Abstract
Situs inversus totalis is an uncommon anatomical congenital anomaly characterized by complete transposition of viscera with right-to-left reversal across the sagittal plane. Consequently, surgery in such cases is more technically challenging and requires a complete reorientation of visual-motor coordination skills. We describe a case of a 50-year-old gentleman with locally advanced lower esophagus carcinoma post-neoadjuvant chemoradiotherapy with situs inversus totalis and treated with minimally invasive McKeown esophagectomy using a left thoracoscopic, laparoscopic-assisted and right cervical approach. The operative procedure and difficulties during surgery are highlighted. Minimal invasive esophagectomy is safe and feasible in situs inversus totalis. Recognition of the anatomy with a meticulous preoperative planning is advocated for an uneventful operative intervention.
Introduction
Situs inversus totalis (SIT) is a rare anatomical congenital deviation characterized by a symmetrical and complete transposition of viscera in context to midline. From a surgeon’s perspective, this condition presents a technical challenge due to the reverse anatomical orientation of organs. We report our experience with minimally invasive esophago-proximal gastrectomy in a patient with carcinoma lower esophagus with SIT.
Case Report
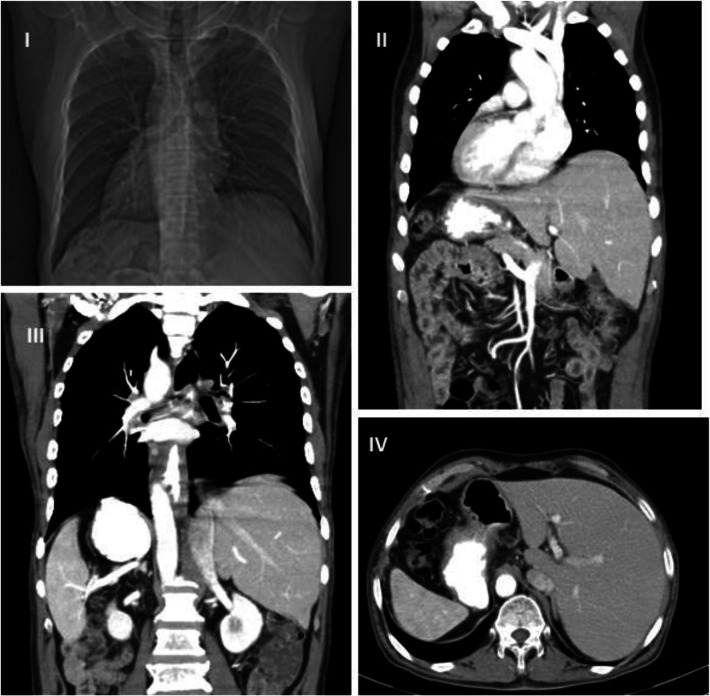
A 58-year-old gentleman with no known comorbidities presented with dysphagia of 3 months’ duration. CT chest and abdomen revealed cT3N + M0 tumor in lower esophagus extending till the gastro-esophageal junction with few periesophageal, paratracheal nodes with situs inversus totalis with right-sided aortic arch, right-sided descending aorta, and left-sided vena cava (Fig. 1). Endoscopy showed growth in the lower esophagus with biopsy well-differentiated squamous cell carcinoma. There was a good response with neoadjuvant chemoradiation, delivered by CROSS-study protocol. He underwent thoraco-laparoscopic assisted esophagectomy, with a complete two-field lymphadenectomy, with gastric pull-up and stapled esophago-gastric cervical anastomosis. Final histopathology revealed complete pathological regression. We discuss the operative details and challenges while operating a case of situs inversus totalis.
Fig. 1.
I—CT scanogram with right-sided heart. II—CT coronal image showing cardiac apex to the right, SVC on left, right-sided arch of the aorta. III—CT coronal section with liver on the left, spleen and stomach on the right side, IVC on left, and aorta on the right side of the midline. It also shows growth in the lower third of the esophagus. IV—CT abdomen axial section showing liver situated on the left, stomach and spleen on the right side, and aorta on the right side of the midline
Operative Details
The entire dissection is divided into three parts, i.e., thoracoscopic, laparoscopic, and cervical.
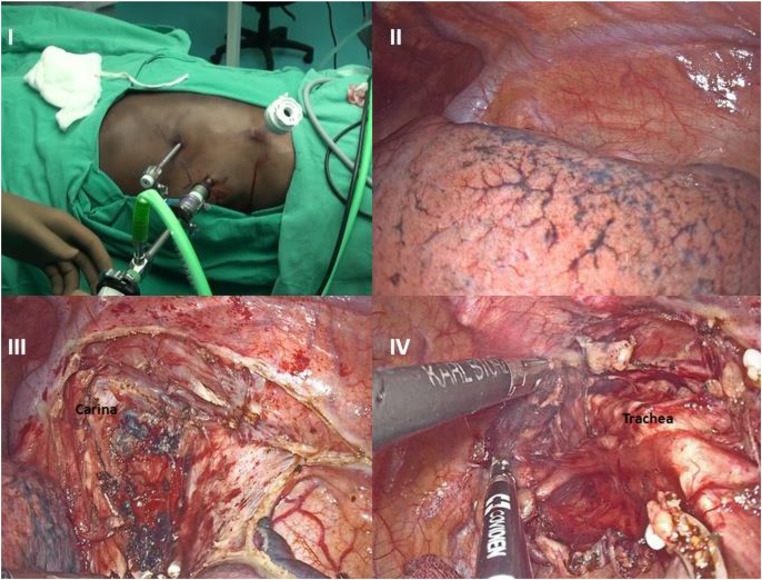
1. Thoracoscopic part—The patient was placed in a prone position. Ventilation was achieved by double lung ventilation. The operating surgeon stood on the left side of the patient opposite to the usual right position for the standard procedure. The camera assistant and second assistant were positioned to the left and right of the operating surgeon and monitor on the right side. The primary 5 mm (surgeon’s left hand working port) was inserted in the 4th intercostal space 2 cm superior and 1 cm medial to scapular tip. CO2 pneumothorax was established by keeping the pressure at 10 mmHg and flow at 10 L/min. Another 10-mm working port (surgeon’s right hand working port) was placed in the 9th intercostal space and a 10-mm camera port in the 7th intercostal space in the anterior axillary line to achieve a triangular configuration (Fig. 2). The left lung was retracted by gentle compression downwards with a gauze held by laparoscopic forceps introduced via another 5 mm assistant port (not shown in fig. 2).
Fig. 2.
I—Placement of ports on the left side of chest reflecting mirror transposition of usual position of ports. II—A left-sided azygos vein. III—Dissection of subcarinal nodes. IV—Dissection of ipsilateral paratracheal nodes
Infra-Azygos Dissection
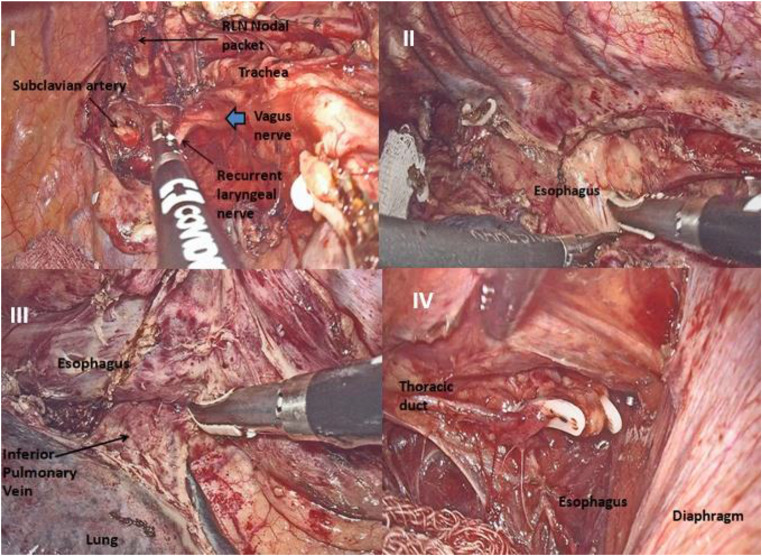
A left-sided azygos vein was seen looping above the left main bronchus to join the left-sided superior vena cava. There was complete reversal of anatomy. The visceral pleural cut was taken with the Harmonic scalpel (Ethicon Endo-surgery India). Vagus was divided distal to its branch to bronchi, thus preserving innervation to bronchi. Right and left bronchial, subcarinal nodes were dissected. Residual thickening was noticed in the lower esophagus. The esophagus was mobilized with periesophageal tissue and periesophageal nodes till esophago-gastric junction. Small branches directly emanating from the aorta were clipped with Hem-o-lock clips. Further dissection was done by Liga-Sure laparoscopic sealer (Covidien Healthcare India Private Limited, Gurgaon, India). With infra-azygos dissection, a complete clearance of subcarinal, paraesophageal, inferior pulmonary ligament, and the hiatal nodes was achieved (Figs. 2 and 3). Right-sided aortic arch and right descending aorta, dextrocardia with the apex of the heart facing to the right were visualized.
Fig. 3.
I—Dissection of ipsilateral recurrent laryngeal nerve (RLN) nodes. II—Dissection of para-esophageal nodes. III—Dissection of inferior pulmonary ligament nodes. IV—Clipping of thoracic duct at caudad aspect of thorax
Supra-Azygos Dissection
Azygos vein was clipped and divided. Dissection was continued cephalad along the esophagus, clearing ipsilateral paratracheal nodes and recurrent laryngeal nodes. Then the nodes along contralateral paratracheal and recurrent laryngeal nerve were dissected. Bilateral bronchial arteries were identified and preserved. Aortopulmonary window lymph nodes were then cleared. The thoracic duct was clipped and resected en bloc with the specimen (Figs. 2 and 3).
2. Laparoscopic part—The patient was then turned to the supine position with legs separated wide. The operating surgeon stood between the legs of the patient. The camera assistant stood on the left side opposite to the right on a usual case, assistant surgeon on the right side. Standard laparoscopic ports were placed: 10-mm umbilical camera port, 10-mm epigastric port for introduction of gauze pieces and retraction of the liver, 5-mm port on mid clavicular line on either side as surgeon’s right- and left-hand working ports, and an additional left subcostal port along the anterior axillary line for stomach retraction. The intraperitoneal pressure was kept at 12 mmHg.
A comprehensive assessment of abdominal viscera was done. There was a complete transposition of viscera, with the spleen and stomach situated on the right side, the right lobe of the liver, gallbladder, appendix, and cecum on the left. When gastro-hepatic ligament was divided, there was a complete reversal of celiac anatomy. The left gastric and splenic artery was lying on the right side, common hepatic and hepatic artery proper on the left side (Fig. 4). A complete D2 lymphadenectomy (except supra, infrapyloric, and splenic hilar nodes) was done. The left gastric artery and vein were divided, and gastric mobilization was performed. Gastrocolic omentum was coagulated and cut by a Liga-Sure laparoscopic sealer with preservation of the “right” gastroepiploic vessels, which would have otherwise been left gastroepiploic vessels in a patient without SIT. The gastric conduit was created intracorporeally by firing three 60-mm endo-GIA staplers. The newly created gastric conduit was left attached to the remnant specimen for extraction via the neck incision.
Fig. 4.
I—Left lobe of liver and stomach situated on the right side. II—Right lobe of liver and gall bladder on the left side. III—Spleen and stomach on the right side. IV—Porta hepatis on the left side and dissection of D2 nodes
3. Cervical part—Neck was turned toward the left side. A right transverse supra-clavicular incision (instead of the usual left-sided incision) was taken. The platysma, investing layer of cervical fascia, medial head of the sternocleidomastoid muscle, strap muscles, and the omohyoid muscles were divided. Plane of dissection was medial to carotid sheath containing the internal jugular vein and carotid artery. The esophagus with the nasogastric tube was lifted from its bed; the nasogastric tube was retracted to the neck level. The esophagus was divided at a lower cervical level. Mobilized esophagus with gastric conduit was retrieved in continuity via neck incision. Distal 3 cm of stapler line was found dehisced (misfired). The conduit was retrieved via a small laparotomy incision and the stapler line was reinforced with sutures. The conduit was brought to the neck through the posterior mediastinum, and side–side stapled esophago-gastric anastomosis was done by modified Collard technique.
Discussion
Situs inversus is a rare congenital anatomical deviation in which organs are mirror transposed from their original sites to the contralateral side of the body. It may involve the only transposition of abdominal organs or the thoracic viscera, and more frequently, both abdominal and thoracic viscera (SI totalis). SIT denotes an entire right-to-left transposition of abdominal and thoracic structures. SIT occurs in the frequency of 1 in 10,000 to 20,000 of total live births [1] and is presumed to originate from genetic deviation culminating in a reversal of polarity [2]. No conclusive causative agent has been recognized. Individuals with SIT typically live a normal life expectancy, and their condition often comes to light when a chest radiograph or ultrasonography for some other condition is done. Situs inversus per se carries no clinical consequences and does not escalate the risk of malignancies. Rarely, cardiac anomalies or bronchiectasis are associated, or Kartagener syndrome may coexist in individuals with SIT [3].
Because of its rarity, a surgeon may infrequently encounter a case of SIT requiring surgery in his career. Therefore, it is of vital importance that an advanced and thorough preparedness for surgery is required when an operative intervention is contemplated and related difficulties are predicted. An in-depth and thorough comprehension of anatomy and detailed preoperative planning are crucial for a surgical intervention. Preoperative radiology to delineate the various venous and arterial deviation assumes vital importance in such cases.
Yoshida et al. in 1992 reported a case of gastric cancer associated with situs inversus totalis treated by total gastrectomy [5]. Om et al. first detailed the usage of laparoscopic cholecystectomy in the patient with situs inversus totalis [4]. Mimae et al. reported a case of open esophagectomy in a patient with carcinoma esophagus with SIT [7]. Yoshida, Yagi, and Peel et al. then described successful cases of video-assisted thoracoscopic surgery esophagectomy with hand-assisted laparoscopic esophagectomy with gastric mobilization [6, 9, 10]. Palanivelu et al. had reported a complete minimally invasive McKeown procedure with a modified three-field lymphadenectomy [11]. Gopal Singh et al. described their experience with minimally invasive Ivor Lewis esophagectomy in SIT [12]. In line with the previous cases, the present study also highlights the successful completion of minimally invasive McKeown esophagectomy with operative details. Table 1 gives an account of patients of situs inversus totalis with esophageal cancer treated by surgery.
Table 1.
Details of patients with situs inversus totalis and esophageal cancer as reported in the literature
| Author/year | Age/sex | Site | Surgery | Conduit type | Route | Associated anomaly | |
|---|---|---|---|---|---|---|---|
| 1 | Yoshida et al. 2004 [6] | 57/M | Distal third | Left video-assisted thoracoscopic surgery/laparotomy | Gastric conduit | Posterior mediastinum | Nil |
| 2 | Mimae et al. 2008 [7] | 65/M | Middle third | Left thoracotomy and laparotomy | Gastric conduit | Posterior mediastinum | Nil |
| 3 | Aoki et al. 2011 [8] | 53/M | Distal third | Left thoracotomy and laparotomy | Gastric conduit | Posterior mediastinum | Nil |
| 4 | Yagi et al. 2012 [9] | 73/M | Mid third | Hybrid thoracoscopic/laparotomy | Gastric conduit | Intrathoracic | Nil |
| 5 | Peel et al. 2014 [10] | 67/M | Distal third | Left video-assisted thoracoscopic surgery | Gastric conduit | Posterior mediastinum | Kartagener syndrome |
| 6 | Palanivelu C et al. 2015 [11] | 62/M | Mid third | Minimally invasive McKeown procedure with a modified three-field lymphadenectomy | Gastric conduit | Posterior mediastinum | Nil |
| 7 | Gopal Singh et al. 2016 [12] | 65/M | GEJ | Minimally invasive Ivor Lewis surgery | Gastric conduit | Intrathoracic | Nil |
| 8 | Ujiie et al. 2016 [13] | 63/M | Mid third | Hybrid thoracoscopic/laparotomy | Gastric conduit | Posterior mediastinum | Nil |
| 9 | Ramanandham 2016 [14] | 54/M | Lower third and GEJ | Open transhiatal esophagectomy | Gastric conduit | Posterior mediastinum | Nil |
| 10 | Nakano et al. 2017 [15] | 82/M | Upper third | Hybrid thoracoscopic/laparotomy | Gastric conduit | NR | Nil |
| 11 | Nakano et al. 2017 [15] | 66/M | Mid third | Hybrid thoracoscopic/laparotomy | Gastric conduit | NR | Intestinal malrotation, polysplenia |
| 12 | Charalabopoulos et al. 2018 [16] | 49/M | Distal third | Hybrid thoracoscopic/laparotomy | Supercharged colon interposition | Posterior mediastinum | Nil |
| 13 | Present case | 58/M | Distal third | Hybrid thoracoscopic/laparoscopic assisted | Gastric conduit | Posterior mediastinum | Nil |
MIE, as compared to traditional open surgery, significantly reduced respiratory morbidity without compromising oncological outcomes [17, 18]. Minimally invasive surgery, which is itself a complex, challenging procedure, in the background of SIT is especially demanding attributed to unfamiliar reverse anatomical orientation. With careful preoperative planning, surgery can be successfully accomplished in individuals with SIT in experienced hands, especially in high-volume institutes. Except for a minor misfiring for a stapler, which required reinforcement of stapled line, we did not encounter any notable event intraoperatively, and the total operative time was 8 h, which was a bit longer than usual.
Conclusion
Minimally invasive esophagectomy in the context of situs inversus totalis is especially demanding ascribed to the unusual reverse orientation of anatomical structures. With a careful preoperative assessment and planning, surgery can be accomplished in individuals with SIT in experienced hands, especially in high-volume institutes.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Financial Support
The authors also declare no grants, honoraria, or financial supports from any agencies.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Syed Nusrath, Email: dr.nusrath2008@gmail.com.
S. Murtaza Ahmed, Email: syedmurtazaahmed@gmail.com
B. Madhunarayana, Email: madhu4smile@gmail.com
K. V. V. N. Raju, Email: drkvvnraju2002@yahoo.co.in
T. Subramanyeshwar Rao, Email: subramanyesh@gmail.com
Sujit Chyau Patnaik, Email: drsujit888@gmail.com.
References
- 1.Mayo CW, Rice RG. Situs inversus totalis: a statistical review of data on seventy-six cases with special reference to disease of the biliary tract. Arch Surg. 1949;58:724–730. doi: 10.1001/archsurg.1949.01240030734014. [DOI] [PubMed] [Google Scholar]
- 2.Niikawa N, Kohsaka S, Mizumoto M, Hamada I, Kajii T. Familial clustering of situs inversus totalis, and asplenia and poly splenia syndromes. Am J Med Genet. 1983;16:43–47. doi: 10.1002/ajmg.1320160108. [DOI] [PubMed] [Google Scholar]
- 3.Bohun CM, Potts JE, Casey BM, Sandor GG. A population-based study of cardiac malformations and outcomes associated with dextrocardia. Am J Cardiol. 2007;100:305–309. doi: 10.1016/j.amjcard.2007.02.095. [DOI] [PubMed] [Google Scholar]
- 4.Oms LM, Badia JM. Laparoscopic cholecystectomy in situs inversus totalis: the importance of being left-handed. Surg Endosc. 2003;17:1859–1861. doi: 10.1007/s00464-003-9051-7. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida Y, Saku M, Masuda Y, Maekawa S, Ikejiri K, Furuyama M. Total gastrectomy for gastric cancer associated with situs inversus totalis. A report of 2 cases. S Afr J Surg. 1992;30:156–158. [PubMed] [Google Scholar]
- 6.Yoshida T, Usui S, Inoue H, Kudo SE. The management of esophageal cancer with situs inversus totalis by simultaneous hand-assisted laparoscopic gastric mobilization and thoracoscopic esophagectomy. J Laparoendosc Adv Surg Tech. 2004;14(6):384–389. doi: 10.1089/lap.2004.14.384. [DOI] [PubMed] [Google Scholar]
- 7.Mimae T, Nozaki I, Kurita A, Takashima S. Esophagectomy via left thoracotomy for esophageal cancer with situs inversus totalis: report of a case. Surg Today. 2008;38(11):1044–1047. doi: 10.1007/s00595-008-3770-2. [DOI] [PubMed] [Google Scholar]
- 8.Aoki Y, Hihara J, Emi M, Sakogawa K, Hamai Y, Okada M. Advanced esophageal cancer with situs inversus totalis successfully treated with chemoradiotherapy followed by esophagectomy: case report. Hiroshima J Med Sci. 2011;60(1):21–24. [PubMed] [Google Scholar]
- 9.Yagi Y, Yoshimitsu Y, Maeda T, Sakuma H, Watanabe M, Nakai M, Ueda H. Thoracoscopic esophagectomy and hand-assisted laparoscopic gastric mobilization for esophageal cancer with situs inversus totalis. J Gastrointest Surg. 2012;16(6):1235–1239. doi: 10.1007/s11605-011-1789-y. [DOI] [PubMed] [Google Scholar]
- 10.Peel J, Darling G. Left video-assisted thoracoscopic surgery esophagectomy in a patient with situs inversus totalis and Kartagener syndrome. Ann Thorac Surg. 2014;98(2):706–708. doi: 10.1016/j.athoracsur.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 11.Chinusamy P, Bansal S, Praveenraj P, Ramakrishnan P. Minimally invasive McKeown esophagectomy with modified three-field lymphadenectomy in case of situs inversus totalis with carcinoma mid esophagus. J Minim Access Surg. 2016;12(1):68–70. doi: 10.4103/0972-9941.171994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh G, Costa J, Bessler M, Sonett J. Minimally invasive Ivor Lewis oesophago-gastrectomy in a patient with situs inversus totalis. Interact Cardiovasc Thorac Surg. 2016;22(2):235–237. doi: 10.1093/icvts/ivv299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ujiie N, Nakano T, Kamei T, Ichikawa H, Miyata G, Onodera K, Ohuchi N. Thoracoscopic esophagectomy for esophageal cancer with situs inversus totalis: a case report and literature review. Gen Thorac Cardiovasc Surg. 2016;64(6):359–362. doi: 10.1007/s11748-016-0639-y. [DOI] [PubMed] [Google Scholar]
- 14.Ramanandham B. Transhiatal oesophagectomy for a patient with carcinoma oesophagus and situs inversus totalis—a unique case report. Univ J Surg Surg Spec. 2016;19:2(3). [Google Scholar]
- 15.Nakano T, Kamei T, Onodera Y, Ujiie N, Ohuchi N. Thoracoscopic surgery in the prone position for esophageal cancer in patients with situs inversus totalis: a report of two cases. Int J Surg Case Rep. 2017;31:43–46. doi: 10.1016/j.ijscr.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charalabopoulos A, Kordzadeh A, Sdralis E, Lorenzi B, Ahmad F. Thoracoscopic total esophagogastrectomy with supercharged colon interposition for the treatment of esophageal adenocarcinoma in situs inversus. Acta Chir Belg. 2019;119(4):259–262. doi: 10.1080/00015458.2018.1438562. [DOI] [PubMed] [Google Scholar]
- 17.Biere SS, Maas KW, Bonavina L, Garcia JR, van Berge Henegouwen MI, Rosman C, Sosef MN, De Lange ES, Bonjer HJ, Cuesta MA, Van Der Peet DL. Traditional invasive vs. minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial) BMC Surg. 2011;11(1):2. doi: 10.1186/1471-2482-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straatman J, Van Der Wielen N, Cuesta MA, Daams F, Garcia JR, Bonavina L, Rosman C, van Berge Henegouwen MI, Gisbertz SS, Van Der Peet DL. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial the TIME trial. Ann Surg. 2017;266(2):232–236. doi: 10.1097/SLA.0000000000002171. [DOI] [PubMed] [Google Scholar]