Abstract
Purpose
The current project was developed to obtain natural history information regarding cisplatin-induced peripheral neuropathy in males with testicular/germ cell cancers and to compare such neuropathy data with similarly obtained data in patients receiving other chemotherapy drugs in similarly conducted clinical trials.
Methods
Patients without baseline neuropathy symptoms, who were initiating cisplatin-based chemotherapy, completed the EORTC CIPN 20 patient-reported instrument to evaluate chemotherapy-induced peripheral neuropathy (CIPN). Results were compared to EORTC CIPN 20 data obtained from independent study sets regarding patients receiving 1) paclitaxel, 2) combined paclitaxel and carboplatin, 3) oxaliplatin, or 4) a combination of doxorubicin and cyclophosphamide (AC). The last study set of patients on AC was selected to evaluate the use of EORTC CIPN 20 data in patients receiving chemotherapy not known to cause CIPN.
Results
Cisplatin-induced neuropathy was more similar to neuropathy in patients receiving oxaliplatin than in those receiving paclitaxel. The cisplatin and oxaliplatin groups exhibited the coasting phenomenon and more prominent upper extremity symptoms than lower extremity symptoms during chemotherapy administration weeks. In contrast, paclitaxel-treated patients did not, on average, exhibit the coasting phenomenon; additionally, lower extremity symptoms were more prominent during the weeks when paclitaxel was administered. Cisplatin-induced neuropathy was less severe than was seen in patients in the other two groups, potentially because the cisplatin-receiving patients were younger. Patients receiving AC did not report substantial EORTC CIPN 20 changes.
Conclusion
Understanding neuropathy similarities and differences with various chemotherapy agents may help elucidate CIPN processes and facilitate means to prevent and/or treat established CIPN.
Keywords: Cisplatin, neuropathy, CIPN, chemotherapy-induced peripheral neuropathy
Structured Abstract
The manuscript provides new insights into the natural history of cisplatin-induced neuropathy via the use of the EORTC CIPN-20 instrument. Through comparing and contrasting to other neurotoxic and non-neurotoxic chemotherapy agents, including oxaliplatin, paclitaxel, and doxorubicin/cyclophosphamide, unique differences in chemotherapy-induced peripheral neuropathy (CIPN) are better understood. Such information should facilitate the evaluation of neuropathy for clinical trials and may help elucidate better means for neuropathy prevention.
Background
Chemotherapy-induced peripheral neuropathy (CIPN) is a major dose-limiting toxicity of many chemotherapeutic regimens. While CIPN is not a major problem with all cytotoxic agents, a prominent subset of chemotherapy drugs is clearly associated with substantial neuropathy. These drugs include taxanes (i.e., paclitaxel, nab-paclitaxel and docetaxel), platinum agents (i.e., cisplatin, oxaliplatin, and carboplatin), vinca alkaloids (i.e., vincristine), epothilones (i.e., ixabepilone), bortezomib, thalidomide and lenalidomide.
To date, efforts to prevent and/or treat established CIPN have fallen short, as evidenced by the most recently published ASCO clinical practice guidelines regarding CIPN.1
While there are many similarities regarding neuropathy caused by different chemotherapy drugs, there are also distinct differences among them. For example, some drugs, such as paclitaxel and oxaliplatin, can cause distinct acute neuropathy syndromes that become quite prominent within hours to days after a single dose of chemotherapy. Acute neuropathy symptoms decrease in intensity over several days and then reoccur with each subsequent dose of chemotherapy (resulting in a sawtooth pattern of neuropathy).2–4 These symptoms generally reverse once chemotherapy is completed. The acute neuropathy symptoms from these two drugs are markedly different from each other. Oxaliplatin is well known for causing cold-induced sensitivities, while paclitaxel causes a more central pain syndrome that for years was labeled as “paclitaxel- associated arthralgia/myalgia”. These acute neuropathy syndromes, despite being quite bothersome, are usually not dose limiting.
While not all neurotoxic chemotherapy drugs cause this acute neuropathy problem, virtually all of them result in a relatively gradual steady worsening of numbness, tingling, and pain (in contrast to the sawtooth pattern associated with the acute neuropathy problems noted above). These neuropathy symptoms, which are usually most prominent in distal extremities and are usually termed as being a more chronic neuropathy, generally initially manifest as sensory symptoms such as paresthesia and dysesthesia (numbness, tingling, abnormal touch sensations). 5 Pain is often reported and may be described as burning, freezing, lancination, shock-like, or electric. Normal touch may be perceived as painful (allodynia), and sensations that would normally be painful can become excruciating (hyperpathia/hyperalgesia). Motor symptoms are less common and usually milder. This constellation of neuropathy symptoms can steadily worsen with each dose of chemotherapy, and often limits the doses of chemotherapy that can be given.
There are also differences in the more chronic dose-limiting neuropathies of these drugs. While a number of manuscripts have reported on characteristics of the chronic neuropathies of different cytotoxic agents, there have not been any substantial comparisons of neuropathies from different chemotherapy drugs using the same measure of neuropathy. Utilizing a similar measure of neuropathy for different drugs may allow an investigator to compare and contrast neuropathy syndromes between them. This may facilitate understanding of the causes of neuropathy and, potentially, facilitate therapeutic management.
Given this gap in the literature, an effort was begun, several years ago, to compare and contrast neuropathies from different cytotoxic drugs, using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-CIPN twenty-item scale (EORTC CIPN 20) instrument as a common measure of chronic neuropathy. This allowed detailed evaluations of neuropathy caused by weekly administered paclitaxel,3 combined paclitaxel and carboplatin in 3- week cycles,6 oxaliplatin,4,7 and a head-to-head comparison of these three treatments.7
The current project (Alliance for Clinical Trials in Oncology A151724) expands this effort by using the EORTC CIPN 20 instrument to help define cisplatin-induced neuropathy in patients with germ cell tumors and to allow comparisons with the clinical manifestations/patterns of paclitaxel- and oxaliplatin-induced CIPN.
Additionally, the specificity of the EORTC CIPN 20 has not been previously evaluated. In other words, is the test reliably negative in patients undergoing chemotherapy known not to cause CIPN? To further validate the EORTC CIPN 20 tool, the current work evaluates how well the EORTC CIPN20 performed in reliably ruling out the presence of CIPN in patients receiving nonneurotoxic chemotherapy drugs, specifically doxorubicin (Adriamycin) and cyclophosphamide (AC).
Materials and Methods
Eligible patients for the cisplatin evaluation part of this project needed to be at least 15 years old and have a diagnosis of testicular or extragonadal germ cell cancer. Participants provided written informed consent and were able to complete questionnaire(s) in English by themselves or with assistance. They were scheduled to receive at least 3 cycles of cisplatin-based chemotherapy (20 mg/m2/d for 5 days) and agreed to continued clinical follow-up at one of two study cancer centers (Indiana University or Mayo Clinic Rochester).
Patients were ineligible if they had a diagnosis (current or previous) of peripheral neuropathy (e.g., from diabetes or other causes) or previous exposure to neurotoxic chemotherapy drugs including taxanes, platinum agents, vinca alkaloids, or epothilones.
Once recruited, the patients completed the EORTC CIPN 20 instrument prior to starting cisplatin-based chemotherapy and prior to each subsequent chemotherapy cycle. Following completion of chemotherapy, the participants completed the EORTC CIPN 20 instrument at 2- month intervals for 18 months.
The EORTC CIPN 20 is a well-established patient-reported outcome (PRO) tool, understanding that PROs are better than clinician-measured tools for a variety of symptoms, including CIPN. The EORTC CIPN 20 is a 20-item self-report questionnaire that contains 9 items assessing sensory function, 8 items assessing motor function, and 3 items assessing autonomic function. Items are scored on a Likert scale from 1 to 4 with 1 representing no symptoms (“not at all”) and 4 representing “very much” symptoms. The EORTC CIPN 20 has been tested in cancer patients receiving a variety of chemotherapy agents, and has been shown to be reliable, valid, and responsive to change. Cronbach’s alpha coefficients for the three subscales are 0.82, 0.73, and 0.76, respectively.8,9
The EORTC CIPN 20 was scored according to standard scoring algorithms and all subscales and individual items were then converted to a 0–100 scale, such that higher scores corresponded to improved quality of life. From a statistical standpoint, descriptive statistics and graphical plots were utilized to summarize the three subscale scores (sensory, motor, and autonomic) and individual items over the course of cisplatin treatment and for 18 months following completion of cisplatin. The change from baseline, in absolute scale and in percentage, was plotted.
To examine the psychometric properties of the EORTC CIPN 20 in a population of patients receiving non-neurotoxic chemotherapy, patients with breast cancer undergoing neoadjuvant or adjuvant standard of care AC chemotherapy were asked to complete the EORTC CIPN 20 instrument prior to initiation of chemotherapy, every 2 weeks during chemotherapy for a total of 4 cycles, and at end of treatment. Questionnaires in this study were not completed in the months following chemotherapy completion. Eligibility criteria included female gender, aged 18 years or older, with an Eastern Clinical Oncology Group (ECOG) performance status of 0–1, with planned treatment of 4 14-day cycles of neoadjuvant or adjuvant doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2. Participants were required to be able to self-complete questionnaires, or with or without assistance. Participants must have had an oral reply of 0/10 to the following question: How much of a problem is numbness, tingling, or shooting/burning pain in your hands or feet, on a scale of 0 to 10? Exclusion criteria included a previous diagnosis of peripheral neuropathy from any cause, diagnosis (current or previous) of fibromyalgia, currently using or having used within last 6 months gabapentin or pregabalin, and prior exposure to neurotoxic chemotherapy. Investigations in Alliance A151724 received institutional internal review board approval per United States federal guidelines. All analyses were based on the study database frozen on November 19, 2018.
Results from the two investigations were compared to data from groups of patients who completed the same tool during and after receiving other chemotherapy agents (i.e., oxaliplatin, weekly paclitaxel, and a combination of paclitaxel/carboplatin every 3 weeks), to compare and contrast neuropathy characteristics with these different neurotoxic agents.3,4,6,7 Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center.
Results
Cisplatin patients
Fifty-four patients were accrued to the cisplatin portion of the study from July 2015 through January 2018. Of these 54 patients, one patient did not complete a baseline questionnaire and therefore was not included in these analyses. Table I depicts on-study patient characteristics.
Table 1:
Demographics for cisplatin patients
| Total (N=54) | |
|---|---|
| Age (years) | |
| N | 54 |
| Mean (SD) | 32.9 (11.48) |
| Median | 30.0 |
| Range | 16.0, 67.0 |
| Gender, n (%) | |
| Male | 54 (100.0%) |
| Race, n (%) | |
| Black or African American | 1 (2%) |
| More than one race | 1 (2%) |
| Native Hawaiian or Other Pacific Islander | 1 (2%) |
| Other | 2 (4%) |
| Unknown | 4 (7%) |
| White | 45 (83%) |
| Ethnicity, n(%) | |
| Hispanic or Latino | 5 (9%) |
| Non-Hispanic | 46 (85%) |
| Unknown | 3 (6%) |
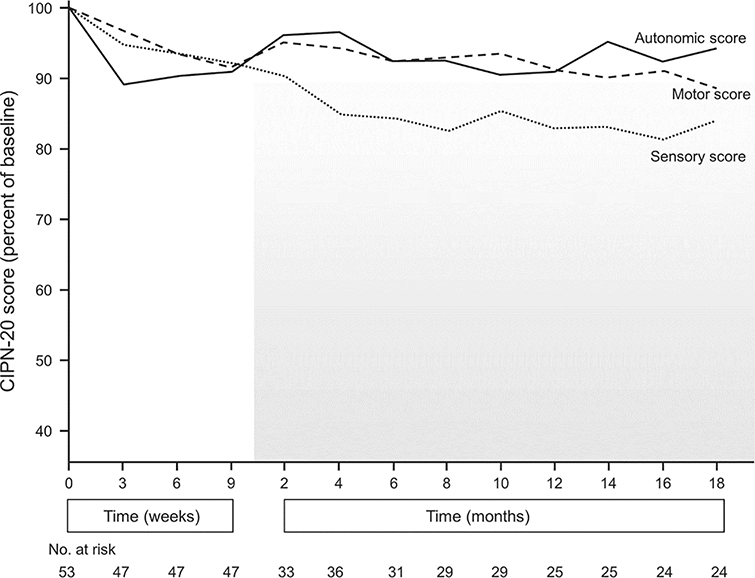
Figure 1 illustrates the EORTC CIPN 20 sensory, motor and autonomic subscale scores during treatment and for 18 months following completion of cisplatin. Most patients received 9 weeks of cisplatin-based chemotherapy. Autonomic, motor and sensory neuropathy scores worsened during chemotherapy, but after cisplatin was stopped, autonomic and motor neuropathy improved, nearly back to baseline. For the sensory neuropathy score, symptoms worsened for several months after finishing chemotherapy, consistent with the so called “coasting”4 phenomenon. Sensory neuropathy, after worsening during the coasting stage, eventually plateaued, although it did not approach the prechemotherapy baseline.
Figure 1:
Sensory, motor and autonomic EORTC CIPN 20 scores while patients were receiving cisplatin and for 18 months following completion of it (shaded area).
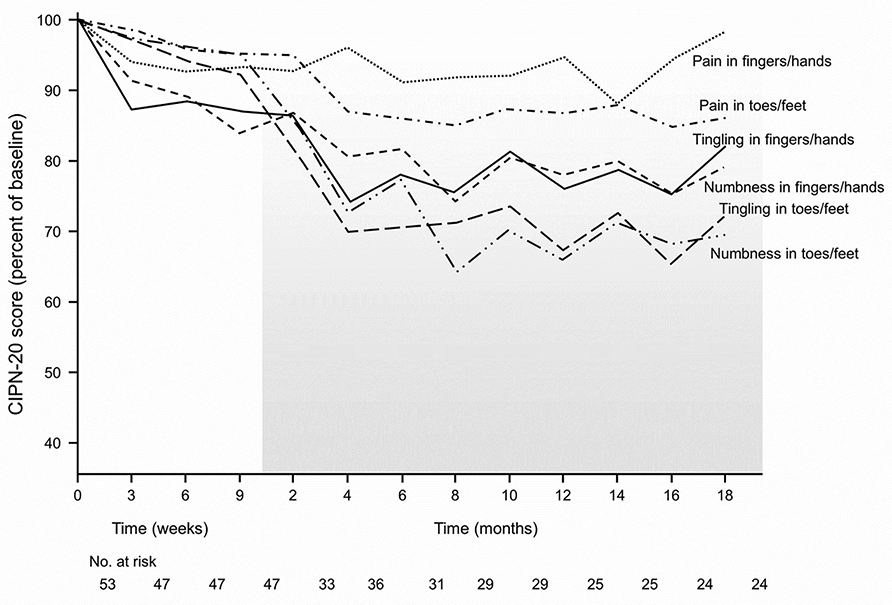
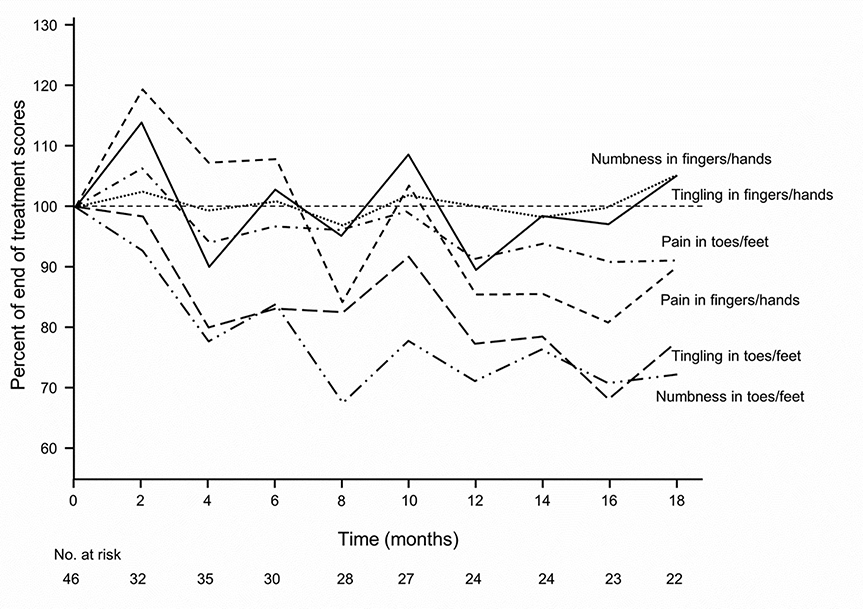
Figure 2 separates the main components of the sensory subscale into the six individual items relating to numbness or tingling or shooting/burning pain, in both upper and lower extremities. This figure highlights a number of key findings. Painful neuropathy in the fingers and hands develops during chemotherapy and then plateaus after stopping chemotherapy. In contrast, painful neuropathy in the toes/feet likewise starts during chemotherapy but then worsens after stopping. Nonetheless, painful neuropathy in both locations is relatively mild. Tingling and numbness in the hands and feet substantially worsen after stopping chemotherapy. It is important to note that neuropathy symptoms of tingling and numbness are notably worse in the toes/feet after discontinuation of chemotherapy than in fingers/hands. This figure illustrates that shooting/burning pain is much less of a problem during treatment and follow-up than is numbness and/or tingling. Figure 3 also illustrates numbness, tingling, and shooting/burning pain in upper and lower extremities in terms of changes in these factors for patients who completed chemotherapy and had at least one additional post-treatment follow-up. This clearly depicts that numbness and tingling in the toes and fingers, on average, worsen after treatment with cisplatin-based chemotherapy and then plateau, but do not return to baseline. These two figures reveal that numbness and tingling are more prominent problems in the upper extremities than in the lower extremities during treatment, but that these two symptoms become more prominent in the lower extremities during the follow-up period.
Figure 2:
Numbness, tingling, and shooting/burning pain EORTC CIPN 20 individual item scores in upper extremities and in lower extremities, for patients who received cisplatin.
Figure 3:
Numbness, tingling, and shooting/burning pain in upper extremities and in lower extremities, in terms of changes in these factors during the months after completion of chemotherapy, for patients who received cisplatin (shaded area)..
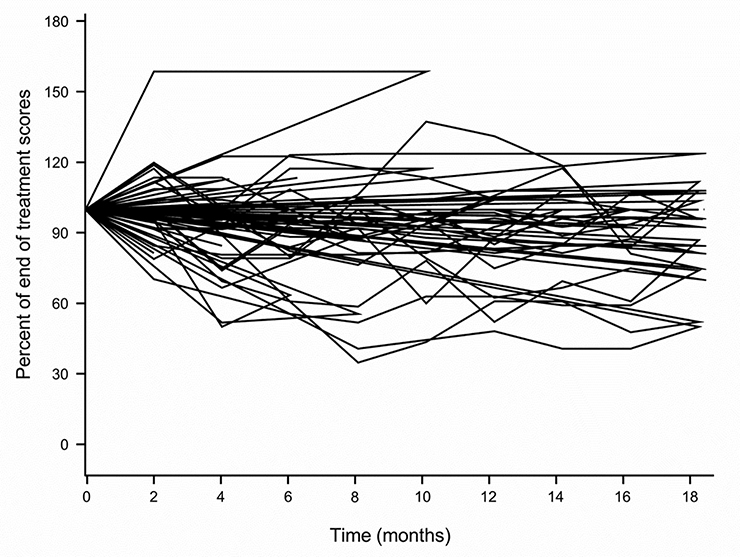
Figure 4 illustrates changes in EORTC CIPN 20 sensory neuropathy scores in individual patients following completion of cisplatin treatment, showing that, while most scores worsened during this period of time, in some patients the symptoms remained stable or improved.
Figure 4:
Changes in EORTC CIPN 20 sensory neuropathy scores following completion of cisplatin treatment, for individual patients.
AC patients
Eighteen patients receiving AC were evaluated. Table II illustrates on-study patient characteristics.
Table II:
Demographics for AC patients
| Total (N=18) | |
|---|---|
| ECOG Performance Score | |
| 0 | 16 (89%) |
| 1 | 2 (11%) |
| Has the patient been diagnosed with fibromyalgia? N (%) | |
| No | 17 (94.4%) |
| Unknown | 1 (5.6%) |
| Has patient used gabapentin or pregabalin in the past 6 months? N (%) | |
| No | 18 (100%) |
| Has the patient been diagnosed with peripheral neuropathy? N (%) | |
| No | 18 (100%) |
| Patient Weight (kg) | |
| Mean (SD) | 77.5 (16.91) |
| Median | 77.0 |
| Range | 50.0, 112.0 |
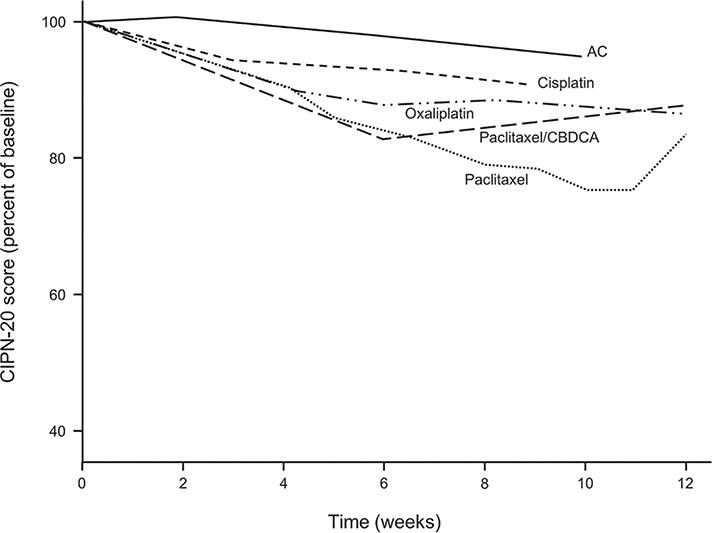
Figure 5 shows data regarding the EORTC CIPN 20 sensory subscale for patients receiving AC chemotherapy on a plot that provides the same data in patients receiving cisplatin in the current study and, additionally, portrays previously published data from groups of patients receiving: 1) oxaliplatin,4 2) weekly paclitaxel,3 and combination paclitaxel/carboplatin every 3 weeks.6 This figure supports that fewer EORTC CIPN 20 abnormalities are seen in patients receiving AC than are seen in patients who receive chemotherapy regimens known to cause chemotherapy neuropathy.
Figure 5:
EORTC CIPN 20 sensory subscale scores for patients who received AC chemotherapy, cisplatin, oxaliplatin, weekly paclitaxel, and a combination of paclitaxel/carboplatin. These data were obtained while patients were receiving chemotherapy or within 2 weeks of their last dose.
Discussion
Noting that the EORTC CIPN 20 scores following treatment with AC are better than any of the scores from the other neurotoxic chemotherapy drugs further confirms that the EORTC CIPN 20 instrument is a measure of chemotherapy-induced neuropathy, as opposed to it just being a measure of generalized toxicity associated with chemotherapy. These data provide evidence of contrasting-group/discriminant validity and adds to the extensive information that supports that this instrument is among the best patient-reported outcome tools for evaluating chronic neuropathy in patients receiving chemotherapy.10
How is cisplatin-induced neuropathy different from oxaliplatin-induced neuropathy? Oxaliplatin is well-known for causing an acute neuropathy syndrome characterized by cold hyperaesthesia that worsens after each dose of treatment and then tends to improve before the next dose of drug is given.4 Of note, cisplatin is not associated with acute neuropathy, and therefore, this was not directly examined in the current study.
An additional finding of note is that the EORTC CIPN 20 autonomic neuropathy score is more substantially suppressed during the time of cisplatin therapy (Figure 1) than was seen in previous reports related to oxaliplatin and taxanes.3,4,6,7 This may be because one of the items that denotes evidence of autonomic neuropathy on the EORTC CIPN 20 scale is impotence, and this may have been related to the testicular cancer process or its associated surgery.
Regarding similarities among cisplatin- and oxaliplatin-induced neuropathies, the coasting phenomenon, which is the continued worsening of neuropathy symptoms for a few months after the neuropathic chemotherapy drug has been stopped, is prominently observed with both cisplatin and oxaliplatin. With both of these drugs, coasting was not prominently observed with shooting/burning pain in either the upper or lower extremities. It was, however, quite prominent with tingling and numbness in the lower extremities.4,7 With oxaliplatin, the coasting phenomenon was also observed with numbness in upper extremities,4 while it was not observed with numbness in the upper extremities in patients who received cisplatin in the current study. Coasting has been reported in other trials evaluating cisplatin neuropathy 11–14 and oxaliplatin neuropathy. 4,15–17 In contrast, coasting has not been observed with groups of patients receiving paclitaxel (although individual patients, who received paclitaxel, can have worsening symptoms after finishing it). 7
Another feature, seen with both cisplatin and oxaliplatin neuropathy, is that upper extremity symptoms are more prominent than lower extremity symptoms during the time of chemotherapy administration. With both of these drugs, the upper extremity symptoms improved more in the months following chemotherapy completion, while lower extremity symptoms worsened. This more prominent problem in lower extremities, in the years following completion of platinum drugs, has also been reported by other authors.17–20 This contrasts with what is seen with patients receiving paclitaxel (alone and/or with carboplatin), wherein lower extremity symptoms are more prominent, than upper extremity symptoms, both while the patient is receiving chemotherapy and during the months following completion of paclitaxel.3
A similarity between cisplatin, oxaliplatin and paclitaxel is that shooting/burning pain is much less prominent than are numbness and tingling, especially during the post-treatment follow-up period.
It is interesting to note that the cisplatin-treated patients appeared to have less neuropathy than was seen with oxaliplatin (Figure 5) in a previous trial using the same EORTC CIPN 20 instrument.4 One potential explanation for this phenomenon is that the cisplatin-treated patients were much younger (median age 30 years) than were patients in the trial who had received oxaliplatin (median age 56 years).21 Another possibility might be related to gender, as all of the cisplatin-treated patients were male.
It is hoped that the currently described similarities and differences between cisplatin, oxaliplatin, and paclitaxel will provide clues to the pathophysiological mechanisms that cause neuropathies, and will lead to means to counteract CIPN without inhibiting anticancer activity. One theoretical possibility is that the difference in clinical manifestations related to upper versus lower extremity neuropathy might be related to platinum effects on the dorsal root ganglion, which anatomically is closer to the tips of upper extremity nerve fibers. This may explain why, with platinum- induced neuropathy, upper extremity symptoms are more impaired initially. With taxanes, the pathology is thought to be more from microtubule injury, impairing transport of nutrients to the nerves. Thus, dying back may be experienced at the tips of the longest nerves first, potentially explaining why more lower extremity problems are seen in the early neuropathy process.
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), UG1CA233160, UG1CA233196, UG1CA233339, and UG1CA232760; https://acknowledgments.alliancefound.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Potential conflicts of interest: Dr. Einhorn owns Amgen and Biogenidec stock; Dr. Loprinzi reports personal fees from PledPharma, personal fees from Disarm Therapeutics, personal fees from Asahi Kasei, personal fees from Metys Pharmaceuticals, personal fees from OnQuality, personal fees from NKMax, and personal fees from Mitsubishi Tanabe, outside the submitted work; Dr. Wagner-Johnston reports personal fees from Regeneron, ADC Therapeutics, CALIB- R, Verastem, outside the submitted work; None of the other authors noted any potential conflicts of interest.
Clinicaltrials.gov Identifier NCT02677727
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Hershman DL, Lacchetti C, Dworkin RH, et al. : Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:1941–67, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Loprinzi CL, Maddocks-Christianson K, Wolf SL, et al. : The Paclitaxel acute pain syndrome: sensitization of nociceptors as the putative mechanism. Cancer J 13:399–403, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Loprinzi CL, Reeves BN, Dakhil SR, et al. : Natural history of paclitaxel- associated acute pain syndrome: prospective cohort study NCCTG N08C1. J Clin Oncol 29:1472–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pachman DR, Qin R, Seisler D, et al. : Clinical course of patients with oxaliplin- associated neuropathy: N08CB (Alliance). J Clin Oncol 32:3595, 2014 [Google Scholar]
- 5.Wolf SL, Barton DL, Qin R, et al. : The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Support Care Cancer 20:625–32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves BN, Dakhil SR, Sloan JA, et al. : Further data supporting that paclitaxel- associated acute pain syndrome is associated with development of peripheral neuropathy: North Central Cancer Treatment Group trial N08C1. Cancer 118:5171–8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pachman DR, Qin R, Seisler D, et al. : Comparison of oxaliplatin and paclitaxel- induced neuropathy (Alliance A151505). Support Care Cancer 24:5059–5068, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavoie Smith EM, Barton DL, Qin R, et al. : Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual Life Res 22:2787–99, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postma TJ, Aaronson NK, Heimans JJ, et al. : The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ- CIPN20. Eur J Cancer 41:1135–9, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Gewandter JS, Brell J, Cavaletti G, et al. : Trial designs for chemotherapy-induced peripheral neuropathy prevention: ACTTION recommendations. Neurology 91:403–413, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunberg SM, Sonka S, Stevenson LL, et al. : Progressive paresthesias after cessation of therapy with very high-dose cisplatin. Cancer Chemother Pharmacol 25:62–4, 1989 [DOI] [PubMed] [Google Scholar]
- 12.LoMonaco M, Milone M, Batocchi AP, et al. : Cisplatin neuropathy: clinical course and neurophysiological findings. J Neurol 239:199–204, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Podratz JL, Knight AM, Ta LE, et al. : Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis 41:661–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quasthoff S, Hartung HP: Chemotherapy-induced peripheral neuropathy. J Neurol 249:9–17, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Choi J, Kong K, Mozaffar T, et al. : Delayed oxaliplatin-associated neurotoxicity following adjuvant chemotherapy for stage III colon cancer. Anticancer Drugs 17:103–5, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Lehky TJ, Leonard GD, Wilson RH, et al. : Oxaliplatin-induced neurotoxicity: acute hyperexcitability and chronic neuropathy. Muscle Nerve 29:387–92, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kidwell KM, Yothers G, Ganz PA, et al. : Long-term neurotoxicity effects of oxaliplatin added to fluorouracil and leucovorin as adjuvant therapy for colon cancer: results from National Surgical Adjuvant Breast and Bowel Project trials C-07 and LTS-01. Cancer 118:5614–22, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mols F, Beijers T, Lemmens V, et al. : Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 31:2699–707, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Land SR, Kopec JA, Cecchini RS, et al. : Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol 25:2205–11, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Tofthagen C, McAllister RD, McMillan SC: Peripheral neuropathy in patients with colorectal cancer receiving oxaliplatin. Clin J Oncol Nurs 15:182–8, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Loprinzi CL, Qin R, Dakhil SR, et al. : Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol 32:997–1005, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]