Abstract
The outcome of surgery for signet ring adenocarcinoma of rectum is suboptimal with high predilection for locoregional and peritoneal metastases. Lack of intercellular adhesion due to focal loss of epithelial cell adhesion molecule (EpCAM) may account for this. In such patients, whether minimal invasive surgery carries a high risk of dissemination by pneumoperitoneum and tumor implantation remains uncertain. The aim of this study was to compare the outcomes of patients undergoing minimally invasive surgery (MIS) versus open surgery in patients with signet ring cell adenocarcinoma of rectum. A retrospective study was conducted at a tertiary care center over 3 years on 39 patients undergoing open surgery and 40 patients undergoing MIS diagnosed with signet ring cell carcinoma (SRCC) identified from our surgical database. Patient characteristics in terms of demographics, clinicoradiological staging, neoadjuvant therapy, and type of surgery with morbidity were compared in the two groups. Data on patients undergoing adjuvant therapy and 3 years disease-free survival (DFS) and overall survival (OS) were analyzed. Recurrence patterns in both groups were separately identified as locoregional, peritoneal, or systemic. The number of patients undergoing surgery in the two arms was 40 (MIS) and 39 (open). In the MIS arm, mean DFS was 29 months whereas in the open arm, it was 25.8 months. The mean OS was 33.65 months for the MIS arm and that for the open arm was 36.34 months. This retrospective study reveals no significant difference in outcomes of surgery for signet ring cell rectal cancers with either MIS or open approach.
Keywords: Rectal cancer, Signet ring cell adenocarcinoma, Minimally invasive surgery, Peritoneal recurrences, Minimal access surgery of rectal cancers
Background
Colorectal cancer is a common cancer worldwide. It is the third most common in men and second most common in women with more than 14 lakh cases diagnosed every year (Torre LA, 2012) [1]. Most of the cases are seen in the developed countries, but mortality is higher in developing countries, which have inadequate resources. The mortality rates are significantly decreasing in the West due to factors such as early detection by screening and improved treatment outcomes (Center MM, 2010) [2].
Age-standardized rate of colorectal cancer in India is low but in absolute numbers, in our population, it translated to significant numbers (Globocan 2018) [3]. The 5-year survival rate in colorectal cancer is quite low; in fact, the CONCORD 2 study (S. Benitez Majano, 2018) has reported a decrease in 5-year survival rate [4]. These may be considered as pointers to fallacies in diagnosis and treatment protocols whereas one needs to analyze data on the presentation and pathological features of population to search for a reason for the same.
Patients in India tend to be younger, have more advanced disease at presentation, and have a higher frequency of signet ring cell type of adenocarcinoma (Patil P, 2017) [5]. Signet ring cell rectal cancers are associated with higher rate of locoregional and peritoneal failures compared to other histological subtypes of adenocarcinomas (Vallam KC, 2016) [4]. This may explain the relatively poor survival in Indian population. At the molecular level, the loss of epithelial cell adhesion molecule (EpCAM) is considered to be a reason for early dissemination and peritoneal recurrences of this subtype.
Signet ring cancer of rectum has an aggressive biology due to many of the abovementioned factors. In addition, laparoscopic surgery with its associated pneumoperitoneum may facilitate tumor spread and peritoneal metastasis. Keeping this in mind, retrospective analysis was performed to evaluate the outcomes of open and minimally invasive surgery (MIS) in rectal cancers showing signet ring cell histology. Although the efficacy and non-inferiority of MIS techniques used for rectal cancers are studied in multiple trials, a direct comparison of MIS with open techniques in cases with signet ring cell cancers of rectum is lacking due to a very low frequency in Western databases. However, with an alarming rate of rise in colorectal cancers in young in the West, the incidence of signet ring cell cancers may increase (Vuik, 2019) [6].
Methods
Patients with signet ring cell carcinoma (SRCC) of rectum who underwent surgical resection from 2014 to 2017 were identified from a prospectively maintained surgical database at a tertiary care center in Mumbai. In accordance with the definition of World Health Organization, SRCC is diagnosed when a colorectal tumor contains at least 50% signet ring cells (JASS JR, 1989) [7] . Patients who did not undergo surgery or treated with palliative intent were excluded from the analysis. All patients underwent magnetic resonance imaging (MRI) of the pelvis, contrast-enhanced computed tomography (CECT; thorax + abdomen), and colonoscopy (if scope is passable). Proctoscopy and sigmoidoscopy-guided biopsies were performed before initiating any definitive treatment.
The baseline characteristics include demographics, location of tumor (upper/mid/lower rectum), clinicoradiological stage (AJCC, 7th edition) prior to initiation of neoadjuvant therapy, carcinoembryonic antigen (CEA) levels, type of neoadjuvant therapy either long-course chemoradiation (LCRT) or short-course radiotherapy, and four cycles of capecitabine + oxaliplatin, type of surgery, and adjuvant therapy. Some patients received salvage chemotherapy if R0 resection was not considered likely on response evaluation of MRI scan. The decision regarding type of surgery (TME, extended TME, or beyond TME) was taken by a multidisciplinary team. The decision of approach (open or laparoscopic/robotic) was taken by surgeons on the team.
All patients were followed up using a standard protocol; three monthly CEA, annual CECT scan of TAP (thorax, abdomen, and pelvis), and colonoscopy as per NCCN (National Comprehensive Cancer Network) recommendations. Patients were followed up as close as possible based on electronic medical record entries or telephonic follow-up.
Recurrence, if any, and location of recurrence (locoregional or distant) were recorded. All patients who had been included had undergone curative resection. The pathological diagnosis of signet ring type adenocarcinoma of the rectum was made by specialist pathologists. Disease-free survival (DFS) was determined from the date of last day of primary treatment (surgery or adjuvant therapy) to the date of recurrence. Overall survival (OS) was duration between the date of completion of treatment and the date of death or date of last follow-up.
Results
In all the patients who were operated between January 2014 and December 2017, a total of 79 patients had signet ring cell adenocarcinoma. Among them, 39 underwent open resection and 40 patients had MIS. In the MIS group, 9 patients underwent robotic surgery and 31 underwent laparoscopic surgery. Demographics and tumor characteristics were comparable, as seen in Table 1.
Table 1.
Baseline characteristics
| MIS (n = 40) | Open (n = 39) | |
|---|---|---|
| Age < 40 years | 29 | 29 |
| Median age | 36 | 33 |
| M:F | 29:11 | 19:20 |
| Tumor location | ||
| Upper | 2 | 1 |
| Mid | 8 | 10 |
| Lower | 30 | 28 |
| Pretreatment T stage | ||
| T2 | 5 | 1 |
| T3 | 31 | 31 |
| T4 | 4 | 7 |
| CRM status at presentation | ||
| Free | 16 | 14 |
| Threatened | 6 | 3 |
| Involved | 18 | 22 |
| Neoadjuvant therapy | ||
| None (upfront surgery) | 4 | 1 |
| LCRT + chemo (concurrent) | 19 | 15 |
| LCRT + chemo f/b additional chemo | 13 | 19 |
| SCRT only | 2 | 1 |
| SCRT f/b chemotherapy | 2 | 3 |
| MIS types | NA | |
| Lap: 31 | ||
| Robotic: 9 | ||
Table 2 shows that higher number of patients in open arm required extended total mesorectal excision (TME) and beyond TME (included patients who needed additional resection for circumferential resection margin (CRM); e.g., pelvic nodes or vaginal wall or bladder peritoneum as part of the main specimen). In the open-arm group, three patients underwent posterior exenteration and one underwent total pelvic exenteration. One patient in the open-arm group underwent additional retroperitoneal lymph node dissection and inguinal lymph node dissection. One patient in the open-arm group died during postoperative period due to sudden cardiac arrest and another in the open-arm died during the follow-up period due to non-oncological reasons.
Table 2.
Treatment and post treatment outcomes
| Type of surgery | MIS (n = 40) | Open (n = 39) |
|---|---|---|
| ISR | 8 | 5 (2 patients beyond TME) |
| Prone APR | 1 | 2 |
| ELAPR | 7(3 beyond TME) | 18(6 beyond TME, 1 with i/l ILND) |
| AR | 24 | 9 (3 beyond TME) |
| AR + PLND + RPLND | 0 | 1 |
| Total Pelvic exenteration | 0 | 1 |
| Posterior exenteration | 0 | 3 |
| From HPR | ||
| Early T stage (pCR, pT1, pT2) | ||
| Mean DFS (months) | 34 | 28.53 |
| Late T stage (pT3, pT4) | ||
| Mean DFS | 24.86 | 22.50 |
| Early T stage (pCR, pT1, pT2) | ||
| Mean OS | 39.5 | 38.12 |
| Late T stage (pT3, pT4) | ||
| Mean OS | 28.95 | 32.6 |
| Overall | ||
| Mean DFS (months) | 29 | 25.8 |
| Overall | ||
| Mean OS (months) | 33.65 | 36.34 |
| Median duration of follow-up (months) | 30 | 32 |
In the MIS arm, three patients had CRM involved (CRM positive) in the final HPR compared to five patients in the open arm. Distal margin was involved with disease in one patient in the MIS arm and two in the open arm.
While comparing patients who presented with cT3 disease prior to start of any treatment protocols, mean DFS was 24.4 months for open surgery and 28.4 months for the MIS arm (p = 0.13) whereas the OS was 34.6 and 32.8 months, respectively (p = 0.59).
While comparing the data of results across the various parameters, we did not observe any significant difference between both arms. Patients undergoing upfront surgery in view of early stage disease and those with better response to neoadjuvant therapy (pCR, pT1, pT2) had better survival rates in both arms compared to those with less responsive disease.
With regard to recurrences and their pattern, 8 patients in the MIS arm and 10 patients in the open-surgery arm had peritoneal recurrence on follow-up. Median duration of follow up for the MIS group is 30 months and for the open group, it was 32 months. At the end of follow-up, 16 patients in the open arm are alive and disease free whereas 21 patients in the MIS arm are alive and disease free.
Recurrences (see Table 3)
Table 3.
Recurrences
| Locoregional only | Distant only | Both | Peritoneal | |
|---|---|---|---|---|
| Open | 4 | 13 | 6 | 10 |
| MIS | 4 | 9 | 5 | 8 |
In all, 23 patients (58.9%) in the open arm and 18 patients (45%) in the MIS arm had recurrences. Rate of peritoneal recurrences was 20% for the MIS and 25.6% for the open-arm group. One patient in the open-arm died during the postoperative period due to sudden cardiac arrest and another died due to non-oncological reasons. A few patients in both arms developed recurrence but were later lost to follow-up while receiving palliative chemotherapy. Both these groups were distinct and had not been randomized or propensity-matched, yet no significant difference was noted between outcomes in both arms.
Discussion
SRCC of the colorectum was first reported in 1951. It is a relatively rare and distinctive type. Its prognosis is known to be poorer compared to other subtypes.
SRCC incidence accounts for 0.2–2.6% of all colorectal cancer cases. This rate goes up to 19% in some series (Anthony T, 1996) [7]. SRCCs have been studied by multiple teams across the globe and universally found to have poorer outcomes compared to non-signet ring cell tumors (Hu, 2012) [8]. An audit of cases seen in Tata Memorial Hospital, Mumbai, showed that 13.4% cases diagnosed had signet ring cell histology. It was more commonly seen in patients aged less than 40 years. A higher proportion of SRCC presented at higher T stage (Patil P, 2017) [5].
Symptoms usually present later, leading to diagnosis at later stages (KIm JH, 2013) [9], Bonello et al. [10] reported that delay in diagnosis was due to intramucosal tumor spread with relative sparing of mucosa (JC & SH, 1980). However, absence of lymphovascular invasion and decreased depth of invasion were associated with better prognosis.
The work of Vallam et al. showed the difference in outcome of colorectal malignancies based on the histological subtype (Vallam KC, 2016) [4]. Presence of SRCC was an independent poor risk factor in case of advance cancer (stages III and IV) although the difference was not significant in early stage (Sang-Oh et al., 2017). [11]. This study had its limitations. It was a single-center, retrospective, non-randomized study and protocols of treatment were selected on the basis of surgeons’ choice and included colon and rectum cases.
The pattern of metastasis in these cases was also different, as signet ring and mucinous tumors are generally seen with metastasis to peritoneum and the ovaries compared to tumors lacking these components which have visceral metastatic disease, mainly in the liver and lung. Thus, these patients are less likely to be screen-detected and have a poorer prognosis. Owing to higher incidence in a resource-poor condition, it is more likely to recur and has poorer survival with a different pattern of treatment failure (Jinn-Shiun Chen, 2010) [12].
SRCC management protocols need to be discussed and optimized instead of offering patients the standard management protocols reserved for the rest of the cases in view of these specific reasons. For example, with regard to neoadjuvant therapy for locally unresectable or metastatic yet resectable SRCC cases, short-course radiation therapy (SCRT) followed by chemotherapy and surgery in responsive cases may be a valid treatment option that can reduce the treatment duration and allow patients to undergo definitive surgery earlier compared to traditional treatment protocols (O & A, 2019) [13]. Hence, we have changed treatment protocol to SCRT followed by chemotherapy for cases with signet ring cell rectal cancer, which was shown to be equivalent in a study by the Polish Colorectal Cancer Study Group (Cisel B, 2019) [14]. In addition, it has been shown that rectal cancers with poor differentiation and higher grade are more likely to progress while receiving neoadjuvant therapy, both locally and systemically (Singhal N, 2016, June) [15]. This can be used to justify restaging with multi-detector computed tomography with or without laparoscopy in cases of signet ring cancers of rectum post neoadjuvant chemotherapy radiotherapy. In this study, only 30% of all cases with SRCC of rectum underwent surgery.
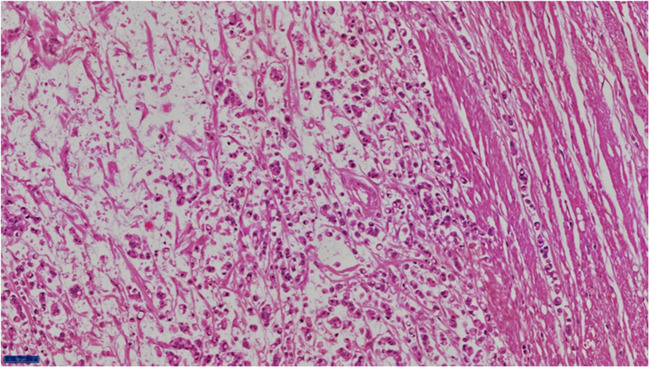
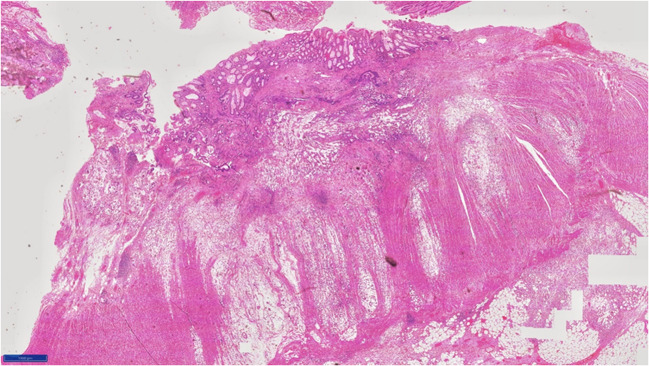
The serrated adenoma–carcinoma pathway has been postulated for development of these tumors. Terada et al. [16], in their immunohistochemical study of primary signet ring of the stomach and colorectum, found that epithelial membrane antigen was downregulated in colorectal SRCC. Kim (Kim, 2014) [17] showed that focal loss of EpCAM was associated with development of SRCC in colonocytes. These molecular changes may be related to preferential peritoneal spread of this subtype. These tumors produce mucin, which can be within the cells or be secreted [18]. Extracellular mucin dissects through tumor wall aiding local extension (Figs. 1 and 2) (Green JB, 1993) [19]. This may be the reason for dissemination or increased recurrence in signet. Knowing this, we should study how MIS is better than open surgery in cases of signet ring cell adenocarcinoma.
Fig. 1.
Histological appearance of signet ring cell adenocarcinoma of rectum mention magnification 400x
Fig. 2.
Histological appearance of signet ring cell adenocarcinoma of rectum. Magnification 100x
Although a number of studies are available on the incidence and outcomes of SRCC, there is a paucity of literature comparing MIS and open-arm surgery. As such, cases are more likely to be diagnosed at advanced stages; the chances of tumor handling and tumor cell dissemination during surgery are to be considered. Thus, optimal mode of surgery (minimally invasive vs open surgery) should be evaluated for patients with this histology. Multiple large multicenter trials have shown that laparoscopic surgery is not inferior to open surgery. Some important trails among these were the European COLOR II (A Randomized Trial of Laparoscopic versus Open Surgery for Rectal Cancer, 2015) [20] and South Korean COREAN trial (Jeong SY, 2014) [21]. The ROLARR trial (David Jayne, Alessio Pigazzi, Helen Marshall, & al, 2017) [22] found robotic rectal surgery comparable to laparoscopic surgery. One must note that the ACOSOG Z6051 (Fleshman J, 2015) [23] and ALaCaRT (Stevenson ARL, 2019) [24] failed to show that laparoscopic surgery is not inferior to open surgery. However, with respect to patient convenience with smaller incisions, lower pain score, early ambulation, early alimentation, and early discharges, MIS has been considered to achieve improvement in short-term postoperative outcomes. There has been no specific comparison between minimal access and open approaches in patients with SRCC.
In our group, the median age of patients was 36 years in the MIS arm and 33 years in the open arm. In the patients undergoing open surgery, a higher proportion needed resection to go “beyond TME” to achieve a negative CRM. In the open arm, more often patients had higher disease burden, poorer radiological response to neoadjuvant therapy, and hence, worse disease biology whereas patients with better response to neoadjuvant therapy or lower disease burden upfront were more likely to undergo MIS. This could affect the survival in both the arms. However, R0 resection rates in both arms were comparable.
Both these groups were neither randomized nor propensity-matched. No significant differences on outcomes were noted in both arms. Ten patients in the open arm and five patients in the MIS arm developed recurrence and were later lost to follow-up while receiving palliative chemotherapy.
With regard to recurrence rates, studies have consistently found a higher rate of locoregional and systemic recurrence in SRCC (Nitsche U, 2013) [25] . Retrospective data from our own institution as well as the world over have shown a higher rate of peritoneal cancer recurrence (Tamhankar, 2016) [26]. However, the frequency of peritoneal recurrences was lower in our case series.
SRCC has been associated with peculiar genomic changes such as high-degree microsatellite instability (MSI-high) (up to 40%), high frequency of CpG island methylator phenotype, higher methylation level of long interspersed nucleotide element 1, and frequent BRAF mutation and low COX-2 expression (Gopalan V, 2011) (Ogino S, 2006) (Tanaka, 2006) [26–28]. However, these included cases with colon cancer along with rectal cancer. In our own institute, the incidence of MSI-H was significantly lower in cohorts of only rectal cancers (Ostwal V, Feb 2019) [29].
Partial loss of EpCAM is associated with invasive front, poor differentiation, signet ring histology, tumor budding, lymphovascular invasion/perineural invasion, locoregional/distant metastases, and poor DFS. EpCAM loss was frequently observed in invasive front and in tumor buds of colorectal cancers. This could be due to reduced cell–cell adhesions between invasive cancer cells (Gosens MJ, 2007) [30].
While looking at CO2 pneumoperitoneum to be the cause of increased dissemination of tumor cells within peritoneum, the available evidence suggests no increase in risk (Jingli C, 2006; Barbulescu M, 2012), [31, 32], but animal models have shown increased dissemination following laparoscopic procedures. In our study, very few patients underwent upfront surgery (usually with low volume, early-stage disease) and most cases received neoadjuvant therapy, both factors that could reduce chances of dissemination. Further research would be needed to get better answers in this direction.
There is a significantly higher incidence of signet ring cell rectal cancer in the Indian subcontinent despite being a relatively low prevalence in the region. However, due to high population, these numbers are still significantly higher. These patients are younger and less likely to be screen-detected in an early stage, contributing to poorer survival. On comparison between the MIS and open arms, there was no significant difference with respect to survival, with a non-significant trend of better results in the MIS arm, which could more likely be due to these patients having less bulky and less extensive disease. Our results show that even after neoadjuvant chemoradiation, extended resection may be required. However, no difference in recurrence patterns was seen between the open arm and MIS. So, although efforts to improve outcomes in signet rectal cancers are required, MIS is not a contraindication for such cases.
With surgeons now pushing boundaries of MIS, it may be time to compare both these arms in an RCT to get a clearer answer. Other directions in which research on treatment of signet ring cell adenocarcinoma of rectum can be considered include use of prophylactic hyperthermic intraperitoneal chemotherapy procedure in view of poor survival and likelihood of peritoneal recurrences and of early postoperative intraperitoneal chemotherapy.
Conclusion
In a country with high incidence of signet ring cancer, minimal access surgery appears to be safe and equivalent to open approach in terms of outcomes for signet ring cell adenocarcinoma of the rectum.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre LA, Bray F. Global cancer statistics 2012. CA Cancer J Clin. 2012;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancers. CA Cancer J Clin. 2010;59(6):366–378. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 3.Globocan. (2018). Fact sheets by population-CRC India ASRs [Online]. Available: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed October 2019
- 4.Vallam KC, D. A. Adenocarcinoma of the rectum- a composite of three different subtypes with varying outcomes? Clin Colorectal Cancer. 2016;15:e47–e52. doi: 10.1016/j.clcc.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Patil P, Saklani A, Gambhire P, Mehta S, Engineer R, De'Souza A, et al. Colorectal cancer in India: an audit from tertiary cancer centre in a low prevalence area. Indian J Surg Oncol. 2017;8(4):484–490. doi: 10.1007/s13193-017-0655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vuik NB. Increasing incidence of colorectal cancer in young adults in Europe over last 25 years. Gut. 2019;68:1820–1826. doi: 10.1136/gutjnl-2018-317592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthony T, George R, Rodriguez-Bigas M, Petrelli NJ. Primary signet ring cell carcinoma of colon and rectum. Ann Surg Oncol. 1996;3(4):344–348. doi: 10.1007/BF02305663. [DOI] [PubMed] [Google Scholar]
- 8.Hu JR-Y. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the national cancer data base. Ann Surg Oncol. 2012;19(9):2814–2821. doi: 10.1245/s10434-012-2321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Park SJ. Early stage primary signet ring cell carcinoma of the colon. World J Gastroenterol. 2013;19:3895–3898. doi: 10.3748/wjg.v19.i24.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonello JC, Quan SH, Sternberg SS. Primary linitis plastica of the rectum. Dis Colon Rectum. 1980;23(5):337–42U. doi: 10.1007/BF02586841. [DOI] [PubMed] [Google Scholar]
- 11.Yun S-O, et al. Clinical significance of Sigmnet ring cell colorectal cancer as a prognostic factor. Annals of Coloproctology. 2017;33(6):232–238. doi: 10.3393/ac.2017.33.6.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JS, Hsieh PS, et al. Clinical outcomes in mucinous adenocarcinoma and signet ring cell carcinoma of colon. Chang Gung Med J. 2010;33(1):51–57. [PubMed] [Google Scholar]
- 13.Ostwal V, Kapoor A, Engineer R, Saklani A, deSouza A, Patil P, Arya S, Ankathi SK, Chopra S, Patil M, Jain S, Ramaswamy A. Systemic chemotherapy and short-course radiation in metastatic rectal cancers: a feasible paradigm in unresectable and potentially resectable cancers. South Asian J Cancer. 2019;8(2):92–97. doi: 10.4103/sajc.sajc_174_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cisel B, Pietrzak L, Michalski W, Wyrwicz L, Rutkowski A, Kosakowska E. Long-course preoperative chemoradiation vs. 5 x 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol. 2019;30(8):1298–1303. doi: 10.1093/annonc/mdz186. [DOI] [PubMed] [Google Scholar]
- 15.Singhal N, Vallam KC. Restaging after neoadjuvant chemoradiation in rectal cancers: is histology the key in patient selection? J Gastrointest Oncol. 2016;7(3):360–364. doi: 10.21037/jgo.2016.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terada T. An immunohistochemical study of primary signet-ring of the stomach and colorectal. Int J Clin Exp Pathol. 2013;6(4):613–621. [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JH, Bae JM, Kim KJ, Rhee YY, Kim Y, Cho NY, Lee HS, Chang MS, Kang GH. Differential features of microsatellite unstable colorectal carcinomas depending on EPCAM status. Korean J Pathol. 2014;48(4):276–282. doi: 10.4132/KoreanJPathol.2014.48.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jass JR, Sobin L. Histological typing of intestinal tumours. Berlin: Springer; 1989. [Google Scholar]
- 19.Green JB, Timmcke AE, Mitchell WT. Mucinous carcinoma - just another colon cancer ? Dis Colon Rectum. 1993;36(1):49–54. doi: 10.1007/BF02050301. [DOI] [PubMed] [Google Scholar]
- 20.Jaap Bonjer H, Deijen CL, Abis GA, Cuesta MA, van der Pas Martijn HGM, de Lange-de Klerk Elly SM, Lacy AM, Bemelman WA, Anderson J. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–1332. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 21.Jeong SY, Park JW. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet : Oncology. 2014;5(7):767–774. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 22.David Jayne M, Alessio Pigazzi P, Helen Marshall M, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer. JAMA. 2017;318(16):1569–1580. doi: 10.1001/jama.2017.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleshman J, Branda M. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal Cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314:1346–1355. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson ARL, Solomon MJ, et al. Disease-free survival and local recurrence after laparoscopic-assisted resection or open resection for rectal cancer: the Australasian laparoscopic cancer of the rectum randomized clinical trial. Ann Surg. 2019;269(4):596–602. doi: 10.1097/SLA.0000000000003021. [DOI] [PubMed] [Google Scholar]
- 25.Nitsche U, Zimmermann A, Späth C, Müller T, Maak M, Schuster T, Slotta-Huspenina J, Käser SA, Michalski CW, Janssen KP, Friess H, Rosenberg R, Bader FG. Mucinous and signet ring cell colorectal cancers differ from classical adenocarcinomas in tumour biology and prognosis. Ann Surg. 2013;258(5):775–782. doi: 10.1097/SLA.0b013e3182a69f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamhankar IP. Signet ring cell colorectal carcinoma : do we need to improve the treatment algorithm. World J Gastrointest Oncol. 2016;8(12):819–825. doi: 10.4251/wjgo.v8.i12.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogino S, Brahmandam M, Cantor M, Namgyal C, Kawasaki T, Kirkner G, Meyerhardt JA, Loda M, Fuchs CS. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19(1):59–68. doi: 10.1038/modpathol.3800482. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka D, Deng G, Matsuzaki K, Kakar S, Kim GE, Miura S, et al. BRAF mutation, CpG island methylator phenotype and microsatellite instability occur more freqauently and concordantly in mucinous than non-mucinous colorectal cancer. Int J Cancer. 2006;118(11):2765–2771. doi: 10.1002/ijc.21701. [DOI] [PubMed] [Google Scholar]
- 29.Ostwal V, Pandey NS, Engineer R, Saklani A, deSouza A, Ramadwar M, et al. Low prevalence of deficient mismatch repair (dMMR) protein in locally advanced rectal cancers (LARC) and treatment outcomes. J Gastrointest Oncol. 2019;10(1):19–29. doi: 10.21037/jgo.2018.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gosens MJ, v. K. Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod Pathol. 2007;20:221–232. doi: 10.1038/modpathol.3800733. [DOI] [PubMed] [Google Scholar]
- 31.Jingli C, Rong C. Influeince of colorectal laparoscopic surgery on dissemination and seeding of tumor cells. Surg Endosc. 2006;20:1759–1761. doi: 10.1007/s00464-005-0694-4. [DOI] [PubMed] [Google Scholar]
- 32.Barbulescu M, Alecu L. Port-site metastasis after laparoscopic surgery for colorectal cancer--still a real concern? Case report and review of the literature. Chirurgia. 2012;107(1):103–107. [PubMed] [Google Scholar]