Abstract
Introduction
There is a paucity of data that can be used to guide the management of critically ill patients with COVID-19. In response, a research and data-sharing collaborative—The COVID-19 Critical Care Consortium—has been assembled to harness the cumulative experience of intensive care units (ICUs) worldwide. The resulting observational study provides a platform to rapidly disseminate detailed data and insights crucial to improving outcomes.
Methods and analysis
This is an international, multicentre, observational study of patients with confirmed or suspected SARS-CoV-2 infection admitted to ICUs. This is an evolving, open-ended study that commenced on 1 January 2020 and currently includes >350 sites in over 48 countries. The study enrols patients at the time of ICU admission and follows them to the time of death, hospital discharge or 28 days post-ICU admission, whichever occurs last. Key data, collected via an electronic case report form devised in collaboration with the International Severe Acute Respiratory and Emerging Infection Consortium/Short Period Incidence Study of Severe Acute Respiratory Illness networks, include: patient demographic data and risk factors, clinical features, severity of illness and respiratory failure, need for non-invasive and/or mechanical ventilation and/or extracorporeal membrane oxygenation and associated complications, as well as data on adjunctive therapies.
Ethics and dissemination
Local principal investigators will ensure that the study adheres to all relevant national regulations, and that the necessary approvals are in place before a site may contribute data. In jurisdictions where a waiver of consent is deemed insufficient, prospective, representative or retrospective consent will be obtained, as appropriate. A web-based dashboard has been developed to provide relevant data and descriptive statistics to international collaborators in real-time. It is anticipated that, following study completion, all de-identified data will be made open access.
Trial registration number
ACTRN12620000421932 (http://anzctr.org.au/ACTRN12620000421932.aspx).
Keywords: intensive & critical care, respiratory infections, epidemiology, public health, epidemiology
Strengths and limitations of this study.
This protocol is of a pragmatic international, multicentre, observational clinical study of patients with confirmed or suspected SARS-CoV-2 infection admitted to intensive care units (ICUs) around the world.
This is an evolving clinical registry, which will facilitate the characterisation of patients and their management and provide real-time information on associated characteristics and outcomes.
These data will assist clinicians in deriving evidence-based practices for the care of critically ill patients infected by SARS-CoV-2.
Patients will not receive identical treatments and care.
While this will limit some aspects of data analysis, it will also give breadth to the scope of the investigation, as data on laboratory and patient characteristics, interventions and adjunct therapies and outcomes will be available.
This study relies on clinicians and support staff to accurately record data during a time of increased patient influx and ICU workload, raising concerns over data input error and completeness.
Introduction
The world is currently witnessing a viral pandemic. Cases of atypical pneumonia first emerged in Wuhan, China, in December 2019.1 Investigation has identified the cause as a novel betacoronavirus, ultimately named SARS-CoV-2.2 The virus, and the disease it causes—COVID-19—has since spread internationally. WHO declared the outbreak a ‘Public Health Emergency of International Concern’ on 30 January 2020, and a ‘pandemic’ on 12 March 2020. There have now been >39 million confirmed infections globally, resulting in 1.1 million deaths (as of 17 October 2020).3
SARS-CoV-2, COVID-19 and critical illness
The mortality rate of COVID-19 among patients admitted to the intensive care unit (ICU) has been reported around 30%4 and substantially higher for mechanically ventilated patients.5–9 Early data and clinical experience indicate that this is caused primarily by acute hypoxaemic respiratory failure (AHRF).10 11 These same data have also prompted some authors to suggest that the pathobiology of COVID-19-associated AHRF may differ from that of acute respiratory distress syndrome (ARDS).12 13 This assertion hinges on reports of patients with severe COVID-19-associated AHRF and high pulmonary compliance, a presentation not thought to be typical of ARDS. Much has also been made of the high incidence of thromboembolic events in critically ill patients.14 15 However, many reports are limited by either small numbers of patients or by geographic restrictions. These fail to account for variations in practices or for the variations between countries in patient, systemic and organisational factors. Consequently, much of our current practice is driven by anecdotal cases or by limited case series.
Rationale for developing a worldwide registry of patients with COVID-19 admitted to ICUs
We aim to improve conclusions robustness regarding the management, interventions and treatment of critically-ill patients with COVID-19 around the world. We aim to do this by using combined data sets which detail a wide variety of patients entering the ICU at multiple stages of COVID-19 illness from diverse geographic locations. This ongoing research effort will aid in developing best practices based on evidence from a wide variety of ICUs throughout the world. This is especially important as there is currently a paucity of evidence-based guidelines and limited clinical resources globally. This data will also aid decision-making of clinicians working in healthcare systems that are currently managing or yet to face a surge in COVID-19 cases.
Methods and analysis
Study design
This is an international, multicentre, prospective, observational study. The study protocol V.1.2.8 appears in online supplemental 1.
bmjopen-2020-041417supp001.pdf (143.4MB, pdf)
Study eligibility
The inclusion criteria are: (1) clinically suspected (as determined by attending physician) or laboratory-confirmed SARS-CoV-2 infection (by real-time PCR and/or next-generation sequencing) and (2) admission to an ICU. Patients admitted to an ICU for a reason other than SARS-CoV-2 infection are excluded. In addition, patients who were recently diagnosed with SARS-CoV-2 infection and later admitted to the ICU for reasons not related to the SARS-CoV-2 infection will be excluded. Patients of all ages from infants through adults can be enrolled into the study.
Enrolment and participating sites
This study commenced on 1 January 2020. There is no fixed end date for the study. Currently, 350 centres are included, spanning 48 countries (online supplemental 2), coordinated by regional leads and assistants (online supplemental 3) and the operating team at the coordinating site (online supplemental 3). Co-enrolment with other studies, including interventional trials, is permitted.
Outcome measures
A summary of variables recorded by the study case report form (CRF) is presented in table 1.
Table 1.
Assessment schedule
| Screening | ICU admission | Start MV | Start ECMO | Daily | Outcomes | |
| Eligibility criteria | x | |||||
| Demographics | x | |||||
| Comorbidities | x | |||||
| Severity scoring | x | |||||
| Symptoms | x | |||||
| ABG and biochemistry | x | x | x | x | ||
| Respiratory support | x | x | x | |||
| Adjunctive therapies | x | x | x | |||
| ECMO parameters | x | x | ||||
| Pulmonary mechanics | x | x | ||||
| Microbiology | x | |||||
| Blood transfusion | x | |||||
| Length of stay | x | |||||
| Survival | x |
ABG, arterial blood gas; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; MV, mechanical ventilation.
Data collection
Data collection methods
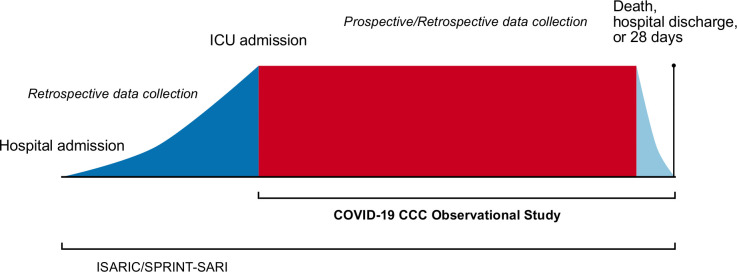
Streamlined data collection instruments and procedures are used to minimise the workload at study centres. Data can be collected and entered prospectively (preferred) or retrospectively dependent on the participating site’s resources. Data collection begins at the time of hospital admission using the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) and Short Period Incidence Study of Severe Acute Respiratory Illness (SPRINT-SARI) data tools (https://isaric.tghn.org/COVID-19-CRF/). Data collection for the COVID-19 Critical Care Consortium (CCC) observational study commences at the time of a patient’s admission to an ICU, using a study-specific adaptation of the ISARIC/SPRINT-SARI COVID-19 CRF (online supplemental 4). Figure 1 outlines the schedule of assessments used for patients included in the COVID-19 CCC study. De-identified study data are collected and managed using the REDCap electronic data capture tool hosted at the University of Oxford, UK.16 Data will not be used for any purpose other than those described in the study protocol. Each site’s principal investigator is responsible for ensuring data integrity. Regular written and web-based training is provided. In countries unable to upload data into a centralised database, the ability to retain a local database on a national server is available, with aggregated anonymised data exported centrally for analysis.
Figure 1.
Schematic study overview. The figure shows in detail periods of data collection into the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) case report form (dark blue), COVID-19 Critical Care Consortium (COVID-19 CCC) case report form (red) and for both case report forms (light blue). As shown, data for the COVID-19 CCC can be collected and entered prospectively (preferred) or retrospectively dependent on the participating site’s resources. The study ends at death, hospital discharge/transfer or 28 days, whichever occurs latest. ICU, intensive care unit; SPRINT-SARI, Short Period Incidence Study of Severe Acute Respiratory Illness.
Interhospital transfer
If a patient is transferred from a facility participating in the COVID-19 CCC and ISARIC/SPRINT-SARI to another participating centre, the patient’s previously allocated unique identifier transfers with them. However, sites will not have access to study data collected outside their hospital. It is the responsibility of each hospital to enter data pertaining to their component of the patient’s hospital admission. If a patient is transferred to a non-participating hospital, there will be no further data collection. All sites will be asked to include a COVID-19 CCC and ISARIC/SPRINT-SARI study information sheet in any outgoing patient’s documentation.
Data management
Several procedures are in place to optimise data quality and completeness. These include: (1) a detailed data dictionary, (2) quality assurance within the data management system, (3) quality assurance of key variables within the CRF and (4) regular written and web-based training for local study investigators. A compendious CRF is fundamental to the success of this study. Extensive efforts have been made to limit data collection to essential variables. It is hoped that this will contribute to more complete data entry with a reduced burden on participating centres. Information that is not available to the investigator will not be treated as missing, and no assumptions will be made for missing data. An audit will be conducted on a randomly selected sample (approximately 5%) of cases. In-person site visits will not be feasible, given the nature of the study and pandemic. Substudy projects will be accessed via the main CRF platform. Specific extensions will be used to collect additional variables, limiting the overall burden on data collectors, but allowing centres involved in sub-studies to enter data in the single REDCap format.
Data access
The coordinating team will have access to all collected data to assure integrity, provide oversight and conduct the main study analyses. Individual sites will have access to all the data they collect. A multinational steering committee (online supplemental 1) oversees registry operations worldwide and approves investigator-initiated or site-specific substudies, external requests for data and reviews suggestions by participants. To date, several substudies have been initiated focusing on the impact of COVID-19 on the brain, heart, kidneys, management and risks of ECMO, coagulation and thrombosis risks and long-term effects, all involving multicentre participation. Once approval is obtained, relevant de-identified data will be made available. It is anticipated that, following study completion, all de-identified data will be made open access.
Statistical considerations
Initial characterisation will be descriptive, including all eligible patients at participating centres enrolled within defined timeframes. Where analysis is hypothesis-driven, sample size calculations and power analysis (where appropriate) will depend on the specific outcome or end point under consideration and will be predefined. Results that aim to show an association or test a hypothesis will include 95% CIs. These intervals and associated means will be interpreted in terms of their clinical and statistical significance, and discussion may include whether a comparison is under-powered.
For discharge, mortality and length-of-stay outcomes, we will use a survival analysis with competing risks approach.17 We will graphically depict the risks of death and discharge over time using cumulative incidence plots. We will estimate which patient variables influence the risk of death and discharge using Cox regression, with separate models for death and discharge. In addition to Cox models, we will construct non-linear predictive models for both outcomes using Random Forest models, which will be externally validated on a hold-out test set. Comparison of the predictive performance of both the Cox regression and Random Forest modelling approaches will be made using: (1) a Brier score,18 (2) area under the receiver operating characteristic curves using a two-sided DeLong test and (3) calibration plots, characterised by visual inspection and reporting of slope and intercept.18 For the Random Forest models, a Shapley Tree Explainer will be used to identify variables that are highly predictive of each outcome.19 This analysis will follow the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis reporting guideline for prediction model development and validation.20
To show within-patient trends, we will plot continuous longitudinal variables over time using line plots. We will summarise each trend using daily averages and will estimate trends over time and the influence of patient variables using a linear mixed model with a random intercept per patient to control for repeated data. For binary variables, we will use panel bar charts to show the average change over time, and will model these variables using a generalised linear mixed model with a binomial distribution. A smooth estimation using cubic spline will be explored to estimate potential non-linear trends of the continuous longitudinal variables and binary variables.
Patient and public involvement in research
The data collection methodology of this study has been designed without patient or public input due to the urgent need for inclusion of prospective data from critically ill COVID-19. However, a consultative approach is planned via structured interviews, workshops and surveys to develop research questions, refine methods and ensure public voice helps to shape consumer-focused outcomes.
Ethics and dissemination
Ethical considerations
Chief investigators and the study management team are responsible for ensuring that the study is conducted in accordance with both the protocols, Declaration of Helsinki and the Principles of Good Clinical Practice. The study management team will continue to work with local principal investigators to ensure that the study adheres to all relevant national regulations, and that the necessary approvals are in place before a site may contribute data. The principal investigator at each site is responsible for maintaining a securely held enrolment log, linking each patient’s hospital record number with the COVID-19 CCC study number, if required. The original protocol and subsequent amendments will be translated into the main language of the collaborating institutions and submitted for institutional review board approval or an equivalent. Patients will not be enrolled under the conditions of an amended protocol, until after approval has been granted.
It is expected that this study will not require informed consent in most jurisdictions. This study is, in effect, a large-scale clinical audit, as all data are collected routinely. This may justify a waiver of consent. Any jurisdiction that deems informed consent necessary may use forms provided on our websites (https://www.elso.org/COVID19/ECMOCARD.aspx). Within such jurisdictions, patients who meet the eligibility criteria will be approached directly. If this is not possible, due to the patient’s incapacity, a model of retrospective or representative consent may be used, per local requirements.
Dissemination
Due to the evolving nature of the pandemic and the uncertainty surrounding its impact, this study was designed to be responsive to the international call for swift characterisation of patients with COVID-19. Hence, in collaboration with University of Queensland and extramural collaboration with IBM Australia (St. Leonard’s, Australia), a web-based dashboard has been developed to provide relevant data and descriptive statistics to international collaborators in real-time. The collected data will also eventually be made available and shared on a public open access platform once core research questions have been answered.
Discussion
Herein, we have described the rationale and design of an international, multicentre, observational registry of patients with COVID-19 admitted to an ICU. To date, the characterisation of patients admitted to ICUs with COVID-19 has been limited to national or single-centre series. This study, using a large collaborative network, attempts to overcome the limitations induced by small patient numbers and geographic restrictions, by providing real-time global data. In a pandemic of an emerging pathogen, high-quality, real-time information is crucial to guide an optimal response. The speed of this response and cumulative experience of ICUs worldwide offer the best framework for determining evidence-based best practices and, therefore, improving outcomes for those requiring critical care.
The design of the COVID-19 CCC study has several strengths. First, the care of patients admitted to the ICU, specifically those who are mechanically ventilated, is dependent on regional resources and may vary.21 22 This potential heterogeneity is mitigated by the international composition of the consortium. In addition, we are planning to further characterise individual ICUs, collecting data on nurse/doctor-to-patient ratio, capacity and potential expanded capacity. Second, the study leverages novel data acquisition methods, which may improve and expedite data collection. Third, the registry-based, collaborative and open-source approach of the study lends itself to the conduct of multiple prospective substudies. Fourth, the study incorporates the provision of a web-based dashboard, which provides real-time data in an accessible format.
Limitations
Patients will not receive identical treatments and care. While this will limit some aspects of data analysis, it will also give breadth to the scope of the investigation, as data on laboratory and patient characteristics, interventions and adjunct therapies, and outcomes will be available.
This study relies on clinicians and support staff to accurately record data during a time of increased patient influx and ICU workload, raising concerns over data input error and completeness. To overcome this, coordinators at each site have access to regular training, as well as ‘drop-in’ query sessions on-line.
This study will provide inclusive global characterisation of critically ill patients with COVID-19. As the study is open-ended, continued data accrual will result in increased power to answer hypothesis-led questions over time and guide the development of evidence-based patient management tools to improve outcomes.
Supplementary Material
Footnotes
Twitter: @aidybarnett, @SebColombo
GLB and JS contributed equally.
Contributors: We hereby confirm that all authors listed below have provided substantial contributions to either the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published. In addition, all authors listed below agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Concept and design: GLB, JS, SC, HD, JFF. Planning: GLB, JS, AGB, AC, IL, SH, SS, JFF. Acquisition, analysis or interpretation of data: GLB, JS, AGB, AC, JM, JF, IL, SC, KW, SL, GA, SH, BL, SS, HD, JFF. Drafting of the manuscript: GLB, JS, JM, JF, KW, SL, GA. Critical revision of the manuscript for important intellectual content: GLB, JS, AGB, AC, IL, SC, SH, BL, SS, HD, JFF. Statistical analysis: AGB, SH, BL, SS. Reporting: GLB, JS, AC, JM, JF, IL, SC, KW, SL, GA, SH, SS.
Funding: This work is supported by the Common Good, an initiative of the Prince Charles Hospital Foundation; and, Wesley Medical Research, UnitingCare Health.
Competing interests: GLB and JFF received research funds, through their affiliated institution, from Fisher & Paykel for studies related to high-flow oxygen therapy.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya AE, Baker SC, Baric RS, et al. Severe acute respiratory syndrome-related coronavirus – the species and its viruses, a statement of the coronavirus Study Group. bioRxiv 2020. 10.1038/s41564-020-0695-z [DOI] [Google Scholar]
- 3.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985. 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrasa H, Rello J, Tejada S, et al. SARS-CoV-2 in Spanish intensive care units: early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med 2020;39:553–61. 10.1016/j.accpm.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med 2020;382:2012–22. 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574–81. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med 2020;8:853–62. 10.1016/S2213-2600(20)30316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–8. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 Does Not Lead to a "Typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2020;201:1299–300. 10.1164/rccm.202003-0817LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA 2020;323:2329. 10.1001/jama.2020.6825 [DOI] [PubMed] [Google Scholar]
- 14.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–7. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 2020;7:e438–40. 10.1016/S2352-3026(20)30145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolkewitz M, Cooper BS, Bonten MJM, et al. Interpreting and comparing risks in the presence of competing events. BMJ 2014;349:g5060. 10.1136/bmj.g5060 [DOI] [PubMed] [Google Scholar]
- 18.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–38. 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundberg SM, Erion G, Chen H, et al. From local explanations to global understanding with explainable AI for trees. Nat Mach Intell 2020;2:56–67. 10.1038/s42256-019-0138-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMC Med 2015;13:1. 10.1186/s12916-014-0241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788. 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 22.Rhodes A, Ferdinande P, Flaatten H, et al. The variability of critical care bed numbers in Europe. Intensive Care Med 2012;38:1647–53. 10.1007/s00134-012-2627-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041417supp001.pdf (143.4MB, pdf)