limitations. First, this study was carried out at a single center. However, the research team believe that the study provides valuable clinical information for assessing ICU outcomes of using nebulized heparin and salbutamol in AECOPD patients, as the study population was disease-specific. Second, the results of this study were evaluated at day 14 and not at day 28 because 28 days would be a relatively long duration considering that most of AECOPD patients would be extubated before this. Additionally, mortality cases were omitted from the assessment. We assumed a timeframe of 14 days would be enough. Our results show that nearly 20% of patients remained ventilated after the 14-days timeframe (Fig. 2) which makes the timeframe (and not the VFDs) a limitation in our study. The research team recommends increasing the timeframe of VFDs evaluated in future studies regarding mechanically ventilated AECOPD. Third, a larger sample size is required to achieve significant differences in the side effects encountered. Fourth, the time from admission to hospital discharge, which is important outcome because of its economic implications, was not examined. This should be considered in future randomized clinical trials.
Nevertheless, this study also has some strengths. First, all the patients were subjected to prolonged (MV) along with its potential of lung damage. Second, the randomized and double-blind design decreased the possibility of bias. Third, the use of VFDs as an outcome provided added value because of its statistical power to detect treatment effects rather than the binary outcome measure of mortality [36]. Survivors of respiratory failure from COPD tend to return to baseline lung function very slowly (i.e., weeks to months). However, the risk for re-hospitalization and re-intubation for patients with COPD is increased markedly after an episode of respiratory failure requiring MV. COPD patients continue to experience significantly increased rates of severe exacerbations and use of healthcare resources, indicating a potential unmet need in this group of patients [37]. The need for new pharmaceutical therapies and protocols of treatments to reduce severe exacerbations is evident and may in the future be of benefit for this high-risk population.
Possible roles of heparin and salbutamol nebulization in target¬ing the pathophysiology of AECOPD were presented in Fig. 5 [1,6,17,38–40].
In conclusion, the co-administration of nebulized heparin and salbutamol, compared with salbutamol alone, significantly increased VFDs among mechanically ventilated AECOPD patients without increasing bleeding risks.
Acknowledgments
The research team would like to thank the residents and nursing staff working in the internal medicine intensive care unit of Ain Shams University Hospital for their valuable help in this work.
Footnotes
Funding Statement
Study equipment was provided by Ain-Shams University Hospital. This research did not receive any specific grant from funding agencies in the public, commercial, not-for-profit sectors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Tarek Mohamed Ashoor (Conceptualization; Investigation; Writing – original draft; Writing – review & editing)
Ahmad Mahmoud Hasseb (Formal analysis; Writing – review & editing)
Ibrahim Mamdouh Esmat (Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing)
References
- 1.Søgaard M, Madsen M, Løkke A, Hilberg O, Sørensen HT, Thomsen RW. Incidence and outcomes of patients hospitalized with COPD exacerbation with and without pneumonia. Int J Chron Obstruct Pulmon Dis. 2016;11:455–65. doi: 10.2147/COPD.S96179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Said AF, Ewis AA, Omran AA, Magdy ME, Saleeb MF. Prevalence and predictors of chronic obstructive pulmonary disease among high-risk Egyptians. Egypt J Bronchol. 2015;9:27–33. [Google Scholar]
- 3.Honrubia T, García López FJ, Franco N, Mas M, Guevara M, Daguerre M, et al. Noninvasive vs conventional mechanical ventilation in acute respiratory failure: a multicenter, randomized controlled trial. Chest. 2005;128:3916–24. doi: 10.1378/chest.128.6.3916. [DOI] [PubMed] [Google Scholar]
- 4.Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(1):CD004104. doi: 10.1002/14651858.CD004104.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Roisin R. COPD exacerbations.5: management. Thorax. 2006;61:535–44. doi: 10.1136/thx.2005.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas R, Brooks T. Common oligosaccharide moieties inhibit the adherence of typical and atypical respiratory pathogens. J Med Microbiol. 2004;53:833–40. doi: 10.1099/jmm.0.45643-0. [DOI] [PubMed] [Google Scholar]
- 7.Darien BJ, Fareed J, Centgraf KS, Hart AP, MacWilliams PS, Clayton MK, et al. Low molecular weight heparin prevents the pulmonary hemodynamic and pathomorphologic effects of endotoxin in a porcine acute lung injury model. Shock. 1998;9:274–81. doi: 10.1097/00024382-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Yildiz-Pekoz A, Ozsoy Y. Inhaled heparin: therapeutic efficacy and recent formulations. J Aerosol Med Pulm Drug Deliv. 2017;30:143–56. doi: 10.1089/jamp.2015.1273. [DOI] [PubMed] [Google Scholar]
- 9.Glas GJ, Muller J, Binnekade JM, Cleffken B, Colpaert K, Dixon B, et al. HEPBURN - investigating the efficacy and safety of nebulized heparin versus placebo in burn patients with inhalation trauma: study protocol for a multi-center randomized controlled trial. Trials. 2014;15:91. doi: 10.1186/1745-6215-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glas GJ, Serpa Neto A, Horn J, Cochran A, Dixon B, et al. Nebulized heparin for patients under mechanical ventilation: an individual patient data meta-analysis. Ann Intensive Care. 2016;6:33. doi: 10.1186/s13613-016-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed SM, Athar M. Mechanical ventilation in patients with chronic obstructive pulmonary disease and bronchial asthma. Indian J Anaesth. 2015;59:589–98. doi: 10.4103/0019-5049.165856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Global Initiative for Chronic Obstructive Lung Disease . Fontana: COLD; Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease [Internet] [updated 2015; cited Oct 24, 2020]. Available from http://www.goldcopd.org/ [Google Scholar]
- 13.Adriance SM, Murphy CV. Prophylaxis and treatment of venous thromboembolism in the critically ill. Int J Crit Illn Inj Sci. 2013;3:143–51. doi: 10.4103/2229-5151.114274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon B, Schultz MJ, Smith R, Fink JB, Santamaria JD, Campbell DJ. Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: a randomized controlled trial. Crit Care. 2010;14:R180. doi: 10.1186/cc9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machin D, Campbell MG, Tan SP, Tan SH. Sample Size Tables for Clinical Studies. 3rd ed. Chichester: John Wiley & Sons, Ltd; 2009. pp. 47–57. [Google Scholar]
- 16.Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015:507151. doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwig RJ. Therapeutic use of heparin beyond anticoagulation. Curr Drug Discov Technol. 2009;6:281–9. doi: 10.2174/157016309789869001. [DOI] [PubMed] [Google Scholar]
- 18.Törkvist L, Thorlacius H, Sjöqvist U, Bohman L, Lapidus A, Flood L, et al. Low molecular weight heparin as adjuvant therapy in active ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1323–8. doi: 10.1046/j.1365-2036.1999.00599.x. [DOI] [PubMed] [Google Scholar]
- 19.de Vroege R, van Oeveren W, van Klarenbosch J, Stooker W, Huybregts MA, Hack CE, et al. The impact of heparin-coated cardiopulmonary bypass circuits on pulmonary function and the release of inflammatory mediators. Anesth Analg. 2004;98:1586–94. doi: 10.1213/01.ANE.0000114551.64123.79. [DOI] [PubMed] [Google Scholar]
- 20.Dixon B, Opeskin K, Stamaratis G, Nixon I, Yi M, Newcomb AE, et al. Pre-operative heparin reduces pulmonary microvascular fibrin deposition following cardiac surgery. Thromb Res. 2011;127:e27–30. doi: 10.1016/j.thromres.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed T, Garrigo J, Danta I. Preventing bronchoconstriction in exercise-induced asthma with inhaled heparin. N Engl J Med. 1993;329:90–5. doi: 10.1056/NEJM199307083290204. [DOI] [PubMed] [Google Scholar]
- 22.Miller AC, Rivero A, Ziad S, Smith DJ, Elamin EM. Influence of nebulized unfractionated heparin and N-acetylcysteine in acute lung injury after smoke inhalation injury. J Burn Care Res. 2009;30:249–56. doi: 10.1097/BCR.0b013e318198a268. [DOI] [PubMed] [Google Scholar]
- 23.Ghiasi J, Sadeghian M, Emami M, Kiaie BA, Mousavi S. A pilot study of nebulized heparin for prevention of ventilator induced lung injury: comparative effects with an inhaled corticosteroid. Indian J Crit Care Med. 2017;21:634–639. doi: 10.4103/ijccm.IJCCM_183_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi X, Li H. Anticoagulation therapy in patients with chronic obstructive pulmonary disease in the acute exacerbation stage. Exp Ther Med. 2013;5:1367–70. doi: 10.3892/etm.2013.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian Y, Xie H, Tian R, Yu K, Wang R. Efficacy of low molecular weight heparin in patients with acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. COPD. 2014;11:171–6. doi: 10.3109/15412555.2013.831062. [DOI] [PubMed] [Google Scholar]
- 26.Kashefi NS, Nahan JI, Dissanaike D. Does a nebulized heparin/N-acetylcysteine protocol improve outcomes in adult smoke inhalation? Plast Reconstr Surg Glob Open. 2014;2:e165. doi: 10.1097/GOX.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon B, Santamaria JD, Campbell DJ. A phase 1 trial of nebulised heparin in acute lung injury. Crit Care. 2008;12:R64. doi: 10.1186/cc6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shute JK, Calzetta L, Cardaci V, di Toro S, Page CP, Cazzola M. Inhaled nebulised unfractionated heparin improves lung function in moderate to very severe COPD: a pilot study. Pulm Pharmacol Ther. 2018;48:88–96. doi: 10.1016/j.pupt.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Jaques LB, Mahadoo J, Kavanagh LW. Intrapulmonary heparin. A new procedure for anticoagulant therapy. Lancet. 1976;2:1157–61. doi: 10.1016/s0140-6736(76)91679-2. [DOI] [PubMed] [Google Scholar]
- 30.Bendstrup KE, Chambers CB, Jensen JI, Newhouse MT. Lung deposition and clearance of inhaled (99m)Tc-heparin in healthy volunteers. Am J Respir Crit Care Med. 1999;160:1653–8. doi: 10.1164/ajrccm.160.5.9809123. [DOI] [PubMed] [Google Scholar]
- 31.Dixon B, Schultz MJ, Hofstra JJ, Campbell DJ, Santamaria JD. Nebulized heparin reduces levels of pulmonary coagulation activation in acute lung injury. Crit Care. 2010;14:445. doi: 10.1186/cc9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang C, Zhu H, Shen N, Han X, Chen Y, He B. Utility of the combination of serum highly-sensitive C-reactive protein level at discharge and a risk index in predicting readmission for acute exacerbation of COPD. J Bras Pneumol. 2014;40:495–503. doi: 10.1590/S1806-37132014000500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;175:250–5. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 34.Kumar R, Nigam P. C-reactive protein in chronic obstructive pulmonary disease, its correlation with lung function and the role of statin in chronic obstructive pulmonary disease. Int J Sci Study. 2015;3:168–171. [Google Scholar]
- 35.Gupta R, Kaur R, Singh V, Dahiya K, Gupta A, Kumawat M. Serial estimation of serum CRP levels in patients of COPD with acute exacerbation. Glob J Med Public Health. 2012;1:1–10. [Google Scholar]
- 36.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200:828–36. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995;274:1852–7. [PubMed] [Google Scholar]
- 38.Palmieri TL. Use of beta-agonists in inhalation injury. J Burn Care Res. 2009;30:156–9. doi: 10.1097/BCR.0b013e3181923bc3. [DOI] [PubMed] [Google Scholar]
- 39.McSuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004;32:1470–6. doi: 10.1097/01.ccm.0000129489.34416.0e. [DOI] [PubMed] [Google Scholar]
- 40.Darien BJ, Fareed J, Centgraf KS, Hart AP, MacWilliams PS, Clayton MK, et al. Low molecular weight heparin prevents the pulmonary hemodynamic and pathomorphologic effects of endotoxin in a porcine acute lung injury model. Shock. 1998;9:274–81. doi: 10.1097/00024382-199804000-00007. [DOI] [PubMed] [Google Scholar]