Abstract
Rotator cuff supraspinatus tendon injuries are common with high rates of anatomic failure after surgical repair. The purpose of the study was to define clinically relevant features of a mouse model of supraspinatus tendon injury to determine painful, functional, and structural outcomes; we further investigated two cell populations mediating healing using genetic lineage tracing after full detachment and repair of the supraspinatus tendon in mice. Pain was assessed using the mouse grimace scale and function by gait analysis and tensile testing. Histological and microCT analyses were used to determine enthesis/tendon and bone structure, respectively. Lineage tracing was carried out using inducible Cre lines for ScxCreERT2 (tendon cells) and αSMACreERT2 (myofibroblasts and mesenchymal progenitors). Mice only expressed pain transiently after surgery despite long-term impairment of functional and structural properties. Gait, tensile mechanical properties, and bone properties were significantly reduced after injury and repair. Lineage tracing showed relatively few Scxlin tendon cells while αSMAlin cells contributed strongly to scar formation. Despite surgical reattachment of healthy tendon, lineage tracing revealed poor preservation of supraspinatus tendon after acute injury and loss of tendon structure, suggesting that tendon degeneration is also a key impediment of successful rotator cuff repair. Scar formation after surgery is mediated largely by αSMAlin cells and results in permanently reduced functional and structural properties.
Keywords: rotator cuff, tendon injury, repair, pain, function
Introduction
With aging and/or chronic overuse, the rotator cuff tendons can degenerate and rupture.1; 2 Delayed treatment or no treatment leads to a sequela of irreversible damage to the musculoskeletal unit, including fatty atrophy of muscle, tendon retraction, and bone loss.3-7 Although rotator cuff and shoulder injuries are common, accounting for more than 4.5 million physician visits annually, treatment options remain limited.8 To date, surgical repair of ruptured tendon back to its bony attachment is the leading treatment choice with best outcomes, however failure rates range from 13–94% depending on the patient population.9-11 In elderly patients especially, failure is high due to the poor quality of the tendon and overall reduced healing capacity of the patient.12; 13
Intrinsic mechanisms of cuff degeneration include degeneration within the cable region and morphological age related change in tendon composition.14; 15 Although injury and rupture is initiated within the tendon, surgical repair failures are largely due to an inability to regenerate a functional attachment of tendon back to bone. In addition, the healing tendon is unable to regenerate tendon tissue with similar strength and viscoelastic properties to normal tendon.16 After tendon injury and repair, the surgical reattachment site heals by disorganized scar tissue, which is unable to recapitulate this key mechanical function.17; 18 It is not known which tissues and cell lines contribute to tendon healing, and how they remodel in final phases of healing.
To identify new therapies to augment rotator cuff repair, we and others have developed rotator cuff tendon injury models in mice.18-22 Like the rat, the mouse rotator cuff has several features that are similar to that of the human rotator cuff, including an intra-articular supraspinatus tendon that passes beneath the acromion and interdigitated collagen architecture at the insertion site.23; 24 Although the miniature scale of the mouse supraspinatus tendon is challenging for testing surgical repair techniques or devices, the availability of genetic tools for mouse is highly attractive for mechanistic studies that address basic biological questions. Using Cre-LoxP recombination tools and fluorescent reporters, the cellular populations that orchestrate rotator cuff tendon and enthesis healing are beginning to be elucidated.20; 25-27 In a previous study for example, we employed two different supraspinatus tendon injury models and showed distinct cellular mechanisms of healing depending on the injury induced.18 However, in that study, functional analyses of healing were limited and the effects of the tendon injury on the underlying bone were not assessed.
In this study, we focused on our model of full supraspinatus tendon detachment with surgical repair to comprehensively define functional healing and effects on bone, as well as further evaluate which cell lines contribute to the formation of healing tendon. Specifically, we investigated the participation of myofibroblast and mesenchymal progenitor cells (αSMACreERT2)25 as well as tendon cells (ScxCreERT2)18 during healing using genetic lineage tracing. Functional outcomes in this model were assessed by forelimb gait analysis and biomechanical testing of the tendon while bony changes were determined by microCT quantification. We further utilized the mouse grimace scale in order to determine whether pain correlated to functional results.28 We hypothesized that tendon healing after repair will lead to persistent pain, impaired function, and recruitment of non-Scxlin scar-forming cells.
Material and Methods
Mice
All procedures were carried out according to IACUC guidelines at the Icahn School of Medicine at Mount Sinai. Mice were housed in virus-free animal facilities and veterinary care provided by the Center of Comparative Medicine and Surgery at Mount Sinai. For pain and functional outcome measurements, C57BL6 wild type mice were used. For lineage tracing, existing mouse lines for ScxCreERT2 (generated by Dr. Ronen Schweitzer), αSMACreERT229, and Ai14 Rosa26-TdTomato Cre reporter (RosaT)30 were used.
Experimental Design
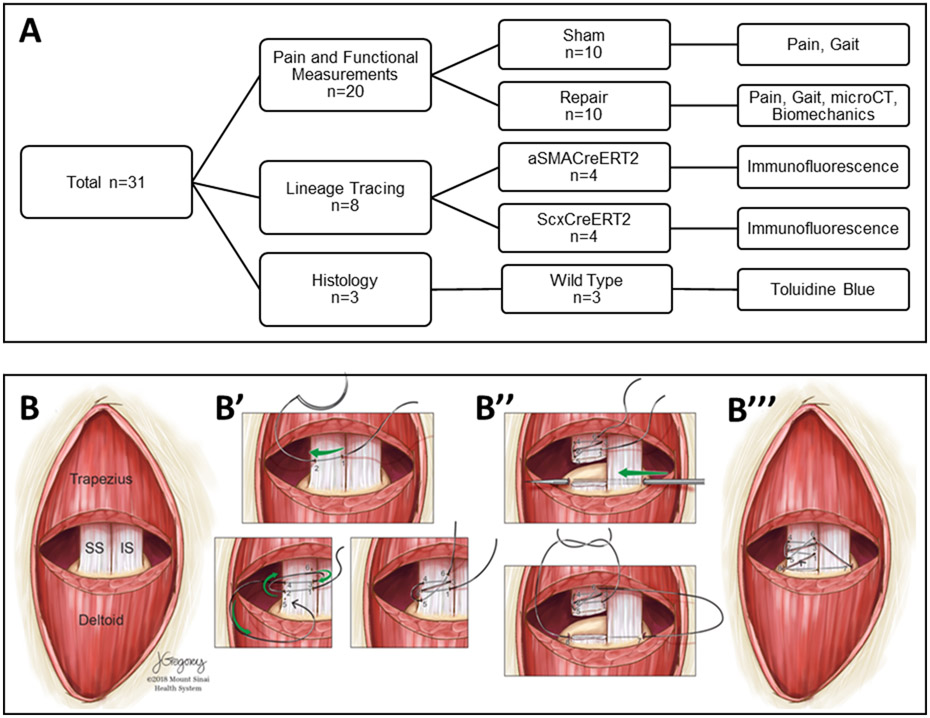
For these studies, a total of n=31 mice were used for assays, including: pain and functional outcome measurements (n=20), lineage tracing (n=8), and histology (n=3) (Figure 1A). To reduce variability due to size, only male mice were used for these studies. Sham control animals were separately generated for pain and gait analyses while contralateral uninjured limbs were used as controls for other assays. Since pain and gait measurements are non-terminal, these assays were carried out on the same animals (pain: Pre-Op, 3h, 6h, 24h, 48h; gait: Pre-Op, d3, d7, d14, d28). All mice were sacrificed at d28. Shoulders were then harvested for microCT imaging followed by mechanical testing. Shoulders for lineage tracing were processed for cryosectioning and shoulders for histology processed for plastic sectioning.
Figure 1: Experimental design and surgical overview.
(A) Flow chart of experimental design, assays, and sample sizes. (B) Schematic of supraspinatus tendon detachment and repair surgery. (B’) Suture is placed in supraspinatus tendon followed by (B”) detachment of the tendon at the attachment site. A bone tunnel is created with needle and the suture passed through to (B’”) reattach the tendon.
Supraspinatus Tendon Detachment and Repair Surgery
For all surgeries, the side of the surgical procedure was alternated between left and right shoulders to avoid potential bias; alternating shoulders were carried out consecutively. The mean age and weight of injured mice used was 157.5 ±11.1 d and 31.17±1.25 g, respectively. The mean age and weight of sham control mice used was 119.6±15.6 d and 28.25±1.78 g, respectively. Age and weight were not significantly different between groups. Animals were anesthetized with 4% isoflurane inhalation, which was maintained at 2% throughout the surgical procedure. The supraspinatus tendon detachment and repair was carried out as described previously.18; 19 Mice were positioned in the decubitus position with the forelimb fixed in external rotation. The skin over the acromion was shaved and disinfected with povidone-iodine and cleansed with alcohol. A 1cm incision was made over the acromion to expose the deltoid muscle. The deltoid muscle was detached from the acromion and lifted gently to expose the supraspinatus tendon beneath it. The supraspinatus tendon was then secured using an 8–0 Prolene suture (Ethicon) close to the musculotendinous junction (Figure 1B). After detachment from the humeral head, the tendon stub was fixed to its insertion with the sutures running through the humeral head via a bone tunnel created with a 26G needle. The bone tunnel was created from the lateral edge of the infraspinatus enthesis to the medial edge of the supraspinatus footprint, avoiding the tuberosity as well as the growth plate. Finally, the deltoid muscle was reattached to the acromion (8–0 Prolene, Ethicon) and the skin closed (6–0 Polypropylene, Henry Schein). In sham control animals, the deltoid muscle was detached and reattached while the supraspinatus tendon was left intact. For mice undergoing pain assessment, no buprenorphine was given after the surgery. Pain management was provided to all other mice (0.05 mg/kg buprenorphine) . All mice exhibited normal cage activity following surgeries with good body condition.
Mouse Grimace Scale (MSG) Scoring
All mice were placed in a 5×5×9cm transparent box and filmed continuously over a 30 min period. Screenshots of the face were then obtained every 3 min for analyses (10 images per mouse and time point). Each image was scored by a blinded observer for the following parameters according to the established mouse grimace scale: orbital tightening, nose bulge, cheek bulge, ear position, and whisker change (Figure 2A).28 Point values were given for each parameter: 0=not present, 1=moderate, 2=severe. All values were then summed for each image (combining parameters) and averaged for each animal.
Figure 2: Mouse grimace score shows transient increase in pain after rotator cuff surgery.
(A) Example images of mouse grimace parameters used to calculate pain scores. Representative images are taken from repaired mice except the ‘no pain’ image which is from a Pre-Op example. (B) Quantitation of mouse grimace scores after sham and detachment/repair surgeries shown as box plots. Blue bars indicate significant differences from Pre-Op scores p<0.05.
Gait Analysis
Gait analysis was carried out using the DigiGait Imaging System (Mouse Species Inc., Quincy, MA). Mice were gaited at 10 cm/s for 4 s on a transparent treadmill. A high-speed digital camera captured the position of each paw, and the footage was analyzed using DigiGait Analysis Software (DigiGait 12.4). The measurements obtained for each forelimb (contralateral control and sham/repair) were used to calculate % Stance Stride and Paw Area. We and others previously identified these parameters as outcome measurements associated with tendon injuries.18; 19; 31
MicroCT Imaging
Right and left humeri were dissected leaving only the supraspinatus tendon and muscle intact. Individual bones were potted in 1% agarose (Fisher Scientific), up to the mid-diaphysis, in 1.5 mL cryotubes. The tubes were mounted upside down so the tendon and humeral head were suspended freely in air and scanned using a SkyScan 1272 system (Bruker) using the following settings (X-ray tube potential 60 kV, X-ray intensity 166 μA, 0.25 mm aluminum filter, frame averaging 3, rotation step 0.2°, resolution 5.0 μm). Analysis for tendon cross-sectional area and humeral head bone morphometry were performed using SkyScan software. First, reconstructed image stacks were rotated in 3D (DataViewer, Bruker) to approximately the same position such that the tendon was aligned along the vertical axis. Two new image stacks were generated; one specific to tendon and the other the humeral head. Tendon cross-sectional area was then determined by finding the minimal cross-sectional area (CTan, Bruker) between the humeral head and the supraspinatus muscle. Bone morphometry was determined by manually segmenting an ROI around the humeral head cortex and growth plate. Specifically, the ROI was the proximal epiphysis of the humeral head defined by the periosteal and articular surfaces to the growth plate. Since the drill hole was distal to the growth plate, the hole was excluded from analyses. A semi-automated segmentation algorithm was used to threshold, shrink-wrap the ROI to the bone surface, and separate the cortical and trabecular bone layers. Outcome measures for cortical bone were cortical thickness (Ct.Th), cortical porosity (Ct.Po), and cortical tissue mineral density (TMD); and for trabecular bone were total volume (TV), bone volume (BV), bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular spacing (Tb.N), and trabecular bone mineral density (BMD).
Biomechanical Testing
After microCT scanning, mechanical testing was performed using an ElectroForce 3230 Series III (TA Instruments). The samples were prepared for testing by entirely removing the supraspinatus muscle and adhering a small piece of tissue paper (Kimwipe, Kimberly-Clark) to the myotendinous junction using cyanoacrylate (Ultragel Control, Loctite), to increase gripping surface area. Slippage was not observed during testing for any samples. Samples were then placed in 3D printed molds which encapsulate the humerus but allow access to the supraspinatus tendon. The molds were gripped using custom tooling, and the supraspinatus tendon was pulled until failure at a rate of 0.1% strain/second, parallel to the long-axis of the humerus shaft. System displacement and force were recorded at 100 Hz. The structural properties determined were maximum force, yield force, stiffness, and work. The stiffness was determined from the maximally linear region of the force/displacement data using random sample consensus (RANSAC) implemented in a custom Matlab (Mathworks) script. Work was determined by computing the area under the force/displacement curve. Engineering stress and strain were computed using the cross-sectional area measured by μCT and the initial specimen gauge length measured from the enthesis to the tendon grip tooling, respectively. From these data, the material properties determined were ultimate stress, yield stress, Young’s Modulus, and Modulus of Resilience.
Lineage Tracing
Lineage tracing using inducible Cre lines was carried out by three consecutive daily tamoxifen injections given by intraperitoneal injection (100 mg/kg, Sigma) one week before the injury. All ScxCreERT2/RosaT (age 113±0 d and weight 27.65±3.28 g) and αSMACreERT2/RosaT (age 104.5±32.1 d and weight 22.55±3.14 g) mice were sacrificed at d28 after surgery with no other assessments.
Histology, Immunofluorescence, and Fluorescence Imaging Analysis
Shoulders were fixed in 4% paraformaldehyde, decalcified in EDTA, infiltrated with 5% and 30% sucrose and frozen in OCT. Alternating coronal cryosections (12um) through the shoulder were collected and stained with DAPI for cell nuclei. Immunostaining was carried out using an antibody against αSMA (C6198, Sigma) with Cy5 secondary detection (711–175-152, Jackson Immuno Research) and DAPI counterstaining. Samples for plastic sections were fixed in zinc formalin, dehydrate and infiltrated with methacrylate monomer and embedded. The sections were acquired at 6um and stained with Toluidine Blue. All images were acquired using the Zeiss AxioImager microscope, with Apotome for optical sectioning of fluorescent images. Image processing was carried out using Adobe Photoshop CS6.
Statistical analysis
For gait analysis, we compared injury as well as sham control to uninjured controls using a linear mixed effect model with side (injury vs uninjured control; sham control vs uninjured control) and timepoint (day 3, 7, 14, 28) as independent variables, and gait parameters (stance stride or paw area) as dependent variables. The mouse identity was set as a random variable to control for repeated measurements. For pain analysis, we compared the MSG score of the repair animals as well as the sham control animals over time using an analysis of variance with time point as the independent variable and the MSG Score as the dependent variable. The mouse identity was set as a random variable to control for repeated measurements. We computed the pairwise comparisons between the means at all the time points using contrasts tests and used the algorithm described in Piepho (2004)32 to determine the statistical groups (p<0.05). For biomechanical and microCT analyses, paired t-tests were used to compare the contralateral control and repaired shoulders. Statistical analyses were performed using R software (version 3.4.4) or GraphPad Prism 7.33 Linear mixed effects models were calculated using the lme4 package.34 Data was tested for normality using a D’Agostino-Pearson Omnibus test.
Results
Mouse Grimace Score for pain is transiently increased after repair and sham surgery
Pain is seldom tested as an outcome measure in animal models. We therefore tested whether pain can be detected in mice after supraspinatus tendon detachment and repair using the established mouse grimace scale.28 In sham control animals, pain was significantly increased at 3 hours after surgery compared to the uninjured Pre-Op state; by 6 hours, pain scores were no longer significant, although there was wide variation (Figure 2B). In repair animals, increased pain scores were detected at 3 and 6 hours, before returning to baseline levels by 24 hours.
Gait function is impaired after supraspinatus tendon injury and repair
To determine the functional consequences of full supraspinatus tendon detachment and repair, we gaited the mice prior to surgery (Pre-Op) and from d3 to d28 after injury. Paw area was significantly reduced in both injured and uninjured forelimbs at almost every timepoint relative to Pre-Op measurements, in both repair and sham animals (Figure 3A). %Stance Stride however, showed a significant reduction in the repair injured limb relative to contralateral control from d3 through d14, while sham injured limbs were only transiently impaired at d3 relative to contralateral control (Figure 3B). Comparison to Pre-Op measurements showed a significant increase in the non-injured limb at d3 after repair, suggesting compensation at this timepoint. A significant decrease in %Stance Stride for the repair limb vs Pre-Op was only detected at d7 (p<0.05). By contrast, sham control forelimbs were not different from Pre-Op at any timepoint. By d28, Stance Stride had returned to baseline levels for the repair cohort and differences could no longer be detected between forelimbs. When injured limbs were normalized to their respective uninjured limbs, we found no significant differences between sham and repair groups for either paw area or Stance Stride (Figure S1). However, differences were still observed between timepoints for repair animals. Collectively, these results suggest that paw area function may be largely due to injury to the deltoid muscle rather than supraspinatus tendon while Stance Stride is a more sensitive and specific parameter for supraspinatus tendon injury and repair.
Figure 3: Gait analysis indicates reduced function after supraspinatus tendon injury and repair.
(A) Paw area and (B) Stance Stride parameters acquired using Digigait System. Solid blue bars indicate significant difference of both limbs relative to Pre-Op values (p<0.05). Dotted blue bars indicate significant difference for indicated limb relative to Pre-Op values (p<0.05). Asterisks indicate significant difference between surgical limb and contralateral intact limb (p<0.05). ns indicates no statistical difference detected.
Supraspinatus tendon mechanical properties is not recovered after injury
Biomechanical testing of dissected supraspinatus tendons at d28 showed permanent loss of all tensile properties. Repaired tendons were consistently weaker than intact controls, with increased cross-sectional area (consistent with scar formation), and reduced maximum force, stiffness, and work to yield properties (Figure 4A-E). Material properties including ultimate stress, Young’s Modulus, and Resilience were also dramatically reduced (Figure 4F-H). All repairs failed within the soft-tissue mid-substance between the humerus and the grips.
Figure 4: Tensile mechanical properties are persistently impaired at d28.
(A) Force displacement curves for all samples tested at d28. (B) Cross-sectional area, (C) Maximum force, (D) Stiffness, and (E) Work to yield for control and repaired tendons. The material properties (F) Ultimate stress, (G) Young’s Modulus, and (H) Resilience for control and repaired tendons were calculated from stress strain curves. Asterisks indicate significant differences * p<0.05 *** p<0.001 **** p<0.0001.
MicroCT Scan
To determine bony changes to the underlying attachment site, microCT imaging was used at d28 and cortical and trabecular bone properties were quantified (Figure 5A-5B’). Although cortical thickness was unchanged, cortical porosity increased and cortical tissue mineral density decreased, suggesting cortical bone loss (Figure 5C). Similarly, significant differences in trabecular bone properties (including thickness, number, and bone mineral density) were detected in repair vs control groups, indicative of trabecular bone loss (Figure 5D). These measurements show that supraspinatus tendon detachment results in permanent cortical and trabecular bone loss despite surgical repair.
Figure 5: Bone properties are reduced at d28 after supraspinatus tendon injury and repair.
Representative microCT images of (A) control and (B) repaired humerus at d56. A’ and B’ are higher magnification images from boxes in A and B. Red dotted lines highlight the lower mineral density region of the supraspinatus enthesis. (C) Cortical and (D) trabecular bone properties quantified from microCT images. Asterisks indicate significant differences * p<0.05 *** p<0.001. Scalebars: 500 μm.
Genetic lineage tracing shows an increase of aSMAlin cells within the scar tissue and reduction of Scxlin tendon cells
To examine tissue morphology, we acquired histological sections through the supraspinatus tendon and enthesis, using the acromion as an anatomical guide to identify the correct location. Toluidine blue staining of control shoulders showed distinctive tendon tissue (aligned structure with tenocytes arranged in linear arrays) and organized enthesis gradient of tendon to fibrocartilage to bone) (Figure 6A, 6A’). After injury and repair, the tendon structure was no longer observed; instead the area adjacent to the enthesis was dominated by massive, hypercellular scar, that did not stain for fibrocartilage (Figure 6B, 6B’). The original enthesis was still easily visible, however the orientation appeared disrupted (no characteristic angled orientation). Lineage tracing for myofibroblasts/progenitors (αSMAlin) labeled by tamoxifen prior to injury showed strong contribution of αSMAlin cells to scar (Figure 6C, 6D). In control shoulders, αSMAlin cells were not detected in the tendon (consistent with our previous results), but were found in the periosteum surrounding the acromion, within the bursa, and within the deltoid muscle. To determine whether αSMA was still expressed at this timepoint, immunostaining was performed. Analysis of cryosections showed little staining within the scar region while staining was detected in the marrow cavities of the acromion and humeral head, as well as in the deltoid muscle (Fig S2). Lineage tracing of tenocytes (Scxlin) showed little or no Scxlin cells in the control enthesis with labeling of tenocytes away from the enthesis. Although Scxlin cells at d28 were detected in repaired shoulders near the enthesis (suggesting that retraction of the tendon had not occurred), distinctive tendon structure and organization was absent and the region was highly occupied by non-labeled cells. These results indicate tendon degeneration (Figure 6E, 6F). Images for remaining lineage tracing mice can be found in Figure S3 and S4.
Figure 6: Supraspinatus tendon heals by massive scar formation and tendon degeneration after detachment and repair.
(A, B) Toluidine blue staining of plastic mouse shoulder sections. A’ and B’ are higher magnification images from boxes in A and B. Red dotted outlines indicate enthesis regions. Black scalebars: 500 and 100 μm as shown in images. (C, D) Lineage tracing of αSMAlin cells and (E, F) Scxlin cells is shown in cryosections. Yellow dotted outlines indicate enthesis regions. A: acromion, D: deltoid muscle; T: supraspinatus tendon, E: supraspinatus enthesis; H: humerus; G: glenoid; S: scar. Yellow arrows indicate sutures. White scalebars: 100 μm.
Discussion
In this study, we found that healing does not originate in the tendon proper itself, but rather from surrounding tissues. Lineage tracing of Scxlin cells showed relatively few cells present after injury and repair and loss of characteristic tendon morphology. In a previous study of partial supraspinatus tendon detachment without repair, we found that although the localized injury site was devoid of Scxlin cells, the tendon was otherwise well-preserved with characteristic aligned architecture and retention of Scxlin tenocytes.18 Using the current model, our results suggest that the surgical repair was either not able to mechanically recreate the proper tension required to maintain Scxlin tenocytes after full detachment or the drilling of bone to create a tunnel for surgical repair may have induced an excessive inflammatory environment leading to loss of the cells. It is well established that inflammation is a feature of tendinopathy and prolonged or excessive inflammation can result in tendon damage and loss of matrix and mechanical properties.35; 36 The loss of mechanical loading and excessive inflammation may also explain the loss in cortical and trabecular bone at the insertion site. This is consistent with findings in human cadaver shoulders showing impaired bone volume and trabecular properties with torn rotator cuff tendons compared to intact.37 These results indicate that while restoration of the tendon to bone interface remains a critical problem, the loss of tendon is also a key clinical challenge that cannot be overcome by surgical repair alone. In the clinical setting, this challenge is exacerbated by supraspinatus tendons that are more likely aged and pathological at the time of repair versus the young, healthy tendons used in these studies. In human patients, the shoulder is also immobilized after surgical repair and weight-bearing is limited; although this is difficult to replicate in mice, future studies may better approximate post-operative procedures in our mouse model to determine these effects on healing. Future studies will also assess the specific role of inflammation on maintenance of tenocytes after surgery.
The loss of tendon and tendon cells was coupled with massive hypercellular scar formation. In our previous study, we found that Axin2lin cells formed a large proportion of scar-forming cells after tendon detachment and repair.18 Here, lineage tracing of aSMAlin cells also showed strong contribution of these cells to the scar. Although it is likely that aSMAlin cells and Axin2lin cells comprise both overlapping and distinct scar populations, it is difficult to definitively determine using current genetic tools since both lines use Cre-LoxP recombination. The generation of Flp-FRT recombination lines would allow dual analysis in the same mice.38; 39 To date, the source of these cells also remains an open question; potential sources include released bone marrow or bursal cells.20 However, in the absence of specific markers for bone marrow/bursa or live imaging methods, this can be challenging to define. In our partial tear model of rotator cuff enthesis injury, we did not observe aSMAlin cells recruited in the scar, however abundant aSMAlin cells were recruited after full detachment and repair injury. This may be due to release of bone marrow cells from drilling since the partial tear injury did not significantly penetrate into the marrow cavity. Immunostaining as well as lineage tracing showed many aSMA+ cells in the marrow. However, these differences between models may also be due to differences in mechanical loading since the partial tear model retained most of the tendon-bone attachment. Other factors include enhanced inflammation generated by the more severe injury and intervention, which may activate resident aSMAlin cells compared to the partial tear injury. In addition to bone marrow, a previous lineage tracing study also showed that aSMAlin cells derived from the bursa expand after a partial rotator cuff tendon injury.18; 20 Similarly, we also observe expansion of the bursa after detachment and repair injury; it is possible these cells may be recruited into the injury site. A recent study also showed that implanted bursal tissues may improve cellularity during rotator cuff tendon healing, although mechanical properties were not improved.40 Thus, retention of the bursa during rotator cuff tendon repair surgeries may help to support scar-mediated healing.
To date, almost all studies of tendon healing using animal models focus on functional measurements of recovery (such a biomechanics and structural integrity), however pain is also a critical factor in terms of patient outcomes. A search of the available literature revealed that very few studies have evaluated pain measures in rodent rotator cuff models, despite the importance of pain clinically. The very few studies that have attempted to study pain used gene or protein expression of neuropeptide or inflammatory markers as indicators for pain, and are therefore less direct compared to the grimace scoring used here.41; 42 Our results using MGS (which to our knowledge has never before been used in a rotator cuff injury model) showed only an early and transient painful response in mice undergoing surgery, which is inconsistent with the clinical setting in which patients report longer-term pain after rotator cuff injury. Although there are alternative methods for detecting painful responses in rodents (including the Von Frey test, or hot and cold plate tests)43; 44, these methods rely on experimenter-evoked pain to measure changes in sensitivity. The advantage in using MGS is the ability to detect spontaneously expressed pain. Interestingly, the original description of MGS reported that ‘pain face’ was more likely to be detected in noxious stimuli of moderate duration (10 min – 4h); in cases of longer stimulation (days), MGS was no longer sensitive. This was similar to the range of time we detected elevated MGS scores after tendon injury and repair (3–6h), with no change detected at 24h or 48h. This limitation of MGS may be due to the unique ability of rodents to suppress ‘pain face’ over longer duration stimuli as an evolutionary adaptation to mask weakness from potential threats. Future studies will test the ability of other tests such as the Von Frey test for mechanical allodynia to determine painful responses over longer durations, which is likely more clinically relevant for rotator cuff tendon injuries.
To determine functional recovery, we used gait analysis to assess overall forelimb function as well as direct mechanical testing of the supraspinatus tendon. In our previous study, we established key gait parameters associated with supraspinatus tendon injury, including paw area and %Stance Stride.18 Interestingly, although we previously found that paw area was permanently increased relative to non-injured mice, we now find that paw area is actually reduced after tendon injury and subsequent repair. In the current study, we compared to pre-operative baseline values of the same animals rather than separate non-injured animals, which likely accounts for this important difference. This was observed for both paws, suggesting that mice shifted weight-bearing to hindlimbs after injury, rather than to the uninjured contralateral forelimb. Surprisingly, sham injuries also elicited a dramatic and permanent reduction in paw area, suggesting that this parameter is likely associated with injury to the deltoid muscle, rather than supraspinatus tendon. By contrast, %Stance Stride was more specific to tendon injury, since repair mice showed prolonged changes in this parameter between control and injured paws, relative to a more modest change in sham mice that was resolved by day 7 post-injury. However, this may also be due to the bone tunnel created during the repair, rather than the tendon injury itself. Direct mechanical testing showed very poor functional healing at d28 despite recovery of %Stance Stride at this timepoint. Both structural and material tensile properties were reduced. This is consistent with numerous studies in rat16; 44-47 and emerging studies in mouse19; 21; 48 showing poor functional recovery of supraspinatus tendon after injury, which is not recovered despite surgical repair.
Although it remains possible that tendon retraction occurred, we did not observe evidence for this in cryosections through the tissue, even in regions close to the supraspinatus muscle. In addition, while ScxCreERT2 successfully targeted tendon cells, not all cells in the control tendons were labeled at these adult stages. This is consistent with recent work showing that ScxCreERT2 labels ~60% of adult tenocytes in flexor tendons.49 Due to this incomplete recombination, some of the unlabeled cells in the repair may therefore derive from the tendon. The majority of the analyses was also limited to a single timepoint; while this timepoint was chosen to represent the ‘healed’ status, early timepoints would be interesting to capture evolving biomechanical properties and the cellular dynamics related to expression of markers, proliferation, and recruitment. In a recent paper, temporal labeling of Scxlin cells by tamoxifen in a mouse flexor tendon injury model showed that Scxlin cells turned off Scx in the early timepoints after injury before re-activating Scx expression, suggesting that healing occurred by de-differentiation and re-differentiation of Scxlin cells.49 Temporal injection of tamoxifen will therefore be carried out in future studies to elucidate the dynamics of aSMAlin cell activation and recruitment. Another limitation is the repair technique did not fully recapitulate the original anatomic footprint since the very small size of the mouse limited repair methods. This may lead to eccentric loading patterns and contribute to poor functional healing and tendon degeneration observed. Future studies will compare our repair technique with a recently published Bunnell stitch techinique using two bone tunnels in mouse that better matches the native anatomy and tissue tension.22 Finally, we used detachment and reattachment of the deltoid muscle as our sham control, which was not able to distinguish the effects of the bone tunnel. While we observed a significant effect on gait with this sham, an additional sham control group that includes bone drilling without supraspinatus detachment would be highly informative.
In conclusion, these studies show that despite surgical reattachment of healthy tendon, there is poor preservation of supraspinatus tendon cells after acute injury, suggesting that tendon degeneration is a key impediment of successful rotator cuff repair. We further find that scar formation after surgery is mediated largely by aSMAlin and healing involves cells from surrounding tissues rather than being initiated by the tendon itself.
Supplementary Material
Figure S1: Normalized gait analyses show no significant differences between sham and repaired limbs. (A) Paw area and (B) Stance Stride parameters acquired using Digigait System were normalized (injured/uninjured). Black bars indicate significant difference of repair limbs relative to indicated timepoints (p<0.05). ns indicates no statistical difference detected (blue bars, p>0.05).
Figure S2: αSMA immunostaining reveals minimal αSMA expression in scar region at d28 after repair. A: acromion, D: deltoid muscle; H: humerus; S: scar. Yellow dotted outlines indicate enthesis regions. Scalebars: 100 μm.
Figure S3: Additional images from remaining ScxCreERT/RosaT lineage tracing animals at d28. A: acromion, D: deltoid muscle; T: supraspinatus tendon, S: scar. Yellow dotted outlines indicate enthesis regions. Scalebars: 100 μm.
Figure S4: Additional images from remaining αSMACreERT/RosaT lineage tracing animals at d28. A: acromion, D: deltoid muscle; T: supraspinatus tendon, E: supraspinatus enthesis; H: humerus; S: scar. Yellow dotted outlines indicate enthesis regions. Scalebars: 100 μm.
Acknowledgements
We thank Dr. Ronen Schweizer for providing the ScxCreERT2 line and Dr. Ivo Kalajzic for the αSMACreERT2 line. We also acknowledge Jill K. Gregory for illustrations. Further we thank Dr. Simon Garnier for assistance with the statistical analysis. This study was supported by funding from the NIH/NIAMS (R01AR069537, R01AR055580, R01AR057836) and a fellowship from the Hans Neuenschwander Fond, Inselspital Bern to HM. Dr. Zumstein and Dr. Galatz also serve as consultants for Medacta.
Footnotes
Disclaimer: none
References
- 1.Soslowsky LJ, Thomopoulos S, Tun S, et al. 2000. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg 9:79–84. [PubMed] [Google Scholar]
- 2.Economopoulos KJ, Brockmeier SF. 2012. Rotator cuff tears in overhead athletes. Clin Sports Med 31:675–692. [DOI] [PubMed] [Google Scholar]
- 3.Goutallier D, Postel JM, Bernageau J, et al. 1994. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res:78–83. [PubMed] [Google Scholar]
- 4.Melis B, DeFranco MJ, Chuinard C, et al. 2010. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Relat Res 468:1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melis B, Nemoz C, Walch G. 2009. Muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1688 cases. Orthop Traumatol Surg Res 95:319–324. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons MC, Singh A, Anakwenze O, et al. 2017. Histological Evidence of Muscle Degeneration in Advanced Human Rotator Cuff Disease. J Bone Joint Surg Am 99:190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung SW, Oh JH, Gong HS, et al. 2011. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med 39:2099–2107. [DOI] [PubMed] [Google Scholar]
- 8.Oh LS, Wolf BR, Hall MP, et al. 2007. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res 455:52–63. [DOI] [PubMed] [Google Scholar]
- 9.Galatz LM, Ball CM, Teefey SA, et al. 2004. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 86-A:219–224. [DOI] [PubMed] [Google Scholar]
- 10.Harryman DT 2nd, Mack LA, Wang KY, et al. 1991. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am 73:982–989. [PubMed] [Google Scholar]
- 11.Mather RC 3rd, Koenig L, Acevedo D, et al. 2013. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am 95:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rashid MS, Cooper C, Cook J, et al. 2017. Increasing age and tear size reduce rotator cuff repair healing rate at 1 year. Acta Orthop 88:606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh JH, Kim SH, Kang JY, et al. 2010. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med 38:672–678. [DOI] [PubMed] [Google Scholar]
- 14.Cho NS, Moon SC, Hong SJ, et al. 2017. Comparison of Clinical and Radiological Results in the Arthroscopic Repair of Full-Thickness Rotator Cuff Tears With and Without the Anterior Attachment of the Rotator Cable. Am J Sports Med 45:2532–2539. [DOI] [PubMed] [Google Scholar]
- 15.Connizzo BK, Grodzinsky AJ. 2018. Multiscale Poroviscoelastic Compressive Properties of Mouse Supraspinatus Tendons Are Altered in Young and Aged Mice. J Biomech Eng 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Killian ML, Cavinatto LM, Ward SR, et al. 2015. Chronic Degeneration Leads to Poor Healing of Repaired Massive Rotator Cuff Tears in Rats. Am J Sports Med 43:2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomopoulos S, Genin GM, Galatz LM. 2010. The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing. J Musculoskelet Neuronal Interact 10:35–45. [PMC free article] [PubMed] [Google Scholar]
- 18.Moser HL, Doe AP, Meier K, et al. 2018. Genetic lineage tracing of targeted cell populations during enthesis healing. J Orthop Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell R, Taub P, Cagle P, et al. 2015. Development of a mouse model of supraspinatus tendon insertion site healing. J Orthop Res 33:25–32. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida R, Alaee F, Dyrna F, et al. 2016. Murine supraspinatus tendon injury model to identify the cellular origins of rotator cuff healing. Connect Tissue Res 57:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebaschi A, Nakagawa Y, Wada S, et al. 2017. Tissue-specific endothelial cells: a promising approach for augmentation of soft tissue repair in orthopedics. Ann N Y Acad Sci 1410:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Liu X, Davies MR, et al. 2018. A Mouse Model of Delayed Rotator Cuff Repair Results in Persistent Muscle Atrophy and Fatty Infiltration. Am J Sports Med 46:2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derwin KA, Baker AR, Iannotti JP, et al. 2010. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng Part B Rev 16:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soslowsky LJ, Carpenter JE, DeBano CM, et al. 1996. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg 5:383–392. [DOI] [PubMed] [Google Scholar]
- 25.Dyment NA, Hagiwara Y, Matthews BG, et al. 2014. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One 9:e96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz AG, Long F, Thomopoulos S. 2015. Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development 142:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz AG, Galatz LM, Thomopoulos S. 2017. Enthesis regeneration: a role for Gli1+ progenitor cells. Development 144:1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langford DJ, Bailey AL, Chanda ML, et al. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. [DOI] [PubMed] [Google Scholar]
- 29.Grcevic D, Pejda S, Matthews BG, et al. 2012. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells 30:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madisen L, Zwingman TA, Sunkin SM, et al. 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howell K, Chien C, Bell R, et al. 2017. Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Sci Rep 7:45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piepho HP AB, Richter C. 2004. A Mixed Modelling Approach for Randomized Experiments with Repeated Measures. Journal of Agronomy and Crop Science 190:230–247. [Google Scholar]
- 33.R Core Team (2018). 2018. R: A language and environment for statistical computing. In: Vienna: ARFfSC; editor. [Google Scholar]
- 34.Bates D, Mächler M., Bolker B., and Walker S. 2015. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67:1–48. [Google Scholar]
- 35.Abraham AC, Shah SA, Golman M, et al. 2019. Targeting the NF-kappaB signaling pathway in chronic tendon disease. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millar NL, Gilchrist DS, Akbar M, et al. 2015. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat Commun 6:6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zumstein MA, Raniga S, Labrinidis A, et al. 2016. Optimal Lateral Row Anchor Positioning in Posterior-Superior Transosseous Equivalent Rotator Cuff Repair: A Micro-Computed Tomography Study. Orthop J Sports Med 4:2325967116671305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hara T, Verma IM. 2019. Modeling Gliomas Using Two Recombinases. Cancer Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schonhuber N, Seidler B, Schuck K, et al. 2014. A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat Med 20:1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dyrna F, Zakko P, Pauzenberger L, et al. 2018. Human Subacromial Bursal Cells Display Superior Engraftment Versus Bone Marrow Stromal Cells in Murine Tendon Repair. Am J Sports Med 46:3511–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagura N, Kenmoku T, Uchida K, et al. 2019. Nerve growth factor continuously elevates in a rat rotator cuff tear model. J Shoulder Elbow Surg 28:143–148. [DOI] [PubMed] [Google Scholar]
- 42.Yamazaki H, Ochiai N, Kenmoku T, et al. 2014. Assessment of pain-related behavior and pro-inflammatory cytokine levels in the rat rotator cuff tear model. J Orthop Res 32:286–290. [DOI] [PubMed] [Google Scholar]
- 43.Chaplan SR, Bach FW, Pogrel JW, et al. 1994. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. [DOI] [PubMed] [Google Scholar]
- 44.Yalcin I, Charlet A, Freund-Mercier MJ, et al. 2009. Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. J Pain 10:767–773. [DOI] [PubMed] [Google Scholar]
- 45.Hettrich CM, Gasinu S, Beamer BS, et al. 2014. The effect of mechanical load on tendon-to-bone healing in a rat model. Am J Sports Med 42:1233–1241. [DOI] [PubMed] [Google Scholar]
- 46.Hettrich CM, Gasinu S, Beamer BS, et al. 2013. The effect of immobilization on the native and repaired tendon-to-bone interface. J Bone Joint Surg Am 95:925–930. [DOI] [PubMed] [Google Scholar]
- 47.Kim HM, Galatz LM, Das R, et al. 2011. The role of transforming growth factor beta isoforms in tendon-to-bone healing. Connect Tissue Res 52:87–98. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Tan H, Lebaschi AH, et al. 2018. Kartogenin Enhances Collagen Organization and Mechanical Strength of the Repaired Enthesis in a Murine Model of Rotator Cuff Repair. Arthroscopy 34:2579–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Best KT, Loiselle AE. 2019. Scleraxis lineage cells contribute to organized bridging tissue during tendon healing and identify a subpopulation of resident tendon cells. FASEB J 33:8578–8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Normalized gait analyses show no significant differences between sham and repaired limbs. (A) Paw area and (B) Stance Stride parameters acquired using Digigait System were normalized (injured/uninjured). Black bars indicate significant difference of repair limbs relative to indicated timepoints (p<0.05). ns indicates no statistical difference detected (blue bars, p>0.05).
Figure S2: αSMA immunostaining reveals minimal αSMA expression in scar region at d28 after repair. A: acromion, D: deltoid muscle; H: humerus; S: scar. Yellow dotted outlines indicate enthesis regions. Scalebars: 100 μm.
Figure S3: Additional images from remaining ScxCreERT/RosaT lineage tracing animals at d28. A: acromion, D: deltoid muscle; T: supraspinatus tendon, S: scar. Yellow dotted outlines indicate enthesis regions. Scalebars: 100 μm.
Figure S4: Additional images from remaining αSMACreERT/RosaT lineage tracing animals at d28. A: acromion, D: deltoid muscle; T: supraspinatus tendon, E: supraspinatus enthesis; H: humerus; S: scar. Yellow dotted outlines indicate enthesis regions. Scalebars: 100 μm.