Abstract
Cholangiocarcinoma (CCA) is an aggressive and heterogeneous malignancy of the biliary tree. A typical hallmark of CCA is that cancer cells are embedded into a dense stroma containing fibrogenic cells, lymphatics and a variety of immune cells. Functional roles of the reactive tumor stroma are not fully elucidated; however, recent studies suggest that the tumor microenvironment plays a key role in the progression and invasiveness of CCA. CCA cells exchange autocrine/paracrine signals with other cancer cells and the infiltrating cell types that populate the microenvironment. This crosstalk is under the control of signals mediated by various cytokines, chemokines, and growth factors. In addition, extracellular vesicles (EVs), exosomes and microvesicles, containing cargo mediators, such as proteins and RNAs, play a key role in cell-to-cell communication, and particularly in epigenetic regulation thanks to their content in miRNAs. Both cytokine- and EV-mediated communications between CCA cells and other liver cells provide a potential novel target for the management of CCA. This review summarizes current understandings of the tumor microenvironment and intercellular communications in CCA and their role in tumor progression.
Keywords: Biliary cancer, tumor microenvironment, fibroblasts, macrophages, extracellular vesicles
Introduction
Cholangiocarcinoma (CCA) is a malignancy with features of the biliary epithelium. Although CCA is recognized as a rare cancer, it is the second most common primary liver tumor after hepatocellular carcinoma (HCC), and accounts for 10-20% of the deaths in hepatobiliary malignancies (1). Excluding carcinoma of the gallbladder, CCA can be categorized into 3 major classes according to their anatomical origin : intrahepatic CCA (iCCA), hilar CCA (hCCA), or extrahepatic CCA (eCCA) (2). The histogenesis of CCA is therefore heterogeneous as it may derive from: i) Cholangiocytes; ii) Hepatic progenitor cells; iii) Liver stem cells; and iv) Biliary transdifferentiation of periportal hepatocytes (3). These types of CCA differ in their clinical manifestations, risk factors, natural history, genomics, and responsiveness to chemotherapy. The early diagnosis of CCA is challenging, and the cancer is aggressive which leads to poor prognosis, high mortality rates, and limited treatment options (4). Even patients who undergo potentially curative surgical resection experience a high rate of recurrence and early local or distant metastases (5).
A typical histological feature of CCA is desmoplasia, i.e. the presence of abundant fibrotic stroma that surrounds and infiltrates the tumor structures and a rich tumor microenvironment (Figure 1) (6). Instructed by CCA cells, several other cell types populate the CCA microenvironment such as: cancer-associated fibroblasts (CAFs), endothelial and lymphatic cells, tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), regulatory T lymphocytes (Tregs), and natural killer (NK) cells. These cells contribute to CCA progression and metastases through various mechanisms such as increased migration, suppression of immune responses, and induction of angiogenesis and lymphangiogenesis (6). This review summarizes the current understandings of the different cell types that dwell in the CCA microenvironment and the signals they exchange to support the evidence that the tumor microenvironment modulates the invasiveness and progression of CCA.
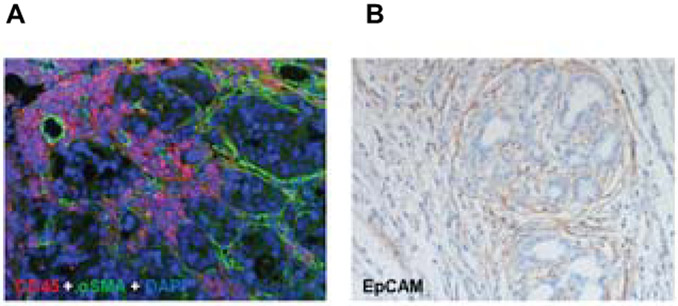
Figure 1. Spatial relationship of stromal cells populating the tumor microenvironment in CCA.
A) Tumoral ducts are embedded in a dense stroma, populated by CAFs identified by α-SMA (green). Inflammatory cells are recognized by their expression of CD45 (red) including macrophages and neutrophils. Nuclei are stained by DAPI (blue) (dual immunofluorescence, original magnification: 100X). B) A rich lymphatic bed decorated by the antibody recognizing the lymphatic endothelial cell marker EpCAM (clone D2-40) closely aligns the periphery of the tumoral areas (immunohistochemistry, original magnification: 100X). Histological sections were obtained from a surgical sample of a patient with intrahepatic cholangiocarcinoma undergoing hepatic resection.
Functional roles of the CCA microenvironment
Cancer-associated fibroblasts (CAFs)
CAFs are a group of activated myofibroblasts expressing several phenotypic markers such as α-smooth muscle actin (α-SMA), the mucin-like transmembrane glycoprotein podoplanin, and the cell surface metalloprotease CD10 (7). As shown in Figure 1, immunohistochemistry shows high numbers of α-SMA-positive myofibroblasts in CCA tissues. It has been shown that high α-SMA expression correlates to poor survival rates of patients (8). CAFs are the major cells which contribute to tumoral fibrogenesis and most likely to tumor progression in CCA (9). The origin of CAFs in CCA is heterogeneous and still controversial. Using lineage-tracing experiments, CAFs were shown to originate from liver resident hepatic stellate cells (HSCs) (10), portal fibroblasts (11), or bone marrow-derived circulating mesenchymal cells (12). On the other hand, their origin from epithelial-to-mesenchymal transition of CCA cells has been ruled out (13-15).
CAFs release a number of paracrine mediators including transforming growth factor β1 (TGF-β1), hepatocyte growth factor (HGF), epidermal growth factor (EGF), connective tissue growth factor (CTGF), stromal cell–derived factor-1 (SDF-1), extracellular matrix (ECM) components, such as periostin, tenascin-C, and osteopontin, and a variety of matrix metalloproteases (MMPs), such as MMP1, MMP2, MMP3, and MMP9 (16). These mediators perform a range of pro-oncogenic cell functions and can shape the microenvironment to become more supportive to tumor growth and invasiveness (Figure 2). This concept is confirmed by transcriptomic analysis of the stromal component of CCA showing that the expression levels of profibrogenic and proinflammatory cytokines, TGF-β1 and tumor necrosis factor alpha (TNFα), are negatively correlated with clinical outcome (17). CCA cells and CAFs engage in an extensive network of mutual communications, which sustain and enhance the malignant properties of tumor cells. This negative circle is exemplified by the production of heparin-binding (HB) EGF by CAFs, which binds to EGF receptor (EGFR) on CCA cells, resulting in the transcriptional activation of β-catenin signaling via extracellular signal-regulated kinase (ERK) 1/2 and signal transducer and activator of transcription 3 (STAT3) promoting tumor cell motility and invasion (18). Upon EGFR activation, CCA cells secrete TGF-β1, further inducing myofibroblast activation and secretion of HB-EGF (18). SDF-1 is another mediator originating from CAFs, which interacts with the C-X-C chemokine receptor 4 (CXCR4) expressed by CCA cells, and stimulates tumor cell invasion via ERK1/2 and AKT signaling (19).
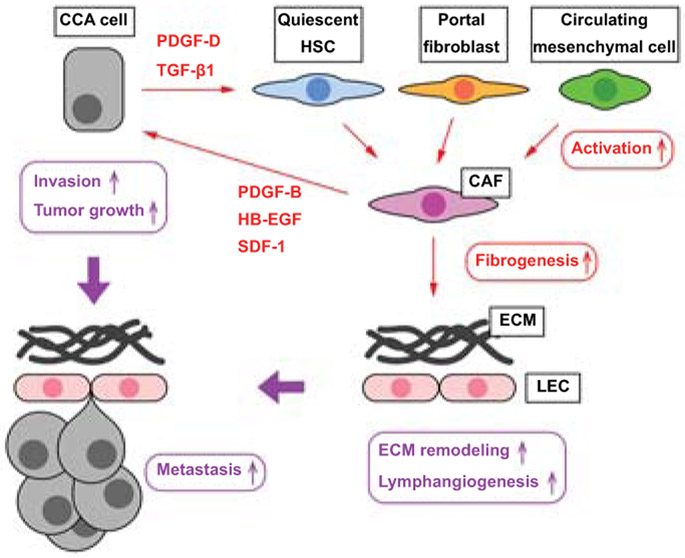
Figure 2. The crosstalk between CCA cells and CAFs.
CCA cells secrete mediators such as PDGF-D and TGF-β1, which induce differentiation of hepatic stellate cells, portal fibroblasts, or circulating mesenchymal cells into activated CAFs. CAFs secrete mediators including PDGF-B, HB-EGF, and SDF-1 leading to CCA tumor growth and invasion. CAFs also contribute to fibrogenesis, leading to ECM remodeling, and to lymphangiogenesis, promoting CCA invasion through the lymphatic endothelial cell (LEC) barrier. This tumor microenvironment is proficient to CCA progression and metastases.
CCA tumor cells secrete TGF-β1 as well as platelet-derived growth factor D (PDGF-D), thereby triggering fibroblast recruitment. PDGF-D signals through its cognate receptor PDGFRβ that is expressed by activated fibroblasts, and through the downstream effectors, Rho GTPase and c-Jun N-terminal kinase (JNK) (20). PDGF-D induces CAFs to secrete vascular endothelial growth factors (VEGF)-A/C, which promote tumor lymphangiogenesis and increase the ability of CCA cells to cross the endothelial wall invading the lymphatics (21). PDGF-B, another member of the PDGF family, is released by CAFs and interacts with PDGFRβ in CCA cells (22). PDGF-B has been shown to activate Hedgehog signaling, which stimulates tumor cell resistance to TNFα-related apoptosis inducing ligand (TRAIL) (22). In an orthotopic syngeneic rat model of CCA, Hedgehog blockade by cyclopamine suppressed tumor growth by inducing CCA cell apoptosis (22).
The tumor-promoting functions by CAFs in CCA are supported by in vivo studies performed in an experimental rat model of syngeneic CCA in which CAF depletion was achieved by inducing their apoptosis with the BH3 mimetic, navitoclax (23). Selective targeting of CAFs by navitoclax is reliant on their unique profile of the Bcl-2 proteins regulating apoptosis (23). CAF depletion reduced the tumor growth in the primary site, the tumor lymphatic vascularization, and the metastases to the regional lymph nodes and the peritoneum (21, 23).
CAFs also modulate the functions of immune cells. A recent study has highlighted the importance of the interaction between CAFs and myeloid-derived suppressor cells (MDSCs), a population of circulating cells displaying strong immunosuppressive functions (24). A CAF subset is also identified by elevated expression of the fibroblast activation protein (FAP), and FAP-overexpressing CAFs display an active pro-inflammatory secretory profile, which is sustained by CCL2-STAT3 signaling, leading to the inhibition of T cell proliferation and MDSC infiltration in murine CCA models (25). Immunosuppressive activities of CAFs are also related to their functional interactions with dendritic cells (DCs), which represent the connection between innate and immune cell responses. CAFs attract DCs and dampen the expression of antigen-presenting HLA molecules, thereby, impairing the capability to activate tumor-infiltrating lymphocytes (TILs), and to stimulate immunosuppressive functions (26).
Tumor-associated macrophages (TAMs)
There are two types of hepatic macrophages: the liver resident Kupffer cells and bone marrow-derived macrophages. During liver injury such as cholestatic liver injury, both types of macrophages can be activated as classic inflammatory M1 or alternative anti-inflammatory M2 subsets (27). Activated macrophages secrete cytokines including IL-6, TNFα, and TGF-β1, which trigger cholangiocyte proliferation, fibrogenesis, and biliary carcinogenesis (28). In CCA tumor tissues, high infiltration of macrophages (TAMs) has been associated with poor outcomes (29). Although functional differences between Kupffer cells and bone marrow-derived macrophages are still undefined, a study has demonstrated that Kupffer cells express increased levels of TNFα near the iCCA lesions leading to cholangiocyte proliferation as well as carcinogenesis via activation of JNK signaling (30). Bone marrow-derived macrophages are recruited by various mediators released by tumoral and stromal cells, such as cytokines (IL-1β, IL-10, IL-13, and IL-4), monocyte chemoattractant protein-1 (MCP-1), colony stimulating factor 1 (CSF-1), and VEGF-A (31). Macrophage recruitment and differentiation into TAMs is driven by a specific subgroup of CCA cells mediated by IL-13, IL-34, and osteoactivin (32). A previous study showed that infiltrated hepatic macrophages were activated predominantly as M2 subsets in patients with iCCA (33). In the cancer stem cell niche, TAMs express both M1 and M2 phenotypes, and show increased adhesive and invasive functions in vitro suggesting that TAMs have multiple functions within the tumor mass (32). TAMs can exert pro-invasive effects at multiple levels (Figure 3). First, TAMs induce tumoral angiogenesis by secreting pro-angiogenic factors, such as VEGFs and angiopoietins (34). Second, TAMs contribute to CCA cell proliferation by activating the Wnt/β-catenin pathway upon release of Wnt3a and Wnt7b (35, 36). Third, TAMs can suppress anti-tumor functions of T cells and contribute to tumor growth via increased expression of hypoxia inducible factor-1 (HIF-1α), which is associated with increased metastases and poor survival rates in CCA patients (37, 38).
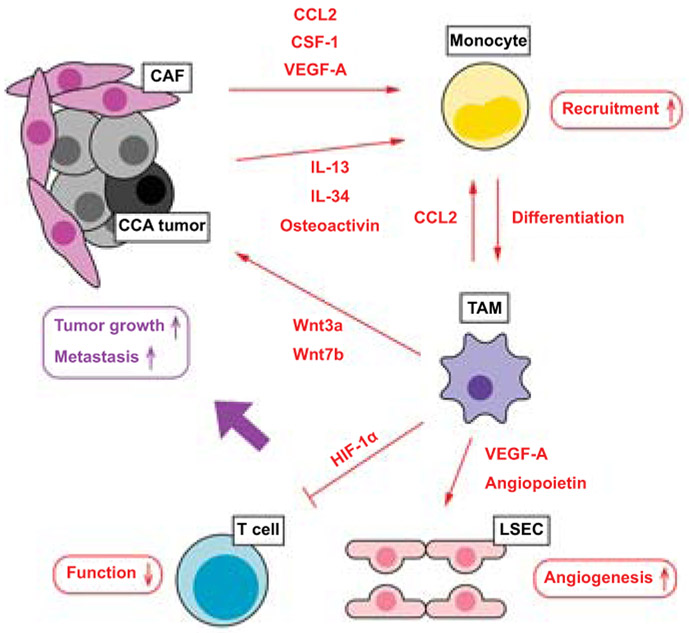
Figure 3. The interplay between CCA cells with CAFs and TAMs.
CCA tumor cells and CAFs secrete various mediators, such as CCL2, CSF-1, and VEGF-A, which attract circulating monocytes into the tumor area. A subset of CCA cells promote differentiation of monocyte-derived TAMs by secreting IL-13, IL-34 and osteoactivin. These mediators especially CCL2 induce monocyte differentiation into TAMs. Activated TAMs educate the tumor microenvironment to become more permissive to tumor growth and invasion at different levels. TAMs stimulate angiogenesis by secreting VEGF-A and angiopoietin, which act on liver sinusoidal endothelial cell (LSEC). TAMs induce tumor growth directly by secreting Wnt proteins (Wnt3a and Wnt7b). TAMs also dampen anti-tumor functions of T cells by inducing expression of HIF-1α.
Tumor-associated neutrophils (TANs)
Although hepatic neutrophils generally play a role in inflammatory responses and pathogen removal during infections and other liver injuries, a recent study has shown that neutrophils (TANs) also play a vital role in the development and progression of cancer (39). Analysis of eCCA tissues identified infiltrated Tregs and TANs, and elevated numbers of these cells were correlated with poor survival rates in patients (40). Accumulation of TANs at tumor area also correlates with increased aggressiveness in iCCA (41). Previous studies have demonstrated that the increased neutrophil-to-lymphocyte ratio in peripheral blood is correlated with survival rates for both iCCA and eCCA patients (42, 43). CXCL5 secreted by tumoral and stromal cells is a major chemoattractant of TANs through activation of PI3K-AKT and ERK1/2 pathways, leading to TAN accumulation and tumor metastases in CCA (44). Although the role of TANs is likely to become a major field of research, current studies in CCA are limited and further evidence is required to elucidate functions of TANs in the CCA microenvironment.
Natural killer (NK) cells
NK cells are lymphocytes phenotypically characterized by CD3−CD56+ in humans and CD3−NK1.1+ in mice and cooperate with cytotoxic T cells to build up a strong primary defense line against tumors. These cells share the capability to recognize and eliminate arising tumor cells by cell lysis, induced by emission of cytotoxic granules, as they do to control microbial infections (immune surveillance) (45). However, tumor cells may adopt several strategies to evade the tumoricidal activity of NK cells (immune escape). They can display MHC class I antigens, or shed ligands for the activating NK receptor (NKG2DLs), or secrete immunomodulatory molecules that antagonize NK cell activity (TGF-β, prostaglandin E2) (45). Upregulation of these proteins suppress the local immune aggression, which allows for uncontrolled tumor growth. The immune mechanisms regulated by NK cells are particularly developed within the liver, where NK population accounts for 30-40% of all tissue lymphocytes (46). Transplantation of NK cells facilitates cytotoxicity against cancer cells, as previous studies have demonstrated promising results for HCC patients (47, 48). Although current studies are limited for CCA, an in vitro study has shown that the combination of cetuximab and NK cells inhibits human CCA cell growth (49). A study using xenograft mouse models with the human CCA cell line HuCCT-1 demonstrated that transplantation of human NK cells induced in vivo cytotoxic activity of NK cells against HuCCT-1 tumors and inhibited tumor growth, suggesting that the increase of NK cell numbers or functions may be a promising approach for the treatment of CCA (50).
Tumor-infiltrating lymphocytes (TILs)
TILs are an heterogeneous group of immune cells including CD20+ B lymphocytes, CD8+ cytotoxic T cells, and CD4+CD25highFOXP3+ Tregs (51). In CCA, immunogenic tumor-associated antigens have been detected, in conjunction with a variable localization of TIL subtypes across the tumor. Whereas CD4+ TILs are mostly confined to the peritumoral area, CD8+ TILs prevail in the core of the tumor (52, 53). Higher population of CD4+ and CD8+ lymphocytes are associated with a better prognosis, in terms of overall survival, lymph node metastases, and venous and perineural invasion (54, 55). Antigen-presenting DCs prime T cell activities against the tumor, and their accumulation at the periphery of the lesion correlates with the amount of CD4+ and CD8+ TILs in the bulk of the tumor with better prognosis in iCCA (56). Although data on the functional relevance of CD20+ B lymphocytes in CCA is limited, elevated population of B cells have been observed in the lymphoepithelioma-like CCA, a rare type of iCCA associated with Epstein-Barr virus infection (57).
Tregs induce immunosuppressive responses against NK and cytotoxic T cells, and these effects are mediated by the secretion of IL-10 and TGF-β1 (58). As for TAMs and TANs, increased Tregs in the tumor area are correlated with poor survival in eCCA patients (40). Moreover, Tregs overexpress FoxP3, a transcription factor associated with the up-regulation of CTLA-4 on the cell surface. CTLA-4 binds to CD80 expressed by antigen-presenting cells and inhibit cytotoxic T cell activation, and in iCCA, expression of both CTLA-4 and CD80 is higher at the tumor-host interface correlating with tumor recurrence and chemo-resistance (59). PD-1 is another immune checkpoint protein that binds to PD-L1 promoting peripheral T cell exhaustion (Figure 4). About 6% of patients with eCCA have overexpression of PD-1 and PD-L1 in the tumor area (60), and PD-1/PD-L1 overexpression is associated with increased tumor progression and metastases, especially when accompanied by low CD3+ or CD8+ T cell infiltration (54). Although current studies on immunotherapy in CCA are limited, pembrolizumab, which is an anti-PD-1 antibody, showed promising results in patients with advanced CCA and is approved for and microsatellite instability/mismatch unresectable or metastatic solid cancers, including hepatobiliary cancers (61, 62). A study using laser capture microdissection with 78 iCCA cases has identified four immune subtypes in the tumor microenvironment (63). These subtypes had different population and profiles of infiltrating cells as well as different gene expression profiles in inflammatory and immune checkpoint pathways, indicating that effects of immunotherapy such as PD-1 antibodies may be limited to a certain percentage of CCA patients (63). Cases with massive infiltration of T-lymphocytes may be more responsive to immune therapy, but, at this time, this is a still speculative issue.
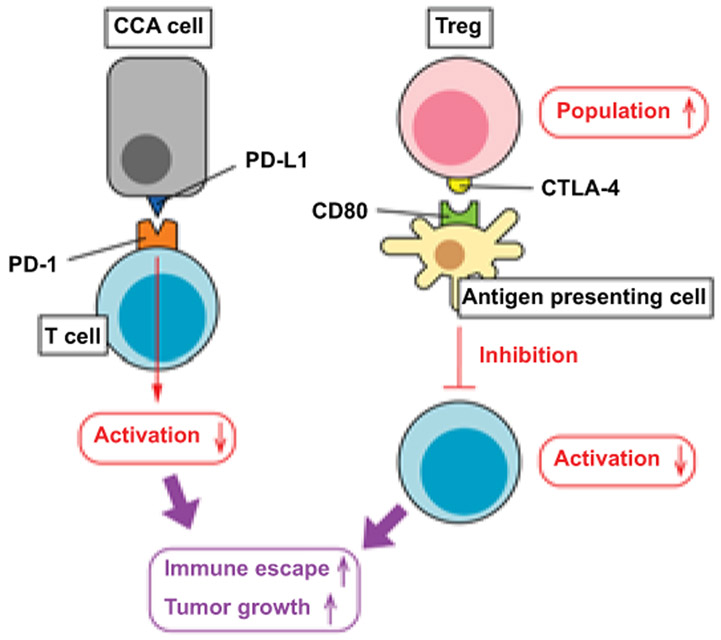
Figure 4. Immune checkpoints regulated by CCA tumors and the microenvironment.
CCA tumor cells express high levels of PD-L1, which binds to PD-1 expressed in T cells. Activation of PD-1 signaling inhibits T cell activation and anti-cancer functions. The CCA microenvironment contains high population of Tregs and these CCA-associated Tregs express high levels of CTLA-4. Antigen presenting cells detect CTLA-4 by its receptor CD80 and this CTLA-4/CD80 signaling also inhibits T cell activation as anti-cancer cells. The tumor microenvironment expressing high levels of PD-L1/PD-1 and CTLA-4/CD80 pathways promotes immune escape of CCA cells leading to tumor growth and metastases.
Extracellular matrix (ECM)
As other desmoplastic cancers, CCA is characterized by an intense remodeling of the ECM through the synthesis of new components, such as periostin, tenascin-C, and osteopontin, and the secretion of several proteolytic enzymes, including MMPs (64, 65). Overexpression of periostin, tenascin-C, and osteopontin has clinical significance, being correlated with an increase in tumor size and lymph node metastases with poor survival rates (66-68). By interacting with integrin α5 expressed by CCA cells, periostin activates the phosphoinositide 3-kinase (PI3K)/AKT signaling, which stimulates tumor cell proliferation and invasion (69). Tenascin-C contains adhesive and anti-adhesive sequences that enable its interactions with multiple other ECM components, soluble factors, and cell surface receptors (66). In desmoplastic tumors, such as colorectal cancers, tenascin-C produced by CAFs accumulates within the ECM to generate specialized paths that direct cancer cell invasion and dissemination through c-MET- and EGFR-dependent mechanisms (70). Osteopontin is a key factor for the development of NK cells and survival of T cells, and therefore has a potential role in immune responses and CCA progression (71, 72). The functional role of osteopontin in CCA is however controversial. Low level osteopontin expression in iCCA tissues was associated with lymph node metastasis and poor survival rates, and low levels of circulating osteopontin were associated with multiple tumors (73). A prior study has shown that elevated osteopontin expression in stromal but not in tumor cells is significantly associated with increased tumor size, invasion, metastases, and advanced staging in CCA patients (74). Osteopontin promotes iCCA growth and metastasis via the activation of MEK/MAPK1 and Wnt/β-catenin signaling (75). Molecular profiling of stroma by laser-capture microdissection obtained from human iCCA showed significantly increased osteopontin mRNA expression compared with non-tumor tissue, and elevated levels of osteopontin were correlated with poor survival (76). Although osteopontin expressed by CAFs may locally affect the CCA microenvironment and its function may differ from circulating osteopontin, further studies are required to elucidate the role of osteopontin and its therapeutic potentials in CCA progression.
Extracellular vesicles in CCA
Extracellular vesicles (EVs) play a vital role in intercellular crosstalk during liver diseases as a carrier of miRNAs (77). EVs are small membrane-bound vesicles secreted from cells. EVs can be categorized into three classes based on their biogenesis: i) Exosomes (50-100nm in diameter) are formed in endosomes and released from multivesicular bodies by fusion with the plasma membrane; ii) Microvesicles (0.1-1μm) are formed directly by outward budding and fission of the plasma membrane; iii) Apoptotic bodies (>1μm) are larger vesicles formed from cells undergoing apoptosis (78). Smaller EVs, exosomes and microvesicles, carry cargo mediators, such as proteins, DNAs, and RNAs, and deliver these mediators from donor into recipient cells regulating physiological cell events (77). Cholangiocytes secrete EVs and regulate cell events in other cholangiocytes when activated (79). This EV-mediated cell-to-cell communication may be essential also in CCA.
Mesenchymal stem cells (MSCs) can differentiate into CAFs or myofibroblasts within the tumor microenvironment, to promote tumor growth in various cancers (80). Haga et al. isolated EVs from culture media of CCA cell line KMBC cells and incubated bone marrow-derived MSCs with those KMBC EVs (81). CCA-derived EVs induced expression of fibrogenesis markers such as α-SMA in MSCs and increased MSC migration (81). KMBC-derived EVs elevated the secretion of various cytokines and chemokines that are associated with cancer progression including IL-6, and culture media of MSCs treated with KMBC-derived EVs induced cell proliferation of KMBC cells, indicating the role of EVs in the activation of CAFs and microenvironment development (81).
Non-coding RNAs and extracellular vesicles
Non-coding RNAs, such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), can regulate gene expression and play a key role in proliferation and metastasis in many cancer types. Previous studies have identified a number of candidate miRNAs and lncRNAs in CCA tissues (82, 83). In addition to CCA cells, non-coding RNAs may regulate gene expression of all cells populating the tumor microenvironment. In CAFs isolated from CCA tissues, miR-15a was downregulated as compared to normal skin fibroblasts (84). Furthermore, miR-15a inhibits the expression of plasminogen activator inhibitor-2 (PAI-2), which promotes the migration of CCA cells, indicating the therapeutic significance of miRNAs in the regulation of CAFs and CCA progression (84).
Extracellular vesicles as a therapeutic target for CCA
A recent study has shown that expression levels of miR-30e are decreased in the human CCA cell line HuCCT1 compared to normal cholangiocytes (85). HuCCT1 were transfected with miR-30e mimic, and EVs were isolated from culture media. EVs containing miR-30e mimic decreased cell proliferation and migration of non-transfected HuCCT1 cells by regulating the miR-30e target Snail, which is a transcription factor involved in cell migration (85). Coculture of HuCCT1 cells with the human HSC line LX2 cells induced the downregulation of a number of miRNAs including miR-195 in LX2 cells (86). HuCCT1 cells incubated in the culture media of LX2 cells transfected with miR-195 mimic showed decreased cell proliferation and increased levels of miR-195 in HuCCT1 cells (86). Furthermore, injection of miR-195-enriched EVs decreased tumor size and increased survival rates in xenograft CCA rat models, indicating that injected HSC-derived EVs can be delivered into CCA tumors and regulate CCA progression by delivering cargo miR-195 (86).
Conclusions and future perspectives
Available evidence indicates that the tumor microenvironment plays a relevant role in CCA progression and metastases. These studies also suggest that cells populating the tumor microenvironment, such as CAFs and TAMs, are additional potential targets to devise novel treatments of CCA. Drugs that selectively induce apoptosis or cytotoxicity in CAFs or TAMs are of great interest. For example, depletion of CAFs using navitoclax and depletion of TAMs using liposomal clodronate inhibited tumor growth in animal models (23, 30). Current studies also indicate that targeting the crosstalk between cells populating the tumor microenvironment and CCA cells may provide another interesting therapeutic approach. Inhibition of secretion of cytokines, growth factors, or their cognate receptors by small molecules or antibodies could lead to novel treatment paradigms. Immunotherapy, which targets immune checkpoints including PD-L1/PD-1 and CTLA-4/CD80 pathways such as anti-PD-1 antibodies, has demonstrated promising anti-cancer effects in various cancers. Although current studies in CCA are limited, immunotherapy may have potential for future CCA treatments. However, CCA treatments are challenging because of its marked heterogeneity. CCA can be caused by various mutations, and only small percentages of patients can be responsive to chemotherapy or inhibitors targeting genes with mutations or aberrations (87). Since the CCA microenvironment is also heterogeneous with different gene expression profiles for immune checkpoint pathways (63), effects of immunotherapy may be limited to small numbers of patients. There is great interest in combination therapies, where immune check point blockade is coupled with existing or experimental drugs with a different mechanism of action or even with loco-regional treatments (88). Novel therapeutic approaches are required for the development of universal CCA treatments that are not limited to phenotypes of CCA tumors or microenvironment. EVs have potential to be utilized as a therapeutic tool to deliver cargo mediators or drugs into CCA tumors or cells in the tumor microenvironment. In theory, selective cargo delivery via EVs might induce more effective responses in the target cells with less side effects or unwanted reactions in other cells although further studies are required to refine the methodology for selective EV delivery into cells in CCA tumors and the microenvironment.
Acknowledgements:
We thank Dr. Massimiliano Cadamuro, Department of Molecular Medicine, University of Padua, Italy, for kindly providing the histopathological micrographs of the tumor microenvironment and to Madison Adamo, Yale Liver Center, and Department of Internal Medicine for reading and checking the language.
Financial Support
This work was supported by: The Senior Research Career Scientist Award to Dr. Alpini (1IK6BX004601), and Research Career Scientist Award to Dr. Alpini (5I01BX000574) from the United States Department of Veteran’s Affairs Biomedical Laboratory Research and Development Service; The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs; The Hickam Endowed Chair, Division of Gastroenterology and Hepatology, Department of Medicine, Indiana University School of Medicine to Dr. Alpini; The project described was supported by the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative; Dr. Alpini acknowledges the support of the NIH (R01 DK115184) and PSC Partners Seeking a Cure Foundation (460933-00001). Dr. Strazzabosco acknowledges the support of the NIH grants DK101528-05 and DK034989, Silvio O. Conte Digestive Diseases Research Core Center (Cellular, Molecular, and Clinical/Translational cores), and a grant from PSC Partners Seeking a Cure Foundation (AWD0002203, Proposal ID: 18-004707). Dr. Fabris acknowledges the support of the University of Padua, ‘Progetti di Ricerca di Dipartimento’ (PRID) 2017.
Abbreviations
- α-SMA
α-smooth muscle actin
- CAFs
cancer-associated fibroblasts
- CCA
cholangiocarcinoma
- CSF-1
colony stimulating factor 1
- CTGF
connective tissue growth factor
- CXCR4
C-X-C chemokine receptor 4
- DCs
dendritic cells
- eCCA
extrahepatic CCA
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- EVs
extracellular vesicles
- FAP
fibroblast activation protein
- HB
heparin-binding
- HCC
hepatocellular carcinoma
- hCCA
hilar CCA
- HIF-1α
hypoxia inducible factor-1
- HSCs
hepatic stellate cells
- iCCA
intrahepatic CCA
- HGF
hepatocyte growth factor
- IL
interleukin
- JNK
c-Jun N-terminal kinase
- lncRNAs
long non-coding RNAs
- MCP-1
monocyte chemoattractant protein-1
- MDSCs
myeloid-derived suppressor cells
- miRNAs
microRNAs
- MMPs
matrix metalloproteases
- MSCs
mesenchymal stem cells
- NK
natural killer
- PAI-2
plasminogen activator inhibitor-2
- PDGF
platelet derived growth factor
- PI3K
phosphoinositide 3-kinase
- SDF-1
stromal cell–derived factor-1
- STAT3
signal transducer and activator of transcription 3
- TANs
tumor-associated neutrophils
- TAMs
tumor-associated macrophages
- TGF-β1
transforming growth factor β1
- TILs
tumor-infiltrating lymphocytes
- TNFα
tumor necrosis factor-α
- TRAIL
TNFα-related apoptosis inducing ligand
- Tregs
regulatory T lymphocytes
- VEGF
vascular endothelial growth factor
References
- 1.Alsaleh M, Leftley Z, Barbera TA, Sithithaworn P, Khuntikeo N, Loilome W, Yongvanit P, et al. Cholangiocarcinoma: A guide for the nonspecialist. Int J Gen Med 2019;12:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato K, Glaser S, Alvaro D, Meng F, Francis H, Alpini G. Cholangiocarcinoma: Novel therapeutic targets. Expert Opin Ther Targets 2020:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bragazzi MC, Ridola L, Safarikia S, Matteo SD, Costantini D, Nevi L, Cardinale V. New insights into cholangiocarcinoma: multiple stems and related cell lineages of origin. Ann Gastroenterol 2018;31:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez-Merino N, Aix SP, Cortes-Funes H. Chemotherapy for cholangiocarcinoma: An update. World J Gastrointest Oncol 2013;5:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, et al. Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261–280. [DOI] [PubMed] [Google Scholar]
- 6.Fabris L, Perugorria MJ, Mertens J, Bjorkstrom NK, Cramer T, Lleo A, Solinas A, et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int 2019;39:63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishihara Y, Aishima S, Hayashi A, Iguchi T, Fujita N, Taketomi A, Honda H, et al. CD10+ fibroblasts are more involved in the progression of hilar/extrahepatic cholangiocarcinoma than of peripheral intrahepatic cholangiocarcinoma. Histopathology 2009;55:423–431. [DOI] [PubMed] [Google Scholar]
- 8.Chuaysri C, Thuwajit P, Paupairoj A, Chau-In S, Suthiphongchai T, Thuwajit C. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep 2009;21:957–969. [DOI] [PubMed] [Google Scholar]
- 9.Sirica AE, Campbell DJ, Dumur CI. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol 2011;27:276–284. [DOI] [PubMed] [Google Scholar]
- 10.Okabe H, Beppu T, Hayashi H, Horino K, Masuda T, Komori H, Ishikawa S, et al. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol 2009;16:2555–2564. [DOI] [PubMed] [Google Scholar]
- 11.Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology 2010;51:1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, et al. The bone marrow functionally contributes to liver fibrosis. Gastroenterology 2006;130:1807–1821. [DOI] [PubMed] [Google Scholar]
- 13.Vaquero J, Guedj N, Claperon A, Nguyen Ho-Bouldoires TH, Paradis V, Fouassier L. Epithelial-mesenchymal transition in cholangiocarcinoma: From clinical evidence to regulatory networks. J Hepatol 2017;66:424–441. [DOI] [PubMed] [Google Scholar]
- 14.Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology 2011;53:1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology 2010;139:987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;9:44–54. [DOI] [PubMed] [Google Scholar]
- 17.Tanimura Y, Kokuryo T, Tsunoda N, Yamazaki Y, Oda K, Nimura Y, Naing Mon N, et al. Tumor necrosis factor alpha promotes invasiveness of cholangiocarcinoma cells via its receptor, TNFR2. Cancer Lett 2005;219:205–213. [DOI] [PubMed] [Google Scholar]
- 18.Claperon A, Mergey M, Aoudjehane L, Ho-Bouldoires TH, Wendum D, Prignon A, Merabtene F, et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology 2013;58:2001–2011. [DOI] [PubMed] [Google Scholar]
- 19.Gentilini A, Rombouts K, Galastri S, Caligiuri A, Mingarelli E, Mello T, Marra F, et al. Role of the stromal-derived factor-1 (SDF-1)-CXCR4 axis in the interaction between hepatic stellate cells and cholangiocarcinoma. J Hepatol 2012;57:813–820. [DOI] [PubMed] [Google Scholar]
- 20.Cadamuro M, Nardo G, Indraccolo S, Dall’olmo L, Sambado L, Moserle L, Franceschet I, et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2013;58:1042–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadamuro M, Brivio S, Mertens J, Vismara M, Moncsek A, Milani C, Fingas C, et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J Hepatol 2019;70:700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fingas CD, Bronk SF, Werneburg NW, Mott JL, Guicciardi ME, Cazanave SC, Mertens JC, et al. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology 2011;54:2076–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, Gustafson MP, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res 2013;73:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol 2018;19:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, Dang Y, et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res 2016;76:4124–4135. [DOI] [PubMed] [Google Scholar]
- 26.Cheng JT, Deng YN, Yi HM, Wang GY, Fu BS, Chen WJ, Liu W, et al. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis 2016;5:e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato K, Hall C, Glaser S, Francis H, Meng F, Alpini G. Pathogenesis of kupffer cells in cholestatic liver injury. Am J Pathol 2016;186:2238–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato K, Meng F, Giang T, Glaser S, Alpini G. Mechanisms of cholangiocyte responses to injury. Biochim Biophys Acta 2018;1864:1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subimerb C, Pinlaor S, Khuntikeo N, Leelayuwat C, Morris A, McGrath MS, Wongkham S. Tissue invasive macrophage density is correlated with prognosis in cholangiocarcinoma. Mol Med Rep 2010;3:597–605. [DOI] [PubMed] [Google Scholar]
- 30.Yuan D, Huang S, Berger E, Liu L, Gross N, Heinzmann F, Ringelhan M, et al. Kupffer cell-derived Tnf triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell 2017;31:771–789 e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol 2011;41:2522–2525. [DOI] [PubMed] [Google Scholar]
- 32.Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D, Chiorino G, et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol 2017;66:102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF, Beppu T, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci 2010;101:1913–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy S, Glaser S, Chakraborty S. Inflammation and progression of cholangiocarcinoma: Role of angiogenic and lymphangiogenic mechanisms. Front Med 2019;6:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, Ridgway RA, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest 2015;125:1269–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loilome W, Bungkanjana P, Techasen A, Namwat N, Yongvanit P, Puapairoj A, Khuntikeo N, et al. Activated macrophages promote Wnt/beta-catenin signaling in cholangiocarcinoma cells. Tumour Biol 2014;35:5357–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thongchot S, Yongvanit P, Loilome W, Seubwai W, Phunicom K, Tassaneeyakul W, Pairojkul C, et al. High expression of HIF-1α, BNIP3 and PI3KC3: Hypoxia-induced autophagy predicts cholangiocarcinoma survival and metastasis. Asian Pac J Cancer Prev 2014;15:5873–5878. [DOI] [PubMed] [Google Scholar]
- 38.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 2010;70:7465–7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol 2019;16:601–620. [DOI] [PubMed] [Google Scholar]
- 40.Kitano Y, Okabe H, Yamashita YI, Nakagawa S, Saito Y, Umezaki N, Tsukamoto M, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer 2018;118:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao ZY, Zhu GQ, Xiong M, Ren L, Bai L. Prognostic value of neutrophil distribution in cholangiocarcinoma. World J Gastroenterol 2015;21:4961–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buettner S, Spolverato G, Kimbrough CW, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, et al. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery 2018;164:411–418. [DOI] [PubMed] [Google Scholar]
- 43.Kitano Y, Yamashita YI, Yamamura K, Arima K, Kaida T, Miyata T, Nakagawa S, et al. Effects of preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios on survival in patients with extrahepatic cholangiocarcinoma. Anticancer Res 2017;37:3229–3237. [DOI] [PubMed] [Google Scholar]
- 44.Zhou SL, Dai Z, Zhou ZJ, Chen Q, Wang Z, Xiao YS, Hu ZQ, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis 2014;35:597–605. [DOI] [PubMed] [Google Scholar]
- 45.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 2016;16:7–19. [DOI] [PubMed] [Google Scholar]
- 46.Bjorkstrom NK, Ljunggren HG, Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol 2016;16:310–320. [DOI] [PubMed] [Google Scholar]
- 47.Alnaggar M, Lin M, Mesmar A, Liang S, Qaid A, Xu K, Chen J, et al. Allogenic natural killer cell immunotherapy combined with irreversible electroporation for stage IV hepatocellular carcinoma: Survival outcome. Cell Physiol Biochem 2018;48:1882–1893. [DOI] [PubMed] [Google Scholar]
- 48.Xie S, Wu Z, Zhou L, Liang Y, Wang X, Niu L, Xu K, et al. Iodine-125 seed implantation and allogenic natural killer cell immunotherapy for hepatocellular carcinoma after liver transplantation: a case report. Onco Targets Ther 2018;11:7345–7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morisaki T, Umebayashi M, Kiyota A, Koya N, Tanaka H, Onishi H, Katano M. Combining cetuximab with killer lymphocytes synergistically inhibits human cholangiocarcinoma cells in vitro. Anticancer Res 2012;32:2249–2256. [PubMed] [Google Scholar]
- 50.Jung IH, Kim DH, Yoo DK, Baek SY, Jeong SH, Jung DE, Park SW, et al. In vivo study of natural killer (NK) cell cytotoxicity against cholangiocarcinoma in a nude mouse model. In Vivo 2018;32:771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298–306. [DOI] [PubMed] [Google Scholar]
- 52.Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, Joehrens K, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer 2013;109:2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kasper HU, Drebber U, Stippel DL, Dienes HP, Gillessen A. Liver tumor infiltrating lymphocytes: comparison of hepatocellular and cholangiolar carcinoma. World J Gastroenterol 2009;15:5053–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim YJ, Koh J, Kim K, Chie EK, Kim B, Lee KB, Jang JY, et al. High ratio of programmed cell death protein 1 (PD-1)+/CD8+ tumor-infiltrating lymphocytes identifies a poor prognostic subset of extrahepatic bile duct cancer undergoing surgery plus adjuvant chemoradiotherapy. Radiother Oncol 2015;117:165–170. [DOI] [PubMed] [Google Scholar]
- 55.Miura T, Yoshizawa T, Hirai H, Seino H, Morohashi S, Wu Y, Wakiya T, et al. Prognostic impact of CD163+ macrophages in tumor stroma and CD8+ T-cells in cancer cell nests in invasive extrahepatic bile duct cancer. Anticancer Res 2017;37:183–190. [DOI] [PubMed] [Google Scholar]
- 56.Takagi S, Miyagawa S, Ichikawa E, Soeda J, Miwa S, Miyagawa Y, Iijima S, et al. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Hum Pathol 2004;35:881–886. [DOI] [PubMed] [Google Scholar]
- 57.Jeng YM, Chen CL, Hsu HC. Lymphoepithelioma-like cholangiocarcinoma: An Epstein-Barr virus-associated tumor. Am J Surg Pathol 2001;25:516–520. [DOI] [PubMed] [Google Scholar]
- 58.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 2012;12:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghidini M, Cascione L, Carotenuto P, Lampis A, Trevisani F, Previdi MC, Hahne JC, et al. Characterisation of the immune-related transcriptome in resected biliary tract cancers. Eur J Cancer 2017;86:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, Hama N, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003–1010. [DOI] [PubMed] [Google Scholar]
- 61.Alshari OM, Dawaymeh TA, Tashtush NA, Aleshawi AJ, Al Manasra ARA, Obeidat KA. Completely resolved advanced biliary tract cancer after treatment by pembrolizumab: a report of two cases. Onco Targets Ther 2019;12:5293–5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–952. [DOI] [PubMed] [Google Scholar]
- 63.Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, Elarouci N, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nissen NI, Karsdal M, Willumsen N. Collagens and cancer associated fibroblasts in the reactive stroma and its relation to cancer biology. J Exp Clin Cancer Res 2019;38:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fabris L, Cadamuro M, Cagnin S, Strazzabosco M, Gores GJ. Liver matrix in benign and malignant biliary tract disease. Semin Liver Dis 2020. [DOI] [PubMed] [Google Scholar]
- 66.Aishima S, Taguchi K, Terashi T, Matsuura S, Shimada M, Tsuneyoshi M. Tenascin expression at the invasive front is associated with poor prognosis in intrahepatic cholangiocarcinoma. Mod Pathol 2003;16:1019–1027. [DOI] [PubMed] [Google Scholar]
- 67.Utispan K, Thuwajit P, Abiko Y, Charngkaew K, Paupairoj A, Chau-in S, Thuwajit C. Gene expression profiling of cholangiocarcinoma-derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Mol Cancer 2010;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sirica AE, Almenara JA, Li C. Periostin in intrahepatic cholangiocarcinoma: Pathobiological insights and clinical implications. Exp Mol Pathol 2014;97:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Utispan K, Sonongbua J, Thuwajit P, Chau-In S, Pairojkul C, Wongkham S, Thuwajit C. Periostin activates integrin α5β1 through a PI3K/AKTdependent pathway in invasion of cholangiocarcinoma. Int J Oncol 2012;41:1110–1118. [DOI] [PubMed] [Google Scholar]
- 70.De Wever O, Nguyen QD, Van Hoorde L, Bracke M, Bruyneel E, Gespach C, Mareel M. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J 2004;18:1016–1018. [DOI] [PubMed] [Google Scholar]
- 71.Chung JW, Kim MS, Piao ZH, Jeong M, Yoon SR, Shin N, Kim SY, et al. Osteopontin promotes the development of natural killer cells from hematopoietic stem cells. Stem Cells 2008;26:2114–2123. [DOI] [PubMed] [Google Scholar]
- 72.Stromnes IM, Goverman JM. Osteopontin-induced survival of T cells. Nat Immunol 2007;8:19–20. [DOI] [PubMed] [Google Scholar]
- 73.Zhou KQ, Liu WF, Yang LX, Sun YF, Hu J, Chen FY, Zhou C, et al. Circulating osteopontin per tumor volume as a prognostic biomarker for resectable intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2019;8:582–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laohaviroj M, Chamgramol Y, Pairojkul C, Mulvenna J, Sripa B. Clinicopathological significance of osteopontin in cholangiocarcinoma cases. Asian Pac J Cancer Prev 2016;17:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng Y, Zhou C, Yu XX, Wu C, Jia HL, Gao XM, Yang JM, et al. Osteopontin promotes metastasis of intrahepatic cholangiocarcinoma through recruiting MAPK1 and mediating Ser675 phosphorylation of beta-Catenin. Cell Death Dis 2018;9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sulpice L, Rayar M, Desille M, Turlin B, Fautrel A, Boucher E, Llamas-Gutierrez F, et al. Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology 2013;58:1992–2000. [DOI] [PubMed] [Google Scholar]
- 77.Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. J Hepatol 2016;65:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato K, Meng F, Venter J, Giang T, Glaser S, Alpini G. The role of the secretin/secretin receptor axis in inflammatory cholangiocyte communication via extracellular vesicles. Sci Rep 2017;7:11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 2009;4:e4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles 2015;4:24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song W, Miao DL, Chen L. Comprehensive analysis of long noncoding RNA-associated competing endogenous RNA network in cholangiocarcinoma. Biochem Biophys Res Commun 2018;506:1004–1012. [DOI] [PubMed] [Google Scholar]
- 83.Li Z, Shen J, Chan MT, Wu WK. The role of microRNAs in intrahepatic cholangiocarcinoma. J Cell Mol Med 2017;21:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Utaijaratrasmi P, Vaeteewoottacharn K, Tsunematsu T, Jamjantra P, Wongkham S, Pairojkul C, Khuntikeo N, et al. The microRNA-15a-PAI-2 axis in cholangiocarcinoma-associated fibroblasts promotes migration of cancer cells. Mol Cancer 2018;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ota Y, Takahashi K, Otake S, Tamaki Y, Okada M, Aso K, Makino Y, et al. Extracellular vesicle-encapsulated miR-30e suppresses cholangiocarcinoma cell invasion and migration via inhibiting epithelial-mesenchymal transition. Oncotarget 2018;9:16400–16417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li L, Piontek K, Ishida M, Fausther M, Dranoff JA, Fu R, Mezey E, et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology 2017;65:501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simile MM, Bagella P, Vidili G, Spanu A, Manetti R, Seddaiu MA, Babudieri S, et al. Targeted therapies in cholangiocarcinoma: Emerging evidence from clinical trials. Medicina 2019;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loeuillard E, Conboy CB, Gores GJ, Rizvi S. Immunobiology of cholangiocarcinoma. JHEP Rep 2019;1:297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]