Abstract
Purpose
To evaluate the clinical usefulness of the endometrial receptivity array (ERA) and the preimplantation genetic test for aneuploidy (PGT-A) in patients with severe and moderate recurrent implantation failure (RIF).
Design
A retrospective multicenter cohort study was conducted in patients who failed to achieve implantation following transfer of 3 or more or 5 or more embryos in at least three single embryo transfers; patients were classified as moderate or severe RIF, respectively. Patients with previous RIF were compared based on the testing they received: PGT-A, ERA, or PGT-A+ERA versus a control group with no testing. Mean implantation rate and ongoing pregnancy rates per embryo transfer were considered primary outcomes. Multiple logistic regression analysis was performed and adjusted ORs were calculated to control possible bias.
Results
Of the 2110 patients belonging to the moderate RIF group, those who underwent transfer of euploid embryos after PGT-A had a higher implantation rate than those who did not. Additionally, the PGT-A group had a significantly higher rate of ongoing pregnancy. The same outcomes measured for the 488 patients in the severe RIF group did not reveal any statistically significant improvements. The use of the ERA test did not appear to significantly improve outcomes in either group.
Conclusions
PGT-A may be beneficial for patients with moderate recurrent implantation failure but not for severe cases. At its current level of development, ERA does not appear to be clinically useful for patients with RIF.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01948-7) contains supplementary material, which is available to authorized users.
Keywords: Recurrent implantation failure, Endometrial receptivity, Window of implantation, PGT-A, Infertility, Embryo transfer, ERA test, Pregnancy, Implantation rate, Ongoing pregnancy rate, IVF cycles
Introduction
Recurrent implantation failure (RIF) is one of the most common conditions affecting IVF outcomes and is diagnosed after the failure of embryo transfer (ET) [1, 2]. Although there are disparities in RIF definitions based on varying numbers of ET, the quality of embryos and endometrium status are considered important factors in implantation [3]. Preimplantation genetic testing for aneuploidy (PGT-A) informs the transfer of normal embryos based on chromosomal analysis [4] to avoid chromosomal aneuploidies, which can cause pregnancy loss and implantation failure [5]. Patients most likely to benefit from PGT-A are infertile women of advanced maternal age [6, 7], while patients with a history of recurrent pregnancy loss or RIF may not benefit from PGT-A [8]. Although embryonic aneuploidy is likely a major contributor to human implantation failure, the proportion of euploid embryos failing to implant is approximately 30% [9, 10], suggesting that endometrial receptivity plays a central role in the persistent infertility of these couples.
Implantation is initiated by attachment of the blastocyst to the epithelial layer of the endometrium [11]. Attachment and invasion are optimal during the window of implantation (WOI) [12]. The WOI is a short period of the menstrual cycle in which the endometrium acquires a functional, but transient, status that supports blastocyst acceptance in a synchronic way. This window was classically diagnosed by endometrial histology [13], but this step is somewhat subjective and has recently been excluded from infertility workup [14, 15]. The introduction of the endometrial receptivity array (ERA) based on the expression of 238 endometrial genes may objectively diagnose receptivity more reliably than histology [16]. The ERA test improves the detection of temporal displacement of the WOI [17, 18] over endometrial histology. Personalized ET with ERA could result in better pregnancy rates [19, 20]; RIF may result from displacement of the WOI and/or its disruption by molecular pathologies independent of timing [21]. In RIF patients asynchrony (displacement) and pathology (disruption) are independently or together present in the same patient [22–24], RIF patients with displacement could benefit from a personalized embryo transfer day while patients with disrupted WOI should be identified for the research and development of new treatments [24]. Considering the multifactorial etiology of RIF, we used a large cohort to retrospectively evaluate the effectiveness of testing for endometrial (using ERA) and embryonic quality (using PGT-A) to improve clinical outcomes in IVF.
Material and methods
Patients
This observational, retrospective, multicenter study evaluated ART results from couples with RIF between 2013 and 2018 using data from IVIRMA clinics in Europe. Infertile patients between 18 and 45 years old who experienced RIF after repeated ART with their own or donated oocytes were included in the study. Patients who failed to achieve a clinical pregnancy after transfers of at least three good-quality embryos in different single fresh or frozen embryo transfers were considered RIF. Patients who lacked evidence of prior implantation events, including previous births, voluntary interruptions of pregnancy, or clinical miscarriages, were included in the study. Patients with an abnormal karyotype such as translocation or an inversion carrier and with thrombophilia, either congenital or acquired, were excluded. Patients with severe metabolic or endocrine disorders and patients with atrophic endometrium were not included in the study. Submucous myomas or polyps, previous ET with high difficulty, and/or bleeding without previous hysteroscopy correction were excluded [20]. Previous use of PGT-A was a criterion for exclusion.
Only embryos of good quality and day-5 embryos (blastocysts) were transferred. Embryos were graded according to expansion and quality of the inner cell mass and trophectoderm [25]. Good-quality embryos were defined as having the correct number of cells corresponding to the day of development.
A moderate RIF (M-RIF) group consisted of patients who first received at least three embryos transferred in different single embryo transfers (SET) without achieving implantation and without having received PGT-A or ERA. Subsequent ETs were categorized depending on the treatment received. Severe RIF (S-RIF) patients underwent at least five embryo transferred summed across consecutive cycles without ERA or PGT-A testing. In a previous study, 94.9% of patients with three euploid embryos transferred achieved clinical pregnancy [26]; therefore, we divided our population into a group who had at least three embryos transferred and a group who had a least five embryos transferred in at least three ET sessions. Classification was inclusive; patients included as S-RIF were also analyzed in the analysis of M-RIF. This classification demonstrates that the severity of RIF could depend on the number of embryos transferred, so different therapeutic approaches could be considered. Subsequent ET were categorized after ERA, PGT-A, or both. Patients who underwent frozen ET had either natural or hormonal cycles.
Ethical approval
This study was approved by the Ethics Committee of the Instituto Valenciano de Infertilidad (IVI), (identification code # 1801-FIVI-048-AP).
Outcomes measures
We determined the benefit of determining each patient’s and each group’s mean implantation rate as (a) the mean implantation rate per patient defined as the number of gestational sacs with heart beats divided by the number of embryos transferred, (b) the mean implantation per study group defined as the total number of gestational sacs with heart beats divided by the total number of embryos transferred within the group, and (c) ongoing pregnancy rate per transferred embryo defined as a positive pregnancy beyond 20 weeks gestation confirmed by ultrasound with fetal heart activity divided by the number of embryos transferred.
Endometrial receptivity analysis
The ERA (iGenomix, Valencia, Spain) is a transcriptomic analysis combined with artificial intelligence technology for dating the WOI [16]. The test assesses the expression of 238 genes that are biomarkers of endometrial dating. The ERA may personalize the timing of ET, synchronizing embryonic development with the endometrial WOI of the patient [27], and is used to determine endometrial receptivity in a sample obtained 7 days after the LH serum peak in a natural cycle or 5 days after progesterone administration in a hormone replacement cycle. Endometrial biopsies were collected from the uterine fundus, and samples were analyzed by iGenomix according to their protocol [16, 17, 20, 27]. Endometria were classified by expression profile as receptive or pre- or post-receptive [28].
Preimplantation genetic screening
Chromosomal analysis was performed by array comparative genomic hybridization (aCGH) or next-generation sequencing (NGS). Per iGenomix procedures and as specified by the manufacturer (Illumina), the 24sure aCGH platform has an effective 10-Mb resolution; therefore, only full chromosomal aneuploidies and segmental aneuploidies affecting chromosomal fragments larger than 10 Mb were identified [7, 29]. Embryos were vitrified and transferred in subsequent natural or hormonal cycles.
Statistical analysis
Categorical and continuous variables are presented as percentages or means with standard deviations or 95% confidence intervals (95% CI). To compare means, ANOVA tests were used with Bonferroni post-hoc tests. To control possible bias in multivariate analyses and to calculate adjusted ORs, logistic regression analysis was performed considering the proportion of ET that used donated oocytes, the day of ET, the mean number of embryos transferred per procedure, age, fresh/frozen status of the embryo, and the mean of the prior number of embryos transferred per patient as clinically relevant variables. Because this study was conducted over a 5-year period and couples may have had consecutive ET with different diagnostic techniques (e.g., PGT-A and then ERA+PGT-A), the groups lack independence and there is potential correlation between data from each group. Accordingly, we used generalized estimating equations to estimate the parameters of a generalized linear model with a possible unknown correlation between outcomes. Statistical significance was established at P < 0.05. Calculations were made with R version 3.5.0 (R core team) [30]. Estimation of statistical power was conducted to define the probability that a given test rejects a false null hypothesis to better interpret results, give context, and focus the discussion.
Results
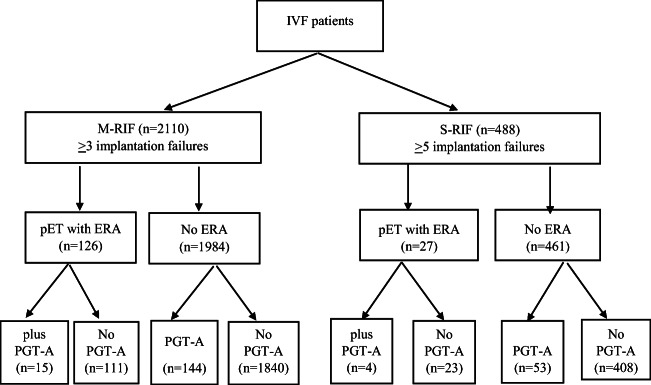
We identified 2110 patients with M-RIF and 488 with S-RIF (Fig. 1). The general and clinical features of the cycles and patients included in the study are shown in Table 1. Table 2 summarizes the parameters of the test-guided IVF cycles among the different groups of patients. The retrospective nature of our study resulted in the differential distribution of some relevant variables (Tables 1 and 2). To avoid bias, we included parameters with statistically significant differences and/or clinical relevance within a multivariate analysis model using generalized estimating equations. Some patients included in the M-RIF and S-RIF groups had infertility associated with uterine factors (Supplemental Table 1). Patients who received the ERA test had a higher percentage of uterine pathologies than control patients or those undergoing PGT-A (Supplemental Table 1). Patients scheduled for personalized ET after the ERA are shown in Supplemental Table 2. A large percentage of patients had an asynchronous or displaced WOI, particularly in the M-RIF group. The percentages of personalized ET (pET) were 25.7% and 39.3% in the S-RIF and M-RIF groups, respectively. The rate of euploid embryos in M-RIF for patients with PGT-A and ERA+PGT-A was 48.13%, 95% CI (46.56–49.69) and 39.35%, 95%CI (34.74–43.96), respectively, P = 0.225. In the S-RIF group, the rate of euploid embryos with PGT-A and ERA+PGT-A was 57.18%, 95%CI (54.71–59.65) and 62.13%, 95%CI (53.57–70.70), respectively, P = 0.708.
Fig. 1.
Patient selection flowchart. IVF in vitro fertilization, M-RIF moderate recurrent implantation failure, S-RIF severe recurrent implantation failure, pET personalized embryo transfer, ERA endometrial receptivity assay, PGT-A preimplantation genetic testing for aneuploidy
Table 1.
Number of embryo transfers (fresh and frozen) and general characteristics of patients before undergoing ERA, PGT-A, or ERA+PGT-A. Data are expressed as means or percentages with corresponding 95% confidence intervals within brackets. Superscripts in the P value column indicate statistically significant differences between groups with the same letter. The P values without superscripts indicate ANOVA results. Patients with no ERA, PGT-A, or ERA+PGT-A cycles are presented in the standard group
| M-RIF (N = 2110) | S-RIF (N = 488) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Standard | PGT-A | ERA | ERA+PGT-A | P value | Standard | PGT-A | ERA | ERA+PGT-A | P value | |
| Age (mean) |
37.9 [37.7–38.1] a,c |
38.2 [38.0–38.5] a,d |
38.6 [38.3–38.9] d,f |
38.5 [38.1–39.0] c,f |
< 0.001 a< 0.001 c0.0013 d< 0.001 f< 0.001 |
38.5 [38.2–38.7] a,c |
38.3 [38.1–38.6] a,d,e |
38.9 [37.8–41.9] d,f |
37.9 [37.7–38.5] c,e,f |
< 0.001 a< 0.001 c< 0.001 d0.0082 e< 0.001 f< 0.001 |
| Years of infertility |
2.6 [2.5–2.7]a |
2.2 [1.9–2.5] a |
2.6 [2.2–3] - |
1.9 [1.3-2.8] - |
0.0178 a0.0216 |
2.7 [2.4–2.9] c |
2.1 [1.8–2.5] - |
2.2 [1.7–2.9] - |
1.3 [0.9–2] c |
0.0016 c0.0059 |
| Body mass index (kg/m2) |
23.2 [23–23.4] - |
22.6 [21.9–23.2] - |
22.7 [22.1–23.2] - |
23.6 [21.2–25.9] - |
0.1196 |
23.2 [22.8–23.6] - |
22.2 [21.1–23.3] - |
22.9 [21.5–24.2] - |
24.2 [19.8–28.6] - |
0.3272 |
| Mean number of previous fresh embryos transferred per patient |
2.22 [2.22–2.24] b |
2.24 [2.17–2.31] d |
2.4 [2.31–2.49] b,d |
2.33 [2.11–2.58] - |
0.0015 b< 0.001 d0.0412 |
3.31 [3.25–3.37] - |
3.19 [3.06–3.32] - |
3.35 [3.13–3.58] - |
3.25 [2.85–3.70] c |
0.3708 |
| Mean number of previous frozen embryos transferred per patient |
1.35 [1.32–1.38] a,b |
1.55 [1.44–1.66] a |
1.57 [1.44–1.71] b |
1.64 [1.35–2.01] - |
<0.001 a< 0.001 b0.0029 |
1.82 [1.73–1.91] a |
2.16 [1.95–2.4] a |
1.86 [1.854–2.26] - |
2.25 [1.86–2.72] - |
0.0046 a0.0101 |
Table 2.
Characteristics of IVF cycles in patients who underwent ERA, PGT-A, or ERA+PGT-A or standard IVF treatments. Data are expressed as means or percentages with corresponding 95% confidence intervals in brackets. Superscripts in the P value column indicate statistically significant differences between groups with the same letter. The P values without superscripts indicate ANOVA results. Patients with no ERA, PGT-A, or ERA+PGT-A cycles are presented in the standard group
| M-RIF | S-RIF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Standard | PGT-A | ERA | ERA+PGT-A | P value | Standard | PGT-A | ERA | ERA+PGT-A | P value | |
| Number of total embryo transfers | 2636 | 183 | 160 | 21 | 591 | 72 | 35 | 6 | ||
| Embryo transfers with donated oocytes | 1593 | 15 | 124 | 1 | 427 | 9 | 26 | 0 | ||
| Percentage of donated oocyte-derived embryos among all embryos |
60.43 [58.06–62.75] a,b,c |
8.2 [4.04–15.91] a,d |
77.5 [68.41–84.56] b,d,f |
4.76 [0.64–27.8] c,f |
< 0.001 a< 0.001 b0.0038 c0.0057 d< 0.001 f< 0.001 |
72.25 [67.54–76.52] a,c |
12.5 [4.4–30.71] a,d,e |
74.29 [50.55–89.09] d,f |
0 [0–0] c,e,f |
< 0.001 a< 0.001 c< 0.001 c< 0.001 e< 0.001 f< 0.001 |
| Number frozen embryos transferred | 1774 | 183 | 124 | 21 | 407 | 72 | 23 | 6 | ||
| Percentage of transferred frozen embryos among all transferred embryos |
67.3 [65.52–69.03] a,b,c |
100 [100–100] a,e |
77.5 [70.97–82.91] b,f |
100 [100–100] c,e,f |
< 0.001 a< 0.001 b0.0216 c< 0.001 e< 0.001 f< 0.001 |
68.87 [65.09–72.41] a,c |
100 [100–100] a,d,e |
65.71 [52.0–77.2] d,f |
100 [100–100] c,e,f |
< 0.001 a0.0182 c< 0.001 d0.039 e< 0.001 f< 0.001 |
| Mean number of oocytes retrieved or donated |
14.1 [13.71–14.51] - |
14.58 [13.1–16.23] - |
14.6 [13.28–16.06] - |
14.07 [10.98–18.03] - |
0.8405 |
15.26 [14.38–16.2] - |
15.65 [13.42–18.26] - |
13.18 [11.31–15.36] - |
15.25 [9.22–25.21] - |
0.3376 |
| Mean number of MII oocytes inseminated |
12.48 [12.18–12.78] - |
12.26 [11.08–13.58] - |
13.09 [12.13–14.13] - |
11.87 [9.54–14.76] - |
0.6261 |
13.77 [13.1–14.48] - |
12.94 [11.09–15.11] - |
13.17 [11.47–15.13] - |
13.25 [8.49–20.67] - |
0.8318 |
| Fertilization rate per inseminated oocyte |
76.59 [75.65–77.55] - |
76.16 [73.34–79.09] - |
75.29 [71.86–78.89] - |
77.53 [65.83–91.3] - |
0.9024 |
75.89 [74.16–77.66] |
77.11 [72.83–81.65] |
77.89 [71.71–84.59] |
79.25 [70.38–89.23] |
0.8022 |
| Mean number of embryos available per oocyte pick-up |
5.05 [4.83–5.27] a,c |
3.29 [2.78–3.9] a,e |
4.28 [3.54–5.17] f |
1.47 [1.18–1.82] c,e,f |
<0.001 a< 0.001 c< 0.001 e< 0.001 f< 0.001 |
5.31 [4.84–5.82] a,c |
3.17 [2.46–4.08] a,d |
6.17 [4.47–8.53] d,f |
1.5 [0.85–2.64] c,f |
< 0.001 a< 0.001 b0.8163 c< 0.001 d0.007 e0.0782 f< 0.001 |
| Mean endometrial thickness (mm) |
8.9 [8.8–9] a,b |
9.4 [9.1–9.8] a,d |
8.3 [8–8.6] b,d |
8.7 [8.2–9.3] - |
< 0.001 a0.0301 b0.0013 d< 0.001 |
8.8 [8.6–9] - |
9.2 [8.7–9.7] - |
8.5 [7.6–9.4] - |
8.5 [7.2–9.8] - |
0.4423 |
| Mean number of days for endometrial preparation |
11.1 [10.9–11.3] b |
11.1 [10.5–11.7] d |
12.3 [11.7–12.9] b,d |
11.4 [10.5–12.4] - |
0.002 b< 0.001* d0.038* |
13.7 [12.3–15.2] c |
11.4 [10.7–12.1] e |
11.8 [10.7–13.1] - |
13.7 [12.3–15.2] c,e |
0.0095 c0.0052 e0.0128 |
Univariate ANOVA of the M-RIF group revealed a statistically significant difference in the overall mean implantation rates of the subgroups (P = 0.0053). The use of PGT-A yielded a better implantation rate per transfer (45.9%) than standard IVF (35.9%) with an OR of 1.34, 95% CI: 1.17–1.55, P < 0.001. Implantation rates per transfer were not improved over standard rates by ERA, OR 1.03, 95% CI: 0.85–1.24, P = 0.9926, and ERA+PGT-A, OR 0.94, 95% CI: 0.53–1.65, P = 0.9954. Significant differences were not detected between subgroups subjected to different tests (ERA vs PGT-A, ERA vs. PGT-A+ERA, PGT-A vs. PGT-A+ERA). Logistic regression models adjusted for control variables confirmed that within the M-RIF group, only the PGT-A test yielded significant improvement (AdjOR 1.22, 95% CI: 1.14–1.30, P < 0.001) over standard treatment, without statistical significance over ERA and ERA+PGT-A (Supplemental Table 3). When comparing the other subgroups after adjusting for control variables, we found a significant difference between the ERA+PGT-A and ERA (OR 0.84, 95% CI: 0.77–0.92, P < 0.001), but no other subgroup comparison reached significance.
Univariate ANOVA of the implantation rate per transfer as calculated by the number of gestational sacs per number of embryos transferred revealed statistically significant differences between the M-RIF subgroups (P = 0.005). The highest implantation rate was in PGT-A (47.2%) versus the control group (35.8%), ERA (35.6%), and ERA+PGT-A (31.82%). For M-RIF patients, the implantation rate per transfer was higher after PGT-A testing than after standard IVF (OR 1.61, 95% CI: 1.24–2.11, P = 0.002). There were no statistically significant differences between standard over ERA (OR 1.00, 95% CI: 0.74–1.34, P = 1) and ERA+ PGT-A (OR 0.84, 95% CI: 0.34–2.07, P = 0.9797). When the ORs were adjusted by logistic regression models for control variables, the PGT-A subgroup was significantly different from the control subgroup (AdjOR 2.69, 95% CI: 1.99–3.66, P < 0.001), while no statistically confirmed differences were identified between ERA and ERA+PGT-A and controls (Supplemental Table 3). In addition, the ERA subgroup significantly differed from the PGT-A subgroup (AdjOR 0.40, 95% CI: 0.26–0.62, P < 0.001).
Table 3 shows the ongoing pregnancy rates for all study groups based on the number of embryos transferred. Univariate ANOVA revealed statistically significant differences between M-RIF subgroups (P = 0.05). Post hoc testing between M-RIF subgroups revealed that only the PGT-A subgroup differed from the control group (OR 1.51, 95% CI: 1.12–2.05, P = 0.029). There were no significant differences between the standard group and ERA (OR 1.02, 95% CI: 0.73–1.42, P = 0.9997) and ERA+PGT-A (OR 0.89, 95% CI: 0.36–2.22, P = 0.9942). The PGT-A group differed from the control group (AdjOR 2.19, 95% CI: 1.55–3.07, P < 0.0001) when multivariate analysis was applied to adjust for control variables, while no statistical differences were detected for ERA and ERA+PGT-A (Supplemental Table 3). The ERA group showed lower ongoing pregnancy rates compared with the PGT-A group (AdjOR 0.51, 95% CI: 0.31–0.83, P < 0.0284). No statistically significant differences emerged in the comparisons between other subgroups.
Table 3.
Number of ongoing pregnancies and rates of ongoing pregnancies expressed with 95% confidence intervals for all M-RIF and S-RIF patients. Patients who underwent no testing are presented as standard
| Number of ongoing pregnancies | Ongoing pregnancy % (CI) | |||
|---|---|---|---|---|
| M-RIF | S-RIF | M-RIF | S-RIF | |
| Standard | 946 | 201 | 35.89% (34.05–37.75) | 34.01% (30.19–37.99) |
| ERA | 58 | 14 | 36.25% (28.81–44.21) | 40.00% (23.87–57.89) |
| PGT-A | 84 | 26 | 45.90% (38.53–53.41) | 36.11% (25.12–48.29) |
| PGT-A+ERA | 7 | 2 | 33.33% (14.59–56.97) | 33.33% (4.33–77.72) |
Univariate analysis of the mean implantation rate of the S-RIF subgroups revealed no statistically significant differences. The implantation rate per patient was 34.2% (95% CI: 30.68–37.81) for the control, 40% (95% CI: 25.40–54.60) for ERA, 38.2% (95% CI: 28.02–48.37) for PGT-A, and 33.3% (95% CI: 0–68.59) for GT-A+ERA groups. Logistic regression models with adjusted OR for control variables revealed no statistically significant differences between test and control groups or for multiple comparisons between subgroups (Supplemental Table 3).
Univariate analysis did not detect any statistically significant differences in the implantation rate calculated per S-RIF subgroup considering the number of gestational sacs and the number of embryos transferred. The implantation rates were 34.8% (95% CI: 31.63–37.99) for control, 37% (95% CI: 23.21–52.45) for ERA, 39.8% (95% CI 29.78–50.46) for PGT-A, and 33.3% (95% CI 4.33–77.72) for PGT-A+ERA patients. A logistic regression model with adjusted OR for potentially confounding variables revealed no statistically significant differences for any group or the multiple comparisons between all groups (Supplemental Table 3). No statistical significance was detected when comparing ERA vs PGT-A, ERA vs ERA+PGT-A, or PGT-A vs PGT-A+ERA.
Table 3 shows the rates of ongoing pregnancy for all study groups based on the number of embryos transferred. Univariate analysis was not statistically significant for contrasts between S-RIF subgroups. Multivariate analysis did not detect statistically significant differences between treatments (PGT-A, ERA, or PGT-A+ERA) and the control group (Supplemental Table 3) or in the multiple comparisons between the subgroups.
Discussion
Our results suggest that PGT-A could be a useful tool for assessing chromosomal viability in RIF patients to avoid the transfer of aneuploidy embryos in M-RIF. In S-RIF, the use of PGT-A does not improve ongoing pregnancy rate, although this group included a small number of embryo transfers. We also determined that the ERA test does not benefit RIF patients. The assessment of PGT-A in M-RIF confirming the euploid status of embryos significantly improves sustained implantation rates and ongoing pregnancy over selecting embryos based on morphology alone [31]. Patients with RIF have increased numbers of embryo anomalies [32], including translocations, mosaicism, inversions, and deletions, which can be resolved with the use of PGT-A [33]. In some cases, even euploid embryos are unable to implant [9], indicating an etiology independent of embryonic genetics. Although PGT-A assists the selection of embryos with a higher probability of implantation, any euploid embryos that do not implant suggest that chromosomal status is not the only factor contributing to infertility. In the case of S-RIF, the use of PGT-A was insufficient to improve IVF outcomes, suggesting that different tools may be needed to assess embryo quality and account for endometrial factors. Even though PGT-A can mitigate maternal age in IVF patients, patients in the oldest group (> 42 years) had different implantation rates than those in younger groups (< 35–42 years) [34]. Additionally, groups with limited numbers of cases, like S-RIF, may bias results then caution is necessary in their interpretation.
Although PGT-A is an important tool for patients with advanced maternal age, the standard use of PGT-A is actively debated [35]. PGT-A may not improve overall pregnancy outcomes and may decrease the number of embryo transfers necessary for achieving the same live birth rate [36]. Indeed, Munne et al. showed a significant increase in the ongoing pregnancy rate per ET with the use of PGT-A in women aged 35–40 years with two or more embryos that could be biopsied [37]. The methodology of the STAR study [37] was questioned by recent opinion; in fact in contrast to the overall study that had been performed with reference point cycle start (intent-to-treat), their post hoc analysis was performed with reference embryo transfer [38]. The use of PGT-A should be addressed according to clinical history, as in advanced maternal age, recurrent pregnancy loss, and severe male infertility; moreover, the use of PGT-A in patients with RIF could improve live birth rates per embryo transfer compared with patients with no PGT-A [8]. Yet, there were no significant differences in live birth rates per patient given PGT-A (35.7%) or not (26.0%) [8].
In addition, the cost for conducting PGT-A is important from a patient perspective. PGT-A could be effective after age 35 in referral centers that rely on embryologists with expertise in trophectoderm biopsy and that follow a policy of extended embryo culture and single embryo transfer [39]. Ideally, PGT-A should reduce healthcare costs, shorten treatment time, and reduce the risk of failed embryo transfer and clinical miscarriages compared to normal IVF cycles [40]. Based on our evaluation, PGT-A seems to be beneficial for M-RIF patients but is not effective for S-RIF patients, although we cannot rule out the possibility that an unrecognized uterine disorder could influence implantation. In addition, the limited number of cases included in the S-RIF group complicates generalization of the results.
The temporal window of endometrial receptivity to blastocysts is limited, and most of the histologic criteria using markers of endometrial receptivity are subjective and lack accuracy and predictive value [15]. The ERA was created for more accurate endometrial dating throughout the luteal phase [17], because endometrial status could not be assessed with endometrial thickness through transvaginal ultrasound together and luteal phase hormone levels. In our study, numerous M-RIF and S-RIF patients had displaced WOIs and qualified for pET. This is consistent with the previous studies that report a 25–30% contribution of the endometrial factor to implantation failure [41, 42]. The higher percentage of pET in the M-RIF group could be explained by the higher percentage of uterine pathologies, diagnosed by ultrasound, which could affect ERA. Unfortunately, ERA did not improve implantation or ongoing pregnancy rates, consistent with a study in which a personalized adjustment of progesterone did not improve pregnancy outcomes in RIF patients receiving euploid embryos [42]. The first randomized controlled trial on the use of ERA showed that the ERA test did not improve live birth rate after first embryo transfer; rather, the ERA test increased the cumulative live birth rate compared with frozen embryo transfer or fresh embryo transfer after 12 months [43].
Demonstrating embryo health is necessary before considering whether the endometrium might contribute to implantation failure, but chromosomal status does not provide enough information. Novel embryo assessment and selection procedures, such as time-lapse imaging and metabolomics, may help better evaluate embryo quality and viability [44]. These data should be evaluated for their usefulness in RIF as well as chromosomal information, certainly PGT-A should be considered in RIF [45]. In addition, more data on PGT-A in S-RIF is warranted. Based on our results, RIF may not be related to endometrial dating by current ERA, so deep characterization of endometrial pathology is needed to fully evaluate the endometrial factor in IVF cycles.
Strengths and limitations
To our knowledge, this study is the largest to evaluate the clinical usefulness of PGT-A and ERA for RIF patients. Importantly, the severe RIF group is especially difficult to study because few patients do not achieve pregnancy after five embryo transfers (thus, the “severe” group is a small subset). However, our study’s retrospective nature and strict inclusion criteria for defining subpopulations mean that some comparisons were carried out with moderate sample sizes and a limited number of transferred embryos. This particularly affected the S-RIF PGT-A, ERA, and PGT-A+ERA subgroups. From a clinical perspective, this affects how the data should be interpreted. When comparing M-RIF subgroups, our study was powered to detect a 10% effect of using ERA results to guide ET. For the S-RIF subgroups, which had even lower numbers of patients, our study was powered to detect an approximate 20% effect. Our work is underpowered for detecting smaller differences, and as more data is collected, the clinical benefit may become clearer.
Conclusions
The major findings of this study are that M-RIF patients can benefit from PGT-A, while S-RIF patients may not. This difference perhaps reflects the small sample size of S-RIF patients but could also reflect a different cause of implantation failure, potentially related to endometrium quality. Chromosomal screening should be considered for M-RIF patients to overcome infertility. On the other hand, we did not find clinical evidence of benefit from ERA, in terms of live birth rate after first embryo transfer. Further, ERA could not identify the most appropriate time for embryo transfer or detect uterine diseases affecting implantation; new technologies may be necessary to assess the endometrial aspect of implantation. A more thorough investigation of the effect of pET on reproductive outcomes could also shed light on the role of the endometrium. Well-powered prospective studies in RIF patients are needed to fully evaluate whether ERA has a clinical benefit. Although S-RIF is less common than M-RIF in IVF patients, further study is warranted because designing treatments for this condition will likely prove challenging.
Electronic supplementary material
(DOCX 20 kb)
Acknowledgments
We would like to thank the clinical directors of the participating IVI centers for their contribution to the data of the database analyzed. We also thank the team of Fresh Eyes Editing, LLC., especially Dr. Sheila Cherry, for their professional assistance in manuscript preparation.
Authors’ contribution
Mauro Cozzolino: study design, data analysis, interpretation, and manuscript writing. Patricia Díaz-Gimeno: data analysis and interpretation, critical review, and final manuscript approval. Antonio Pellicer: study design, critical review and final manuscript approval. Nicolas Garrido: study design, statistical analysis, critical review, and final manuscript approval.
Funding
This study was funded by IVIRMA global. No additional external funding was received for this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
The study was approved to IVI Valencia Ethic Committee on 3rd March 2019 with number 1806-FIVI-048-AP.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zeyneloglu HB, Onalan G. Remedies for recurrent implantation failure. Semin Reprod Med. 2014;32:297–305. doi: 10.1055/s-0034-1375182. [DOI] [PubMed] [Google Scholar]
- 2.Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, Cutting R, Ong K, Sallam H, Li TC. Recurrent implantation failure: definition and management. Reprod BioMed Online. 2014;28:14–38. doi: 10.1016/j.rbmo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Simon A, Laufer N. Assessment and treatment of repeated implantation failure (RIF) J Assist Reprod Genet. 2012;29:1227–1239. doi: 10.1007/s10815-012-9861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coughlan C. What to do when good-quality embryos repeatedly fail to implant. Best Pract Res Clin Obstet Gynaecol. 2018;53:48–59. doi: 10.1016/j.bpobgyn.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatirnaz S, Ozer A, Hatirnaz E, Atasever M, Basaranoglu S, Kanat-Pektas M, et al. Pre-implantation genetic screening among women experiencing recurrent failure of in vitro fertilization. Int J Gynaecol Obstet. 2017;137:314–318. doi: 10.1002/ijgo.12135. [DOI] [PubMed] [Google Scholar]
- 7.Rubio C, Rodrigo L, Mir P, Mateu E, Peinado V, Milan M, et al. Use of array comparative genomic hybridization (array-CGH) for embryo assessment: clinical results. Fertil Steril. 2013;99:1044–1048. doi: 10.1016/j.fertnstert.2013.01.094. [DOI] [PubMed] [Google Scholar]
- 8.Sato T, Sugiura-Ogasawara M, Ozawa F, Yamamoto T, Kato T, Kurahashi H, Kuroda T, Aoyama N, Kato K, Kobayashi R, Fukuda A, Utsunomiya T, Kuwahara A, Saito H, Takeshita T, Irahara M. Preimplantation genetic testing for aneuploidy: a comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum Reprod. 2019;34:2340–2348. doi: 10.1093/humrep/dez229. [DOI] [PubMed] [Google Scholar]
- 9.Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100:100–7.e1. doi: 10.1016/j.fertnstert.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5:24. doi: 10.1186/1755-8166-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassil R, Casper R, Samara N, Hsieh TB, Barzilay E, Orvieto R, Haas J. Does the endometrial receptivity array really provide personalized embryo transfer? J Assist Reprod Genet. 2018;35:1301–1305. doi: 10.1007/s10815-018-1190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kliman HJ, Frankfurter D. Clinical approach to recurrent implantation failure: evidence-based evaluation of the endometrium. Fertil Steril. 2019;111:618–628. doi: 10.1016/j.fertnstert.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 14.Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, McGovern P, Schlaff WD, Carr BR, Steinkampf MP, Silva S, Vogel DL, Leppert PC, NICHD National Cooperative Reproductive Medicine Network Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264–1272. doi: 10.1016/j.fertnstert.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 15.Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, Zeng D, Fritz MA. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81:1333–1343. doi: 10.1016/j.fertnstert.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Gimeno P, Horcajadas JA, Martinez-Conejero JA, Esteban FJ, Alama P, Pellicer A, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95:50–60. doi: 10.1016/j.fertnstert.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martinez-Conejero JA, Alama P, et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril. 2013;99:508–517. doi: 10.1016/j.fertnstert.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Diaz-Gimeno P, Ruiz-Alonso M, Sebastian-Leon P, Pellicer A, Valbuena D, Simon C. Window of implantation transcriptomic stratification reveals different endometrial subsignatures associated with live birth and biochemical pregnancy. Fertil Steril. 2017;108:703–710.e3. doi: 10.1016/j.fertnstert.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Alonso M, Galindo N, Pellicer A, Simon C. What a difference two days make: “personalized” embryo transfer (pET) paradigm: a case report and pilot study. Hum Reprod. 2014;29:1244–1247. doi: 10.1093/humrep/deu070. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Gomez E, Fernandez-Sanchez M, Carranza F, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818–824. doi: 10.1016/j.fertnstert.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Koot YE, van Hooff SR, Boomsma CM, van Leenen D, Groot Koerkamp MJ, Goddijn M, et al. An endometrial gene expression signature accurately predicts recurrent implantation failure after IVF. Sci Rep. 2016;6:19411. doi: 10.1038/srep19411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macklon N. Recurrent implantation failure is a pathology with a specific transcriptomic signature. Fertil Steril. 2017;108:9–14. doi: 10.1016/j.fertnstert.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Valdes CT, Schutt A, Simon C. Implantation failure of endometrial origin: it is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil Steril. 2017;108:15–18. doi: 10.1016/j.fertnstert.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 24.Sebastian-Leon P, Garrido N, Remohi J, Pellicer A, Diaz-Gimeno P. Asynchronous and pathological windows of implantation: two causes of recurrent implantation failure. Hum Reprod. 2018;33:626–635. doi: 10.1093/humrep/dey023. [DOI] [PubMed] [Google Scholar]
- 25.Cutting R, Morroll D, Roberts SA, Pickering S, Rutherford A, BFS and ACE Elective single embryo transfer: guidelines for practice British Fertility Society and Association of Clinical Embryologists. Hum Fertil (Camb) 2008;11:131–146. doi: 10.1080/14647270802302629. [DOI] [PubMed] [Google Scholar]
- 26.Pirtea P, De Ziegler D, Marin D, Sun L, Zhan Y, Ayoubi JM, Seli E, Franasiak JM, Scott RT., Jr The rate of true recurrent implantation failure (RIF) is low: results of three successive frozen euploid single embryo transfers (SET) Fertil Steril. 2019;112(3):e438–e439. doi: 10.1016/j.fertnstert.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Garrido-Gomez T, Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Vilella F, Simon C. Profiling the gene signature of endometrial receptivity: clinical results. Fertil Steril. 2013;99:1078–1085. doi: 10.1016/j.fertnstert.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Blesa D, Ruiz-Alonso M, Simon C. Clinical management of endometrial receptivity. Semin Reprod Med. 2014;32:410–413. doi: 10.1055/s-0034-1376360. [DOI] [PubMed] [Google Scholar]
- 29.Rubio C, Bellver J, Rodrigo L, Bosch E, Mercader A, Vidal C, de los Santos MJ, Giles J, Labarta E, Domingo J, Crespo J, Remohí J, Pellicer A, Simón C. Preimplantation genetic screening using fluorescence in situ hybridization in patients with repetitive implantation failure and advanced maternal age: two randomized trials. Fertil Steril. 2013;99:1400–1407. doi: 10.1016/j.fertnstert.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. http://www.r-project.org/index.html.
- 31.Dahdouh EM, Balayla J, Garcia-Velasco JA. Impact of blastocyst biopsy and comprehensive chromosome screening technology on preimplantation genetic screening: a systematic review of randomized controlled trials. Reprod BioMed Online. 2015;30:281–289. doi: 10.1016/j.rbmo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Raziel A, Friedler S, Schachter M, Kasterstein E, Strassburger D, Ron-El R. Increased frequency of female partner chromosomal abnormalities in patients with high-order implantation failure after in vitro fertilization. Fertil Steril. 2002;78:515–519. doi: 10.1016/s0015-0282(02)03298-3. [DOI] [PubMed] [Google Scholar]
- 33.Stern C, Pertile M, Norris H, Hale L, Baker HW. Chromosome translocations in couples with in-vitro fertilization implantation failure. Hum Reprod. 1999;14:2097–2101. doi: 10.1093/humrep/14.8.2097. [DOI] [PubMed] [Google Scholar]
- 34.Harton GL, Munne S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695–1703. doi: 10.1016/j.fertnstert.2013.07.2002. [DOI] [PubMed] [Google Scholar]
- 35.Munne S. Status of preimplantation genetic testing and embryo selection. Reprod BioMed Online. 2018;37:393–396. doi: 10.1016/j.rbmo.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Verpoest W, Staessen C, Bossuyt PM, Goossens V, Altarescu G, Bonduelle M, Devesa M, Eldar-Geva T, Gianaroli L, Griesinger G, Kakourou G, Kokkali G, Liebenthron J, Magli MC, Parriego M, Schmutzler AG, Tobler M, van der Ven K, Geraedts J, Sermon K. Preimplantation genetic testing for aneuploidy by microarray analysis of polar bodies in advanced maternal age: a randomized clinical trial. Hum Reprod. 2018;33:1767–1776. doi: 10.1093/humrep/dey262. [DOI] [PubMed] [Google Scholar]
- 37.Munne S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071–1079.e7. doi: 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed] [Google Scholar]
- 38.Orvieto R, Gleicher N. Preimplantation genetic testing for aneuploidy (PGT-A)-finally revealed. J Assist Reprod Genet. 2020;37:669–672. doi: 10.1007/s10815-020-01705-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Somigliana E, Busnelli A, Paffoni A, Vigano P, Riccaboni A, Rubio C, Capalbo A. Cost-effectiveness of preimplantation genetic testing for aneuploidies. Fertil Steril. 2019;111:1169–1176. doi: 10.1016/j.fertnstert.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Neal SA, Morin SJ, Franasiak JM, Goodman LR, Juneau CR, Forman EJ, Werner MD, Scott RT., Jr Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertil Steril. 2018;110:896–904. doi: 10.1016/j.fertnstert.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Fox C, Morin S, Jeong JW, Scott RT, Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertil Steril. 2016;105:873–884. doi: 10.1016/j.fertnstert.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan J, Kan A, Hitkari J, Taylor B, Tallon N, Warraich G, Yuzpe A, Nakhuda G. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J Assist Reprod Genet. 2018;35:683–692. doi: 10.1007/s10815-017-1112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simon C, Gomez C, Cabanillas S, Vladimirov I, Castillon G, Giles J, et al. A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reprod BioMed Online. 2020;41:402–415. doi: 10.1016/j.rbmo.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Das M, Holzer HE. Recurrent implantation failure: gamete and embryo factors. Fertil Steril. 2012;97:1021–1027. doi: 10.1016/j.fertnstert.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Cozzolino M. Recurrent implantation failure might be overestimated without PGT-A. Arch Gynecol Obstet. 2020 doi: 10.1007/s00404-020-05775-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 20 kb)