Abstract
BACKGROUND.
Antimicrobial susceptibility testing (AST) of bacterial isolates is a time- and resource-intensive procedure recommended by cystic fibrosis (CF) treatment guidelines for antimicrobial selection for pulmonary exacerbation (PEx) treatment.
METHODS.
We studied relationships between Pseudomonas aeruginosa (Pa) isolate AST results, antipseudomonal PEx treatments, and treatment responses as change in weight and percent predicted forced expiratory volume in 1 sec (ppFEV1) as well as future antimicrobial treatment hazard for PEx occurring at a CF care center from 1999 through 2018. Treatments were categorized by “Pa coverage” as complete (all Pa isolates susceptible by AST to at least one administered agent), none (no isolates susceptible), incomplete (some, but not all isolates susceptible), and indeterminant (administered antipseudomonals not evaluated by AST). Weight and ppFEV1 responses were compared across Pa coverage categories using unadjusted and adjusted general estimating equations; hazard of future treatment was assessed by Cox and logistic regression.
RESULTS.
Among 3820 antimicrobial PEx treatment events in 413 patients with Pa, 62.6% (2390) had complete Pa coverage; 8.9% (340), 2.4% (99), and 26.2% (1000), had no, incomplete, and indeterminant Pa coverage, respectively. Mean baseline to follow-up weight change was +0.74 kg [95% CI 0.63, 0.86]; ppFEV1 change was +1.60 [1.29, 1.90], Pa coverage category was not associated with significant differences in weight or ppFEV1change or with future antimicrobial treatment hazard.
CONCLUSIONS.
We did not observe superior responses for AST-defined complete Pa coverage treatments versus lesser coverage treatments, suggesting that AST may be of little utility in choosing antimicrobials for CF PEx treatment.
Keywords: cystic fibrosis, pulmonary exacerbation, susceptibility testing, Pseudomonas aeruginosa
INTRODUCTION
People with CF are prone to airway bacterial infection and experience pulmonary exacerbations (PEx), periods of acute increases in respiratory signs and symptoms often coupled with an acute lung function drop, throughout their lives.[1,2] PEx are considered important clinical events;[1–3] guidelines recommend treatment with increased airway clearance, antimicrobial therapy, and nutritional and psychosocial support,[4–5] with antimicrobials chosen by in vitro antimicrobial susceptibility testing (AST) of patient bacterial isolates.[4] The utility of AST for this task has been questioned in a recent systematic literature review[6] and a recent Delphi analysis among CF experts showed little consensus with respect to the utility of AST in choosing antimicrobial treatments.[7]
We studied PEx antimicrobial treatment outcomes from 1999 through 2018 among patients followed at the LeRoy Matthews CF Care Center in Cleveland, OH for associations with their Pseudomonas aeruginosa (Pa) isolate AST results available at treatment start. Antipseudomonal treatment outcomes where all Pa isolates (complete Pa coverage), no isolates (no coverage), and where some isolates (incomplete coverage) were susceptible to at least one administered antipseudomonal were compared. Treatment responses were assessed as lung function and weight changes from a 6-month pretreatment baseline average to a 3-month post-treatment maximum and as future antimicrobial treatment hazard.
METHODS
Antimicrobial treatments from 1999 through 2018 identified by clinicians as for PEx were grouped into “treatment events” of ≥3 days which could include multiple antimicrobial agents delivered by any route and which were considered completed when no antimicrobials were administered for 5 consecutive days; treatments initiated ≥6 days after the end of a treatment event were considered part of a new treatment event. To be included in analyses, treatment events required Pa isolation from the patient’s most recent respiratory culture, a Pa isolate AST conducted ≥6 days but ≤365 days prior to treatment start, ≥1 weight and height measure ≤6 months before treatment, ≥1 patient clinic visit >5 days after treatment end, and no history of lung transplant until >3 months after treatment end. In addition, included treatments had to have ≥1 ppFEV1 (percent predicted forced expiratory volume in 1 sec) measure both >1 year before and ≤6 months before treatment. The earlier ppFEV1 requirement ensured availability of prior-year PEx treatment history (an important covariate for predicting future PEx hazard [8]), the latter provided baseline ppFEV1 (as the average of all measures recorded ≤6 months prior to treatment).
Lung function response analyses included treatment events with ≥1 ppFEV1 measure ≤3 months after treatment stop (follow-up); weight responses were studied among events that had ≥1 weight measure at follow-up. When available, ppFEV1 and weight changes from baseline to treatment start were estimated using measures recorded <4 days before to <2 days after treatment start. Time-to-next treatment and treatment hazards were determined using the first treatment event in the observation period for each patient. ppFEV1 values were generated with Global Lung Initiative normative equations.[9]
Pa “coverage” was defined by the patient’s most recent Pa isolate AST results. Coverage was “complete” if ≥1 antipseudomonal agent was administered to which every Pa isolate was susceptible; susceptibility of all isolates to a single administered agent was not required. Antimicrobial treatments to which no Pa isolates were susceptible, or when no antipseudomonal agents were administered, were considered to provide “no” Pa coverage. “Incomplete” Pa coverage resulted when at least one Pa isolate was susceptible to treatment and at least one other was not. When treatment included antipseudomonals which lacked AST results (i.e., the antipseudomonal had not been tested or was administered by inhalation), Pa coverage was “indeterminant”. A hierarchy of complete > indeterminant > incomplete > none was established: when (additional) untested or inhaled antipseudomonal agents were included it was possible to have complete coverage, but not incomplete or no coverage. Treatment events for which one Pa isolate was susceptible and susceptibility for a second isolate was indeterminant were defined as indeterminant.
Lung function and weight recovery differences between Pa coverage groups were assessed as mean changes from baseline (average of measures ≤6 months before treatment) to follow-up (maximum of measures <3 months after cessation). Weight and ppFEV1 changes from baseline to follow-up were estimated on absolute scales with generalized estimating equations (GEE) to account for repeated antimicrobial treatment events in individuals. Weight and ppFEV1 changes from baseline to treatment initiation (<4 days prior to <2 days after) were also determined by GEE. Weight and ppFEV1 change differences between Pa coverage categories were assessed in unadjusted models and models adjusted for elapsed time from AST to treatment initiation, treatment duration, intravenous (IV) antipseudomonal treatment, combined beta-lactam and aminoglycoside antimicrobial administration, administration of antifungal therapies, sex, age, baseline ppFEV1, baseline body mass index (BMI), and number of prior-year PEx treatment events. Sensitivity analyses were conducted for events a) including IV antipseudomonal antimicrobial administration, b) occurring in the first half versus the second half of the observation period, and c) including internal gaps of <12 days without antimicrobial treatment in the treatment event definition. Additionally, unadjusted and adjusted weight responses were studied in the adult cohort (age ≥18 years). Adjustment covariates were selected a priori and overall tests of each factor were conducted with the Score Test and two-sided p-values reported. 95% confidence intervals (CI) of each estimate relative to the referent group are reported from the GEE models (SAS version 9.4 Cary, NC).
Future antimicrobial treatment hazard was assessed by log-rank and by Cox proportional hazards modeling and odds of retreatment within 30 days post-treatment were assessed by logistic regression of the first antimicrobial treatment event per patient, again unadjusted or adjusted for covariates as above (MedCalc Statistical Software version 19.0.7, Ostend, Belgium). Area-proportional graphing of antimicrobial administration routes was performed with eulerAPE [10] Data analyses were approved by the University Hospitals Institutional Review Board (UH IRB; # 20190855), with data collected between 1998 and 2018 from patient encounters. Patients had previously provided consent for data collection (UH IRB # 11-67-200).
RESULTS
From January 1999 through December 2018, 572 people with CF followed at the LeRoy Matthews CF Care Center received >33,000 antimicrobial treatments for PEx comprising 11,762 treatment events and AST was performed on >22,000 Pa clinical isolates from >600 individuals (Figure 1). Numbers of Pa AST results collected within a year prior to treatment events among .Pa-positive patients and numbers of treatment events meeting inclusion criteria for analyses are provided in Figure 1.
Figure 1.
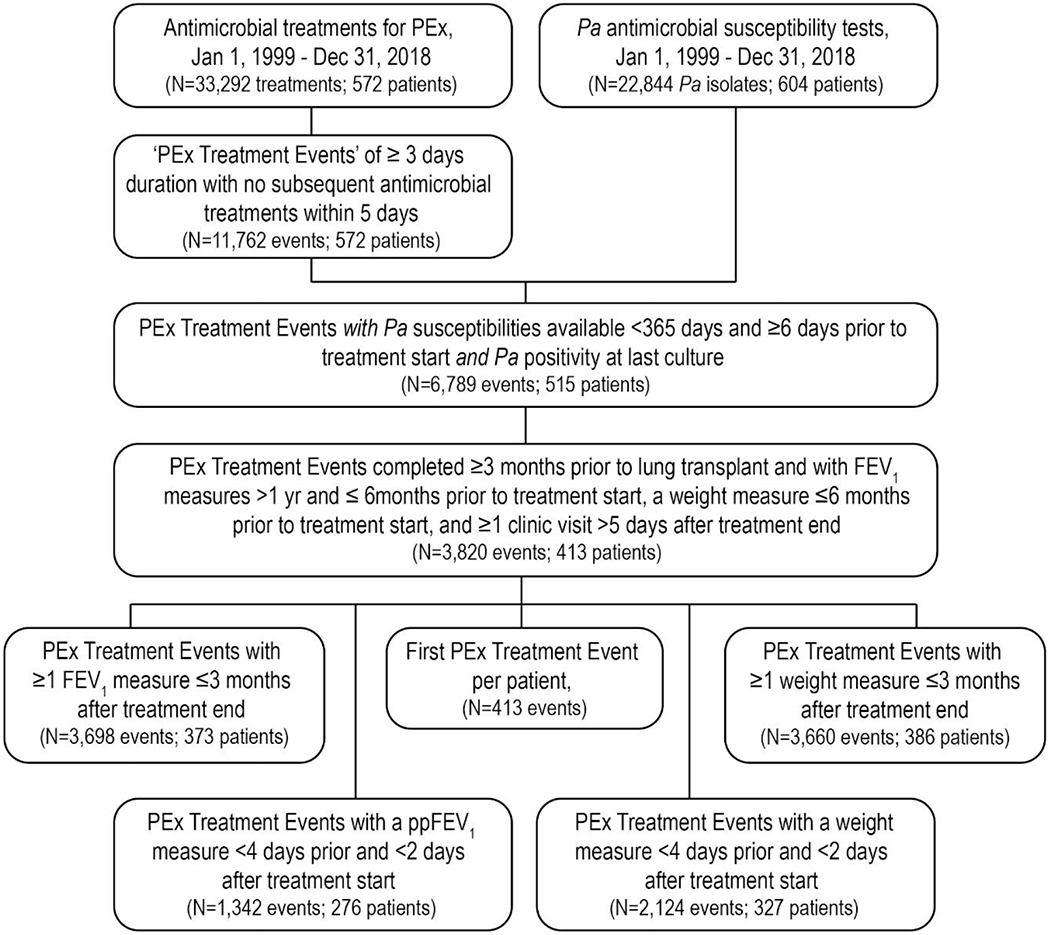
STROBE Diagram of antimicrobial treatment event inclusion
Adults experienced 70% of qualifying treatment events, with baseline average ppFEV1 and BMI of 61.3 (SD=23.2) and 20.3 (3.8) kg/m2, respectively (Table 1). More than 6 in 10 treatment events included oral antipseudomonal agents, almost half included IV antipseudomonal treatments, about 3 in 10 included inhaled antipseudomonals, and less than 1 in 25 did not include any antipseudomonal agents (Table 1 and Supplemental Figure S1). Treatment event durations ranged broadly, with a median of 22 days [interquartile range 18 to 34 days]; the median IV antipseudomonal treatment span was 18 days [14 to 26 days] (Table 1). Nearly two-thirds of events had complete Pa coverage, less than 1 in 10 had no coverage, less than 1 in 50 had incomplete coverage, and about a quarter had indeterminant coverage (Table 1 and Supplemental Table S2).
Table 1.
Pulmonary exacerbation treatment event demographics by Pa coverage category
| Pa Coverage | Complete | None | Incomplete | Indeterminant | All |
|---|---|---|---|---|---|
| Pulmonary exacerbation treatment events | |||||
| N (%)a | 2390 (62.6%) | 340 (8.9%) | 90 (2.4%) | 1000 (26.2%) | 3820 |
| Males, N (%) | 1163 (48.7%) | 138 (40.6%) | 52 (57.8%) | 420 (42.0%) | 1773 (46.4%) |
| Age at exacerbation, years | |||||
| Mean (SD) | 25.6 (11.8) | 27.9 (13.2) | 27.8 (11.5) | 24.7 (12.2) | 25.7 (12.0) |
| Median (IQR) | 23.5 (17.1, 32.3) | 25.1 (17.0, 37.0) | 24.7 (18.4, 36.9) | 22 (15.8, 31.1) | 23.4 (16.8, 32.6) |
| <18 years, N (%) | 710 (29.7%) | 96 (28.2%) | 20 (22.2%) | 332 (33.2%) | 1158 (30.3%) |
| 18 to 24 years, N (%) | 591 (24.7%) | 69 (20.3%) | 27 (30.0%) | 253 (25.3%) | 940 (24.6%) |
| 25 to 39 years, N (%) | 784 (32.8%) | 103 (30.3%) | 26 (28.9%) | 289 (28.9%) | 1202 (31.5%) |
| 40+ years, N (%) | 305 (12.8%) | 72 (21.2%) | 17 (18.9%) | 126 (12.6%) | 520 (13.6%) |
| Average (6-month) pre-treatment ppFEV1 | |||||
| Mean (SD) | 58.1 (23.2) | 60.7 (20.8) | 58.5 (20.9) | 69.2 (22.4) | 61.3 (23.2) |
| Median (IQR) | 56.4 (39.2, 75.3) | 60.6 (44.2, 76.9) | 58.3 (42.7, 70.4) | 71.1 (51.9, 87) | 62.3 (42.5, 78.7) |
| 100+, N (%) | 103 (4.3%) | 9 (2.6%) | 5 (5.6%) | 70 (7.0%) | 187 (4.9%) |
| 70 to <100, N (%) | 651 (27.2%) | 112 (32.9%) | 18 (20.0%) | 446 (44.6%) | 1227 (32.1%) |
| 40 to <70, N (%) | 1022 (42.8%) | 152 (44.7%) | 48 (53.3%) | 371 (37.1%) | 1593 (41.7%) |
| <40, N (%) | 614 (25.7%) | 67 (19.7%) | 19 (21.1%) | 113 (11.3%) | 813 (21.3%) |
| Average (6-month) pre-treatment BMI, kg/m2 | |||||
| Mean (SD) | 20.1 (3.7) | 20.5 (3.5) | 21.4 (3.3) | 20.7 (3.9) | 20.3 (3.8) |
| Median (IQR) | 19.7 (17.4, 21.9) | 20.2 (18.2, 22.1) | 21.3 (19.4, 23) | 20.2 (18.2, 22.5) | 19.9 (17.8, 22.1) |
| Antimicrobial treatment duration, days | |||||
| Mean (SD) | 34.6 (30.3) | 19.7 (12) | 21.4 (12.8) | 28 (16.1) | 31.3 (26.1) |
| Median (IQR) | 24 (17, 40) | 21 (14, 21) | 21 (14, 21) | 23 (21, 30) | 22 (18, 34) |
| 3 to 8 days, N (%) | 37 (1.5%) | 47 (13.8%) | 6 (6.7%) | 18 (1.8%) | 108 (2.8%) |
| 9 to 16 days, N (%) | 521 (21.8%) | 79 (23.2%) | 22 (24.4%) | 137 (13.7%) | 759 (19.9%) |
| 17 to 21 days, N (%) | 489 (20.5%) | 130 (38.2%) | 43 (47.8%) | 312 (31.2%) | 974 (25.5%) |
| 22 to 42 days, N (%) | 804 (33.6%) | 67 (19.7%) | 14 (15.6%) | 416 (41.6%) | 1301 (34.1%) |
| 43+ days, N (%) | 539 (22.6%) | 17 (5.0%) | 5 (5.6%) | 117 (11.7%) | 678 (17.7%) |
| Antipseudomonal antimicrobial administration routeb | |||||
| Oral, N (%) | 1314 (55.0%) | 183 (53.8%) | 69 (76.7%) | 783 (78.3%) | 2349 (61.5%) |
| Inhaled, N (%) | 767 (32.1%) | 0 (0.0%) | 0 (0.0%) | 444 (44.4%) | 1211 (31.7%) |
| Intravenous, N (%) | 1757 (73.5%) | 18 (5.3%) | 22 (24.4%) | 84 (8.4%) | 1881 (49.2%) |
| Mean span, days (SD) | 25.1 (24.1) | 16.6 (5.8) | 15.1 (7.2) | 21.4 (17.1) | 24.8 (23.6) |
| Median span, days (IQR) | 19 (14, 27) | 16 (13, 18) | 13.5 (12, 20) | 17 (12, 23) | 18 (14, 26) |
| Nonec, N (%) | - | 141 (41.5%) | - | - | 141 (3.7%) |
| Elapsed time from treatment end to maximum (3-month) post-treatment ppFEV1 | |||||
| N (%) | 2315 (96.9%) | 326 (95.9%) | 82 (91.1%) | 970 (97.0%) | 3693 (96.7%) |
| Mean (SD) | 60.5 (23) | 58.1 (23.6) | 57.8 (21.8) | 58.2 (23.6) | 59.7 (23.2) |
| Median (IQR) | 65 (44, 80) | 62 (42, 79) | 61 (42, 76) | 63 (42, 78) | 64 (43, 79) |
Percentages are row totals relative to all treatment events studied
Treatment events could include more than one route of antimicrobial administration
Treatment events consisting entirely of antimicrobials considered inactive against P. aeruginosa
SD = standard deviation, IQR = interquartile range, ppFEV1=percent predicted forced expiratory volume in 1 sec. Percentages are column totals unless otherwise noted
Lung Function and Weight Change from Baseline to Follow-up
Mean ppFEV1 difference from baseline to follow-up was +1.60 [95% CI 1.29, 1.90] (Figure 2A). No differences were observed between mean ppFEV1 changes for treatment events with complete Pa coverage by AST (N=2320; mean=1.58) and those with either no (N=328; mean=1.91; P=.46), incomplete (N=82; mean=2.25; P=.42), or indeterminant (N=968; mean=1.47; P=.75) Pa coverage (Table 2). Adjustment for elapsed time from AST test to treatment initiation, antimicrobial treatment duration, IV antipseudomonal administration, combination β-lactam/aminoglycoside administration, antifungal administration, sex, age, baseline ppFEV1, baseline BMI, and number of prior-year PEx did not materially affect these observations (Figure 3, Table 2, and Supplemental Figure S2). Among 1825 events that included IV antipseudomonal treatment, mean ppFEV1 change from baseline to follow-up was +1.80 [1.33, 2.26] (Figure 2A); no significant differences in unadjusted or adjusted mean ppFEV1 change were observed as a function of Pa coverage among this subset of events (data not shown). In a sensitivity analysis where gaps of <12 days without antimicrobial treatment were included in the treatment event definition, mean ppFEV1 difference from baseline to follow-up was slightly less, +1.55 [95% CI 1.23, 1.86], but again no ppFEV1 response differences were observed between Pa coverage groups in unadjusted or adjusted analyses. No ppFEV1 response differences were observed between treatment events occurring in the first half versus the second half of the observation period (data not shown).
Figure 2. Mean ppFEV1 and weight changes from baseline to treatment start and follow-up.
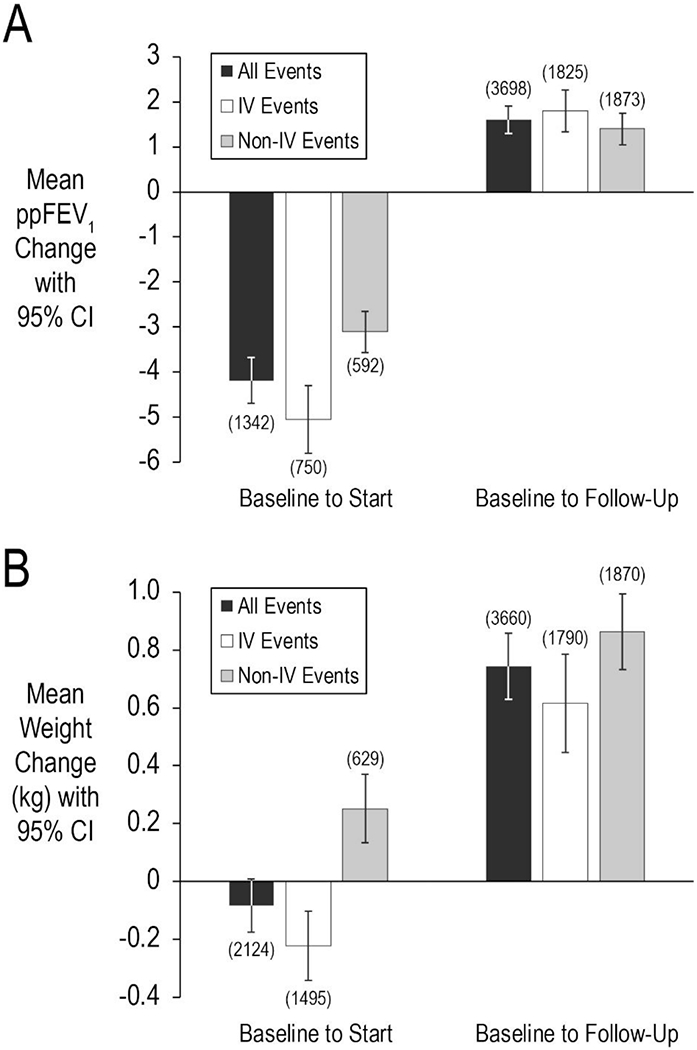
Panel A, mean ppFEV1 changes from baseline to treatment start (left) and follow-up (right). Panel B, mean weight changes (in kg) from baseline to treatment start (left) and follow-up (right). Means were derived from General Estimating Equations (GEE) to account for repeated measures. Means for all treatment events are shown in black, events including IV antipseudomonal treatments are shown in white, and events without IV treatments are in shown gray. Sample sizes are shown in parentheses. Bars represent 95% confidence intervals (CI).
Table 2.
Mean ppFEV1 and weight changes from baseline to follow-up by Pa coverage category
| Complete Pa Coverage | No Pa Coverage | Incomplete Pa Coverage | Indeterminant Pa Coverage | |
|---|---|---|---|---|
| Unadjusted Mean ppFEV1 Change [95%CI] | ||||
| Change from Baseline to Follow-Up | 1.58 [1.19, 1.97] | 1.91 [1.10, 2.72] | 2.25 [0.66, 3.85] | 1.47 [0.94, 2.00] |
| Difference from Complete Pa Coverage | - | 0.33 [− 0.55, 1.21] | 0.68 [−0.96, 2.31] | −0.11 [−0.75, 0.54] |
| Adjusted Mean ppFEV1 Change [95%CI]* | ||||
| Change from Baseline to Follow-Up | 1.13 [0.12, 2.13] | 1.38 [0.07, 2.69] | 1.75 [−0.10, 3.60] | 1.51 [0.30, 2.72] |
| Difference from Complete Pa Coverage | - | 0.25 [− 0.70 1.21] | 0.62 [−0.99, 2.24] | 0.26 [−0.69, 1.21] |
| P-value (change difference) | - | 0.60 | 0.45 | 0.29 |
| Unadjusted Mean Weight Change, kg [95%CI] | ||||
| Change from Baseline to Follow-Up | 0.69 [0.56, 0.83] | 0.90 [0.64, 0.99] | 0.78 [0.24, 1.31] | 0.80 [0.62, 0.99] |
| Difference from Complete Pa Coverage | 0.21 [−0.09, 0.50] | 0.08 [−0.47, 0.64] | 0.11 [−0.11, 0.32] | |
| Adjusted Mean Weight Change, kg [95%CI]* | ||||
| Change from Baseline to Follow-Up | 0.55 [0.26, 0.84] | 0.79 [0.47, 1.10] | 0.79 [0.18, 1.40] | 0.70 [0.38, 1.03] |
| Difference from Complete Pa Coverage | - | 0.24 [−0.07, 0.56] | 0.24 [−0.33, 0.82] | 0.16 [−0.06, 0.38] |
| P-value (change difference) | - | 0.14 | 0.40 | 0.16 |
Means and 95% confidence intervals (CI) generated with General Estimating Equations to account for repeated measures
Adjusted for prior-year pulmonary exacerbation treatment number, baseline ppFEV1, age, sex, treatment duration, IV antipseudomonal administration, beta-lactam/aminoglycoside
co-administration, antifungal administration, time from AST test to treatment start, and BMI.
Figure 3. Baseline to follow-up changes in ppFEV1 and weight by Pa coverage category.
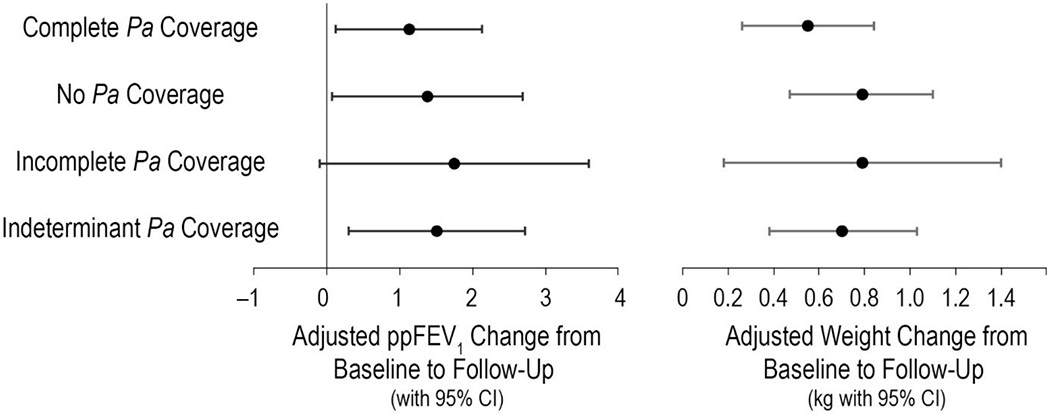
Left panel, mean ppFEV1 changes by coverage category. Right panel, mean weight changes in kg by coverage category. Means were derived from general estimating equations (GEE) to account for repeated measures, with adjustment for prior-year PEx treatment number, baseline ppFEV1, age, sex, treatment duration, IV antipseudomonal administration, beta-lactam/aminoglycoside co-administration, antifungal administration, time from AST test to treatment start, and BMI. Bars are 95% confidence intervals (CI).
The mean weight difference from baseline to follow-up was +0.74 kg [0.63, 0.86] (Figure 2B). As with lung function, no differences were observed between mean weight changes for events with complete Pa coverage (mean=0.69 kg) and those with either no (mean=0.90 kg; P=. 17), incomplete (mean=0.77 kg; P=.77), or indeterminant (mean=0.80 kg; P=.30) Pa coverage; adjustment for covariates noted above did not materially affect these observations (Figure 3, Table 2, and Supplemental Figure S2). Mean weight change among events that included IV antipseudomonal administration was +0.62 kg [0.45, 0.79] (Figure 2B), with no significant differences observed in unadjusted or adjusted mean weight change as a function of Pa coverage (data not shown). As with ppFEV1 response, modification of the treatment event definition to include gaps without treatment of <12 days resulted in a slightly lower weight response, +0.73 kg [0.61, 0.85], but no response differences were observed between Pa coverage groups in unadjusted or adjusted analyses. Mean weight response among adults (age ≥ 18 years) was + 0.34 kg [0.22, 0.46], with no differences in response observed by Pa coverage group after adjustment. No weight response differences were observed between treatment events occurring in the first half versus the second half of the observation period (data not shown).
Among treatment events in which ppFEV1 and weight measures were available around the time of treatment start, mean GEE estimates of ppFEV1 change from baseline to treatment start were −4.2 [−4.7, −3.7] for ppFEV1 (N=1342) and −0.08 kg [−0.17, 0.01] for weight (N=2124) (Figures 2A and 2B). Changes in ppFEV1 and weight from baseline to treatment start were −5.0 [−5.8, −4.3] and −0.22 kg [−0.34, −0.10], respectively, among events treated with IV antipseudomonal antimicrobials (Figures 2A and 2B).
Time to Next Antimicrobial Treatment Event
Among the 413 patients included in time-to-next antimicrobial treatment analyses, 401 (97.1%) experienced a second antimicrobial treatment event during the observation period, with a median time to next treatment of 108 days [95% CI 95, 127] (Figure 4). No statistically significant relationship was observed between Pa coverage and time to next antimicrobial treatment (Log rank P=.7713). Both unadjusted (not shown) and adjusted (Supplemental Figure S3) Cox proportional hazard models for future antimicrobial treatment hazard suggested no significant associations with Pa coverage category. Of 413 patients, 83.0% had a subsequent IV treatment event during the observation period, with median time to IV treatment of 407 days [95% CI 327, 560] (Supplemental Figure S4A). Median times to IV treatment were more variable across Pa coverage groups (Log rank P=.0315), with no suggestion that complete Pa coverage was associated with an increased median time to IV treatment (Supplemental Figure S4B).
Figure 4. Time to next antimicrobial treatment by Pa coverage category.
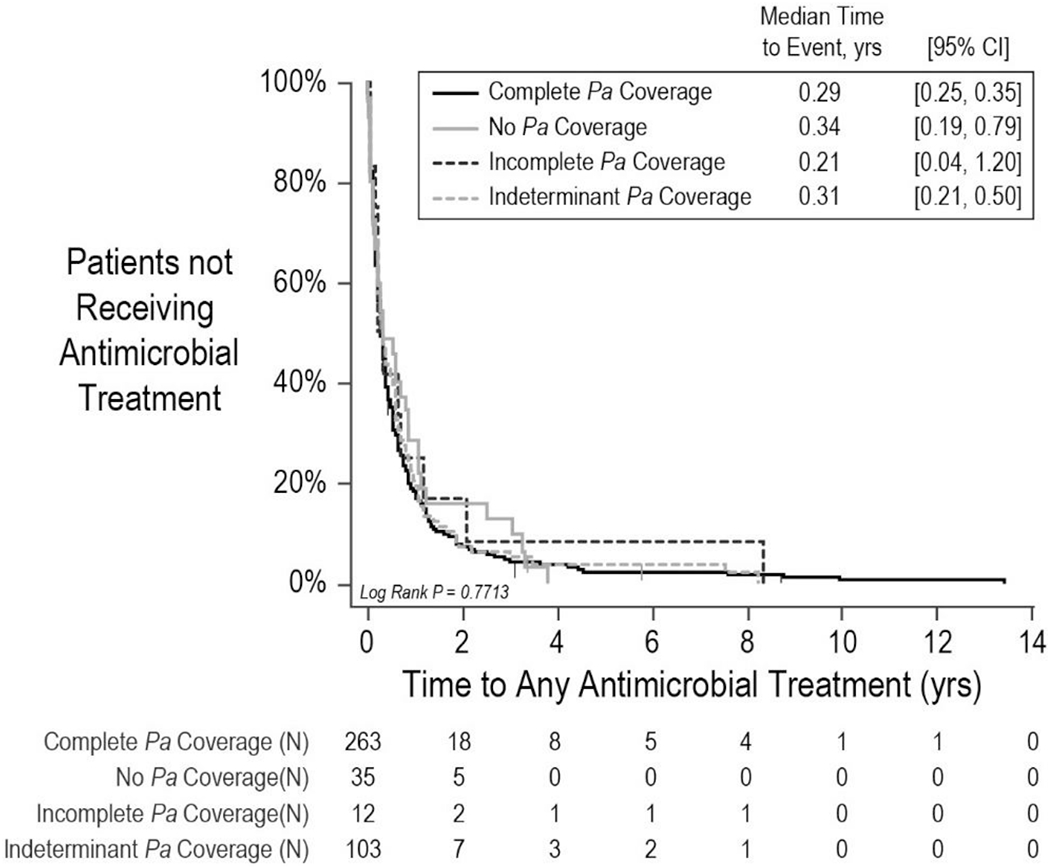
Solid black line, patients with complete Pa coverage. Solid gray line, patients with no Pa coverage. Dashed black line, patients with incomplete Pa coverage. Dashed gray line, patients with indeterminant Pa coverage. Numbers of patients at risk are shown below. CI, confidence interval.
Seventy-one of 413 patients (17.2%) were retreated with antimicrobials for PEx within 30 days of the end of their first treatment event, but only 36 (6.3%) were retreated with IV antimicrobials within the same period. No meaningful differences were observed in odds of retreatment with IV antimicrobials within 30 days as a function of Pa treatment coverage by unadjusted (not shown) or adjusted (Supplemental Figure S3) logistic regression.
DISCUSSION
Despite the clinical importance of PEx,[2] objective data with respect to their diagnosis [1,3] and optimal treatment [6] are limited, resulting in tremendous variation of care between clinicians [11] and across care facilities.[12] Although use of AST to guide selection of PEx antimicrobial treatment is common [7] and found in CF treatment guidelines,[4] the approach has never been prospectively shown to result in superior outcomes to other possible antimicrobial selection methods in CF. On the contrary, a recent systematic literature review concluded that there was “scant evidence that AST is predictive of CF antimicrobial treatment response” and suggested a need for “comprehensive retrospective analyses of relationships between bacterial isolate susceptibility, antimicrobial treatment, and treatment response using care center records” to improve the evidence base.[6] Further, a Delphi survey of CF experts revealed lack of consensus as to the role of AST in choosing antimicrobials for PEx treatment.[7]
If antimicrobial PEx treatments were improved by AST-based antimicrobial selection, then AST-defined differences in antimicrobial coverage should be associated with treatment response differences. Our analyses could not detect antipseudomonal coverage-associated differences in ppFEV1 or weight changes across PEx, or differences in future antimicrobial treatment hazard.
There are distinctions between our current analyses, for which the unit of analysis is PEx antimicrobial treatment independent of delivery route, and our previous analyses which have focused on IV PEx treatments.[8] Less than half of PEx treatment events reported here (49.2%) included IV antimicrobial administration, a finding similar to a previous analysis of >45,000 PEx treatment events among >13,000 North American CF patients from 2003 through 2005.[13] In that same analysis, nearly a quarter of PEx treatments included inhaled antimicrobials, as did about 3 in 10 events in our analysis. Inclusion of non-IV treatment events in time-to-event analyses increased the number of observed events per unit time compared to analyses of only those events in which IV antimicrobials were administered, resulting in a relatively shorter median time to next PEx treatment: 108 days versus 407 days (Supplemental Figure S4A). In addition, PEx treatment events that ultimately include IV treatments commonly begin with oral and/or inhaled treatments,[13] with a result that time to a next antimicrobial treatment independent of route can be less than the time to a next IV treatment for the same next treatment event.
To our knowledge, this is the first PEx treatment response analysis to include weight change across PEx treatments, despite weight loss being a common diagnostic criterion across published PEx definitions.[3] Advantages of weight change over ppFEV1 change as a treatment response measure include lower variance and less chance of underlying disease-associated weight decline from baseline to follow-up. However, growth-associated weight gain in children and adolescents from baseline through follow-up introduces a complication with this approach, and it seems likely that nutritional support is more important than antimicrobial treatment for this outcome. Our analyses also assessed associations of β-lactam and aminoglycoside co-administration and antifungal co-administration on treatment outcomes, with neither found to be associated with differences in treatment response (Supplemental Figures S2 and S3). The former observation is consistent with a Cochrane review of the subject [14] but in contradiction to common P. aeruginosa treatment practice in the CF community. We have separated outcomes for treatments with “indeterminant” Pa coverage, in which at least one antimicrobial was not included in AST or for which there were no established AST interpretive criteria (e.g., inhaled antimicrobials). It appears that inhaled antimicrobials are relatively commonly used in CF PEx treatment, being included in about a quarter of treatment events in this study and about the same proportion in the multicenter PEx treatment analysis noted above.[13] Outcomes for these indeterminant Pa coverage events are included for completeness but are not necessarily informative with respect to AST testing utility.
We (arbitrarily) established a period of 5 days with no antimicrobial treatment as defining the end of a treatment event, reasoning that a gap of 5 days in treatment is longer than could be explained by “administrative” gaps due to problems with prescription access or hospital bed availability. We have avoided opining as to whether a subsequent antimicrobial treatment starting at least 6 days after the end of treatment event represents treatment of a “new” PEx or inadequate treatment of a previous event, although retreatment within a few weeks of treatment cessation seems consistent with treatment failure. Interestingly, there was no evidence that the probability of antimicrobial retreatment within 30 days differed across Pa coverage groups. Sensitivity analyses in which we expanded the allowable period without antimicrobial treatment during a given event to 11 days had no effect on our observation of lack of association between Pa coverage and treatment response assessed as ppFEV1 change, weight change, or risk of retreatment.
There are important limitations to these analyses. First, we have limited our analyses to AST results for Pa isolates in a population co-infected with multiple microbial opportunists (Supplemental Table S3), where other organisms and treatments targeted at them could have confounded results. However, CF airway infection complexity is the rule, not the exception, and choosing antimicrobials to treat Pa in complex CF infections is a routine task. Therefore, we believe that studying Pa AST utility in this context is valid. There may be other aspects of airway infection such as relative or absolute Pa abundance in the airway that may influence antimicrobial treatment response that were not routinely captured and were unavailable for analyses. Further, we have not demonstrated that responses among patients receiving antimicrobial treatments for which their Pa isolates were not covered were equivalent to responses among patients with complete Pa coverage. Rather, we have been unable to demonstrate a difference in responses between these groups. Unfortunately, retrospective analyses such as these are prone to indication bias, as individuals were not randomized to be treated with complete, incomplete, or no Pa coverage at treatment initiation. In addition, there is no assurance that individuals did not engage in antimicrobial self-medication during the study period that was not recorded in their medical records. Although we have adjusted models for demographic and treatment covariates known or suspected to affect the outcomes we have studied, we lack the assurance that we have accounted for unknown risk factors that is afforded by random allocation. As might be expected, events treated with IV antipseudomonal antimicrobials appeared to present with relatively greater clinical severity based on mean changes from baseline in ppFEV1 and weight. However, a sensitivity analysis limited to events treated with IV antipseudomonal antimicrobials yielded the same results: no discemable differences in lung function or weight responses were observed by Pa coverage category. Although “return to ppFEV1 baseline” analyses of PEx outcomes are common in CF observational studies, there are statistical complications relating to the inherent variability of ppFEV1, regression to the mean, and underlying disease progression resulting in irreversible ppFEV1 loss that may be independent of PEx and that can complicate interpretation.[15] Use of a 6-month, as opposed to a 1-year baseline period, definition of the baseline as the average ppFEV1 observed as opposed to the maximum during the period (as is suggested in guidelines [4]), and adjustment for known confounders such as age, sex, and lung disease stage are all intended to mitigate these complications, but the analytical approach remains imperfect.[15] Although weight change may be a less variable measure than ppFEV1 for assessing treatment response, baseline to follow-up increases in weight in growing children complicate this analysis, likely accounting for the observed mean increase in weight from baseline to treatment start among treatments without IV antipseudomonal administration. Unfortunately, standardized symptom scores were not available to include as a treatment outcome measure, an important shortcoming given that symptom resolution is considered more important than lung function recovery to people with CF experiencing exacerbations [16] and may not parallel either lung function response, weight response, or future PEx hazard. Finally, this relatively large data set covering 20 years of experience remains a single-center analysis and associations between AST test results and treatment outcomes, as with diagnostic criteria and PEx treatment approaches, may differ from other CF care centers. However, as noted above, proportions of PEx treatments administered as oral, inhaled, IV, and combinations thereof reported here are similar to those of a previous multi-center analysis of >45,000 PEx treatment events,[13] and results observed during the first half of the observation period did not differ from those observed during the subsequent period, (data not shown) suggesting that changes in CF care over the period have not materially affected associations between Pa isolate AST and PEx treatment response.
Despite lack of evidence that AST is relevant to CF airway Pa infection treatment response [6] and suggestions as to why it may not be relevant,[17] AST remains a ubiquitous tool in the CF armamentarium. The question of whether AST improves PEx treatment outcomes is an important one as AST is resource-intensive and the availability of new test results during PEx treatment may drive antimicrobial regimen changes in the absence of clinical need.[18] Unfortunately, most prior analyses assessing possible relationships between AST and antimicrobial treatment response have been retrospective, with many derived from single-center experiences.[6] Although definitive validation of AST use in PEx treatment would be in the best interest of all stakeholders, controlled randomized prospective trials testing AST and treatment response are unlikely to be embraced as ethical by the community, [19] leaving us limited to retrospective observational studies of the type reported here to infer the clinical value of AST for antimicrobial selection for CF PEx treatment.
Supplementary Material
Highlights.
P. aeruginosa susceptibility and CF exacerbation treatment response was studied
Lung function response did not differ by P. aeruginosa antimicrobial coverage
Weight response did not differ by P. aeruginosa antimicrobial coverage
Future risk of exacerbation was not affected by P. aeruginosa antimicrobial coverage
Antimicrobial susceptibility test (AST) results did not predict treatment response
ACKNOWLEDGEMENTS
This project was supported by the Cystic Fibrosis Foundation (KONSTA09Y0) and the National Institutes of Health through the Clinical and Translational Science Collaborative of Cleveland (UL1TR002548) and P30 DK027651
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
None
References
- [1].Ratjen F, Döring G. Cystic fibrosis. Lancet. 2003. February 22;361(9358):681–9. Review. [DOI] [PubMed] [Google Scholar]
- [2].Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62(4): 360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Flume PA, VanDevanter DR. Exacerbations. In: Hodson and Geddes’ Cystic Fibrosis, 4th Edition. Bush A, Bilton D, Hodson M, eds. CRC Press, Taylor & Francis, Abingdon United Kingdom. July 2015. [Google Scholar]
- [4].Treatment of pulmonary exacerbation of cystic fibrosis. Clinical Practice Guidelines for Cystic Fibrosis. Bethesda, MD: Cystic Fibrosis Foundation; 1997. [Google Scholar]
- [5].Flume PA, Mogayzel PJ Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med 2009; 180(9): 802–8. [DOI] [PubMed] [Google Scholar]
- [6].Somayaji R, Parkins MD, Shah A, Martiniano SL, Tunney MT, Kahle JS, Waters VJ, Elborn JS, Bell SC, Flume PA, VanDevanter DR, on behalf of the Antimicrobial Resistance in Cystic Fibrosis International Working Group. Antimicrobial susceptibility testing (AST) and associated clinical outcomes in individuals with cystic fibrosis: A systematic review. J Cyst Fibros. 2019. March;18(2):236–243. [DOI] [PubMed] [Google Scholar]
- [7].Zemanick E, Burgel PR, Taccetti G, Holmes A, Ratjen F, Byrnes CA, Waters VJ, Bell SC, VanDevanter DR, Stuart Elborn J, Flume PA; Antimicrobial Resistance International Working Group in Cystic Fibrosis. Antimicrobial resistance in cystic fibrosis: A Delphi approach to defining best practice. J Cyst Fibros. 2019. October 31. pii: S1569-1993(19)30919-1. doi: 10.1016/j.jcf.2019.10.006. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [8].VanDevanter DR, Morris NJ, Konstan MW. IV-treated pulmonary exacerbations in the prior year: An important independent risk factor for future pulmonary exacerbation in cystic fibrosis. J Cyst Fibros. 2016. May;15(3):372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J; ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012. December;40(6): 1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One. 2014. July 17;9(7):e101717.[10]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kraynack NC, Gothard MD, Falletta LM, McBride JT. Approach to treating cystic fibrosis pulmonary exacerbations varies widely across US CF care centers. Pediatr Pulmonol. 2011. September;46(9):870–81. [DOI] [PubMed] [Google Scholar]
- [12].Schechter MS, VanDevanter DR, Pasta DJ, Short SA, Morgan WJ, Konstan MW; Scientific Advisory Group and the Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Treatment setting and outcomes of cystic fibrosis pulmonary exacerbations. Ann Am Thorac Soc. 2018. February; 15 (2): 225–233. [DOI] [PubMed] [Google Scholar]
- [13].Wagener JS, VanDevanter DR, Pasta DJ, Regelmann W, Morgan WJ, Konstan MW. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2013;48(7):666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Elphick HE, Scott A. Single versus combination intravenous antipseudomonal antibiotic therapy for people with cystic fibrosis. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No.: CD002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wagener JS, VanDevanter DR, Konstan MW, Pasta DJ, Millar SJ, Morgan WJ. Lung function changes before and after pulmonary exacerbation antimicrobial treatment in cystic fibrosis. Pediatr Pulmonol. 2020;55(3):828–834. [DOI] [PubMed] [Google Scholar]
- [16].Heltshe SL, West NE, VanDevanter DR, Sanders DB, Beckett VV, Flume PA, Goss CH; STOP Study Group. Study design considerations for the Standardized Treatment of Pulmonary Exacerbations 2 (STOP2): A trial to compare intravenous antibiotic treatment durations in CF. Contemp Clin Trials. 2017. November 21;64:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Waters VJ, Kidd TJ, Canton R, Ekkelenkamp MB, Johansen HK, LiPuma JJ, Bell SC, Elbom JS, Flume PA, VanDevanter DR, Gilligan P; Antimicrobial Resistance International Working Group in Cystic Fibrosis. Reconciling antimicrobial susceptibility testing and clinical response in antimicrobial treatment of chronic cystic fibrosis lung infections. Clin Infect Dis. 2019. October 30;69(10):1812–1816. [DOI] [PubMed] [Google Scholar]
- [18].Cogen JD, Whitlock KB, Gibson RL, Hoffman LR, VanDevanter DR. The use of antimicrobial susceptibility testing in pediatric cystic fibrosis pulmonary exacerbations. J Cyst Fibros. 2019. November;18(6):851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Szczesniak RD, Cogen JD, Rosenfeld M. Associating antimicrobial susceptibility testing with clinical outcomes in cystic fibrosis: More rigor and less frequency? J Cyst Fibros. 2019. March;18(2): 159–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


