Abstract
Post esophagectomy anastomotic leakage is a crucial factor in determining morbidity and mortality. Good vascularity of the gastric conduit is essential to avoid this complication. This prospective study compares the utility of intraoperative indocyanine green (ICG) fluorescence angiography and visual assessment in assessing the vascularity of gastric conduit and proximal esophageal stump in patients undergoing esophagectomy. Thirteen consecutive patients who underwent esophagectomy for carcinoma middle, lower third esophagus or gastro-esophageal junction from August 2019 to September 2019 were included. Three patients underwent laparoscopic-assisted transhiatal esophagectomy, ten thoraco-laparoscopic-assisted esophagectomy. Reconstruction was done by gastric pull-up via posterior mediastinal route. All patients underwent assessment of perfusion of gastric conduit and proximal esophageal stump by ICG angiography and by visual assessment based on inspection of the color, the palpation of warmth, pulse, and bleeding from the edges. Visual assessment revealed the tip of the gastric conduit was dusky and ischemic in 11 patients, pink and well perfused in two. ICG fluorescence imaging showed inadequate perfusion at the tip of conduit in 12 patients, adequate in one, overall requiring revision in 12 cases. There was a discrepancy in one patient where visual inspection showed adequate perfusion, but ICG disclosed poor vascularity requiring revision of the conduit’s tip. Resection of the devitalized portion of the proximal esophageal stump was needed in 5 patients both by visual and by ICG assessment. The median time to appearance of blush from the time of injection of dye was 15 s (10 to 23 s). In all the cases, the pattern of blush was simultaneous, with the concurrent appearance of ICG blush in the gastric conduit and gastro-epiploic arcade. No anastomotic leaks were noted. Visual inspection of the gastric conduit vascularity can underestimate perfusion and hence can compromise resection of the devitalized part. ICG fluorescence imaging is an accurate and promising means to ascertain the vascularity of gastric conduit during an esophagectomy. But its utility needs to be validated in randomized trials.
Keywords: Indocyanine green angiography, Gastric conduit perfusion, Proximal esophageal stump
Introduction
Esophagectomy is the prime modality of treatment of carcinoma esophagus. It is a technically complex procedure associated with considerable morbidity and mortality [1, 2]. Besides, the procedure is performed in nutritionally debilitated patients, with advanced age. One of the most dreaded complications after esophagectomy is anastomotic leakage, occurring in 10–20% of patients. When preparing the gastric conduit, it is necessary to divide both the short gastric vessels and the left gastric artery to gain length for its reach to the neck for esophagogastric anastomosis. The conduit relies solely on the right gastroepiploic artery for its blood supply. Inadequate blood supply at the tip of the gastric conduit is one of the most critical factors contributing to anastomotic leakage. Traditionally, assessment of the gastric conduit’s perfusion and viability has depended on somewhat unreliable measures such as visual inspection of the stomach’s color, bleeding at the edges, palpation of warmth, and pulse.
Indocyanine green (ICG) dye is a near-infrared fluorochrome, a water-soluble fluorescent compound that emits light on excitation. It has confirmed short- and long-term safety record, and has been extensively used in vascular, cardiac, plastic, oncological, and ophthalmic surgery. ICG fluorescence imaging has been since long utilized for the detection of sentinel lymph nodes in breast, stomach, and skin cancer surgeries, as well as to assess the vascularity after anastomosis during vascular and microvascular surgery to visualize tissue perfusion [3, 4].
Intravascular injection of ICG dye allows visualization of conduit vascular supply and assessment of perfusion to reduce anastomotic leaks potentially. This new technique allows real-time assessment of the vascularity of gastric conduit during esophagectomy [5–9]. However, the correlation between the vascular assessment by ICG fluorescence and the occurrence of esophagogastric anastomotic leakage has not been studied in a randomized controlled trial. This study analyzed the efficacy of perfusion of the gastric conduit as well as proximal esophageal stump by ICG fluorescence imaging against visual inspection during esophagectomy.
Methodology
The study was prospectively done at a tertiary cancer center in the department of surgical oncology. Thirteen consecutive patients who underwent esophagectomy for carcinoma middle, lower third esophagus or gastro-esophageal junction from August 2019 to September 2019 were included. All underwent reconstruction by gastric conduit via posterior mediastinum route.
Preoperative workup included upper gastrointestinal endoscopy, biopsy, and a contrast-enhanced CT scan of chest and abdomen in all patients. Ten patients underwent neoadjuvant chemotherapy and radiation according to CROSS study protocol [10] followed by surgery; one had 5 FU and cisplatin-based neoadjuvant chemoradiation, one was operated upfront, and one had salvage esophagectomy for a recurrent mid-third esophageal cancer post definitive chemoradiation. All patients included in this study consented to the study. The study was approved by the ethics committee of Basavatarakam Indo American Cancer Hospital and Research Institute, Hyderabad, India.
Thoraco-laparoscopic-Assisted Esophagectomy
For patients with middle and lower third esophageal cancer, initial mobilization of the esophagus was done by thoracoscopic route with a standard infrahilar lymphadenectomy in a semi-prone position with double lung ventilation. Ports placement: A 10-mm right-hand working port is placed just inferiorly and laterally to scapular tip, a 5-mm working port (surgeon’s left-hand working port) in the 9th intercostal space in the posterior axillary line, and a 10-mm camera port in the 7th intercostal space in the anterior axillary line to achieve a triangular configuration. A fourth 5-mm working port for assistant is placed in anterior axillary line 2 rib space superior to camera port in a diamond-shaped configuration. The patient is then turned to the supine position. Standard laparoscopic ports are placed: 10-mm umbilical camera port, 10-mm epigastric port for introduction of gauze pieces and retraction of the liver, 5-mm port on mid clavicular line on either side as surgeon’s right- and left-hand working ports, an addition left subcostal port along the anterior axillary line for stomach retraction. The gastro-hepatic ligament is divided and a complete D2 lymphadenectomy is done. Lastly, greater curvature is divided with care not to injure the gastroepiploic arcade.
After esophagectomy, a 3- to 3.5-cm-wide gastric tube is created with interrupted PDS 2-0 sutures or by staplers. The right gastroepiploic vessels and in the majority of the cases right gastric vessels are preserved. The left gastroepiploic vessels and left gastric vessels are ligated and divided at their origin. The gastric tube, after creation, is placed on the chest wall pointing towards the neck.
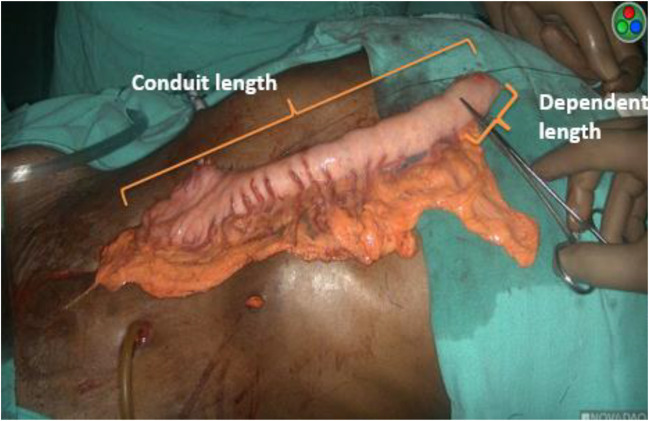
All the parameters concerning the gastric tube, such as the length between the pylorus and tip of the gastric tube (conduit length), the length between the pylorus and last branch of the right gastro-epiploic arcade, the interconnection of the gastro-epiploic arcades, length of gastric conduit from the termination of gastro-epiploic arcade to tip of conduit (dependent length), systolic and mean arterial blood pressure at the time of ICG fluorescence angiography injection, time from injection to blush (Fig. 1), perfusion at tip, ICG dose, pattern of blush (simultaneous or delayed), requirement of revision of conduit tip, length of abnormal conduit segment, and proximal esophageal stump were recorded. After assessment and revision is done by ICG fluorescence angiography (Fig. 2), the gastric tube is brought up to the neck through the posterior mediastinum and doubled stapled side-to-side anastomosis is done using 75- and 100-mm GIA staplers using modified Collard technique.
Fig. 1.
Depicting total and dependent length of conduit. Dependent length is the part of conduit without gastro-epiploic arcade. The part of conduit proximal to artery forceps pointer is dusky and ischemic by visual inspection
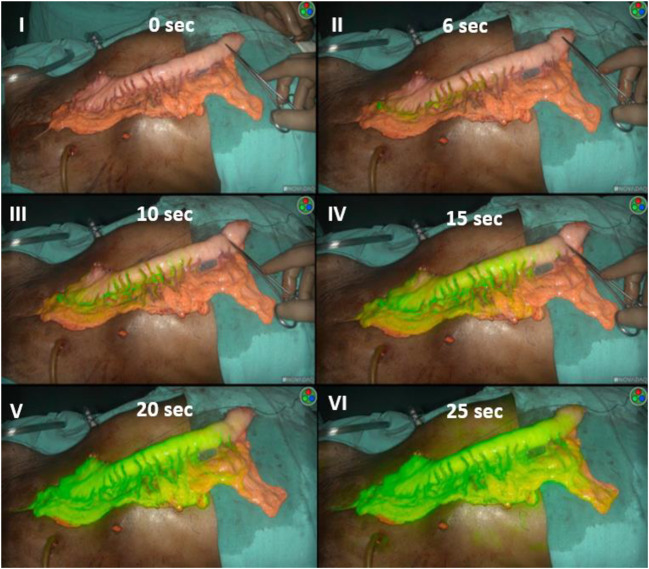
Fig. 2.
Panel no. 1 shows vascularity of gastric conduit assessed under white light. Part of conduit proximal to artery forceps is dusky with doubtful vascularity. Panels II to VI depict the perfusion of conduit at different intervals of time as assessed by ICG fluorescence angiography. Panel II shows first appearance of ICG blush along the arterial arcade at 6 s of injection, whereas panel VI depicts, at 25 s the entire conduit enhancing with ICG fluorescence except its proximal 2 cm nonenhancing part corresponding to same area as visualized under white light
Laparoscopic-Assisted Transhiatal Esophagectomy
Those patients who had transhiatal resection underwent a comprehensive D2 and lower mediastinal nodal dissection laparoscopically followed by stomach mobilization, then a small upper abdominal laparotomy, manual mediastinal dissection then cervical part of dissection, followed by resection of esophagus providing adequate margins to tumor and delivery of specimen by laparotomy incision and reconstruction of conduit as mentioned above.
ICG Protocol
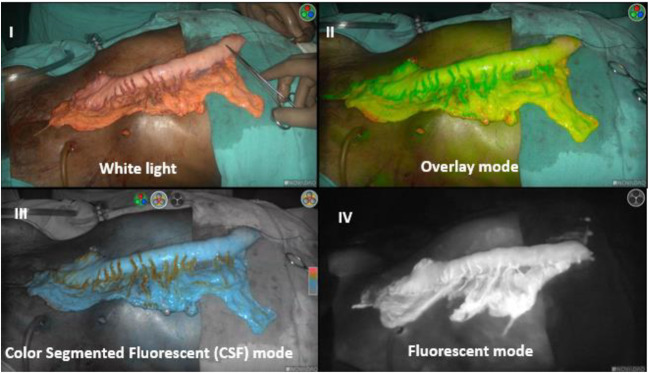
Visual assessment of the stomach conduit vascularity was done by color, pulsation, and bleeding from the edge of the conduit by two senior surgeons independently. Immediately after reconstruction of conduit, indocyanine green dye (Aurogreen, Aurolab, Madurai, India) was injected intravenously as a bolus. Dose of ICG has varied from 2.5 to 15 mg. Blood flow in the gastric tube was assessed with ICG fluorescence imaging using a high-definition camera system PINPOINT endoscopic fluorescence imaging camera (Stryker India Pvt. Ltd., Gurugram, India). This camera system is equipped with a special filter for optimal reproduction during ICG-enhanced fluorescence and standard white light imaging. Serial recording of ICG fluorescence imaging is done to assess the pattern of enhancement (Fig. 2). When illuminated by near-infrared light with a wavelength of 760 to 780 nm, ICG fluorescence emits a light of 800 to 850 nm. As ambient room illumination interferes with the detection of fluorescence, all the operating room lights were turned off for the ICG detection. The PINPOINT camera system has a distinct advantage of recording perfusion in different modes, i.e., white light, onlay mode, color segment fluorescent (CSF) mode, and fluorescent mode (Fig. 3).
Fig. 3.
Visualization of gastric conduit in different modes on Stryker’s PIN POINT camera system
Non-enhancing parts, i.e., those parts of gastric conduit not enhancing with ICG, were considered ischemic and devascularized (see Fig. 1 and panel VI in Fig. 2). Resection of the devitalized portion of the conduit was performed as per clinical or ICG assessment. The primary endpoint was an anastomotic leak at 30 days from the day of surgery. Before anastomosis, the vascularity of proximal stump was also assessed, and a revision was done as dictated by perfusion (Fig. 4).
Fig. 4.
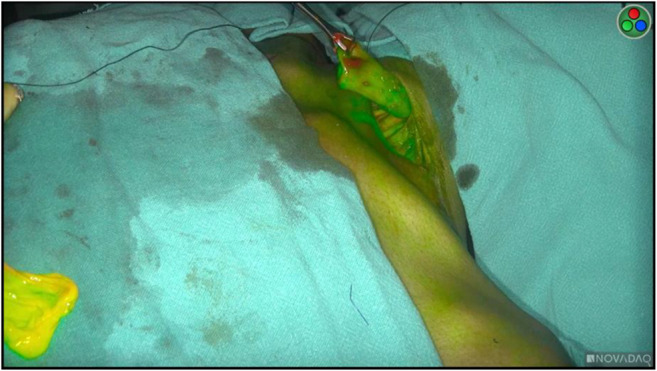
Assessment of proximal esophageal stump perfusion by ICG fluorescence
Results
A total of 13 consecutive patients undergoing esophagectomy were included in this study. There were five male and eight females with a median age of 49 years (range 23 to 73 years). The majority of the cases (n 11) had squamous cell carcinoma histology, while 2 had adenocarcinoma. Four patients were smokers and four had chronic obstructive pulmonary disease. The rest of the patient’s demographic data is detailed in Table 1. Three patients underwent laparoscopic-assisted transhiatal esophagectomy, ten thoraco-laparoscopic-assisted esophagectomy. Reconstruction was done by the gastric pull-up via posterior mediastinal route. Vascularity of gastric conduit was assessed by the near-infrared camera using ICG dye.
Table 1.
Demographic profile of patients
| Case | Age/gender | Site | Histology | Addictions | COPD | Other medical comorbidities | Preoperative therapy | Surgery | Leak | 30 days mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27/F | L | SCC | Nil | No | Nil | C, P + RT | TTE | Nil | Nil |
| 2 | 67/M | L | A | Smoker | Yes | HTN | C, P + RT | TTE | Nil | Nil |
| 3 | 38/F | M | SCC | Nil | No | Nil | Salvage | TTE | Nil | Nil |
| 4 | 32/F | M | SCC | Nil | No | Nil | C, P + RT | TTE | Nil | Nil |
| 5 | 47/F | M | SCC | Nil | No | Nil | 5Fu, Cispl +RT | TTE | Nil | Nil |
| 6 | 40/F | GEJ | SCC | Nil | No | Nil | C, P + RT | THE | Nil | Nil |
| 7 | 43/M | M | SCC | Smoker, alcoholic | Yes | Nil | C, P + RT | TTE | Nil | Nil |
| 8 | 63/M | M | SCC | Smoker | Yes | DM, CVD, HTN | C, P + RT | TTE | Nil | Nil |
| 9 | 58/M | M | SCC | Smoker | Yes | Nil | C, P + RT | TTE | Nil | Nil |
| 10 | 73 /F | GEJ | A | Nil | No | HTN | C, P + RT | THE | Nil | Nil |
| 11 | 54 /F | GEJ | SCC | Nil | No | HTN | C, P + RT | THE | Nil | Nil |
| 12 | 49 /M | L | SCC | Nil | No | Nil | C, P + RT | TTE | Nil | Nil |
| 13 | 68/F | L | SCC | Nil | No | Nil | nil | TTE | Nil | Nil |
L lower third esophageal cancer, M middle third esophageal cancer, GEJ gastro-esophageal junctional cancer. SCC squamous cell carcinoma, A adenocarcinoma, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, CVD cardio-vascular disease, HTN hypertensive, C carboplatin, P paclitaxel, RT radiotherapy, 5Fu 5flouro-uracil, Cispl cisplatin, TTE transthoracic esophagectomy, THE transhiatal esophagectomy, Leak anastomotic leakage
The visual assessment revealed the tip of gastric conduit was dusky in 11 patients, pink and perfused in 2. Details are mentioned in Table 2. ICG fluorescence imaging showed inadequate perfusion at the tip of conduit in 12 patients, adequate in one, requiring revision in 12. There was a discrepancy in one patient where visual inspection showed adequate perfusion, but ICG disclosed poor perfusion requiring revision of the conduit’s tip. The median time to appearance of blush from the time of injection of dye was 15 s (10 to 23 s) (Table 3). In all the cases, the pattern of blush was simultaneous, i.e., the concurrent appearance of blush in the gastric conduit and gastro-epiploic arcade. Resection of the devitalized portion of the proximal esophageal stump was needed in 5 patients by both visual and ICG assessment. No anastomotic leaks were recorded in any patient. The median systolic and mean arterial pressures at the time of injection of ICG dye were 115 and 84 mm of Hg.
Table 2.
Details of gastric conduit and proximal esophageal stump
| Case | Conduit length (cm) | Dependent length (cm) | Vascularity at the tip of gastric conduit by visual inspection | Length of abnormal segment from the tip (in cm) by ICG assessment | Revision of gastric conduit | Proximal segment revision required (cm) | Revision of proximal stump |
|---|---|---|---|---|---|---|---|
| 1 | 28 | 2 | Pink | 0 | No | 1 | Yes |
| 2 | 27 | 4 | Dusky | 2 | Yes | 0 | No |
| 3 | 25 | 3 | Dusky | 2.5 | Yes | 0 | No |
| 4 | 23 | 4 | Dusky | 2 | Yes | 0 | No |
| 5 | 25 | 2 | Dusky | 2.5 | Yes | 1 | Yes |
| 6 | 24 | 2 | Dusky | 2 | Yes | 1 | Yes |
| 7 | 24 | 3 | Dusky | 3 | Yes | 0 | No |
| 8 | 23 | 6 | Dusky | 3 | Yes | 1 | Yes |
| 9 | 22 | 5 | Dusky | 4.5 | Yes | 0 | No |
| 10 | 20 | 6 | Pink | 3 | Yes | 0 | No |
| 11 | 25 | 1 | Dusky | 1 | Yes | 1 | Yes |
| 12 | 21 | 2 | Dusky | 2.5 | Yes | 0 | No |
| 13 | 22 | 7 | Dusky | 2 | Yes | 0 | No |
Table 3.
The timing from injection of ICG dye to appearance and pattern of blush
| Case no. | Time from injection to blush (seconds) | Pattern of blush |
|---|---|---|
| 1 | 15 | Simultaneous |
| 2 | 17 | Simultaneous |
| 3 | 23 | Simultaneous |
| 4 | 19 | Simultaneous |
| 5 | 21 | Simultaneous |
| 6 | 20 | Simultaneous |
| 7 | 12 | Simultaneous |
| 8 | 15 | Simultaneous |
| 9 | 10 | Simultaneous |
| 10 | 18 | Simultaneous |
| 11 | 10 | Simultaneous |
| 12 | 15 | Simultaneous |
| 13 | 15 | Simultaneous |
Postoperatively, one patient had brain hemorrhage in postoperative period, requiring craniotomy and decompression eventually got discharged with residual neurological deficit at postoperative day 28, one had atrial fibrillation managed conservatively, another patient had mild proximal muscle weakness, and self-limiting chyle leak, along with neurapraxia and extrapyramidal symptoms resolved by promethazine. Three cases had pneumonia, one had left pleural effusion with sinus tachycardia, all managed conservatively. The median hospital stay was 8.5 days (range 7–28 days), and ICU stay 4 days (range 4–28 days). There were no 30 days or in-hospital mortality.
Discussion
We did a preliminary study to determine the utility and feasibility of ICG fluorescence imaging to evaluate the gastric conduit perfusion during an esophagectomy. Near-infrared fluorescence imaging using ICG is a novel optical technique for visualizing the blood and lymphatic circulation that has been gradually adopted in various fields of surgery. This imaging appears to be a promising method to determine the blood supply of the gastric conduit. The right gastroepiploic artery remains the sole supply of blood for the constructed gastric conduit during an esophagectomy which makes it of paramount importance that the entire gastric tube is well perfused. Though many factors have a bearing on the development of anastomotic leakage, blood perfusion is one of the most crucial causes. Currently, there is no universally accepted method to assess the perfusion of gastrointestinal anastomoses. The present study deals with the utility of ICG fluorescence angiography against the traditional visual assessment in determining vascularity of gastric conduit and proximal esophageal stump.
Visual Inspection
The viability of the conduit is usually judged by visualization of the color, pulsations of the blood vessels, and bleeding from the cut edges. A study had shown a low predictive value for anastomotic leaks for surgeons’ clinical risk assessment in gastrointestinal surgery. For a precise risk determination of anastomotic leakage, the sensitivity of the surgeon’s prediction was 62% for low colorectal anastomosis and 38% for high anastomosis, with a specificity of 52% and 46%, respectively [11]. Studies have also shown that the visual assessment of the gastric conduit may underestimate perfusion during esophagectomy [12], emphatically underscoring a need for a better diagnostic test to determine conduit perfusion accurately to prevent anastomotic leakage. Conforming previous studies, the present study also shows that visual inspection underestimates the vascularity of the gastric conduit, as seen in one case as a discrepancy, where visual assessment showed adequate perfusion and ICG angiography revealed poor perfusion, requiring revision of conduit tip.
Dose Description
Regarding the optimal dose of ICG, there is no consensus. The dose has been varied from 1.25 to 25 mg per bolus [13]. The lowest dose is one that allows reliable and precise measurement of perfusion. If the signal is not apparent, an extra bolus is given. After a few minutes, a new measurement is also possible. The dose of ICG in the present study has varied from 2.5 to 15 mg. Most of the dose has been given as an extra bolus for clear visualization of perfusion of gastric conduit and also for proximal esophageal stump vascularity.
Assessment of Proximal Esophageal Stump
No studies have reported the utility of ICG for determining the perfusion of the proximal esophageal stump which is equally important as gastric conduit vascularity in determining anastomotic leakage. Our study has demonstrated that ICG has a definite role in determining the perfusion of the proximal esophageal stump, requiring revision in 5 patients.
Timings
The timing of the assessment of gastric conduit perfusion after injection of ICG dye is very crucial. Once the ICG is injected intravenously, the subsequent few seconds are essential in recognizing the conduit perfusion with the maximum possible resolution (Fig. 2). It is of utmost essential to place the gastric conduit in an appropriate position on the chest wall with camera ready in ICG imaging mode. Kumagai et al. proposed a 90-s rule to confirm a good blood perfusion at the anastomosis site. All the anastomoses were created in the area of gastric conduit that enhanced within 90 s from the initial enhancement of the root of the right gastroepiploic artery. Based on this rule, the tip needed resection in 50% (35/70), and the anastomotic site was changed in 18 of those 35 cases. None of the patients underwent anastomosis at a site with delayed enhancement after 90 s. One patient out of 70 cases (1.4%) had an anastomotic leak when the anastomotic site enhanced after 77 s [14]. The present study also had a stringent protocol on the timing of ICG dye injection and visualization of perfusion (Fig. 2). Before the injection of ICG, we ensured the conduit is ready and placed on the chest wall with a PINPOINT high-definition probe ready in ICG imaging mode.
Simultaneous Versus Delayed
Koyanagi et al. demonstrated the utility of blood flow speed by ICG fluorescence in the gastric conduit intraoperatively as useful tool to predict the risk of anastomotic leakage. Based on ICG flow speed, they classified the cohort into two groups, a simultaneous group with similar ICG fluorescence speed in gastric conduit wall and in greater curvature vessels, and a delayed group where the ICG fluorescence flow speed was slower in the gastric conduit wall than the greater curvature vessels (P < 0.001) [15]. Anastomotic leakage developed in none (0/25) in the simultaneous group and 46.7% (7/15) of the cases in the delayed group (P < 0.001). In line with the previous studies, all patients in the present study had a simultaneous pattern of blush on the intravenous injection of ICG, i.e., the concurrent appearance of ICG fluorescence in the gastric conduit and gastro-epiploic arcade. No patient had a delayed pattern. The median time to appearance of blush from the time of injection of dye was 15 s (10 to 23 s). All the anastomoses were placed in the well-perfused area as delineated by ICG fluorescence. There were no leaks in the present study.
Reduction of Anastomotic Leakage
The usage of ICG for vascular assessment of the gastric conduit before anastomosis is secure and leads to a decrease in anastomotic leakage rates since the anastomosis is performed in the well-perfused area. Two meta-analyses found a 70% risk reduction of anastomotic leakages (OR 0.30, 95% CI 0.14–0.63) [13, 16], and one found a similar 70% risk reduction in conduit necrosis in the ICG group [13]. Campbell et al. noted a dramatic decrease in the rate of an anastomotic leak from 20 to 0% after the introduction of intraoperative perfusion assessment of the gastric conduit with ICG fluorescence imaging [17].
Still, there exists a significant chance of anastomotic leakage even after the introduction of ICG perfusion assessment. The meta-analysis by Degett et al. showed that the anastomotic leakage rate in patients with esophageal anastomoses and intraoperative assessment by ICG angiography was 14% [18], whereas Scooter, in their meta-analysis, found the pooled incidence of anastomotic leakage and graft necrosis in the ICG group was 11.10% [13]. Change in management included resection of the poorly perfused part of the gastric conduit and change in anastomotic site. Contrastingly, after a change in management, the pooled incidence of anastomotic leakage and graft necrosis was surprisingly high, i.e., 14.08% (95% CI, 6.55–27.70%) [13].
None of the studies evaluating esophageal anastomoses had a control group without ICG fluorescent angiographic assessment. Furthermore, there are no randomized controlled trials published on this subject. ICG fluorescent angiography seems to be a promising tool to assess perfusion of gastric conduit at the site intended for anastomosis.
Conclusion
Visual assessment of the gastric conduit may underestimate perfusion. ICG fluorescence imaging appears to be a promising method to assess the perfusion of the gastric conduit during esophagectomy. Prospective randomized controlled studies with adequate numbers of patients are needed to define the utility of ICG fluorescence imaging in patients undergoing esophagectomy.
Contributions of Authors
Syed Nusrath, Subramanyeshwar Rao, and Sujith Patnaik perceived the idea of study. Syed Nusrath, Subramanyeshwar Rao, and Ajesh Raj Saksena collected data and wrote the manuscript. All the authors critically reviewed and revised the manuscript. The manuscript has been read and approved by all the authors and each author believes that the manuscript represents honest work.
Compliance with Ethical Standards
The content of the article has not been published or submitted for publication elsewhere. The study was approved by the ethics committee of the Basavatarakam Indo American Cancer Hospital and Research Institute and conformed to the provisions of the Declaration of Helsinki in 1995. Informed consent was obtained from all the subjects, and patients’ anonymity is preserved.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Subramanyeshwar Rao Thammineedi, Email: subramanyesh@gmail.com.
Sujit Chyau Patnaik, Email: drsujit888@gmail.com.
Ajesh Raj Saksena, Email: drajeshraj@gmail.com.
Pratap Reddy Ramalingam, Email: pratapramalingam@gmail.com.
Syed Nusrath, Email: dr.nusrath2008@gmail.com.
References
- 1.Nozaki I, Kato K, Igaki H, Ito Y, Daiko H, Yano M, Udagawa H, Mizusawa J, Katayama H, Nakamura K, Kitagawa Y. Evaluation of safety profile of thoracoscopic esophagectomy for T1bN0M0 cancer using data from JCOG0502: a prospective multicenter study. Surg Endosc. 2015;29:3519–3526. doi: 10.1007/s00464-015-4102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeuchi H, Miyata H, Gotoh M, Kitagawa Y, Baba H, Kimura W, Tomita N, Nakagoe T, Shimada M, Sugihara K, Mori M. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg. 2014;260:259–266. doi: 10.1097/SLA.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 3.Kitai T, Inomoto T, Miwa M, Shikayama T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12:211–215. doi: 10.2325/jbcs.12.211. [DOI] [PubMed] [Google Scholar]
- 4.Tajima Y, Yamazaki K, Masuda Y, Kato M, Yasuda D, Aoki T, Kato T, Murakami M, Kusano M. Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann Surg. 2009;249:58–62. doi: 10.1097/SLA.0b013e3181927267. [DOI] [PubMed] [Google Scholar]
- 5.Nomura S, Ohue M, Seki Y, Tanaka K, Mottori M, Kishi K, Miyashiro I, Ohigashi H, Yano M, Ishikawa O, Miyamoto Y. Feasibility of a lateral region sentinel node biopsy of lower rectal cancer guided by indocyanine green using a near-infrared camera system. Ann Surg Oncol. 2010;17:144–151. doi: 10.1245/s10434-009-0711-2. [DOI] [PubMed] [Google Scholar]
- 6.Taggart DP, Choudhary B, Anastasiadis K, Abu-Omar Y, Balacumaraswami L, Pigott DW. Preliminary experience with a novel intraoperative fluorescence imaging technique to evaluate the patency of bypass grafts in total arterial revascularization. Ann Thorac Surg. 2003;75:870–873. doi: 10.1016/S0003-4975(02)04669-6. [DOI] [PubMed] [Google Scholar]
- 7.Kang Y, Lee J, Kwon K, Choi C. Dynamic fluorescence imaging of indocyanine green for reliable and sensitive diagnosis of peripheral vascular insufficiency. Microvasc Res. 2010;80:552–555. doi: 10.1016/j.mvr.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Shimada Y, Okamura T, Nagata T, et al. Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus. 2011;8:259–266. doi: 10.1007/s10388-011-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishiguro T, Kumagai Y, Ono T, et al. Usefulness of indocyanine green angiography for evaluation of blood supply in a reconstructed gastric tube during esophagectomy. Int Surg. 2012;97:340–344. doi: 10.9738/CC159.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Hagen P, Hulshof MC, Van Lanschot JJ, Steyerberg EW, Henegouwen MV, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 11.Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, Van Dam GM. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Color Dis. 2009;24(5):569–576. doi: 10.1007/s00384-009-0658-6. [DOI] [PubMed] [Google Scholar]
- 12.Schlottmann F, Patti MG. Evaluation of gastric conduit perfusion during esophagectomy with indocyanine green fluorescence imaging. J Laparoendosc Adv Surg Tech A. 2017;27(12):1305–1308. doi: 10.1089/lap.2017.0359. [DOI] [PubMed] [Google Scholar]
- 13.Slooter MD, EshuisWJ CMA, Gisbertz SS, van Berge Henegouwen MI. Fluorescent imaging using indocyanine green during esophagectomy to prevent surgical morbidity: a systematic review and meta-analysis. J Thorac Dis. 2019;11(Suppl 5):S755. doi: 10.21037/jtd.2019.01.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumagai Y, Hatano S, Sobajima J, Ishiguro T, Fukuchi M, Ishibashi KI, Mochiki E, Nakajima Y, Ishida H (2018) Indocyanine green fluorescence angiography of the reconstructed gastric tube during esophagectomy: efficacy of the 90-second rule. Dis Esophagus 31(12) [DOI] [PubMed]
- 15.Koyanagi K, Ozawa S, Oguma J, Kazuno A, Yamazaki Y, Ninomiya Y, Ochiai H, Tachimori Y (2016) Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: new predictive evaluation of anastomotic leakage after esophagectomy. Medicine 95(30) [DOI] [PMC free article] [PubMed]
- 16.Ladak F, Dang JT, Switzer N, Mocanu V, Tian C, Birch D, Turner SR, Karmali S. Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc. 2019;33(2):384–394. doi: 10.1007/s00464-018-6503-7. [DOI] [PubMed] [Google Scholar]
- 17.Campbell C, Reames MK, Robinson M, Symanowski J, Salo JC. Conduit vascular evaluation is associated with reduction in anastomotic leak after esophagectomy. J Gastrointest Surg. 2015;19(5):806–812. doi: 10.1007/s11605-015-2794-3. [DOI] [PubMed] [Google Scholar]
- 18.Degett TH, Andersen HS, Gögenur I. Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbeck's Arch Surg. 2016;401(6):767–775. doi: 10.1007/s00423-016-1400-9. [DOI] [PubMed] [Google Scholar]