Abstract
Purpose
To compare growth factor and cytokine profiles in the endometrial secretions of patients with and without endometriosis to determine whether a particular protein profile is predictive of the disease.
Methods
Patients undergoing laparoscopic gynecologic surgery for benign indications were recruited for this prospective cohort study. Prior to surgery, endometrial fluid was aspirated and multiplex immunoassay was used to quantify 7 cytokines and growth factors. During surgery, each patient was staged according to the ASRM staging system for endometriosis. Cytokines and growth factors were evaluated using the Mann-Whitney and Kruskal-Wallis tests. Combinations of cytokines were evaluated using logistic regression analysis, and ROC curves were generated to evaluate the predictive capacity of the assay.
Results
Endometrial secretions were analyzed from 60 patients. Nineteen had stage 3–4 endometriosis, 19 had stage 1–2 disease, and 22 had no endometriosis. There were no significant differences between controls and stage 1–2 endometriosis; however, levels of IL-1α and IL-6 were significantly increased in women with moderate-to-severe disease. A combination of IL-1α, IL-1β, and IL-6 in endometrial secretions predicts stage 3–4 endometriosis with an AUC of 0.78. A threshold value of 118 pg/mL yields a sensitivity of 75% and specificity of 70%.
Conclusion
Aspiration of endometrial fluid is a safe and effective approach for evaluating the endometrial profile of women with endometriosis. Women with moderate-to-severe endometriosis demonstrate a distinct cytokine profile compared to controls. A combination of IL-1α, IL-1β, and IL-6 in the endometrial secretions is predictive of stage 3–4 endometriosis, but is not predictive of minimal-to-mild disease.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-020-01989-y.
Keywords: Endometriosis, Endometrium, Cytokines, Endometrial fluid profiling
Introduction
Endometriosis is a debilitating estrogen-dependent disease that affects 25 to 50% of infertile women, and 40 to 50% of women with pelvic pain [1–3]. Surgery remains the gold standard for diagnosis of endometriosis; however, the delay from presentation to diagnosis is often as long as 8 to 10 years [4]. Earlier diagnosis is crucial to improve patient experience, avoid side effects associated with unnecessary pharmacologic treatment, and prevent the sequelae of disease progression, including pelvic adhesions and infertility.
There has been great interest in the identification of non-invasive biomarkers of endometriosis in the serum, urine, and endometrial tissue; however, the predictive capacity of most biomarkers has not yet approached the sensitivity and specificity of laparoscopy [5–7]. The unique characteristics of the endometrium in endometriosis, including decreased apoptosis and increased production of steroids, growth factors, and inflammatory cytokines [8], make it an attractive target for non-invasive diagnostic approaches. A recent Cochrane review evaluated 54 studies of endometrial biomarkers for endometriosis, but due to methodological heterogeneity and poor study quality, there was insufficient evidence to recommend any endometrial test in the diagnosis of endometriosis [7]. Most prior studies of endometrial biomarkers have focused on endometrial tissue obtained from a biopsy [7]; however, an endometrial biopsy can be painful, particularly in women with chronic pelvic pain. The aspiration of endometrial fluid is less invasive than a biopsy, requiring only the insertion of an embryo transfer catheter for fluid aspiration. This technique has been previously used to successfully identify protein profiles predictive of pregnancy in IVF cycles [9, 10]. Additionally, Ametzazurra et al. have used this approach to evaluate endometrial protein profiles in women with endometriosis [11, 12].
Cytokines and growth factors play important roles in the pathogenesis of endometriosis. They are thought to regulate mitosis and angiogenesis, and facilitate implantation and growth of ectopic endometrium to the peritoneal surface [8]. A number of studies have demonstrated increased cytokine levels in the peritoneal fluid and serum of women with endometriosis [13–22]; however, they have not previously been evaluated in the endometrial secretions.
In the present study, we evaluate levels of IL-1α, IL-1β, interleukin-6 (IL-6), interleukin-8 (IL-8), vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) in the endometrial secretions of women with and without endometriosis, with the goal of identifying a pattern of cytokines and growth factors that characterizes endometriosis. We aim to (1) evaluate endometrial cytokine levels in women with and without endometriosis and (2) determine whether a particular endometrial fluid profile is predictive of the disease.
Methods
Cohort selection
After approval by the Institutional Review Board, a cohort of pre-menopausal women between the ages of 18 and 45 undergoing laparoscopic surgery for benign indications was recruited from a single academic center. Women with and without a prior diagnosis of endometriosis were included. Women were excluded if they were pregnant, had active or prior malignancy, had evidence of active cervical infection, had used oral estrogen or progesterone within the last 30 days, or received a depo injection of estrogen, progesterone, or a gonadotropin-releasing hormone (GnRH) agonist within the past 3 months. Additionally, patients with radiographic evidence of adenomyosis were excluded.
Finally, patients who received the prostaglandin E1 analogue misoprostol preoperatively were excluded from the primary analysis, as misoprostol is known to modulate cytokine levels [23]. Only patients undergoing surgery for uterine fibroids received preoperative misoprostol as a strategy to reduce intraoperative bleeding and were, therefore, analyzed as a separate cohort.
Sample collection
At the time of surgery, after induction of anesthesia but prior to vaginal prep, secretions were aspirated from the endometrial cavity. Prior to aspiration, 0.5 cc of sterile saline was used to rinse the endometrium, and fluid was aspirated using an embryo transfer catheter attached to a syringe. The aspirated secretions were immediately placed on ice and stored at − 80 °C until the cytokine assay was performed. This sample collection protocol was developed with consideration for the 2014 standard operation procedures (SOPs) and recommendations for endometrial fluid collection developed by the World Endometriosis Society to promote improved comparisons across centers [24]. During surgery, each patient was staged for endometriosis according to the American Society for Reproductive Medicine (ASRM) clinical staging system. The stage of the menstrual cycle was recorded in women with regular cycles based on the date of the last menstrual period. Women with irregular menstrual cycles (n = 4) were excluded from analyses involving cycle phase.
Cytokine assay
Cytokine assays were performed using the Luminex multiplex assay. This is a bead-based immunoassay that uses fluorescently labeled microsphere beads coated with capture antibodies. This approach allows for the parallel analysis of multiple proteins in a small sample volume [25, 26]. Ca# HCYTOMAG-60K was used to analyze endometrial secretion samples. Supernatants treated with analyte-specific antibodies were pre-coated onto magnetic microparticles embedded with fluorophores at set ratios for each unique microparticle region. Microparticles, standards, and samples were pipetted into the wells and the immobilized antibodies bound the analytes of interest. After washing away any unbound substances, a biotinylated antibody cocktail specific to the analytes of interest was added to each well. Following a wash to remove any unbound biotinylated antibody, streptavidin-phycoerythrin conjugate (Streptavidin-PE), which binds to the biotinylated antibody, was added to each well. Final washes removed unbound Streptavidin-PE, and the microparticles were resuspended in buffer and read using the Luminex® 100/200 System.
Statistical considerations
A power calculation was performed to estimate sample size, using the following assumptions: endometriosis prevalence of 33% at laparoscopy and an expected area under the curve (AUC) of at least 0.75 for a predictive combination of cytokines. A sample size of 51 patients (38 endometriosis patients and 13 controls) provides 80% power with a type I error rate of 5%.
Comparisons of the mean of cytokine values between groups were carried out using the Mann-Whitney and Kruskal-Wallis tests with adjustment for false discovery via the Benjamini-Hochberg method (GraphPad Prism 8). Stepwise logistic regression was then carried out using backward elimination (R Foundation for Statistical Computing). Cytokines with P values < 0.25 were included. A receiver operating characteristic (ROC) curve was then generated based on the logistic regression model.
Results
Patient demographics
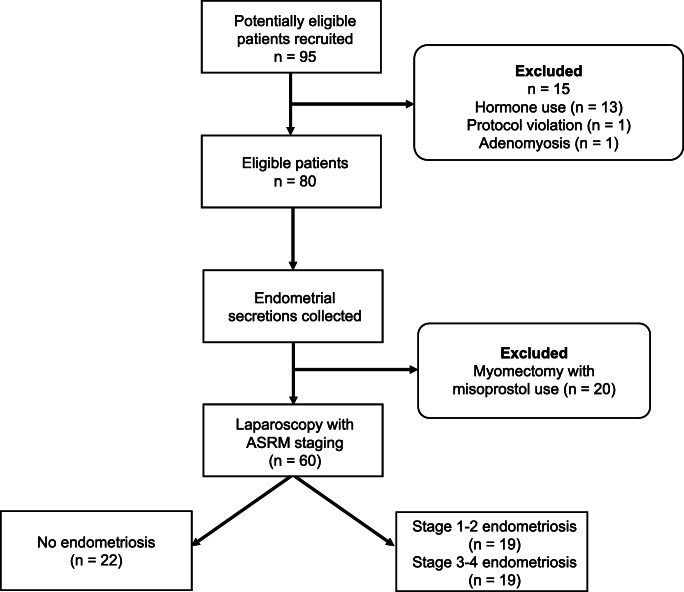
Endometrial secretion samples were obtained from 60 patients undergoing gynecologic laparoscopy for benign indications between March 2017 and October 2018 (Fig. 1). Indications for surgery included pelvic pain, infertility, ovarian cysts, sterilization, and tubal reanastomosis (Table 1). All patients underwent staging for endometriosis at the time of surgery. Nineteen patients were found to have stage 3–4 endometriosis, 19 patients had stage 1–2 disease, and 22 women had no endometriotic lesions. Most patients with stage 1–2 endometriosis had superficial peritoneal disease. The phenotype of stage 3–4 endometriosis was variable; 63% of women with moderate-to-severe disease had endometriomas, 37% had complete posterior cul-de-sac obliteration, and 26% each had rectovaginal septum lesions, bowel or appendiceal lesions, or deep infiltrating lesions of the pelvic sidewall.
Fig. 1.
Patient recruitment flowsheet
Table 1.
Patient demographics and indication for surgery
| No endometriosis (n = 22) | Stage 1–2 endometriosis (n = 19) | Stage 3–4 endometriosis (n = 19) | P value | |
|---|---|---|---|---|
| Mean age + SD | 33 + 5 | 36 + 6 | 35 + 7 | 0.3 |
| BMI | 32.4 | 23.9 | 25.9 | 0.006 |
| Indication for surgery | ||||
| Pelvic pain | 6 | 16 | 17 | |
| Ovarian cyst | 2 | 0 | 2 | |
| Infertility | 8 | 5 | 2 | |
| PCOS | 1 | 0 | 0 | |
| Sterilization | 1 | 0 | 0 | |
| Tubal reanastomosis | 4 | 0 | 0 | |
| Cycle phase | ||||
| Follicular phase | 11 | 9 | 10 | 1 |
| Luteal phase | 8 | 10 | 8 | 0.6 |
| Unknown | 3 | 0 | 1 | 0.4 |
Impact of fibroids on cytokine levels
Samples were initially collected from an additional 20 women with fibroids undergoing myomectomy; however, these patients were subsequently excluded due to a presumed impact of preoperative misoprostol administration on cytokine levels (Supplemental Table 1). Notably, all cytokines were increased among women with fibroids who received misoprostol, but only differences in IL-6 and TNF-alpha reached statistical significance. Because of the use of misoprostol, it is impossible to determine whether cytokine levels were elevated due to the presence of fibroids themselves, or due to the administration of misoprostol.
Cytokine levels and endometriosis stage
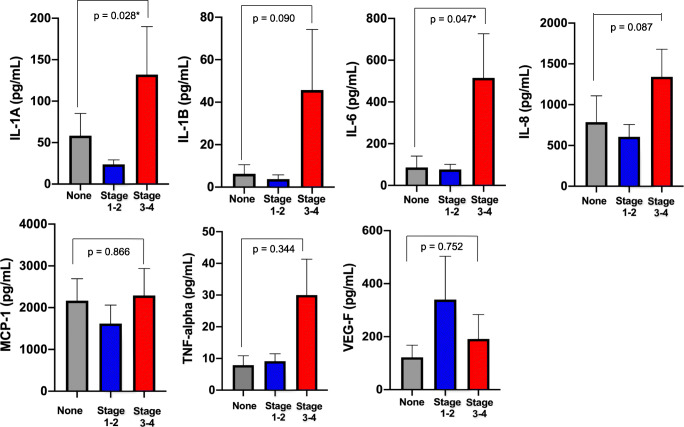
Mean cytokine levels were evaluated according to the endometriosis stage (Fig. 2, Supplemental Table 2). There were no significant differences between controls and stage 1–2 endometriosis; however, levels of IL-1α and IL-6 were significantly increased in the endometrial secretions of women with moderate-to-severe disease. Mean levels of IL-1α were increased by more than twofold in women with advanced endometriosis compared to controls, and levels of IL-6 were increased by nearly sixfold. Levels of IL-1β and IL-8 were also elevated in stage 3–4 endometriosis compared to controls; however, these differences did not reach statistical significance.
Fig. 2.
Mean cytokine and growth factor levels by endometriosis stage. P values are shown for univariate analysis with the Mann-Whitney test. Error bars represent standard error of the mean. Asterisks denote P values that reached statistical significance after adjustment for false discovery
Notably, the typical range of cytokine values in endometrial secretions has not been previously characterized, and these data demonstrate considerable variability in cytokine levels within groups (Supplemental Table 2). The wide variability in cytokine levels in this cohort limited our statistical power.
A logistic regression analysis was performed to evaluate the predictive capacity of a combination of cytokines for identifying patients with stage 3–4 endometriosis. The model with the best predictive capacity included IL-1α, IL-1β, and IL-6. An ROC curve was generated based on this model and demonstrated an area under the curve (AUC) of 0.78 (Fig. 3). A threshold of 118 pg/mL for the additive values of IL-1α, IL-1β, and IL-6 yields a sensitivity of 75% and a specificity of 70%.
Fig. 3.
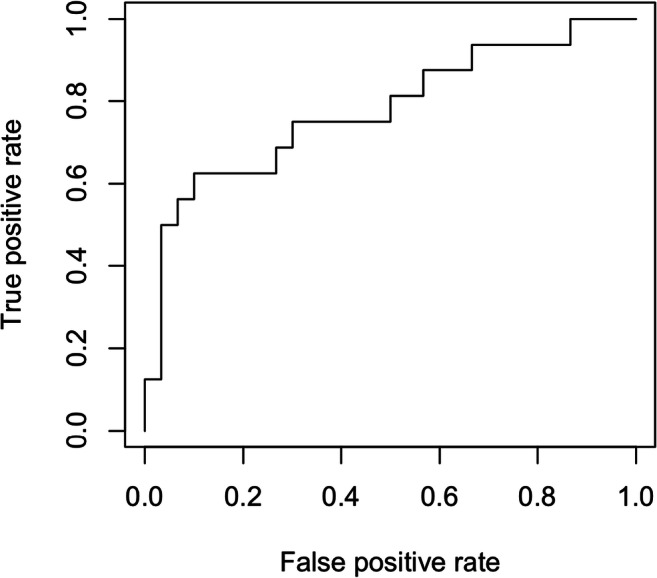
ROC curve including IL-1A, IL-1B, and IL-6 for the prediction of stage 3–4 endometriosis. AUC is equivalent to 0.78
Cytokine levels and cycle phase
Finally, a subgroup analysis was performed to evaluate mean cytokine levels according to the endometriosis stage and cycle phase (Supplemental Fig. 1). As in the overall cohort, mean levels of IL-1α, IL-1β, and IL-6 were increased in stage 3–4 endometriosis compared to controls in both the follicular and luteal phases; however, these differences did not reach statistical significance.
When cytokine values were compared between cycle phases, only TNF-alpha differed significantly between the follicular and luteal phases in the control cohort (9.7 pg/mL in the follicular phase versus 7.6 pg/mL in the luteal phase; p = 0.0041) (Supplemental Fig. 1). There were no significant differences between the follicular and luteal phases in women with endometriosis. Due to the relatively small sample size within each subgroup (8–11 patients), individual logistic regression models could not be created for each cycle phase.
Discussion
Endometrial fluid profiling represents an innovative approach to understanding the endometrial environment and provides insight into changes of the eutopic endometrium associated with moderate-to-severe endometriosis. Levels of IL-1α and IL-6 were significantly increased in the endometrial secretions of women with stage 3–4 disease. Additionally, concentrations of IL-1β and IL-8 were elevated in moderate-to-severe endometriosis, but these differences did not reach statistical significance.
The primary objective of this study was to evaluate the utility of endometrial fluid profiling as a non-invasive approach to the diagnosis of endometriosis. Although we had hoped to identify a combination of cytokines that would be predictive of minimal-to-mild endometriosis, as early disease is not amenable to diagnosis by imaging, the endometrial protein profile investigated was not associated with stage 1–2 disease. A combination of IL-1α, IL-1β, and IL-6 does, however, appear to have predictive capacity for stage 3–4 endometriosis. Increased concentrations of these three cytokines in the endometrial secretions predict stage 3–4 endometriosis with an AUC of 0.78. A threshold value of 118 pg/mL is consistent with a sensitivity of 75% and specificity of 70%. Assuming that the prevalence of endometriosis is approximately 33% in a cohort of women with chronic pelvic pain [27], the use of this screening test would increase the probability of stage 3–4 endometriosis to 55% in case of a positive result (likelihood ratio positive of 2.5), and decrease the likelihood of advanced endometriosis to 15% in case of a negative result (likelihood ratio negative of 0.36). Avoiding surgery in patients who test negative would reduce laparoscopies by 55% with a false-negative rate of 8%; however, this approach would fail to identify patients with minimal-to-mild endometriosis. Therefore, endometrial fluid profiling could be considered in combination with, or an alternative to, imaging to identify patients with advanced disease.
The differences in the endometrial environment according to the endometriosis stage may reflect distinct disease subtypes, ranging from the superficial peritoneal lesions that characterize stage 1–2 disease to the deep infiltrating lesions and endometriomas of advanced disease. The range of endometriosis phenotypes may also provide a potential explanation for the variability in cytokine levels between patients. Mean cytokine levels in endometrial secretions have not previously been characterized, and these results demonstrate large variability in levels between patients. Boomsma et al. tested cytokine levels in the endometrial secretions of women undergoing in vitro fertilization and reported similar variability in results between patients [10]. Future work should define the typical range of cytokine levels in endometrial secretions. Additionally, in order to ensure the validity of this and other tests utilizing endometrial protein profiling, repeat measurements must be performed in the same patient to confirm the reliability of the assay over time.
Levels of IL-6 and TNF-alpha were found to be significantly increased in women with uterine fibroids. Multiple cytokines, including TNF-alpha, IL-1, and IL-6, have been implicated in the pathogenesis of fibroids [28]; therefore, cytokine levels may be increased in the endometrial secretions of women with fibroids. However, women undergoing myomectomy received a dose of the prostaglandin E1 analogue misoprostol preoperatively to minimize intraoperative bleeding. Given that misoprostol is known to modulate cytokine expression, it is unclear whether fibroids, misoprostol, or a combination thereof resulted in increased cytokine levels in these patients.
Some prior studies of endometriosis biomarkers have identified differences in assay performance according to the cycle phase. For instance, Mihalyi et al. showed improved performance of plasma biomarkers as predictors of endometriosis, including IL-6 and IL-8, in the secretory compared to the proliferative phase [29]. Our data show that IL-1α, IL-1β, and IL-6 were increased in stage 3–4 endometriosis compared to controls in both the follicular and luteal phases, as in the overall cohort; however, these differences did not reach statistical significance. The lack of statistical significance is likely due to insufficient power for this subgroup analysis. Further study is needed to better delineate the impact of the cycle phase on test performance.
The results of this study are consistent with previous studies evaluating cytokine levels in the peritoneal fluid and serum of women with endometriosis. Malutan et al. demonstrated significantly increased concentrations of IL-1β and IL-6 in the serum of women with endometriosis compared to controls [22] and Othman et al. showed increased serum levels of IL-6 in endometriosis patients [14]. Additionally, Wickiewicz et al. and Bersinger et al. reported that increased concentrations of IL-6 in the peritoneal fluid are predictive of stage 3–4 endometriosis [16, 18]. Similarly, Mier-Cabrera et al. and Beste et al. showed increased levels of IL-1β and IL-6 in the peritoneal fluid of women with endometriosis [15, 19].
Given the role of cytokines in the pathogenesis of endometriosis, the finding that IL-1 and IL-6 are increased in the endometrial secretions of women with endometriosis is not surprising. A number of cytokines, growth factors, and endometrial factors contribute to the pathogenesis of the disease. Interleukin-1 (IL-1) is a cytokine that is secreted by monocytes, macrophages, T and B lymphocytes, and natural killer (NK) cells, and plays a role in inflammatory and immune responses. In endometriotic tissue, IL-1 sets off an inflammatory cascade, inducing angiogenic factors such as vascular endothelial growth factor (VEG-F), interleukin-6 (IL-6), and interleukin-8 (IL-8) [30]. In addition, it upregulates tumor necrosis factor-alpha (TNF-α), which is thought to facilitate adherence of ectopic endometrium to the peritoneum, and monocyte chemoattractant protein-1 (MCP-1), a recruiter of macrophages. In addition to its role in angiogenesis, IL-8 is an inflammatory cytokine that regulates neutrophil migration and activation, enhances endometrial stromal proliferation, and enhances matrix metalloproteinase activity, which facilitates implantation of ectopic endometrium [31]. It is secreted by endometriotic tissue regardless of the phase of the menstrual cycle [31]. Additionally, in the eutopic endometrial stromal cells of women with endometriosis, IL-1β induces the expression of brain-derived neurotrophic factor (BDNF) and the chemokine regulated on activation normal T cell expressed and secreted (RANTES), both of which are thought to play a role in modulating pain and inflammation in endometriosis [32]. Furthermore, Il-1β in the eutopic endometrium increases the expression of Pak1, a G protein kinase that promotes the viability of endometriotic cells and is increased in the endometrium of women with endometriosis [33].
Although cytokines have previously been identified in the peritoneal fluid of women with endometriosis [13, 15, 16, 18–21], obtaining endometrial secretions is much less invasive, as they can be collected easily at the time of pelvic exam without significant patient discomfort. While there is a theoretical risk of ascending intrauterine infection with entry into the uterine cavity, the exceedingly low rates of infection after hysteroscopy and embryo transfer suggest that this risk is negligible.
Potential clinical applications of endometrial fluid profiling include a screening tool for patients presenting with pain or infertility to evaluate for advanced endometriosis. This could represent a lower-cost alternative to imaging and help to identify patients with moderate-to-severe disease who may benefit from referral to a fertility specialist or an expert endometriosis surgeon, depending on their treatment goals. The availability of a test that can easily be performed at the time of pelvic exam may help to decrease the nearly decade-long delay from presentation to the diagnosis of endometriosis.
Additionally, characterizing the endometrial milieu may further our understanding of both disease pathogenesis and impaired endometrial receptivity seen in women affected by endometriosis. The altered cytokine profile that characterizes advanced endometriosis may contribute to the decreased implantation rates observed in affected women undergoing IVF [34, 35]. Notably, a study evaluating endometrial fluid cytokines at the time of embryo transfer showed that increased endometrial fluid levels of IL-1β were negatively correlated with clinical pregnancy rates [9]. Similarly, cytokine profiles in women with adenomyosis are also altered compared to controls, demonstrating increased concentrations of both IL-1β and MCP-1 [36]. Additionally, data from infertility patients shows that the endometrial cytokine profile is altered by ovarian stimulation for IVF [37], suggesting the potential for modulation of the endometrial environment through medical or surgical treatment of endometriosis. Further studies are required to explore whether the cytokine profile can be normalized by surgical or hormonal therapy, and whether modulation yields improvements in fertility outcomes.
The strengths of this study include the evaluation of an innovative approach to the non-invasive diagnosis of endometriosis, adequate sample size, and the use of a collection protocol in accordance with the recommendations set forth by the World Endometriosis Society. Potential limitations of these data include the possibility that factors related to perioperative stress or anesthesia may contribute to changes in cytokine levels, as samples were collected shortly after anesthesia induction. Although all patients were subjected to similar perioperative conditions, the possibility that differences in anesthetic agents or physiologic response to anesthesia induction may be contributing to cytokine levels, or their inter-patient variability, must be considered. Prospective validation of this endometrial profiling assay is needed in order to further evaluate test performance and delineate the impact of the cycle phase on assay success.
Conclusion
Aspiration of endometrial fluid is a safe and effective approach for evaluating the endometrial protein profile of women with endometriosis. It provides insight into the endometrial milieu that may further our understanding of endometrial receptivity in endometriosis, and provide a target for optimizing implantation rates in affected patients. Additionally, although the proteins evaluated did not have diagnostic utility for early-stage disease, a combination of IL-1α, IL-1β, and IL-6 in the endometrial secretions predicts stage 3–4 endometriosis with acceptable sensitivity and specificity. Endometrial fluid profiling may, therefore, be considered in combination with, or as an alternative to, imaging to identify patients with stage 3–4 disease. Together, these data demonstrate the feasibility of endometrial fluid profiling as a minimally invasive diagnostic approach for the diagnosis of endometriosis and other gynecologic diseases.
Electronic supplementary material
Mean cytokine and growth factor levels by endometriosis stage and cycle phase. P-values shown are for univariate analysis with Mann-Whitney test. Error bars represent standard error of the mean. Asterisk denotes statistically significant difference in luteal-phase and follicular-phase cytokine levels after adjustment for false discovery. (PDF 230 kb)
(DOCX 16 kb)
Acknowledgments
We would like to thank the physicians of the Reproductive Endocrinology and Minimally Invasive Gynecologic surgery divisions at the Cleveland Clinic for facilitating the involvement of their patients in this study. In particular, we would like to acknowledge Drs. Mark Dassel, Tommaso Falcone, Jeffrey Goldberg, Rosanne Kho, and Julierut Tantibhedyangkul. Additionally, we would like to acknowledge the Clinical and Translational Science Collaborative (CTSC) Bioanalyte Core of the Case Western Reserve University for their role in processing data samples.
Funding
Funding for this work was provided by a grant from the Cleveland Clinic Research Program Committee (#274).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Natalia C. Llarena, Email: llarenn@ccf.org
Elliott G. Richards, Email: richare@ccf.org
Anju Priyadarshini, Email: priyadarshini.anju@gmail.com.
David Fletcher, Email: drf@case.edu.
Tracey Bonfield, Email: tlb7@case.edu.
Rebecca L. Flyckt, Email: Rebecca.flyckt2@uhhospitals.org
References
- 1.Mahmood TA, Templeton A. Prevalence and genesis of endometriosis. Hum Reprod. 1991;6(4):544–9. doi: 10.1093/oxfordjournals.humrep.a137377. [DOI] [PubMed] [Google Scholar]
- 2.Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci. 2002;955:11–22. doi: 10.1111/j.1749-6632.2002.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 3.Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Greene R, Stratton P, Cleary SD, Lou Ballweg M, Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91(1):32–9. doi: 10.1016/j.fertnstert.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Nisenblat V, Bossuyt PM, Shaikh R, Farquhar C, Jordan V, Scheffers CS, Mol BW, Johnson N, Hull ML. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2016(5):CD012179. [DOI] [PMC free article] [PubMed]
- 6.Liu E, Nisenblat V, Farquhar C, Fraser I, Bossuyt PM, Johnson N, Hull M. Urinary biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2015;2015(12):CD012019. [DOI] [PMC free article] [PubMed]
- 7.Gupta D, Hull ML, Fraser I, Miller L, Bossuyt PM, Johnson N, Nisenblat V. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;4(4):CD012165. [DOI] [PMC free article] [PubMed]
- 8.Ulukus M, Cakmak H, Arici A. The role of endometrium in endometriosis. J Soc Gynecol Investig. 2006;13(7):467–76. doi: 10.1016/j.jsgi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Boomsma CM, Kavelaars A, Eijkemans MJC, Lentjes EG, Fauser BCJM, Heijnen CJ, Macklon NS. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum Reprod. 2009;24(6):1427–35. doi: 10.1093/humrep/dep011. [DOI] [PubMed] [Google Scholar]
- 10.Boomsma CM, Kavelaars A, Eijkemans MJC, Amarouchi K, Teklenburg G, Gutknecht D, Fauser BJCM, Heijnen CJ, Macklon NS. Cytokine profiling in endometrial secretions: a non-invasive window on endometrial receptivity. Reprod BioMed Online. 2009;18:85–94. doi: 10.1016/S1472-6483(10)60429-4. [DOI] [PubMed] [Google Scholar]
- 11.Ametzazurra A, Matorras R, Garcia-Velasco JA, Prieto B, Gonzalez S, Simon L, Nagore D. Validation of endometriosis markers in the endometrial fluid aspirate. Hum Reprod. 2009;24(suppl_1):i9–i11. [DOI] [PubMed]
- 12.Ametzazurra A, Matorras R, Garcia-Velasco JA, Prieto B, Simon L, Martinez A, Nagore D. Endometrial fluid is a specific and non-invasive biological sample for protein biomarker identification in endometriosis. Hum Reprod. 2009;24(4):954–65. doi: 10.1093/humrep/den450. [DOI] [PubMed] [Google Scholar]
- 13.Podgaec S, Abrao MS, Dias JA, Rizzo LV, de Oliveira RM, Baracat EC. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod. 2007;22(5):1373–9. doi: 10.1093/humrep/del516. [DOI] [PubMed] [Google Scholar]
- 14.Othman EEDR, Hornung D, Salem HT, Khalifa EA, El-Metwally TH, Al-Hendy A. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2008;137(2):240–6. doi: 10.1016/j.ejogrb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Mier-Cabrera J, Jiménez-Zamudio L, García-Latorre E, Cruz-Orozco O, Hernández-Guerrero C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG. 2011;118(1):6–16. doi: 10.1111/j.1471-0528.2010.02777.x. [DOI] [PubMed] [Google Scholar]
- 16.Bersinger NA, Dechaud H, McKinnon B, Mueller MD. Analysis of cytokines in the peritoneal fluid of endometriosis patients as a function of the menstrual cycle stage using the Bio-Plex® platform. Arch Physiol Biochem. 2012;118(4):210–8. doi: 10.3109/13813455.2012.687003. [DOI] [PubMed] [Google Scholar]
- 17.Vodolazkaia A, El-Aalamat Y, Popovic D, MihalyiA, Bossuyt X, Kyama CM, Fassbender A, BokorA, Schols D, Huskens D, Meuleman C, Peeraer K, Tomassetti C, Gevaert O, Waelkens E, Kasran A, De Moor B, D’Hooghe TM. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum Reprod. 2012;27(9):2698–711. [DOI] [PubMed]
- 18.Wickiewicz D, Chrobak A, Gmyrek GB, Halbersztadt A, Gabryś MS, Goluda M, Chełmońska-Soyta A. Diagnostic accuracy of interleukin-6 levels in peritoneal fluid for detection of endometriosis. Arch Gynecol Obstet. 2013;288(4):805–14. doi: 10.1007/s00404-013-2828-6. [DOI] [PubMed] [Google Scholar]
- 19.Beste MT, Pfaffle-Doyle N, Prentice EA, Morris S, Lauffenburger DA, Isaacson KB, Griffit LG. Endometriosis: molecular network analysis of endometriosis reveals a role for c-Jun-regulated macrophage activation. Sci Transl Med. 2014;6(222):222ra16. [DOI] [PMC free article] [PubMed]
- 20.Borrelli GM, Kaufmann AM, Abrão MS, Mechsner S. Addition of MCP-1 and MIP-3β to the IL-8 appraisal in peritoneal fluid enhances the probability of identifying women with endometriosis. J Reprod Immunol. 2015;109:66–73. doi: 10.1016/j.jri.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Kocbek V, Vouk K, Bersinger NA, Mueller MD, Rižner TL. Panels of cytokines and other secretory proteins as potential biomarkers of ovarian endometriosis. J Mol Diagnostics. 2015;17(3):325–34. doi: 10.1016/j.jmoldx.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Malutan AM, Drugan T, Costin N, Ciortea R, Bucuri C, Rada MP, Mihu D. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent Eur J Immunol. 2015;40(1):96–102. doi: 10.5114/ceji.2015.50840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakker R, Pierce S, Myers D. The role of prostaglandins E1 and E2, dinoprostone, and misoprostol in cervical ripening and the induction of labor: a mechanistic approach. Arch Gynecol Obstet. 2017;296(2):167–79. doi: 10.1007/s00404-017-4418-5. [DOI] [PubMed] [Google Scholar]
- 24.Rahmioglu N, et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil Steril. 2014;102(5):1233–43. [DOI] [PMC free article] [PubMed]
- 25.Dorien FO, El Aalamat Y, Waelkens E, De Moor B, D’Hooghe T, Fassbender A. Multiplex immunoassays in endometriosis: an array of possibilities. Front Biosci - Landmark. 2017;22:479–92. doi: 10.2741/4496. [DOI] [PubMed] [Google Scholar]
- 26.Kim H, et al. Identification of serum biomarkers for diagnosis of endometriosis using multiplex immunoassays. Reprod Sci. 2020;27(5):1139–47. doi: 10.1007/s43032-019-00124-2. [DOI] [PubMed] [Google Scholar]
- 27.Guo SW, Wang Y. The prevalence of endometriosis in women with chronic pelvic pain. Gynecol Obstet Invest. 2006;62(3):121–30. doi: 10.1159/000093019. [DOI] [PubMed] [Google Scholar]
- 28.Ciavattini A, et al. Uterine fibroids: pathogenesis and interactions with endometrium and endomyometrial junction. Obstet Gynecol Int. 2013;2013:173184. doi: 10.1155/2013/173184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mihalyi A, et al. Non-invasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum Reprod. 2010;25(3):654–64. doi: 10.1093/humrep/dep425. [DOI] [PubMed] [Google Scholar]
- 30.Lebovic DI, Bentzien F, Chao VA, Garrett EN, Meng YG, Taylor RN. Induction of an angiogenic phenotype in endometriotic stromal cell cultures by interleukin-1β. Mol Hum Reprod. 2000;6(3):269–75. doi: 10.1093/molehr/6.3.269. [DOI] [PubMed] [Google Scholar]
- 31.Akoum A, Lawson C, McColl S, Villeneuve M. Ectopic endometrial cells express high concentrations of interleukin (IL)-8 in vivo regardless of the menstrual cycle phase and respond to oestradiol by up-regulating IL-1-induced IL-8 expression in vitro. Mol Hum Reprod. 2001;7(9):859–66. doi: 10.1093/molehr/7.9.859. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, et al. IL-1β stimulates brain-derived neurotrophic factor production in Eutopic endometriosis stromal cell cultures: a model for cytokine regulation of neuroangiogenesis. Am J Pathol. 2018;188(10):2281–92. doi: 10.1016/j.ajpath.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MY, Kim SH, Ihm HJ, Chae HD, Kim CH, Kang BM. Up-regulation of p21-activated kinase 1 by in vitro treatment with interleukin 1-beta and its increased expression in ovarian endometriotic cysts. Fertil Steril. 2011;96(2):508–11. doi: 10.1016/j.fertnstert.2011.05.082. [DOI] [PubMed] [Google Scholar]
- 34.Pellicer A, Oliveira N, Ruiz A, Remohi J, Simon C. Exploring the mechanism(s) of endometriosis-related infertility: an analysis of embryo development and implantation in assisted reproduction. Hum Reprod. 1995;10(Suppl 2):91–7. doi: 10.1093/humrep/10.suppl_2.91. [DOI] [PubMed] [Google Scholar]
- 35.Simón C, Gutiérrez A, Vidal A, de los Santos MJ, Tarín JJ, Remohí J, Pellicer A. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum Reprod. 1994;9(4):725–9. [DOI] [PubMed]
- 36.Zhihong N, Yun F, Pinggui Z, Sulian Z, Zhang A. Cytokine profiling in the eutopic endometrium of adenomyosis during the implantation window after ovarian stimulation. Reprod Sci. 2016;23(1):124–33. doi: 10.1177/1933719115597761. [DOI] [PubMed] [Google Scholar]
- 37.Boomsma CM, Kavelaars A, Eijkemans MJC, Fauser BCJM, Heijnen CJ, MacKlon NS. Ovarian stimulation for in vitro fertilization alters the intrauterine cytokine, chemokine, and growth factor milieu encountered by the embryo. Fertil Steril. 2010;94(5):1764–8. doi: 10.1016/j.fertnstert.2009.10.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean cytokine and growth factor levels by endometriosis stage and cycle phase. P-values shown are for univariate analysis with Mann-Whitney test. Error bars represent standard error of the mean. Asterisk denotes statistically significant difference in luteal-phase and follicular-phase cytokine levels after adjustment for false discovery. (PDF 230 kb)
(DOCX 16 kb)