Abstract
Background
The aim of this study was to test the hypothesis that the use of inhalational anesthesia leads to higher suppression of the cell-mediated immunity compared to total intravenous anesthesia in patients undergoing kidney cancer surgery under combined low thoracic epidural analgesia and general anesthesia.
Methods
Patients were randomly allocated to either propofol-based (intravenous anesthetic) or sevoflurane-based (volatile anesthetic) anesthesia group with 10 patients in each group, along with epidural analgesia in both groups. Amounts of natural killer (NK) cells, total T lymphocytes, and T lymphocyte subpopulations in the blood samples collected from the patients before surgery, at the end of the surgery and postoperative days 1, 3 and 7 were determined by flow cytometric analysis. The NK cell count served as the primary endpoint of the study, whereas the total T lymphocyte count and cell counts for T lymphocyte subpopulations were used as the secondary endpoint.
Results
Our study showed that there were no significant differences in the amount of NK cells, total T lymphocytes, regulatory T cells, and T-helper cells, cytotoxic T lymphocytes, and their subpopulations between the propofol- and sevoflurane-based anesthesia groups when the anesthesia was administered in combination with epidural analgesia.
Conclusions
The results of this pilot study did not support the hypothesis that the use of inhalational anesthesia leads to higher suppression of the cell-mediated immunity than that of total intravenous anesthesia in patients undergoing kidney cancer surgery under combined low thoracic epidural analgesia and general anesthesia.
Keywords: Anesthesia, Cancer, Epidural analgesia, Immunity, Propofol, Sevoflurane
Introduction
Kidney cancer is the sixth most common cancer among men, and the eighth most common cancer among women [1]. Nephrectomy and surgical resection are the primary methods of choice for the treatment of renal cancer. Perioperative stress is associated with neuroendocrine and immune de-arrangements and contributes to the survival of circulating tumor cells and minimal residual disease [2]. Several in vivo, animal, and retrospective clinical studies showed that anesthesia contributes to the perioperative stress and may affect cancer recurrence and survival [3].
Suppression of cell-mediated immunity has been hypothesized to be associated with poor long-term outcomes of cancer surgery [4]. Several studies demonstrated that the use of volatile anesthetics during cancer surgery is associated with poor outcomes as compared to the use of intravenous anesthetic, propofol [5–7]. These differences in the outcomes are contributed through various effects of anesthetic agents on various immune cells, such as natural killer (NK) cells, cytotoxic T lymphocytes (CTL), and T-helper (Th) cells [8–11].
Although epidural analgesia is routinely used and widely accepted in cancer patients, the selection of an appropriate type of anesthesia (inhalational versus total intravenous anesthesia) remains a matter of debate. Furthermore, studies have so far not standardized the effects of combined anesthetic interventions (e. g., regional and opioid analgesia) on cell-mediated immunity.
The aim of this study was to test the hypothesis that the use of inhalational anesthesia as compared to total intravenous anesthesia leads to the higher suppression of the cell-mediated immunity in patients undergoing kidney cancer surgery under combined low thoracic epidural analgesia and general anesthesia, by conducting a pilot, randomized, controlled trial (RCT).
Materials and Methods
Study population
The RCT was approved by the ethics committee of E. Meshalkin National Medical Research Center, Novosibirsk, Russian Federation (Approval number 14/2018). The trial was registered prior to patient enrollment at clinicaltrials. gov (NCT03514550, Principal investigator: Efremov Sergey, Date of registration: May 2, 2018). Written informed consent was obtained from all participants. All patients were operated in E. Meshalkin National Medical Research Center.
Patients with renal cancer scheduled for open nephrectomy or kidney resection were eligible for the study; inclusion criteria were as follows: surgery for renal cancer and signed informed consent; exclusion criteria: propofol or sevoflurane intolerance, contraindications to epidural analgesia, renal failure, liver failure, congestive heart failure, previous chemotherapy, and co-morbid hematological diseases.
Before anesthesia induction, patients were randomly allocated to either propofol-based (intravenous) or sevoflurane-based (inhalational) anesthesia groups with 10 patients in each group. Random allocation sequence generation, enrollment, and the allocation of the participants to the interventions were performed by an investigator not involved in the intervention and outcome assessment. A simple randomization sequence was generated electronically (https://www.sealedenvelope.com). Patients were allocated to the intervention using numbered-opaque-sealed envelopes, and the procedure for the allocated intervention was performed immediately after opening the envelope. Flank incision was used for surgical access to the kidney.
Clinical characteristics
Demographic and perioperative data were collected and analyzed, including age, height, weight, gender, cancer stage, minimal body temperature during operation, duration of surgery (defined as the period between skin incision and skin closure), duration of anesthesia (defined as the period between induction to anesthesia and end of the administration of the anesthetic agents, sevoflurane or propofol), ventilation time, intensive care unit stay and duration of hospitalization.
Anesthesia
The enrolled patients were not premedicated. Intraoperative monitoring included ECG, pulse oximetry, body temperature, and invasive arterial pressure. Forced-air warming with a blanket covering legs were used for the maintenance of normothermia. Epidural analgesia was performed using an 18-gauge (G) Touhy needle using the median or para-median approach at the T10–11 or T11–12 vertebral level. After confirmation of the needle tip placement, using saline for the loss of resistance technique, catheterization of epidural space with a 20 G epidural catheter was performed, and 7 ml of 0.75% ropivacaine was administered. Epidural block in relevant dermatomes has been confirmed using an alcohol swab test for 20 minutes after ropivacaine administration. Repeated epidural 0.75% ropivacaine injection was performed after 1–3 hours of initial dose in accordance with the anesthesiologist decision.
Anesthesia induction was performed by administering propofol at a dosage of 1.5–2.5 mg/kg intravenously, in the propofol-based anesthesia group, and sevoflurane 5–8% inspired concentration with 50% oxygen, in the sevoflurane-based anesthesia group. Lidocaine (1 mg/kg) and fentanyl (2 µg/kg) were given intravenously, 3 min before intubation to all the patients. After loss of consciousness and confirmation of adequate mask ventilation, rocuronium (0.6 mg/kg) was administered intravenously for muscle relaxation. Tracheal intubation was performed 1–2 min after rocuronium injection. Antibiotic prophylaxis was performed using Cefuroxime (1500 mg, intravenous administration).
Anesthesia was maintained with a dosage of 0.1–0.2 mg/kg/min propofol in the propofol-based group, and with 1 minimal alveolar concentration of sevoflurane in the sevoflurane-based group. Opioids were not used for anesthesia maintenance. During anesthesia, the mean blood pressure was maintained above 60 mmHg. At the end of the surgery, the administration of propofol or sevoflurane was stopped, the patients were transferred to the post-anesthesia care unit, and the epidural analgesia was continued with 0.25% ropivacaine for up to 2 days after surgery. The rate of postoperative epidural ropivacaine infusion was set as 3–8 ml/h and delivered by syringe pump in accordance to individual requirements and tolerability. In the absence of contraindications, non-steroid anti-inflammatory drugs (ketoprofen) and/or acetaminophen were used routinely for multimodal analgesia in all patients. Parenteral opioids were allowed for analgesic rescue postoperatively in cases when multimodal analgesia was insufficient.
Blood samples
Venous blood samples were collected before surgery, at the end of the procedure, and on postoperative days (POD) 1, 3, and 7. Blood samples were collected in ethylenediaminetetraacetic acid tubes. Flow cytometric analysis was performed immediately after blood collection.
Determination of frequencies of natural killer cells and T lymphocytes by flow cytometric analysis
The primary endpoint of the study was NK cell count, and the secondary endpoint was the total T lymphocyte count and counts for the T lymphocyte subpopulations, such as Th cells, CTL, naive, central memory (CM), effector memory (EM), terminally differentiated effector memory (TEMRA) of Th cells and CTL cells, natural killer T cells (NKT), and regulatory T cells (Treg).
Frequencies of NK cells and the different types of T lymphocytes in the blood samples were determined based on the expression of cell-type-specific cluster of differentiation (CD) antigens. Anti-human antibodies specific to the various CD antigens and conjugated to specific fluorophores, allophycocyanin (APC), APC-Alexa Fluor (AF)-700, APC-AF-750, phycoerythrin (PE), phycoerythrin-Texas-Red-x (ECD), phycoerythrin-Cyanin 7 (PC7), phycoerythrin-Cyanin 5 (PC5), pacific blue (PB), were purchased from Beckman Coulter (France), and flow cytometry analysis was performed based on the antibody staining. Investigators, blinded to the allocated intervention, performed the flow cytometry analysis. For that, 100 μl of whole blood samples were stained with the specific fluorophore-conjugated monoclonal antibodies at room temperature (20–25°C), in the dark, for 15 min, in accordance with the manufacturer’s instructions. Red blood cells were lysed using the IQTest 3 Lysing Solution (IM3514, Beckman Coulter, France) at room temperature, in the dark, for 10 min. The various immune cells in the peripheral blood were identified using the Navios flow cytometer (Beckman Coulter, USA), and analysis was performed according to the manufacturer’s protocol. Flow cytometric analysis was performed with at least 10,000 events in every measurement.
The use of antibodies for the determination of circulating NK and NKT cells
The amount of circulating NK and NKT cells in the peripheral blood samples was determined using APC-AF 750-conjugated anti-human CD3 antibody (A66329), PC7-conjugated anti-human CD16 antibody (6607118), and PC7-conjugated anti-human CD56 antibody (A51078). All antibodies were purchased from Beckman Coulter, France. The specific cell types were identified based on the expression of the specific CD antigens: CD3- CD16+ CD56+ for NK cells , CD3+ CD16+ CD56+ for NKT cells.
The use of antibodies for the determination of total T lymphocytes and its subpopulations
The quantity of circulating total T lymphocytes, Th cells, CTL and its subpopulations in the peripheral blood samples were determined using APC-AF 750-conjugated anti-human CD3 antibody (A66329), APC-conjugated anti-human CD8 antibody (IM2469U), PC7-conjugated anti-human CD4 antibody (A6607101), ECD-conjugated anti-human CD62L antibody (IM2713U), PE-conjugated anti-human CD45RO antibody (IM1307U), and PB-conjugated anti-human CD45RA antibody (A82946). All antibodies were purchased from Beckman Coulter, France. The specific cell types were identified based on the expression of the specific CD antigens: CD3+ for mature T lymphocytes ; CD3+ CD4+; CTL, and CD3+ CD8+ forTh cells; CD3+ CD4+ CD62L+ CD45RA- for CM Th cells; CD3+ CD8+ CD62L+ CD45RA- for CM CTL cells; CD3+ CD4+ CD62L- CD45RA- for EM Th cells; CD3+ CD8+ CD62L- CD45RA- for EM CTL cells; CD3+ CD4+ CD62L+ CD45RA+ for naive Th cells; CD3+ CD8+ CD62L+ CD45RA+ for naive CTL cells; CD3+ CD4+ CD62L- CD45RA+ for TEMRA Th cells; CD3+ CD8+ CD62L- CD45RA+ for. TEMRA CTL cells.
The use of antibodies for the determination of circulating regulatory T cells
The amount of circulating regulatory T cells in the peripheral blood samples was determined using APC-AF 750-conjugated anti-human CD3 antibody (A66329), PC7-conjugated anti-human CD4 antibody (A6607101), PC5-conjugated anti-human CD25 antibody (IM2646U), and APC-AF 700-conjugated anti-human CD127 antibody (A71116). All antibodies were purchased from Beckman Coulter, France.
Statistical analysis
Comparisons within each group, as well as between the two anesthesia groups, were performed using mixed-effects model (with time and patient as random effects and group as fixed effects), incorporating multiplicity correction by Tukey’s method. Levene’s test was performed, and sphericity was confirmed to be valid. Qualitative characteristics were compared using the χ2 test and Fisher’s exact test, wherever appropriate. The level of significance was set at P < 0.05 (two-tailed). Statistical analysis was performed using MedCalc statistical software version 13. 1. 0 (MedCalc Software, Belgium). Data were summarized and presented as median and Q1–Q3. Cellular distributions are represented in numbers and percentages.
Results
In total, 20 patients were enrolled from May 2018 to April 2019, and 10 patients were randomly allocated to each the propofol- or sevoflurane-based anesthesia groups (Fig. 1). Groups showed no evidence of differences in terms of baseline and perioperative clinical characteristics (Table 1). Epidural block was successful in all patients. No hemodynamic reactions during skin incision or wound traction were observed. Norepinephrine infusion in order to maintain mean arterial pressure above 60 mmHg was required in (20%, n = 2) patients of propofol and (20%, n = 2) of sevoflurane groups.
Fig. 1.
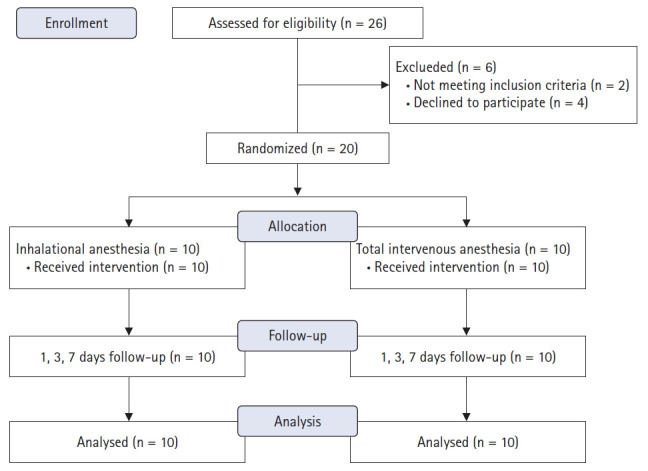
CONSORT flow diagram.
Table 1.
Clinical Characteristics
| Propofol (n = 10) | Sevoflurane (n = 10) | P value | |
|---|---|---|---|
| Age (yr) | 59 (58–67) | 62 (57–62) | >0.999 |
| Sex (F) | 5 (50) | 4 (40) | 0.659 |
| Height (cm) | 168 (159–172) | 166 (164–171) | 0.873 |
| Weight (kg) | 87 (75–97) | 79 (68–85) | 0.271 |
| Cancer stage | |||
| 1 | 3 (30) | 3 (30) | 0.380 |
| 2 | 1 (10) | 0 | |
| 3 | 6 (60) | 5 (50) | |
| 4 | 0 | 2 (20) | |
| Distal metastases at diagnosis | 0 | 2 (20) | 0.138 |
| Invasion to inferior vena cava | 0 | 1 (10) | 0.325 |
| Redo surgery | 0 | 1 (10) | 0.325 |
| ASA score | |||
| II | 6 (60) | 4 (40) | 0.382 |
| III | 4 (40) | 6 (60) | |
| Surgery type | |||
| Nephrectomy | 7 (70) | 8 (80) | 0.611 |
| Kidney resection | 3 (30) | 2 (20) | |
| Intraoperative characteristics | |||
| Total ropivacaine dose (mg) | 75 (53–75) | 75 (53–120) | 0.633 |
| Lowest mean arterial pressure (mmHg) | 57 (51–67) | 65 (61–67) | 0.082 |
| Blood transfusion | 0 (0) | 1 (10) | 0.317 |
| Crystalloids infusion (ml) | 1500 (1000–1500) | 1500 (1500–2500) | 0.153 |
| Diuresis (ml) | 305 (200–470) | 345 (180–420) | 0.970 |
| Minimal body temperature (oC) | 36.0 (35.7–36.1) | 36.0 (35.5–36.5) | 0.559 |
| Duration of surgery (min) | 60 (50–100) | 90 (60–210) | 0.090 |
| Duration of anesthesia (min) | 132 (100–170) | 125 (105–270) | 0.628 |
| Postoperative characteristics: | |||
| Ventilation time (h) | 1.5 (0–2) | 1 (0–2) | 0.973 |
| PACU stay (days) | 1 (1–1) | 1 (1–1) | 0.470 |
| Hospitalization (days) | 7 (6–7) | 6 (5–7) | 0.262 |
Values are presented as median (Q1–Q3) or number (%). PACU: post anesthetic care unit.
The extent of postoperative pain in accordance with 10-points visual analog scale (VAS) showed no evidence of difference in both groups. Thus, VAS values were equal to 2.5 (2–3) and 2 (2–3) at the day of surgery, 2 (2–3) and 3 (3–3) at the first POD 2 (2–3) and 2 (2–2) at the third POD in the propofol and sevoflurane groups, accordingly. One patient in the propofol group and 2 patients in the sevoflurane group needed rescue analgesia with tramadol after discharge from the post-anesthetic care unit.
Detailed cell count data are provided in Table 2. We used NK cell count as the primary endpoint of the study, and our analysis showed that there were no significant differences in the amount of NK cells between the propofol- and sevoflurane-based anesthesia groups. NK cell counts, observed during the different stages and represented as median [interquartile range], were: 169 (105–466) and 187 (147–453) at baseline, 109 (67–262) and 166 (51–245) at the end of the surgery, 116 (69–150) and 128 (71–226) at POD 1, 121 (55–223) and 128 (63–186) at POD 3, 148 (96–245) and 151 (131–206) at POD 7; in the propofol-based and sevoflurane-based anesthesia group, respectively (P = 0.055). The secondary endpoint was the total T lymphocyte count and counts for T-cell subpopulations. Our analysis showed that there were no significant differences in the amount of total T lymphocyte, Treg cells, Th cells, CTL, and their subpopulations between the propofol- and sevoflurane-based anesthesia groups. However, a small reduction in NK cell counts was observed during the first 3 POD in both groups. Significant within-group differences in the total T lymphocytes count (P < 0.001) and Treg cells (P < 0.005) were observed in both groups. Significant within-group differences in the total count of Th cells (P < 0.001), and its subpopulations, naive Th cells (P < 0.001), CM Th cells (P < 0.001), EM Th cells (P < 0.001) were observed during surgery, POD 1, and POD 3 in both groups and the corresponding levels were recovered to the normal by POD 7. Similarly, significant differences in the total count of CTL (P < 0.001), and its subpopulations, naive CTL (P < 0.001), CM CTL (P < 0.005), EM CTL (P < 0. 05) were observed during surgery, POD 1 and POD 3, in both groups and the levels were recovered by POD 7.
Table 2.
T lymphocyte Subtype Counts
| Lymphocyte subpopulation | Group | Baseline | End of surgery | 1 day | 3 days | 7 days | P value* | P value† |
|---|---|---|---|---|---|---|---|---|
| T-cells (cells/μl) | Propofol | 1256 (996–1695) | 957 (857–1032) | 722 (514–806) | 898 (615–1048) | 957 (905–1333) | 0.861 | <0.001 |
| Sevoflurane | 1285 (998–1638) | 958 (572–1513) | 775 (623–921) | 801 (526–1201) | 1307 (1059–1441) | |||
| T-helpers (cells/μl) | Propofol | 727 (556–851) | 591 (520–784) | 413 (330–442) | 533 (416–659) | 698 (659–911) | 0.818 | <0.001 |
| Sevoflurane | 786 (565–961) | 572 (451–868) | 426 (352–582) | 523 (324–734) | 832 (691–872) | |||
| Naive CD4+ cells (cells/μl) | Propofol | 169 (110–215) | 129 (80–178) | 67 (34–99) | 110 (81–150) | 181 (120–255) | 0.859 | <0.001 |
| Sevoflurane | 222 (178–317) | 177 (108–210) | 129 (88–165) | 135 (94–173) | 284 (170–351) | |||
| CM CD4+ cells (cells/μl) | Propofol | 339 (224–535) | 256 (193–364) | 186 (127–217) | 203 (181–347) | 301 (259–417) | 0.314 | <0.001 |
| Sevoflurane | 304 (213–347) | 262 (136–291) | 189 (175–215) | 200 (145–261) | 314 (250–357) | |||
| EM CD4+ cells (cells/μl) | Propofol | 131 (101–245) | 175 (120–188) | 80 (65–111) | 92 (75–171) | 121 (72–178) | 0.613 | <0.001 |
| Sevoflurane | 156 (94–216) | 140 (64–201) | 99 (59–128) | 84 (64–151) | 160 (115–236) | |||
| TEMRA CD4+ cells (cells/μl) | Propofol | 11.6 (5.9–27.7) | 12.1 (4.8–45.4) | 7.7 (2.3–44.1) | 11.2 (6.9–23.5) | 12.6 (7.2–24) | 0.772 | 0.267 |
| Sevoflurane | 14 (6.6–56.1) | 17 (3.6–55.9) | 3.5 (1.9–39.1) | 13.8 (4.4–52.8) | 8.9 (4.1–61.2) | |||
| CTL (cells/μl) | Propofol | 423 (372–573) | 387 (294–452) | 252 (183–359) | 269 (204–431) | 331 (309–404) | 0.488 | <0.001 |
| Sevoflurane | 504 (451–689) | 389 (156–606) | 356 (214–441) | 300 (189–429) | 451 (334–559) | |||
| Naive CTL (cells/μl) | Propofol | 90 (68–132) | 83 (53–127) | 48.1 (36–72) | 62 (49–69) | 75 (63–109) | 0.780 | <0.001 |
| Sevoflurane | 118 (69–147) | 79 (45–93) | 76 (58–110) | 61 (33–117) | 124 (65–142) | |||
| CM CTL (cells/μl) | Propofol | 67.4 (55–104) | 39 (30–104) | 44.1 (28.6–58.5) | 42.7 (30–81) | 54.1 (39.8–107.3) | 0.515 | 0.002 |
| Sevoflurane | 57 (39–83) | 47.3 (22.8–80.1) | 47.5 (26.1–61.6) | 37.4 (28–47.6) | 66.1 (39.1–82.2) | |||
| EM CTL (cells/μl) | Propofol | 102 (65–118) | 79 (39–148) | 59 (37–69) | 58 (47–73) | 65 (44–112) | 0.266 | 0.022 |
| Sevoflurane | 119 (98–125) | 70 (40–124) | 74 (32–138) | 61 (31–107) | 119 (74–146) | |||
| TEMRA CTL (cells/μl) | Propofol | 151 (75–281) | 148 (83–199) | 93 (60–204) | 84 (41–201) | 101 (77–220) | 0.868 | 0.011 |
| Sevoflurane | 152 (108–327) | 106 (46–200) | 110 (49–181) | 115 (45–157) | 160 (50–210) | |||
| Treg (cells/μl) | Propofol | 24.1 (16.5–41.7) | 22.4 (15–36.9) | 18.1 (14.3–23.7) | 20.1 (14.3–35.9) | 34.7 (20.4–53.1) | 0.543 | 0.002 |
| Sevoflurane | 30.2 (24.7–34.8) | 25.3 (19.5–43.4) | 21.7 (15.9–29.3) | 21 (18.7–29.3) | 33.9 (27.9–62.4) | |||
| NK cells (cells/μl) | Propofol | 169 (105–466) | 109 (67–262) | 116 (69–150) | 121 (55–223) | 148 (96–245) | 0.504 | 0.053 |
| Sevoflurane | 187 (147–453) | 166 (51–245) | 128 (71–226) | 128 (63–186) | 151 (131–206) | |||
| TNK cells (cells/μl) | Propofol | 101 (65–168) | 89 (45–103) | 72 (46–125) | 70 (25–130) | 88 (31–110) | 0.753 | 0.174 |
| Sevoflurane | 107 (69–228) | 85 (65–123) | 79 (23–127) | 76 (36–138) | 67 (25–175) |
Values are presented as median (Q1–Q3). EM: effector memory, CM: central memory, TEMRA: terminally differentiated effector memory cells, CTL: cytotoxic T lymphocytes, NK: natural killer, Treg: regulatory T lymphocytes, TNK: natural killer T lymphocytes.
Between-group differences in accordance with a mixed-effects model,
Within-group differences using a mixed-effects model.
Discussion
An important observation from this study is that the suppression of the cell-mediated immunity under the use of inhalational anesthesia was similar to that during total intravenous anesthesia in patients undergoing kidney cancer surgery under combined low thoracic epidural analgesia and general anesthesia. Our analysis showed that there were no significant differences in the amounts of NK cells, total T lymphocytes, regulatory T cells, Th cells, CTL, and their subpopulations between the propofol- and sevoflurane-based anesthesia groups when the anesthesia was administered in combination with epidural analgesia.
NK cells are essential defensive cells for attenuation of cancer progression [12], and due to their anti-tumor activity, which is independent of recognition of tumor-specific antigens that are anyway poorly presented on a majority of the malignant cells, are particularly attractive for cancer treatment. Regulatory T cells inhibit anti-tumor activity of NK cells and CTL, promote cancer growth, recurrence, and metastasis [13].
The results of our study are in accordance with the existing data on the pattern of perioperative-stress-mediated immunosuppression. Several studies have shown that perioperative stress is responsible for suppression of cell-mediated immunity lasting up to 1 week, and the effect is directly related to the extent of surgical stress and incidence of postoperative complications, adversely affecting the long-term outcomes [3].
Furthermore, there are reports on the suppressive effects of volatile anesthetics on lymphocyte proliferation [14,15] and their ability to induce lymphocyte apoptosis [16], whereas propofol was shown to have a neutral effect on the lymphocyte function [17]. Moreover, advantages of using propofol over sevoflurane on the anti-tumor activity of immune cells were investigated in an in vitro study, performed on breast cancer tissue samples. These samples were obtained from the participants of a large ongoing randomized trial aimed to explore the effect of combined use of propofol with paravertebral anesthesia in comparison to sevoflurane with opioid anesthesia (NCT00418457). These pilot studies showed that the combined use of propofol and paravertebral anesthesia increase the levels of NK and Th cell infiltration in the breast cancer tissue samples [18], induce the apoptosis of estrogen receptor-negative breast cancer cells [11], and are associated with increased NK cell cytotoxicity [9] in comparison to sevoflurane. However, in this study, propofol was administered together with paravertebral block, whereas sevoflurane was administered with opioids. Of note, previous studies have demonstrated the dose-dependent detrimental effects of opioids on NK cell cytotoxicity [19], whereas regional anesthesia was shown to attenuate the immunosuppressive effects of surgery by reducing the neuroendocrine stress due to the lesser anesthetic drug requirements.
Further, epidural analgesia is hypothesized to be protective for cancer patients by attenuating inflammation [20], immunosuppression, catecholamine response, sympathetic blockade [21], and eliciting of inhibitory effects on perioperative lymph flow, thus lowering the risk of intraoperative cancer cell dissemination [22]. A meta-analysis of two independent retrospective clinical trials showed a favorable impact of regional anesthesia on the mortality in cancer patients [23,24]. Nevertheless, a study of Myles et al. [25], provided conflicting evidence. Our study did not observe a difference between propofol- and sevoflurane-based anesthesia groups, probably because the immunosuppression, induced by the volatile anesthetics, may be modified when used in combination with regional analgesia.
In a recently published RCT by Oh et al. [10], 201 patients scheduled for breast cancer surgery were randomly assigned to propofol or sevoflurane anesthesia, and no significant differences were observed between the two groups in Treg cells (CD39+, CD73+), Th type 1 and type 17 cells, NK cells, CTL, cytokines, or in the neutrophil-to-lymphocyte ratio. Although the patients enrolled in this study were not administered epidural analgesia, findings of our study are in accordance with these observations.
We have herein discussed some of the essential considerations and strengths of the current study. Our decision on the enrollment of kidney cancer patients in RCT was based on several reasons. First, open nephrectomy is associated with considerable tissue trauma, and perioperative pain is controlled by epidural analgesia in many occasions. Further, none of the patients enrolled in the study received chemotherapy before surgery. The major strength of our study is that the confounders that are capable of influencing cell immunity are uniform between the two groups, and epidural analgesia, opioid-less anesthesia, and maintenance of normothermia were applied in both groups. Furthermore, this is the first study that is assessing the effects of anesthesia on cell-mediated immunity in patients undergoing surgery for kidney cancer. Although several large randomized trials on the investigation of the effects of anesthesia on clinical outcomes among cancer patients are ongoing (NCT01975064, NCT03034096, NCT02813044, NCT03193710, NCT02660411, NCT02840227), it should be noted that none of these trials are designed to administer regional anesthesia to all the participants.
Nevertheless, there are also some limitations of the current study. First, it was a pilot trial with a small number of participants and hence, it is not powered for the primary endpoint. Second, the effect of fentanyl that was administered to all patients during the procedure (a single dose of 2 µg/kg) on cell-mediated immunity is not known. Third, the level of anesthesia using the bispectral index was not monitored during the surgery, and comparative data of anesthesia depth are not provided. However, successful epidural block in all patients, no cases of intraoperative awareness, and end-tidal anesthetic gas guided anesthesia in sevoflurane-based group likely overcome this limitation. Fourth, our study measures cell counts, which may not comprehensively reflect the function and actual difference in cytotoxic activity.
In conclusion, the results of this pilot study do not support the hypothesis that the suppression of cell-mediated immunity is higher under the use of inhalational anesthesia than total intravenous anesthesia in patients undergoing kidney cancer surgery under combined low thoracic epidural analgesia and general anesthesia. Our study indicates that the effect of different types of anesthesia on cell-mediated immunity might be modulated when used in combination with regional analgesia. These findings are of importance for designing clinical trials in the future.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Sergey Mihailovich Efremov (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing)
Victoria Sergeevna Kozireva (Investigation; Writing – original draft)
Gleb Borisovich Moroz (Investigation)
Marat Nikolaevich Abubakirov (Investigation)
Olga Sergeevna Shkoda (Investigation)
Anna Nikolaevna Shilova (Methodology; Supervision)
Sergey Valeriyevich Yarmoshuk (Data curation; Investigation)
Alexandr Alexandrovich Zheravin (Data curation; Investigation)
Giovanni Landoni (Conceptualization; Writing – review & editing)
Vladimir Vladimirovich Lomivorotov (Conceptualization; Supervision; Writing – review & editing)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Coffey JC, Wang JH, Smith MJF, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4:760–8. doi: 10.1016/s1470-2045(03)01282-8. [DOI] [PubMed] [Google Scholar]
- 3.Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15:205–18. doi: 10.1038/nrclinonc.2017.194. [DOI] [PubMed] [Google Scholar]
- 4.Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev. 2017;36:159–77. doi: 10.1007/s10555-016-9647-8. [DOI] [PubMed] [Google Scholar]
- 5.Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124:69–79. doi: 10.1097/ALN.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Kang SH, Kim Y, Kim HA, Kim BS. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol. 2016;69:126–32. doi: 10.4097/kjae.2016.69.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai HC, Lee MS, Lin C, Lin KT, Huang YH, Wong CS, et al. Propofol-based total intravenous anaesthesia is associated with better survival than desflurane anaesthesia in hepatectomy for hepatocellular carcinoma: a retrospective cohort study. Br J Anaesth. 2019;123:151–60. doi: 10.1016/j.bja.2019.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–47. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 9.Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. 2014;113 Suppl 1:i56–62. doi: 10.1093/bja/aeu200. [DOI] [PubMed] [Google Scholar]
- 10.Oh CS, Lee J, Yoon TG, Seo EH, Park HJ, Piao L, et al. Effect of Equipotent Doses of Propofol versus Sevoflurane Anesthesia on Regulatory T Cells after Breast Cancer Surgery. Anesthesiology. 2018;129:921–31. doi: 10.1097/ALN.0000000000002382. [DOI] [PubMed] [Google Scholar]
- 11.Jaura AI, Flood G, Gallagher HC, Buggy DJ. Differential effects of serum from patients administered distinct anaesthetic techniques on apoptosis in breast cancer cells in vitro: a pilot study. Br J Anaesth. 2014;113 Suppl 1:i63–7. doi: 10.1093/bja/aet581. [DOI] [PubMed] [Google Scholar]
- 12.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–37. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Najafi M, Farhood B, Mortezaee K. Contribution of regulatory T cells to cancer: A review. J Cell Physiol. 2019;234:7983–93. doi: 10.1002/jcp.27553. [DOI] [PubMed] [Google Scholar]
- 14.Ferrero E, Ferrero ME, Marni A, Zocchi MR, Stella L, Rugarli C, et al. In vitro effects of halothane on lymphocytes. Eur J Anaesthesiol. 1986;3:321–30. [PubMed] [Google Scholar]
- 15.Salo M, Eskola J, Nikoskelainen J. T and B lymphocyte function in anaesthetists. Acta Anaesthesiol Scand. 1984;28:292–5. doi: 10.1111/j.1399-6576.1984.tb02063.x. [DOI] [PubMed] [Google Scholar]
- 16.Matsuoka H, Kurosawa S, Horinouchi T, Kato M, Hashimoto Y. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology. 2001;95:1467–72. doi: 10.1097/00000542-200112000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263–77. doi: 10.1007/s00540-008-0626-2. [DOI] [PubMed] [Google Scholar]
- 18.Desmond F, McCormack J, Mulligan N, Stokes M, Buggy DJ. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015;35:1311–9. [PubMed] [Google Scholar]
- 19.Beilin B, Shavit Y, Hart J, Mordashov B, Cohn S, Notti I, et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesth Analg. 1996;82:492–7. doi: 10.1097/00000539-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Day AR, Smith RVP, Scott MJP, Fawcett WJ, Rockall TA. Randomized clinical trial investigating the stress response from two different methods of analgesia after laparoscopic colorectal surgery. Br J Surg. 2015;102:1473–9. doi: 10.1002/bjs.9936. [DOI] [PubMed] [Google Scholar]
- 21.Koltun WA, Bloomer MM, Tilberg AF, Seaton JF, Ilahi O, Rung G, et al. Awake epidural anesthesia is associated with improved natural killer cell cytotoxicity and a reduced stress response. Am J Surg. 1996;171:68–72. doi: 10.1016/S0002-9610(99)80076-2. [DOI] [PubMed] [Google Scholar]
- 22.Hiller JG, Ismail HM, Hofman MS, Narayan K, Ramdave S, Riedel BJ. Neuraxial anesthesia reduces lymphatic flow: proof-of-concept in first in-human study. Anesth Analg. 2016;123:1325–7. doi: 10.1213/ANE.0000000000001562. [DOI] [PubMed] [Google Scholar]
- 23.Weng M, Chen W, Hou W, Li L, Ding M, Miao C. The effect of neuraxial anesthesia on cancer recurrence and survival after cancer surgery: an updated meta-analysis. Oncotarget. 2016;7:15262–73. doi: 10.18632/oncotarget.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Li T, Gan TJ. The effects of perioperative regional anesthesia and analgesia on cancer recurrence and survival after oncology surgery: a systematic review and meta-analysis. Reg Anesth Pain Med. 2015;40:589–98. doi: 10.1097/AAP.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 25.Myles PS, Peyton P, Silbert B, Hunt J, Rigg JRA, Sessler DI, ANZCA Trials Group Investigators Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. BMJ. 2011;342:d1491. doi: 10.1136/bmj.d1491. [DOI] [PubMed] [Google Scholar]


