Abstract
Purpose
To compare the effectiveness of starting the ovarian stimulation on the early follicular phase (“Conventional”) with the newer range of non-conventional approaches starting in the luteal phase (“Luteal”), random-start, and studies implementing them in DuoStim (“Conventional”+“Luteal”).
Methods
Systematic review. We searched CENTRAL, PubMed, and Embase, on March 2020. We included randomized and non-randomized controlled trials that compared “Luteal,” random-start ovarian stimulation or DuoStim with “Conventional”; we analyzed them by subgroups: oocyte freezing and patients undergoing ART treatments, both, in the general infertile population and among poor responders.
Results
The following results come from a sensitivity analysis that included only the low/moderate risk of bias studies. When comparing “Luteal” to “Conventional,” clinically relevant differences in MII oocytes were ruled out in all subgroups. We found that “Luteal” probably increases the COH length both, in the general infertile population (OR 2.00 days, 95% CI 0.81 to 3.19, moderate-quality evidence) and in oocyte freezing cycles (MD 0.85 days, 95% CI 0.53 to 1.18, moderate-quality evidence). When analyzing DuoStim among poor responders, we found that it appears to generate a higher number of MII oocytes in comparison with a single “Conventional” (MD 3.35, 95%CI 2.54–4.15, moderate-quality evidence).
Conclusion
Overall, this systematic review of the available data demonstrates that in poor responders, general infertile population and oocyte freezing for cancer stimulation in the late follicular and luteal phases can be utilized in non-conventional approaches such as random-start and DuoStim cycles, offering similar outcomes to the conventional cycles but potentially with increased flexibility, within a reduced time frame. However, more well-designed trials are required to establish certainty.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-020-01966-5.
Keywords: Double ovarian stimulation, DuoStim, Random-start ovarian stimulation, Luteal-phase stimulation, Systematic review
Introduction
Classically, controlled ovarian hyperstimulation (COH) cycles are started during the early follicular phase. During COH, early antral follicles are required to grow synchronically in response to exogenous gonadotropins, in order to accomplish simultaneous maturation [1]. It is challenging to find a consistently efficient ovarian s4timulation protocol [2]. Currently, non-conventional strategies have been developed to retrieve the greatest number of oocytes in the shortest possible time [3–5]. It could be especially important in some specific patients, such as poor responders, and women seeking fertility preservation before oncologic therapy [6–8].
Current evidence suggests that multiple cohorts or waves of antral follicles are recruited continuously during a menstrual cycle [9–11]. This concept helped to develop new approaches, in which the start of the ovarian stimulation is proposed to be initiated not only at the early follicular phase but also during the late follicular phase and in the luteal phase as well. The awareness of the presence of multicyclic development of follicles initially resulted in the appearance of the random-start ovarian stimulation protocols for those requiring urgent egg retrievals such as for fertility preservation. This idea also brought some other approaches such as the luteal-phase ovarian stimulation (“Luteal”), which was presented as a novel strategy for a single stimulation especially for poor responders, or as part of the double stimulation protocol (DuoStim) as well [7, 9].
“Luteal” is a typical COH, but it starts two to 7 days following ovulation or oocyte retrieval. However, as the endometrium misses synchronicity, a fresh embryo transfer is not performed in this case. Otherwise, DuoStim is a back-to-back stimulation protocol within the same menstrual cycle: one in the follicular phase and a second one in the luteal phase of the same cycle [2, 10, 12, 13]. Typically, it is a “Luteal” starting 2 to 5 days after oocyte retrieval. Several DuoStim protocols have been recently described, such as the “New York proposition,” that involves administering clomiphene citrate or letrozole plus FSH/LH in FPS and in “Luteal” [13], the “Shanghai protocol” that includes hMG in both phases, and the “Italian protocol” [7, 14] that uses rFSH and rLH for both FPS and “Luteal.”
Another alternative based on the concept of multiple follicular waves is the random-start ovarian stimulation approach [10, 15], starting not only in the early follicular phase (“Conventional”) or the “Luteal” but also in the late follicular phase [16]. Random-start protocols have been proposed for urgent fertility preservation in oncology patients [6, 17]. If the patient is at the beginning of the follicular or luteal phase, a “Conventional” or “Luteal” protocol is started, similar to those described above. If the patient is in a late follicular phase, the protocol varies according to the presence or not of a dominant follicle.
Our objective was to perform a systematic review of the studies that assessed the effectiveness of the “Luteal,” random-start ovarian stimulation, and DuoStim, in comparison with the usual “Conventional” for women undergoing ART cycles for infertility or for fertility preservation.
Material and methods
A systematic search of all published and unpublished studies until March 2020 with no language restriction was performed. The protocol has been registered on PROSPERO (CRD42019146416) and we followed the Cochrane methods [18] and PRISMA statement for reporting [19].
Inclusion and exclusion criteria
We included randomized controlled trials (RCTs) with parallel design, including the first phases of cross-over trials, before-after studies, and retrospective and prospective cohorts. We excluded those studies of DuoStim that compared the outcomes of “Conventional” with those of the “Luteal” in the same cycle. Participants in the studies were women undergoing COH for ART cycles (both patients and oocyte donors) and patients undergoing COH for oocyte cryopreservation. Each of these populations were analyzed separately as different subgroups. We investigated the following interventions: (a) “Luteal,” defined as a controlled ovarian stimulation that is started in the luteal phase; (b) late follicular phase stimulation (“Late follicular”), defined as a controlled ovarian stimulation that is started in the follicular phase after day 7 of the cycle; (c) DuoStim, defined as a “Conventional” and a “Luteal” in the same menstrual cycle; (d) random-start ovarian stimulation that did not specify where in the cycle was started, defined as COH, started at any time outside of the “Conventional” [11, 13, 20, 21]. In those cases, in which the random-start stimulation evaluated “Late follicular” and “Luteal” as different groups, they were analyzed separately. In all cases, the comparator was an independent group of women that underwent a standard “Conventional.”
When the studied intervention was DuoStim, we excluded studies in which the comparator was the “Conventional” of that DuoStim cycle, as they were not independent groups. To improve the comparability between the interventions, we also excluded those studies in which minimal stimulation was compared with a standard COH protocol. The primary outcome was the number of metaphase II oocytes. Secondary outcomes were the total number of retrieved oocytes, number of fertilized oocytes, number of total and euploid blastocysts, clinical pregnancy rate, live birth rate, cumulative pregnancy rate, multiple pregnancy rate, miscarriage rate, cancelation rate (defined as cycles with incomplete COH, without retrieved oocyte or no embryos available to cryopreserve or transfer), ovarian stimulation length (measured in days), and time to pregnancy.
Search methods for identification of studies
Electronic searches were performed in CENTRAL via the Cochrane Register of Studies Online (CRSO), PubMed, and Embase from inception to March 2020 (Supplemental table 1).
To identify additional studies, we performed hand searches of the reference lists of all relevant publications. We also performed searches to identify ongoing clinical trials in ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). In addition, we searched conference proceedings and some grey literature to identify relevant studies.
Data collection and analysis
A total of four reviewers (DG, RP, MM, KL) screened studies by title and abstract. It was done by pairs of independent reviewers, according to pre-specified criteria. Any disagreements were resolved by consensus. Then, included studies were randomly selected, extracted, and independently assessed the risk of bias of each study. Discrepancies were resolved by consensus. We used the app Covidence for this purpose [22, 23].
Four independent reviewers (DG, RP, MM, KL) performed the data extraction on a data extraction form previously piloted in five studies. Discrepancies were resolved by consensus.
For continuous data, we calculated the mean difference (MD) between treatment groups. For dichotomous data, we calculated odds ratios (ORs) using the numbers of events in the control and intervention groups of each study. We presented 95% confidence intervals for all outcomes.
We performed the analysis per woman randomized in randomized controlled trials. In non-randomized studies, the analysis was performed per included woman (when that information was not available, we analyzed per cycle, which was taken into consideration to classify the risk of bias).
We analyzed data on an intention-to-treat basis whenever possible. If missing or insufficient data were found, we attempted to obtain such data by contacting the first or corresponding authors of the relevant studies. We presented additional information provided by the study authors, when available.
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta-analyses, to provide a clinically meaningful summary. We assessed heterogeneity among the included studies by measuring the I2. An I2 greater than 60% was considered to indicate substantial heterogeneity [24].
We presented information in narrative and structured (table-based) form to describe the results. Analyses were carried out in Review Manager 5.3. We used the GRADE system to assess the quality of evidence of included studies [25].
A subgroup analysis was performed to compare poor responders with the general infertility population, women undergoing oocyte cryopreservation and oocyte donors.
We performed a sensitivity analysis including randomized controlled studies and non-randomized studies, with overall low and moderate risk of bias. We did not include studies with serious and critical limitations.
Risk of bias and certainty of evidence assessment
Two separate review authors (DG, MM) assessed the risk of bias independently for each included study. For randomized controlled trials, we used the Cochrane tool for assessing the risk of bias [26]. For non-randomized studies, this was assessed using a checklist of essential items outlined in ROBINS-I [27]. Study authors were contacted for clarification when questions of methodology relevant to bias assessment were raised [28]. Discrepancies were resolved by consensus.
As there were not enough studies per comparison, we could not use a funnel plot to assess the possibility of publication bias.
We prepared a “Summary of findings” table using the GRADEpro GDT and Cochrane methods [24, 29]. This table evaluated the overall quality of the evidence for some of the primary and secondary outcomes. We assessed the quality of evidence using the GRADE criteria. Two review authors (DG and AC) independently graded the quality of evidence and resolved any disagreements by discussion. We documented and justified our judgments about the quality of the evidence.
Results
Description of the results
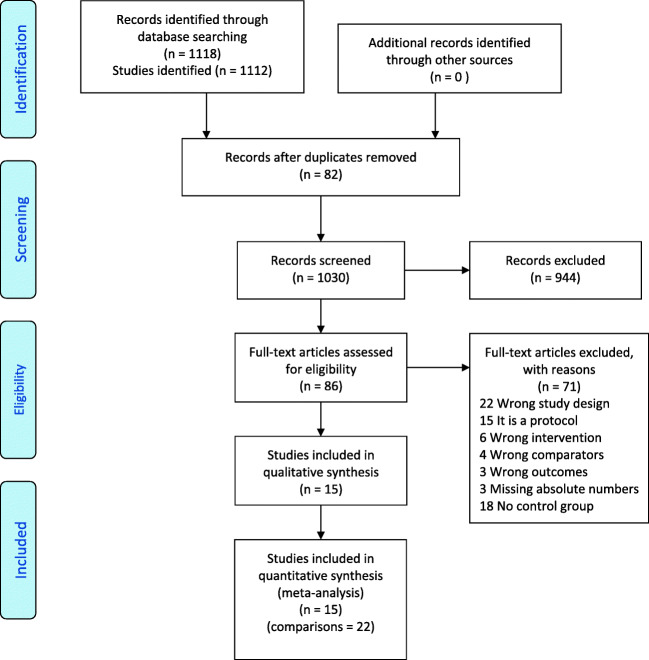
Our search strategy identified 1118 references from 1112 studies. After removing 82 duplicates, we screened 1030 references by title and abstract and 944 were classified as non-relevant, while of the remaining 86 full-text studies evaluated, 71 were excluded and 15 were included (Fig. 1) [6, 14, 30–42]. As some studies compared more than two interventions, we finally made 22 comparisons for four interventions in which one of them was compared with “Conventional”: 11 with “Luteal,” five with “Late follicular,” four with DuoStim and two of them were from random-start stimulation studies that did not specify the starting point in the cycle (Table 1). Eleven of them were analyzed in women undergoing an ART cycle for an infertility treatment (eight in poor responders and three in the general infertile population), two included oocyte donors and eight were performed in women undergoing oocyte cryopreservation due to cancer. Finally, we also found 15 ongoing trials (Supplemental table 2).
Fig. 1.
Flowchart for study selection. Description of the illustration: PRISMA flowchart
Table 1.
Characteristics of included studies
| Studies | Country | Study design | Type of treatment (subgroup of patients) | Participants | Interventions | Comparator |
|---|---|---|---|---|---|---|
| Luteal-phase stimulation | ||||||
| Buendgen 2013 | Germany | Prospective cohort |
IVF (general) |
40 participants (10 LPS and 30 eFPS) Incl crit: 18–36 years; ≤ 3 earlier unsuccessful IVF Excl crit: PCOS, EDT ≥ III and expected poor response |
LPS: uFSH 300 IU-GnRH antagonists Start: days 19–21 |
eFPS: rFSH or hMG 150–225 IU/day-GnRH antagonists |
| Lin 2018 | Taiwan | Prospective cohort |
IVF (poor ovarian reserve) |
60 participants (30 LPS and 30 eFPS) Incl crit: Bologna criteria Excl crit: previous oophorectomy, exposure to cytotoxic or pelvic irradiation for malignancy, positive screening for recurrent pregnancy loss |
LPS: hMG 225 IU/day + CC 100 mg/day - MPA 10 mg/day Start: day 15–18 |
eFPS: rFSH 300 IU/day + rLH 150 IU/day - GnRH antagonists |
| Llacer 2020 | Spain | RCT |
IVF (poor ovarian reserve) |
60 participants (27 LPS and 30 eFPS) Incl crit: Bologna criteria, < 41 years, regular menstrual cycles of 21–35 days, indication for IVF with 300 UI rFSH, presence of both ovaries Excl crit: Follicles > 10 mm in the randomization visit, EDT III/IV, concurrent uterine pathology and concurrent participation in another study |
LPS: rFSH 300 IU/day + rLH 150 IU/day - GnRH antagonists Start: 4 days after an LH positive test |
eFPS: rFSH 300 UI/day + rLH 150 UI/day - GnRH antagonists |
| Wang 2016 | China | Retrospective cohort |
IVF (general) |
2112 participants (727 LPS and 1385 eFPS) | LPS: Letrozole 2.5 mg/day (for 5 days) + hMG 225 IU/day (2–7 days after ovulation) - MPA 10 mg/day | eFPS: hMG 150 IU/day or more - Triptorelin 100 mcg/day |
| Zhang 2018 | China | Retrospective cohort |
IVF (poor ovarian reserve) |
385 participants (154 LPS and 231 eFPS) Incl crit: Bologna criteria |
LPS: CC 50–100 mg/day + hMG 75–150 IU/day (2–7 days after ovulation) - Dydrogesterone 20 mg/day | eFPS: CC 50–100 mg/day (from day 3 to 7) + hMG 75–150 IU/day (from day 8) |
| Double stimulation (DuoStim) | ||||||
|
Martazanova 2018 |
Russia | RCT |
IVF (poor ovarian reserve) |
148 participants (79 Duostim and 72 eFPS) Incl crit: < 43 years; АМH < 1.2 ng/ml; AFC < 6; FSH > 11 IU/ml Excl crit: uterine fibroids, deep EDT, cancer |
DuoStim: not specified Start: day 2 for follicular stim and 4 days after oocyte retrieval |
eFPS: not specified |
| Ubaldi 2015 | Italy | Before-after study |
IVF (poor ovarian reserve) |
34 participants (17 DuoStim and 17 eFPS) Same patient, less than 6 months between the conventional and the double stimulation. Incl crit: < 7 oocytes in previous cycle, AMH ≤ 1.6 ng/ml and antral follicle count ≤ 7 Excl crit: not specified |
DuoStim: Gonadotrophins - GnRH antagonists. Same protocol for both stimulations. After the first oocyte retrieval, GnRH antagonist daily was administrated for 3 days. Start: day 2 for follicular stim and 4 days after oocyte retrieval |
eFPS: not specified |
| Vaiarelli 2020 | Italy | Prospective cohort |
IVF (poor ovarian reserve) |
197 participants (100 DuoStim and 197 eFPS) Incl crit: Bologna criteria for poor responders Excl crit: not specified |
DuoStim: rFSH 300 IU/day + rLH 150 IU/day - GnRH antagonist. Same protocol for both stimulations. Start: day 2 for follicular stim and 5 days after oocyte retrieval |
eFPS: rFSH 300 IU/day + rLH 150 IU/day - GnRH antagonists |
| Random-start stimulation | ||||||
| Muteshi 2018 | UK | Retrospective cohort | Oocyte and embryo cryopreservation (cancer) |
137 participants (24 random-start and 103 eFPS) Incl crit: recently diagnosed with cancer and referred for fertility preservation. Excl crit: not specified |
Random-start: 3 days of cetrorelix 0.25 mg followed by Gonadotrophins on fourth day - GnRH antagonists Start: at any point after menstrual day 5 |
eFPS: rFSH or hMG 250 IU/day – GnRH antagonists |
| More than 2 intervention groups | ||||||
| Cakmak 2013 | USA | Retrospective cohort | Oocyte and embryo cryopreservation (cancer) |
128 participants (13 lFPS, 22 LPS and 93 eFPS) Incl crit: recently diagnosed with cancer and in preparation for chemotherapy/radiotherapy or bilateral oophorectomy Excl crit: history of infertility or previous gonadotoxic treatment |
Gonadotropins ± aromatase inhibitor – GnRH antagonists lFPS: start after day 7 with follicle > 13 mm. LPS: start on day 2–3 after triggering or after high progesterone detection |
eFPS: Gonadotropins ± aromatase inhibitor – GnRH antagonists |
| Cavagna 2018 | Brazil | Retrospective cohort | Oocyte cryopreservation (breast cancer) |
109 participants (42 eFPS, 20 lFPS and 47 LPS) Incl crit: breast cancer with indication of chemotherapy, ≤ 40 years Excl crit: advaced or metastatic disease, ≥ 41 years |
lFPS: hMG 150–300 IU/day + aromatase inhibitor – GnRH antagonists concomitant with gonadotropins. Start with the presence of dominant follicle > 10 mm LPS: rFSH 150–300 IU/day + aromatase inhibitor – GnRH antagonists start when evidence of follicle rupture and endometrium secretory pattern |
eFPS: hMG 150–300 IU/day + aromatase inhibitor – GnRH antagonists start with the absence of dominant follicle > 10 mm |
| Checa 2015 | Spain | RCT | Egg donors |
11 participants (6 lFPS, 5 LPS, and 11 eFPS) All participants had an eFPS cycle followed by either an lFPS or LPS cycle Incl crit: 18–32 years, BMI 12–28, baseline FSH > 10 Excl crit: history of chemotherapy, gonadotoxic drugs, infertility, ovarian surgery, PCOS male factor |
lFPS: Ganirelix 0.25 mg on day 10 until E2 < 60 pg/ml followed by rFSH 225 IU/day – GnRH antagonists LPS: Ganirelix 0.25 mg on day 20 until E2 < 60 pg/ml, followed by daily rFSH 225 IU/day – GnRH antagonists |
eFPS: rFSH 225 IU/day – GnRH antagonists |
| Jin 2018 | China | Retrospective cohort |
IVF (poor ovarian reserve) |
260 participants (132 eFPS, 76 DuoStim, 52 LPS) Incl crit: Bologna criteria Excl crit: basal FSH > 25 mIU/ml, EDT III/IV, BMI < 18 or > 30 kg/m2 |
LPS: CC 50–100 mg/day or letrozole 5 mg/day lasting 5 days + hMG 150–300 UI/day -GnRH antagonists. DuoStim: Start: day 3 for follicular stim and 1–3 days after oocyte retrieval LPS: Start: 1–3 days after natural ovulation |
eFPS: CC 50–100 mg/d or letrozole 5 mg/d (from day 3 to 7) + hMG 150–300 IU/d (from day 8) -GnRH antagonists |
| Qin 2016 | China | Retrospective cohort |
IVF (general) |
150 participants (50 lFPS, 50 LPS and 50 eFPS) Incl crit: <42 years; regular menstrual cycles the previous 3-month; AFC >3 or FSH <12 IU/L, BMI 17–27 kg/m2 Excl crit: AFC <3 or FSH >12, EDT III/IV, PCOS, receipt of hormone treatments within the previous 3-month period, including oral, any contraindications to COH |
lFPS: hMG 150–225 IU/d + CC 25 mg/d + GnRH agonist + MPA 10 mg/d. Start day 6–14 with follicle >10 mm. + E2 > 75. LPS: hMG 150–225 IU/d + CC 25 mg/d. Start after day 14 with P4 > 6.5 mg or corpora luteum |
eFPS: hMG 150–225 IU/d + MPA 10 mg/d + CC 25 mg/d |
| Von Wolf 2016 | Germany | Retrospective cohort | Oocyte cryopreservation (cancer) |
684 participants (109 LFPS 103 LPS and 472 eFPS) Incl crit: Not specified Excl crit: Not specified |
lFPS: rFSH or hMG – GnRH antagonists Start day 6–14 LPS: rFSH – GnRH antagonists. Start after day 14 |
eFPS: rFSH or hMG – GnRH antagonists |
LPS, luteal-phase stimulation. eFPS, early follicular phase stimulation. lFPS, late follicular phase stimulation. AFC, antral follicular count. Spont cycle, spontaneous cycle. Incl crit, inclusion criteria. Excl crit, exclusion criteria. IVF, in vitro fertilization. PCOS, polycystic ovarian syndrome. COH, controlled ovarian hyperstimulation. EDT, endometriosis. BMI, body mass index. E2, estradiol. rFSH, recombinant follicle-stimulating hormone. rLH, recombinant luteinizing hormone. hMG, human menopausal gonadotropin. CC, clomiphene citrate. MPA, medroxyprogesterone
The total number of retrieved oocytes, retrieved MII oocytes, and COH length were the most commonly reported outcomes (Table 2). Only two comparisons reported live birth rates, 11 comparisons reported clinical pregnancy rate, 10 reported miscarriage rates, and six reported cancelation rates. One study reported the number of euploid embryos. Reports of the total number of blastocysts were too heterogeneous to make a description or analysis. Cumulative live birth rate was only reported in 10 patients in one study, and time to pregnancy and multiple pregnancy rate were not reported in any of the included studies.
Table 2.
Outcomes results
| Luteal-phase stimulation | ||||||||
| Studies | MII oocytes (±SD) | Clinical pregnancy rate | Cancelation rate | Days of stimulation (SD) | ||||
| LPS | eFPS | LPS | eFPS | LPS | eFPS | LPS | eFPS | |
| Poor ovarian reserve | ||||||||
| Jin 2018 [33] | NA | NA | 10/25 (40%) | 17/56 (30.4%) | 13/56 (23.2%) | 38/132 (28.8%) | 11.2 ± 3.0 (52) | 8.9 ± 2.4 (132) |
| Lin 2018 [34] | 2.4 ± 1.4 (28) | 1.2 ± 0.8 (23) | 5/28 (17.9%) | 3/23 (13.0%) | 2/30 (6.7%) | 7/30 (23.3%) | 11.5 ± 2.2 (28) | 9.9 ± 2.0 (23) |
| Llacer 2020 [39] | 2.1 ± 2.0 (24) | 2.6 ± 2.2 (27) | NA | NA | 6/30 (20%) | 3/30 (10%) | 8.4 ± 2.8 (24) | 8.2 ± 4.1 (27) |
| Zhang 2018 [40] | NA | NA | 31/109 (28.4%) | 62/163 (38.0%) | NA | NA | 11.3 ± 3.6 (154) | 8.1 ± 2.8 (231) |
| General population | ||||||||
| Buendgen 2013 [30] | 7.2 ± 3.9 (10) | 7.9 ± 4.8 (30) | 1/10 (10%)* | 6/30 (20%)* | NA | NA | 11.7 ± 1.6 (10) | 9.1 ± 1.3 (30) |
| Qin 2016 [37] | 5.2 ± 3.9 (36) | 5.7 ± 3.6 (41) | 14/36 (38.9%) | 17/41 (41.4%) | 8/50 (16%) | 5/50 (10%) | 10.9 ± 3.4 (36) | 8.9 ± 1.4 (41) |
| Wang 2016 [42] | 10.9 ± 7.6 (727) | 9.1 ± 5.5 (1385) | 365/822 (44.4%) | 656/1675 (39.2%) | 90/727 (12.4%) | 138/1385 (10%) | 10.4 ± 1.8 (727) | 8.2 ± 1.7 (1385) |
| Oocyte freezing | ||||||||
| Cakmak 2013 [6] | 10.3 ± 6.3 (22) | 9.7 ± 6.7 (103) | NA | NA | NA | NA | NA | NA |
| Cavagna 2018 [31] | 10.9 ± 7.4 (47) | 8.9 ± 6.8 (41) | NA | NA | NA | NA | NA | NA |
| Von Wolf 2016 [38] | NA | NA | NA | NA | NA | NA | 11.5 ± 2.2 (103) | 10.8 ± 2.4 (472) |
| Oocyte donors | ||||||||
| Checa 2015 [32] | 13.2 ± 5.2 (5) | 12.4 ± 5.2 (5) | 3/5 (60%) | 2/5 (40%) | NA | NA | 10.6 ± 2.1 (5) | 12.2 ± 1.9 (5) |
| Late follicular phase stimulation | ||||||||
| General population | ||||||||
| Qin 2016 [37] | 5.2 ± 3.7 (33) | 5.7 ± 3.6 (41) | 15/33 (45.5%) | 17/41 (41.5%) | 11/50 (22%) | 5/50 (10%) | 11.4 ± 3.1 (33) | 8.9 ± 1.4 (41) |
| Oocyte freezing | ||||||||
| Cakmak 2013 [6] | 9.1 ± 5.1 (13) | 9.7 ± 6.7 (103) | NA | NA | NA | NA | 10.5 ± 1.5 (13) | 9.3 ± 1.5 (103) |
| Cavagna 2018 [31] | 8.0 ± 5.4 (21) | 8.9 ± 6.8 (41) | NA | NA | NA | NA | 9.7 ± 1.3 (21) | 9.9 ± 1.3 (41) |
| Von Wolf 2016 [38] | NA | NA | NA | NA | NA | 10.6 ± 2.7 (109) | 11.6 ± 7.7 (472) | |
| Oocyte donors | ||||||||
| Checa 2015 [32] | 13.0 ± 9.1 (6) | 16.2 ± 4.1 (6) | 6/6 (100%) | 3/6 (50%) | NA | NA | 9.8 ± 0.8 (6) | 10.4 ± 1.5 (6) |
| Double stimulation | ||||||||
| Poor ovarian reserve | ||||||||
| Jin 2018 [33] | NA | NA | 19/52 (36.5%) | 17/56 (30.4%) | 10/76 (13.1%) | 38/132 (28.7%) | NA | NA |
| Martazanova et al. 2018 [35] | 7.4 ± 3.6 (76) | 3.9 ± 2.0 (72) | 39/76 (51.3%) | 30/72 (41.7%) | NA | NA | NA | NA |
| Ubali 2015 [14] | 6.1 ± 3.0 (17) | 3.2 ± 1.5 (17) | NA | NA | NA | NA | NA | NA |
| Vaiarelli 2020 [41] | NA | NA | 15/100 (15%) | 16/197 (8.1%) | NA | NA | NA | NA |
| Random-start stimulation (not specified) | ||||||||
| Oocyte freezing | ||||||||
| Cakmak 2013 [6] | 9.9 ± 6.4 (35) | 9.7 ± 8.4 (109) | NA | NA | NA | NA | 10.9 ± 1.5 (35) | 9.3 ± 1.6 (109) |
| Muteshi 2018 [36] | NA | NA | NA | NA | NA | NA | 12.2 ± 3.6 (24) | 11.5 ± 1.5 (103) |
Continuous outcomes are expressed as mean ± standard deviation. Dichotomic outcomes are expressed as n/N (%). NA, not available. MII, metaphase II. LPS, luteal-phase stimulation. eFPS, early follicular phase stimulation. lFPS, late follicular phase stimulation
*Cumulative pregnancy rate
¶Live birth rate/ongoing pregnancy rate
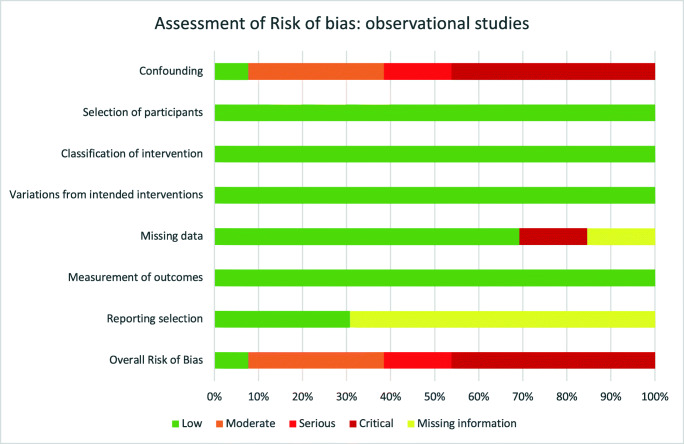
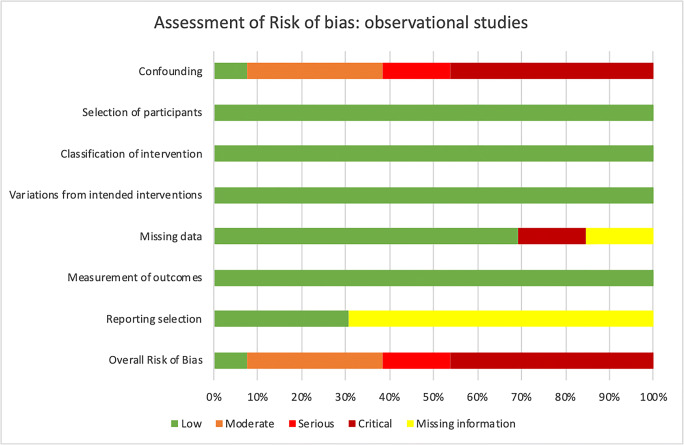
Quality of the evidence of each included study and across them is all shown in Fig. 2 and Table 3.
Fig. 2.
Assessment of risk of bias across observational studies
Table 3.
Assessment of risk of bias of each study
aMore than 10% of missing data
bCOH protocol is different in both groups. And frozen-thawed embryo transfers were done in LPS group while fresh embryo transfer were done in eFPS group
cMost known important variables are balanced
dIt is unclear if some known important variables are balanced or not
eBoth intervention and comparator were performed on the same group of women within a 12-month period. Therefore, variables are balanced
fCOH protocol is different in both groups
gWomen’s age was different in both groups. Only unsuccessful cycles in the eFPS cycles were included
hIt is unclear if some known important variables are balanced or not
iCOH protocol is different in both groups. Besides, denominator are cycles and not women
jCOH protocol is different in both groups and participants in eFPS group are younger
Subgroups (poor responders, general population, and oocyte freezing for cancer) were analyzed separately below. Data from oocyte donors was not included in the quantitative analysis, as they came from a single very small study with 11 participants, and the evidence was very uncertain to withdraw any conclusion (details are described in Tables 1 and 2).
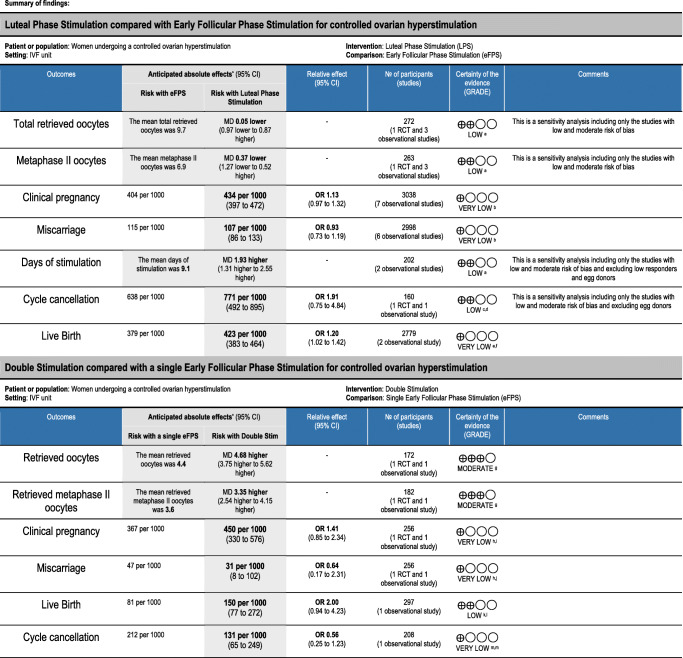
A summary of the results and risk of bias were reported in the Summary of findings tables (Table 4).
Table 4.
Summary of findings table
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
CI, confidence interval; MD, mean difference; OR, odds ratio
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
aOne RCT and 2 retrospective cohorts with low/moderate risk of bias. Main reason for downgrading is the study design
bMost studies are cohorts and before-after, and four with serious/critical risk of bias. Main reasons for downgrading are limitations due to study design and confounding
cOne RCT and one retrospective cohorts with moderate risk of bias. Main reason for downgrading is the study design
dConfidence interval is wide, showing that LPS could reduce slightly the cancelation rate or increase it a lot
eTwo cohort studies with critical risk of bias (the larger with 90% of the weight is retrospective). Main reasons for downgrading are limitations due to study design and unbalanced confounding
fConfidence interval is wide, showing that LPS could increase or have no effect on liver birth rate
gOne RCT with unclear risk for randomization method and allocation concealment and one before-after cohort with critical risk of bias. Main reason for downgrading is the study design
hOne RCT with unclear risk for randomization method and allocation concealment and one retrospective cohort with moderate risk of bias. Main reason for downgrading is the study design
iConfidence interval is wide, showing that Double stimulation could reduce or increase the clinical pregnancy rate
jConfidence interval is wide, showing that Double stimulation could reduce or increase the miscarriage rate
kOne small prospective cohort with moderate risk of bias. Main reason for downgrading is the study design
lConfidence interval is wide, showing that Double stimulation could make little or no increase in live birth rate, or it could be a large increase in live birth rate
mOne small retrospective cohort with critical risk of bias. Main reason for downgrading is the study design and limitations in confounding
nConfidence interval is wide, showing that Double stimulation could reduce or increase the cancelation rate
Luteal-phase stimulation
Poor responders
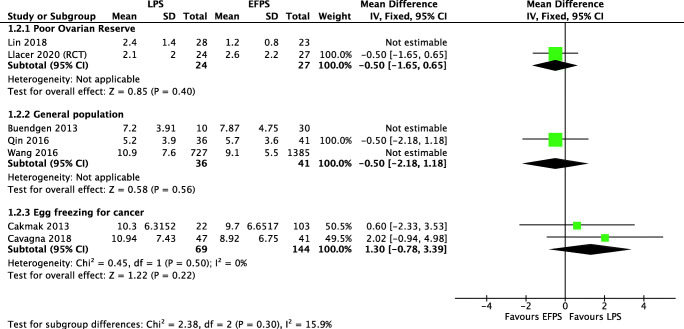
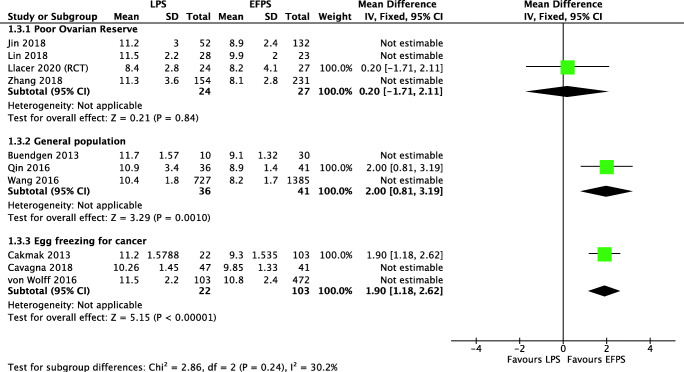
Four studies analyzed 865 women with low ovarian reserve (one RCT, one prospective, and two retrospective cohort studies) evaluated “Luteal” versus “Conventional” [33, 34, 39, 40]. The analysis ruled out a clinically important difference on the total number of retrieved oocytes (MD 0.42, 95% CI 0.07 to 0.76) and MII oocytes (MD 0.83, 95% CI 0.28 to 1.37), which was confirmed when we performed a sensitivity analysis including only low and moderate risk of bias studies, where we have not found clinically important differences for total number of retrieved oocytes (MD 0.09, 95% CI − 1.03 to 1.21) and for MII oocytes (MD − 0.50, 95% CI − 1.65 to 0.65) (Fig. 3). The evidence is very uncertain about the effect of “Luteal” on live birth rate (OR 0.87, 95% CI 0.50 to 1.53), clinical pregnancy rate (OR 0.88, 95% CI 0.57 to 1.36), and miscarriage rate (OR 0.93, 95% CI 0.73 to 1.19). Due to the wide confidence interval and the very-low-quality evidence, we are uncertain if “Luteal” increases the ovarian stimulation length or the cancelation rate in poor responders (Fig. 4 shows the sensitivity analysis including only low/moderate risk of bias).
Fig. 3.
MII oocyte retrieval in LPS and eFPS. Description of the illustration: Sensitivity analysis including best quality studies fails to show any clinically significant
Fig. 4.
Ovarian stimulation length in LPS and eFPS. Description of the illustration: Sensitivity analysis including best quality studies in normo-responders shows that LPS may be some longer than eFPS
General population
Three studies with 2229 women from the general infertile population (one prospective and two retrospective cohort studies) evaluated “Luteal” versus “Conventional” [30, 37, 42]. The analysis ruled out a clinically important difference on the total number of retrieved oocytes (MD − 0.94, 95% CI − 2.56 to 0.67) and MII oocytes (MD 1.44, 95% CI 0.87 to 2.01) (Fig. 3 shows the sensitivity analysis including only low/moderate risk of bias). Due to the very low quality of evidence, we are uncertain about the effect of “Luteal” on live birth rate (OR 1.24, 95% CI 1.05 to 1.49), clinical pregnancy rate (OR 1.18, 95% CI 1.00 to 1.39), and miscarriage rate (OR 0.93, 95% CI 0.73 to 1.19). Both the overall evaluation and the sensitivity analysis that included only the study with low risk of bias studies showed that stimulation is longer in “Luteal” (OR 2.00 days, 95% CI 0.81 to 3.19) (Fig. 4 shows the sensitivity analysis including only low/moderate risk of bias). We are uncertain if cancelation rate is different among both types of stimulations.
Oocyte freezing
Three studies that analyzed 808 women undergoing oocyte freezing (retrospective cohort studies) evaluated “Luteal” versus “Conventional” [20, 31, 38]. “Luteal” may slightly increase the total number of retrieved oocytes (MD 1.85, 95% CI 0.46 to 3.23) but it rules out an important clinical difference in MII oocytes (MD 1.30, 95% CI -0.78 to 3.39) (Fig. 3). We found that “Luteal” probably slightly increases the ovarian stimulation length (MD 0.85 days, 95% CI 0.53 to 1.18) (Fig. 4 shows the sensitivity analysis including only low/moderate risk of bias).
DuoStim
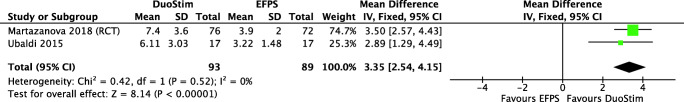
The four included studies (one RCT, one prospective cohort, one retrospective cohort, and one before-after study) compared DuoStim with a single “Conventional,” evaluated only poor responders that underwent IVF cycles [14, 33, 35, 41]. DuoStim showed a higher number of total retrieved oocytes (MD 4.68, 95% CI 3.75 to 5.62) and MII oocytes (MD 3.35, 95% CI 2.54 to 4.15), almost doubling the number obtained in an “Conventional” (Fig. 5). One observational study showed that more women got at least one euploid embryo in a DuoStim in comparison to a single “Conventional” (33% vs 19.3%, p < 0.05) [41]. It is unclear if DuoStim increases the clinical pregnancy rate (OR 1.41, 95% CI 0.85 to 2.34) and miscarriage rate (OR 0.64, 95% CI 0.17 to 2.31) in comparison with a single “Conventional.” Live birth rate was analyzed by a single small prospective cohort study, showing that it may be higher in DuoStim (OR 2.00, 95% CI 0.94 to 4.23) but the confidence interval is wide. The evidence is very uncertain about the effect of DuoStim on the cancelation rate (OR 0.56, 95% CI 0.26 to 1.23).
Fig. 5.
MII oocyte retrieval in double stimulation and eFPS. Description of the illustration: Double stimulation probably increases the number of MII oocytes in comparison with eFPS
We did not find studies evaluating DuoStim in women that were not poor responders or studies evaluating DuoStim versus two separates classical “Conventional.”
Random-start stimulation
Six studies evaluated 11 different comparisons in women undergoing a random-start stimulation (all retrospective cohort studies) [20, 31, 33, 36–38]. Four of them were evaluated in the “Luteal” section. The other started on late follicular phase or did not specify in which part of the cycle the stimulation was started. The evidence is very uncertain about the effect of random-start stimulation on all the analyzed outcomes (see details in Tables 1 and 2).
Discussion
We found no differences in the number of retrieved oocytes when comparing “Luteal” with “Conventional,” neither in oocyte freezing for cancer nor for ART treatments among poor responders or general infertile population. We also found that “Conventional” is probably a day or two shorter than “Luteal.” No high-quality evidence was found for live birth, clinical pregnancy, and miscarriage rates for “Luteal.” No conclusions could be drawn for the late follicular stimulation and random-start stimulation in general, as the evidence is very low quality. Finally, DuoStim, as expected, showed that it may be associated with a higher number of total retrieved oocytes, MII oocytes, and euploid embryos, almost doubling the number obtained in “Conventional.” In terms of live birth, clinical pregnancy, and miscarriage rates, DuoStim may be better than the “Conventional” but, due to the limited number of events, no definitive conclusion can be reached. Unfortunately, we found no studies comparing DuoStim with two separate “Conventional.”
This is the most updated and complete systematic review on different sections of the menstrual cycle when COH was started, including a dual stimulation protocol within the same cycle. The search was performed in the most relevant databases without language restrictions. We not only included those studies in which the intervention was “Luteal,” but we also included those that involved a DuoStim as well as those that were started randomly during any other portion of the menstrual cycle, including “Late follicular.” We limited the inclusion criteria to studies that compared any of the above-mentioned interventions vs the classic “Conventional,” to see how these interventions compared with the standard COH, and to investigate if saving time by starting the COH sooner, rather than waiting for the next cycle could be a valid option. We decided to make a broad approach in order to enhance the generalizability of the results. Therefore, we included ART treatments for general population and poor responders, women undergoing fertility preservation, and oocyte donors as well. To prevent any methodological flaw, we made separate subgroup analysis only, not pooling data from different populations. Quality of the evidence was analyzed systematically by pairs of independent reviewers, we used different types of tools according to the study design, and we provided adequate valuation of the available evidence, which reinforced the need for better primary research on this topic. Given the paucity of randomized trials, we also included non-randomized studies. After performing a sensitivity analysis, we were able to confirm those results. The inclusion of non-randomized trials makes these results more generalizable.
A limitation in our study is that the certainty of the evidence is low to very low. We are aware that combining RCTs with other study designs could be controversial, but we took this approach only in cases that lacked heterogeneity between studies. Only two RCTs were included, and they showed some domains with a high risk of bias. In the rest of the cases, the decision-making process when choosing a stimulation protocol depended arbitrarily on the treating physician, and the lack of random allocation of the patients created a serious to critical overall risk of bias for most studies. Differences observed between the stimulation protocols used in the intervention and the comparator group, as well as the imbalance found between the compared groups, made comparisons difficult. Although this variability, both in the intervention and the comparator, was evident in the heterogeneity of some outcomes, some others such as the number of oocytes were not compromised and pooling of data helped to obtain more precise results. Most of the evidence about the timing on when to start COH was weak, and therefore, we need better quality studies to prove if recommending “Late follicular,” “Luteal,” or DuoStim protocols affect the outcomes when compared with a standard “Conventional.”
We have found one previous systematic review that only included DuoStim in comparison to “Conventional,” this review evaluated five studies with a meta-analytical approach [43]. Although most of the included studies for this comparison were similar to ours, we excluded one of the studies included in that review, because “Conventional” was part of the DuoStim cycle [44], which was an exclusion criterion in our analysis. In agreement with our review, they also found that DuoStim cycles were associated with a higher total number of MII oocytes and embryos, a longer COH length, a higher dose of exogenous gonadotropins, and a lower cycle cancelation rate. They did not find important differences in clinical pregnancy rate, ongoing pregnancy rate, or miscarriage rate either. Similar to our findings, they highlighted that the enrolled populations had a high level of heterogeneity and classified the evidence as low quality. Although these authors also compared “Luteal” with “Conventional,” they only included studies in which both arms came from a single DuoStim cycle. On the contrary, we included studies that compared both interventions (“Luteal” and “Conventional”) but performed in separate cycles. The advantage of comparing separate cycles is that the internal and external validity is higher in order to analyze if “Luteal” outcomes are different from “Conventional.” There is a second systematic review on this topic, published in 2017, which searched only for English-language studies within PubMed [21]. They did not perform a meta-analysis and the main objective of the study was to assess the use of progestins to prevent the LH surge; therefore, its conclusions only refer to that specific topic. We have not found other systematic reviews comparing “Conventional” with “Late follicular,” LFP, random-start ovarian stimulation, or DuoStim. Finally, there is one other study that was only partially included in our review [41]. This study compared 100 patients who underwent a DuoStim with 197 patients that underwent a single conventional stimulation. Out of the 181 that did not get pregnant in the second group, only 17 came for a second single stimulation, with no further pregnancies. It would be useful to have more studies like this but including a larger number of patients with a second classical COH. Information like that would help us to answer an important question: is it more efficient to perform a single DuoStim or two single classical stimulations? Performing a DuoStim cycle allows patients to undergo two oocyte retrievals in a shorter amount of time.
Conclusions
This is the most updated and comprehensive review on this topic. This study shows that starting the COH during any part of the menstrual cycle could be an option for all poor responders, general infertile population, and patients undergoing oocyte freezing for cancer. We also found that performing a DuoStim protocol could achieve a higher number of retrieved MII oocytes in a single menstrual cycle in poor responders. We are fully aware that current evidence comes mainly from observational studies with a high risk of bias, and few randomized controlled trials. However, given the popularity of some of these protocols (i.e., DuoStim), we feel that this manuscript will establish the current status in regard to the scientific evidence and strength behind these alternative stimulation protocols, and encourage the performance of better and more appropriate studies on this topic.
Electronic supplementary material
(DOC 115 kb)
(DOC 48 kb)
Acknowledgements
Thanks, Daniel Comandé, for helping in the search strategy.
Authors’ contributions
DG: participation in study design, execution, analysis, manuscript drafting, and critical discussion.
RP: participation in study design, execution, analysis, manuscript drafting, and critical discussion.
MM: participation in execution, analysis, and manuscript drafting.
CS: participation in study design, manuscript drafting, and critical discussion.
KL: participation in execution, analysis, manuscript drafting, and critical discussion.
AC: participation in study design, analysis, manuscript drafting, and critical discussion.
Data availability
Search strategies are included in supplemental tables.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Demian Glujovsky, Email: demian.glujovsky@gmail.com.
Romina Pesce, Email: romina.pesce@hospitalitaliano.org.ar.
Mariana Miguens, Email: marianamiguens@gmail.com.
Carlos E. Sueldo, Email: drsueldo@hotmail.com
Karinna Lattes, Email: klattes@cirh.es.
Agustín Ciapponi, Email: aciapponi@gmail.com.
References
- 1.Garcia-Velasco JA, Fatemi HM. To pill or not to pill in GnRH antagonist cycles: that is the question! Reprod Biomed Online. 2015;30(1472–6491 (Electronic)):39–42. doi: 10.1016/j.rbmo.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso MCA, Evangelista A, Sartorio C, Vaz G, Werneck CLV, Guimaraes FM, et al. Can ovarian double-stimulation in the same menstrual cycle improve IVF outcomes? JBRA Assist Reprod. 2017;21(3):217–221. doi: 10.5935/1518-0557.20170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Practice Committee of the American Society for Reproductive Medicine Electronic address aao. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112(6):1022–1033. doi: 10.1016/j.fertnstert.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Esteves SC, Carvalho JF, Bento FC, Santos J. A novel predictive model to estimate the number of mature oocytes required for obtaining at least one euploid blastocyst for transfer in couples undergoing in vitro fertilization/intracytoplasmic sperm injection: the ART calculator. Front Endocrinol (Lausanne) 2019;10:99. doi: 10.3389/fendo.2019.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril. 2011;96(5):1058–1061. doi: 10.1016/j.fertnstert.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100(6):1673–1680. doi: 10.1016/j.fertnstert.2013.07.1992. [DOI] [PubMed] [Google Scholar]
- 7.Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, Shoham Z. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol) Reprod BioMed Online. 2014;29(6):684–691. doi: 10.1016/j.rbmo.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Kahraman S, Çil AP, Oğur Ç, Semiz A, Yilanlioglu C. Probability of finding at least one euploid embryo and the euploidy rate according to the number of retrieved oocytes and female age using FISH and array CGH. J Reprod Biotechnol Fertil. 2016;5:2058915816653277. doi: 10.1177/2058915816653277. [DOI] [Google Scholar]
- 9.Vaiarelli A, Cimadomo D, Trabucco E, Vallefuoco R, Buffo L, Dusi L, et al. Double stimulation in the same ovarian cycle (DuoStim) to maximize the number of oocytes retrieved from poor prognosis patients: a multicenter experience and SWOT analysis. Front Endocrinol (Lausanne) 2018;9:317. doi: 10.3389/fendo.2018.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, Trabucco E, Venturella R, Vajta G, Rienzi L. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril. 2016;105(6):1488–1495. doi: 10.1016/j.fertnstert.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Sighinolfi G, Sunkara SK, La Marca A. New strategies of ovarian stimulation based on the concept of ovarian follicular waves: from conventional to random and double stimulation. Reprod BioMed Online. 2018;37(4):489–497. doi: 10.1016/j.rbmo.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Vaiarelli A, Cimadomo D, Argento C, Ubaldi N, Trabucco E, Drakopoulos P, et al. Double stimulation in the same ovarian cycle (DuoStim) is an intriguing strategy to improve oocyte yield and the number of competent embryos in a short timeframe. Minerva Ginecol. 2019;71(5):372–376. doi: 10.23736/S0026-4784.19.04390-9. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J. Luteal phase ovarian stimulation following oocyte retrieval: is it helpful for poor responders? Reprod Biol Endocrinol. 2015;13:76. doi: 10.1186/s12958-015-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ubaldi FM, Vaiarelli A, Alviggi C, Trabucco E, Zullo F, Capalbo A, et al. Double stimulation in a single menstrual cycle increases the number of oocytes retrieved in poor prognosis patients undergoing IVF treatment. Prospective study with historical control. 71st Annual Meeting of the American Society for Reproductive Medicine. Fertil Steril. 2015;104(3):e322. doi: 10.1016/j.fertnstert.2015.07.1007. [DOI] [Google Scholar]
- 15.Tsampras N, Gould D, Fitzgerald CT. Double ovarian stimulation (DuoStim) protocol for fertility preservation in female oncology patients. Hum Fertil (Camb) 2017;20(4):248–253. doi: 10.1080/14647273.2017.1287433. [DOI] [PubMed] [Google Scholar]
- 16.La Marca A, Capuzzo M. Use of progestins to inhibit spontaneous ovulation during ovarian stimulation: the beginning of a new era? Reprod BioMed Online. 2019;39(2):321–331. doi: 10.1016/j.rbmo.2019.03.212. [DOI] [PubMed] [Google Scholar]
- 17.Sonmezer M, Turkcuoglu I, Coskun U, Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril. 2011;95(6):2125. doi: 10.1016/j.fertnstert.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated August 2019). Cochrane 2019. [DOI] [PMC free article] [PubMed]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27(3):215–221. doi: 10.1097/GCO.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 21.Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update. 2017;23(2):211–220. doi: 10.1093/humupd/dmw047. [DOI] [PubMed] [Google Scholar]
- 22.Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation.
- 23.Babineau J. Product review: Covidence (systematic review software). J Can Health Libr Assoc / J Assoc Bibl Santé Can. 2014;35(2). 10.5596/c14-016.
- 24.Higgins J, Green S, (editors). Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Confidence intervals. 2011.
- 25.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011.
- 27.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EPOC Cochrane group. What study designs should be included in an EPOC review? EPOC Resources for review authors, 2017. http://epoc.cochrane.org/resources/epoc-resources-review-authors. Accessed 23 Jan 2018.
- 29.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. 2013.
- 30.Buendgen NK, Schultze-Mosgau A, Cordes T, Diedrich K, Griesinger G. Initiation of ovarian stimulation independent of the menstrual cycle: a case-control study. Arch Gynecol Obstet. 2013;288(4):901–904. doi: 10.1007/s00404-013-2794-z. [DOI] [PubMed] [Google Scholar]
- 31.Cavagna F, Pontes A, Cavagna M, Dzik A, Donadio NF, Portela R, Nagai MT, Gebrim LH. Specific protocols of controlled ovarian stimulation for oocyte cryopreservation in breast cancer patients. Curr Oncol. 2018;25(6):e527–ee32. doi: 10.3747/co.25.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Checa MA, Brassesco M, Sastre M, Gomez M, Herrero J, Marque L, et al. Random-start GnRH antagonist for emergency fertility preservation: a self-controlled trial. Int J Women's Health. 2015;7:219–225. doi: 10.2147/IJWH.S66743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin B, Niu Z, Xu B, Chen Q, Zhang A. Comparison of clinical outcomes among dual ovarian stimulation, mild stimulation and luteal phase stimulation protocols in women with poor ovarian response. Gynecol Endocrinol. 2018;34(8):694–697. doi: 10.1080/09513590.2018.1435636. [DOI] [PubMed] [Google Scholar]
- 34.Lin LT, Vitale SG, Chen SN, Wen ZH, Tsai HW, Chern CU, Tsui KH. Luteal phase ovarian stimulation may improve oocyte retrieval and oocyte quality in poor ovarian responders undergoing in vitro fertilization: preliminary results from a single-center prospective pilot study. Adv Ther. 2018;35(6):847–856. doi: 10.1007/s12325-018-0713-1. [DOI] [PubMed] [Google Scholar]
- 35.Martazanova B, Mishieva N, Bogatyreva K, Veyukova M, Kodileva T, Burmenskaya O, et al. Double stimulation in a single menstrual cycle in patients with reduced ovarian reserve: hormonal characteristics, cumulus cell gene expression, embryological and clinical outcome. Abstracts of the 34rd Annual Meeting of the European Society of Human Reproduction and Embryology. Hum Reprod. 2018;33(Suppl 1):i80. [Google Scholar]
- 36.Muteshi C, Child T, Ohuma E, Fatum M. Ovarian response and follow-up outcomes in women diagnosed with cancer having fertility preservation: comparison of random start and early follicular phase stimulation - cohort study. Eur J Obstet Gynecol Reprod Biol. 2018;230:10–14. doi: 10.1016/j.ejogrb.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Qin N, Chen Q, Hong Q, Cai R, Gao H, Wang Y, Sun L, Zhang S, Guo H, Fu Y, Ai A, Tian H, Lyu Q, Daya S, Kuang Y. Flexibility in starting ovarian stimulation at different phases of the menstrual cycle for treatment of infertile women with the use of in vitro fertilization or intracytoplasmic sperm injection. Fertil Steril. 2016;106(2):334–341. doi: 10.1016/j.fertnstert.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 38.von Wolff M, Capp E, Jauckus J, Strowitzki T, Germeyer A. Ferti Psg. Timing of ovarian stimulation in patients prior to gonadotoxic therapy: an analysis of 684 stimulations. Eur J Obstet Gynecol Reprod Biol. 2016;199:146–149. doi: 10.1016/j.ejogrb.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Llacer J, Moliner B, Luque L, Bernabeu A, Lledo B, Castillo JC, et al. Luteal phase stimulation versus follicular phase stimulation in poor ovarian responders: results of a randomized controlled trial. Reprod Biol Endocrinol. 2020;18(1):9. doi: 10.1186/s12958-020-00570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W, Wang M, Wang S, Bao H, Qu Q, Zhang N, Hao C. Luteal phase ovarian stimulation for poor ovarian responders. JBRA Assist Reprod. 2018;22(3):193–198. doi: 10.5935/1518-0557.20180045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaiarelli A, Cimadomo D, Conforti A, Schimberni M, Giuliani M, D'Alessandro P, et al. Luteal phase after conventional stimulation in the same ovarian cycle might improve the management of poor responder patients fulfilling the Bologna criteria: a case series. Fertil Steril. 2020;113(1):121–130. doi: 10.1016/j.fertnstert.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Wang N, Wang Y, Chen Q, Dong J, Tian H, Fu Y, Ai A, Lyu Q, Kuang Y. Luteal-phase ovarian stimulation vs conventional ovarian stimulation in patients with normal ovarian reserve treated for IVF: a large retrospective cohort study. Clin Endocrinol. 2016;84(5):720–728. doi: 10.1111/cen.12983. [DOI] [PubMed] [Google Scholar]
- 43.Sfakianoudis K, Pantos K, Grigoriadis S, Rapani A, Maziotis E, Tsioulou P, Giannelou P, Kontogeorgi A, Pantou A, Vlahos N, Koutsilieris M, Simopoulou M. What is the true place of a double stimulation and double oocyte retrieval in the same cycle for patients diagnosed with poor ovarian reserve? A systematic review including a meta-analytical approach. J Assist Reprod Genet. 2020;37(1):181–204. doi: 10.1007/s10815-019-01638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Jiang H, Zhang W, Yin H. Double ovarian stimulation during the follicular and luteal phase in women >/=38 years: a retrospective case-control study. Reprod BioMed Online. 2017;35(6):678–684. doi: 10.1016/j.rbmo.2017.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 115 kb)
(DOC 48 kb)
Data Availability Statement
Search strategies are included in supplemental tables.