Abstract
This study assessed the responses of vitamin-D3 intraperitoneally injected to Rohu, Labeo rohita @ of 0 IU/kg bw (only solvent), 100 IU/kg bw and 500 IU/kg bw reared in 20 and 40 ppm of calcium (Ca) enriched water. The cellular changes in Corpuscles of Stannius (CS) gland, serum Ca, and inorganic phosphate (Pi) level were analysed up to the 60th day. Rohu administered with 100 IU/kg bw D3 and exposed to 40 ppm Ca-rich water exhibited notable hyperplasia of CS compared with their control groups. Notable changes with high serum Ca level (13.87 ± 0.3 mg/dl) was detected on the 5th day in fish exposed to 40 ppm Ca-rich water, while related values attained (13.74 ± 0.1 mg/dl) only after 7 days in 20 ppm Ca-rich water of 500 IU/kg bw vitamin D3 injection. Similarly, high serum Pi level (7.66 ± 0.2 mg/dl) in 40 ppm Ca injected with D3 at 500 IU/kg bw. The results demonstrated that the Ca homeostasis of Labeo rohita is influenced by intra-peritoneal vitamin D3. Progressive studies should be conducted by increasing the dose of vitamin D3 to investigate optimum dose/supplement in feed for commercially important aquaculture teleost Labeo rohita for maximum and sustainable absorption of Ca from the variable water Calcium levels to maintain Ca2+ homeostasis.
Keywords: Labeo rohita, Vitamin D3, Ca enriched water, Corpuscles of Stannius, Serum Ca, Inorganic phosphorus
1. Introduction
Freshwater aquaculture in India is dominated by Indian Major Carps (Labeo rohita, Catla catla and Cirrhinus mrigala), by contributing about 87% of the total freshwater fish production (ICLARM, 2001). Among the IMC, Labeo rohita is being widely cultured throughout India and accounts for a majority of the production, and have a great potential in terms of biodiversity as well as consumer preferences.
Corpuscle of Stannius (CS) is endocrine tissue secretes hypocalcemic factor(s), Stanniocalcin to prevent hypercalcemia by reducing branchial and whole body Ca uptake (Fontaine, 1964). Many workers (Fenwick, 1976, Fenwick and Forster, 1972, Wendelaar Bonga and Greven, 1978, Pang et al., 1975). Olivereau, 1964, Johnson, 1972, Gu et al., 2015 reported that CS is more active in seawater (rich in calcium) than in freshwater (poor in calcium) and inferred that calcium content of the surrounding water has a direct impact on the CS activity and transcriptomic responses of CS. Suryawanshi and Mahajan, 1976, Ahmad and Swarup, 1979 have also reported that the activity of CS increases in the fishes kept in calcium-rich freshwater. In past years, a steady flow of papers has advanced evidence that Ca2+ influx in branchial, intestinal (and likely in renal Ca2+ transporting cells) is controlled by stanniocalcin (STC) (Buttler, 1993, Flik et al., 1993, Wendelaar Bonga and Pang, 1986a). STC is a hormone produced by the so-called CS endocrine gland. In gill and intestine STC inhibits Ca2+ entry (Flik et al., 1993, Verbost et al., 1993) and it thus, controls the permeability of Ca2+ of the apical membrane through a secondary messenger (cAMP) dependent pathway most likely a Camp operated calcium channel (Verbost et al., 1993). In the complex epithelium of the gills’ chloride cells, specialized ion transporting cells mediated Ca2+ uptake (Flik et al., 1995, McCormic, 1993, Perry et al., 1992). Stanniocalcin is a glycoprotein hormone important in the maintenance of Ca and Pi homeostasis in fish. Two mammalian related stanniocalcin genes, STC1 and STC2, were found to be expressed in various tissues of fish as paracrine regulators (Luo et al., 2005, Joshi, 2020). Although Stannniocalcin-1 (STC-1) was originally described in fish, it is now known to be present throughout the animal kingdom in both vertebrates and invertebrates (Ishibashi and Imai, 2002, Yoshiko and Aubin, 2004, Tanega et al., 2004, Richards et al., 2012, Palma et al., 2019). The principal sources of STC-1 in bony fish are endocrine glands known as the Corpuscles of Stannius (CS) which are anatomically associated with the kidneys. STC-1 release is stimulated by a rise in serum levels of ionic Ca above the physiological set point through the activation of Ca-sensing receptors (Richards et al., 2012). The hormone then exerts regulatory effects on the epithelial transport of Ca and/or phosphate across the gills, gut, and kidneys to restore normocalcemia as the inhibitory action of STC reduces mucosa to serosa calcium transport, which means that the uptake in gills and intestine and re-uptake from ultra-filtered plasma in the nephron is controlled (Flik and Verbost, 1996). The transcriptomic responses of corpuscle of Stannius gland of Japanese eels (Anguilla japonica) to Changes in Water Salinity are recently reported by Gu et al (2015). The effects of Euphorbia royleana on the alteration of CS of Heteropneustes fossilis are demonstrated by Prasad et al. (2017).
Administration of vitamin D3 and its derivative had been demonstrated in many fishes to make it hypercalcemic and to know the changes in serum Ca and Pi level (Srivastav and Srivastav, 1988, Srivastav and Singh, 1992). Although vitamin D3 is abundantly present in fish liver, its role in Ca homeostasis has been emphatically denied (Rao and Raghuramulu, 1995). It has been reported that vitamin D3 and its metabolites induce hypercalcemia in fishes (Singh and Srivastav, 1996, Swarup and Srivastav, 1982, Srivastav et al., 1985, Srivastav et al., 1998, Hayes et al., 1986, Srivastav and Singh, 1992). However, there is not single literature on the effect of vitamin D3 on the physiological response to Indian major Carps, the most important fishes for aquaculture in India and other neighbouring Asian countries, and changes in serum Ca and Pi level. This study clearly explains the histological change and efficiency of CS and its hormone Stanniocalcin in freshwater fish Labeo rohita. Thus the objective of the present study is to assess the response and behavior of Ca and Pi regulating endocrine gland, Corpuscles of Stannius (CS) in Labeo rohita, most cultured fish in aquaculture system when reared in two levels of Ca enriched water exposed to intra-peritoneal doses of vitamin D3.
2. Materials and methods
2.1. Experimental fish
The experiment was conducted for 60 days at the wet laboratory of ICAR - Central Institute of Fisheries Education (Deemed University), Mumbai. Healthy Labeo rohita (weight: 30 ± 2 g;) fishes were brought from Mahad fish farm, Mumbai in oxygen-filled polythene bags to CIFE, Mumbai. The fish stock was acclimatized for one week in calcium-deficient water under an aerated condition with a basal diet.
2.2. Experimental setup
The experimental setup consists of 18 uniform size plastic rectangular tanks (80 cm × 57 cm × 42 cm, 150 L capacity) covered with perforated lids. Two hundred and sixteen fish were randomly and equally distributed and stocked into experimental tanks with a 2 × 3 factorial design in triplicates. The calcium level in water is enriched using Calcium chloride (CaCl2·2H2O) and the experimental fish were stocked @12 fish/tank into 2 groups, namely group A – 20 ppm Ca, and group B − 40 ppm Ca enriched water. The level of Ca in the experimental tank is measured using EDTA titration method (APHA, 1998). Similarly, water quality parameters like pH, Dissolved Oxygen, Ammonia, Nitrite, Nitrate, and Temperature were estimated periodically as per APHA (1998) and maintained throughout the experiment.
2.3. Intra-peritoneal injection
After stocking fish from both groups were injected intraperitoneally i.e., in the abdomen at a 45° angle between the pelvic fins and anal vent with vitamin D3 at different doses of, (i) (0.0 IU/kg bw only solvent, Arachis oil) (ii) (100 IU/kg bw) and (iii) (500 IU/kg bw). By considering 0.2 ml Vitamin D3 injection for 25 g size fish, the 3Lakh IU Arachitriol (oil-based) was diluted with Arachis oil. Out of 9 tanks from group A with 20 ppm Ca water, 3 tanks were given Vitamin D3 Intraperitoneal injection @ the dose of 0 IU/kg bw (control, only 0.2 ml solvent, Arachis oil), 3 tanks were given Vitamin D3 Intraperitoneal injection @ the dose of 100 IU/kg bw and 3 tanks were given Vitamin D3 Intraperitoneal injection @ the dose of 500 IU/kg bw respectively. A, similar setup is arranged for group B with 40 ppm Ca-rich water. The controls were given only solvent (without vitamin D3 to give similar conditions in control and experimental fishes of both the treatments A & B), thus, the administration of Vitamin D3 was not done to the control group. The doses 100 IU/kg and 500 IU/kg is at par with many authors used in their studies (Swarup and Srivastav, 1982, Swarup and Norman, 1996, Singh and Srivastav, 1996, Srivastav et al., 1998).
2.4. Fish sampling and serum analysis
Sampling was done on Day 1, 2, 3, 5, 7, 9, 11, 13, 15, 30, and 60. In each sampling, one fish from each replicate was sacrificed during the study period and taken for ionic activity, and histological studies. Serum samples were taken by collecting blood in vials using a sterile syringe and they are kept aside without any disturbance for separation of serum from a blood clot. Then the vials were centrifuged for perfect separation of serum without any hemolysis to estimate Ca and inorganic phosphate (Pi) level using Trinder, 1960, Fiske and Subbarow, 1925 methods respectively.
2.5. Histological studies
The Corpuscle of Stannius along with the adjoining portion of the kidney was removed from the fish of each treatment group and they were fixed in aqueous Bouin’s fluid. The fixed samples were processed by standard techniques following a series of alcohol treatment, cleared in xylene, and embedded in paraffin wax. Thin (5 μm) sections were prepared with a microtome and stained with Haematoxylin/Eosin (HE).
2.6. Statistical analysis
The results of each experiment are expressed as mean ± standard deviation and analysed by multivariate analysis of variance (ANOVA) using statistical package SPSS version 16 to test the significance of the difference between the control and experimental groups. A probability level of 0.05 was used to find out the significance in all cases.
3. Results
3.1. Change in serum Ca and inorganic phosphate (Pi)level and cellular changes of Corpuscle of Stannius in two different Ca enriched groups
3.1.1. Control group (A and B)
The serum Ca and Pi level was observed on days; 0, 1st, 2nd, 3rd, 5th, 7th, 9th, 11th, 15th, 30th, and 60th day. And there was no rise in serum Ca and Pi levels from day 1 to day 60 (Table 1, Fig. 1, Fig. 2, Fig. 3, Fig. 4). The corpuscles of Stannius also possess oval or rounded nuclei without any cellular changes (Fig. 5, Fig. 6).
Table 1.
Serum Ca concentration (mg/dl) of L. rohita injected with graded level of Vitamin D3 and reared in group A and B.
| Sr. No. | Day | Control (A) 20 ppm Ca |
Exp.(AL) 100 IU/kg b.w. |
Exp.(AH) 500 IU/kg b.w. |
Control(B) 40 ppm Ca |
Exp.(BL) 100 IU/kg b.w. |
Exp.(BH) 500 IU/kg b.w. |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 8.42 ± 0.4 | 8.44a ± 0.2 | 8.47a ± 0.6 | 8.38 ± 0.2 | 8.39 a ± 0.4 | 8.45a ± 0.4 |
| 2 | 1 | 8.54 ± 0.6 | 8.79a ± 0.5 | 8.92a ± 0.4 | 8.47 ± 0.3 | 8.88 a ± 0.2 | 8.99a ± 0.2 |
| 3 | 2 | 8.52 ± 0.3 | 10.55b ± 0.4 | 12.36b ± 0.2 | 8.49 ± 0.4 | 10.78b ± 0.3 | 11.76b ± 0.3 |
| 4 | 3 | 8.49 ± 0.3 | 10.59b ± 0.1 | 12.98b ± 0.6 | 8.46 ± 0.6 | 10.79b ± 0.4 | 13.55c ± 0.2 |
| 5 | 5 | 8.47 ± 0.1 | 11.45c ± 0.4 | 13.05b ± 0.5 | 8.40 ± 0.2 | 12.48c ± 0.5 | 13.87c ± 0.3 |
| 6 | 7 | 8.48 ± 0.2 | 10.26b ± 0.2 | 13.74c ± 0.1 | 8.37 ± 0.4 | 11.48c ± 0.7 | 13.80c ± 0.7 |
| 7 | 9 | 8.55 ± 0.7 | 9.78a,b ± 0.3 | 12.28b ± 0.2 | 8.44 ± 0.3 | 9.24a,b ± 0.2 | 12.72b ± 0.6 |
| 8 | 11 | 8.37 ± 0.5 | 8.75a ± 0.4 | 9.98a ± 0.3 | 8.42 ± 0.2 | 8.94a ± 0.5 | 10.02b ± 0.4 |
| 9 | 15 | 8.43 ± 0.4 | 8.41a ± 0.1 | 8.49a ± 0.4 | 8.38 ± 0.5 | 8.66a ± 0.2 | 9.06a ± 0.1 |
| 10 | 30 | 8.45 ± 0.7 | 8.36a ± 0.5 | 8.47a ± 0.6 | 8.33 ± 0.1 | 8.74a ± 0.3 | 8.54a ± 0.1 |
| 11 | 60 | 8.49 ± 0.2 | 8.23a ± 0.3 | 8.38a ± 0.4 | 8.40 ± 0.4 | 8.43a ± 0.2 | 8.48a ± 0.2 |
Different superscript in a column differ significantly at 95% confidence limit (P < 0.05) A = Control group (20 ppm Ca) with 0.0 IU Vitamin D3/kg bw (only solvent); AL = Group with low dose IP, 100 IU Vitamin D3/kg bw; AH = Group with high dose IP, 500 IU Vitamin D3/kg bw; B = Control group (40 ppm Ca) with 0.0 IU Vitamin D3/kg bw (only solvent); BL = Group with low dose IP, 100 IU Vitamin D3/kg bw; BH = Group with high dose IP, 500 IU Vitamin D3/kg bw.
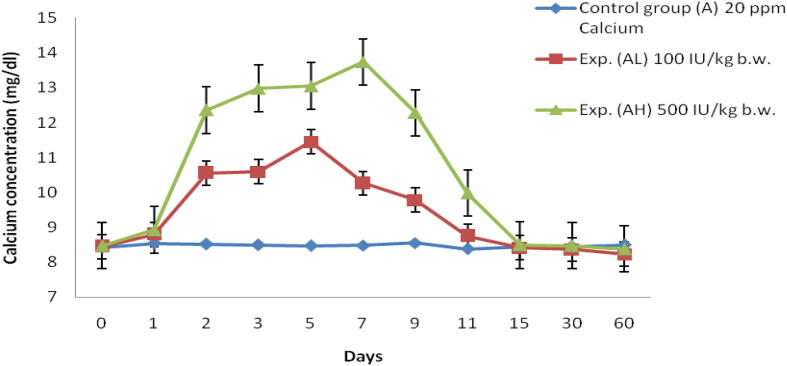
Fig. 1.
Serum Ca concentration (mg/dl) of Labeo rohita injected with graded levels of vitamin D3 and reared in group A (20 ppm Ca).
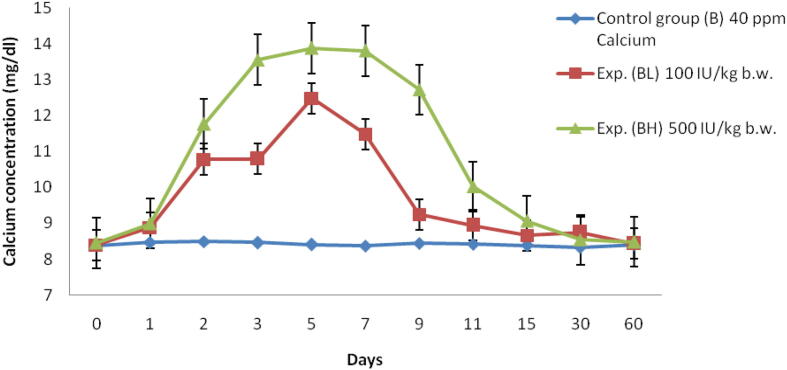
Fig. 2.
Serum Ca concentration (mg/dl) of Labeo rohita injected with graded levels of vitamin D3 and reared in group B (40 ppm Ca).
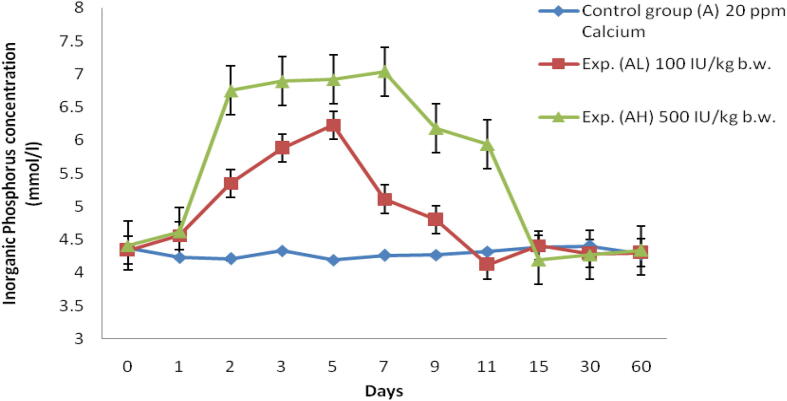
Fig. 3.
Serum inorganic phosphorus concentration (mmol/l) of Labeo rohita injected with graded level of vitamin D3 and reared in group A (20 ppm Ca).
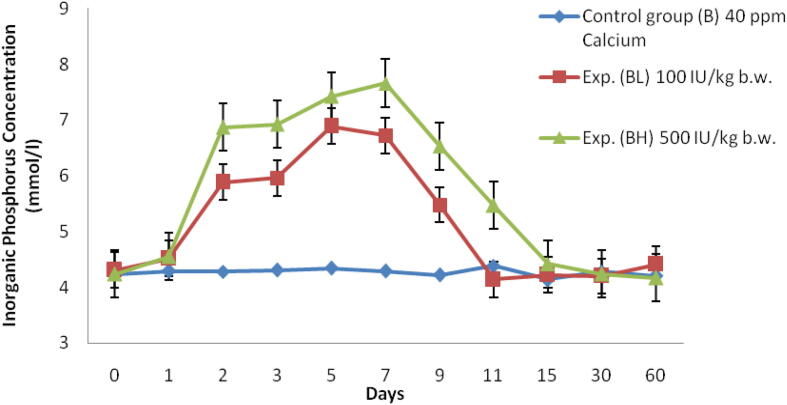
Fig. 4.
Serum inorganic phosphorus concentration (mmol/l) of Labeo rohita injected with graded level of vitamin D3 and reared in group B (40 ppm Ca).
Fig. 5.
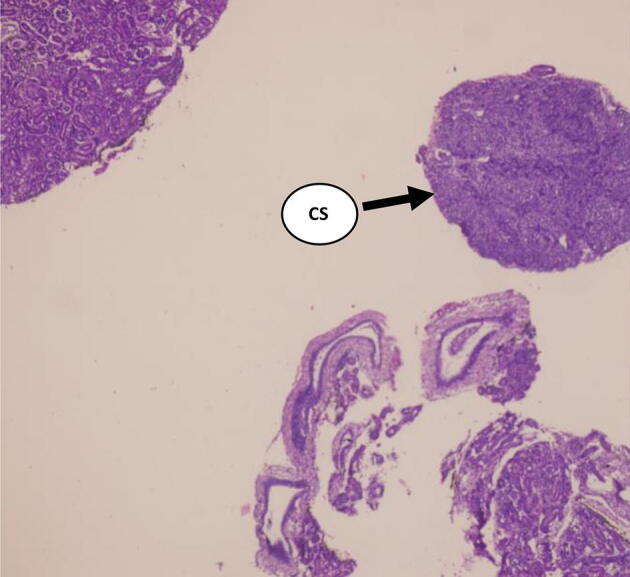
Section of Corpuscles of Stannius (CS) in the fish, Labeo rohita in Control group A (H and E 4X) Day – 0 (Ca 20 ppm and 0.0 IU D3).
Fig. 6.
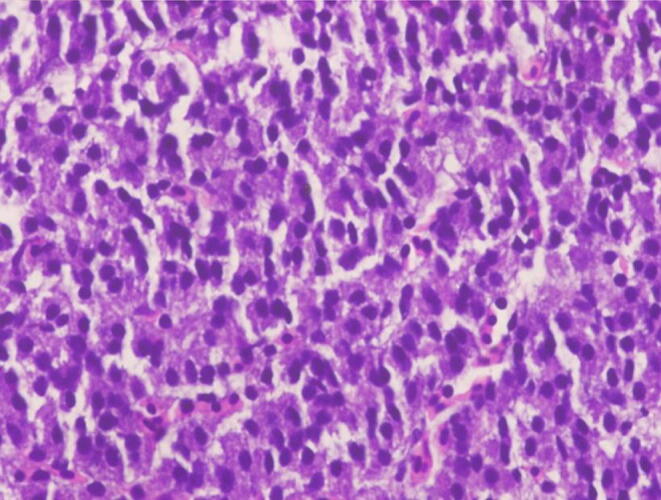
Section of Corpuscles of Stannius in the fish, Labeo rohita in Control Group A (H and E 60X) Day – 0 (Ca 20 ppm and 0.0 IU D3).
3.2. Group A (20 ppm Ca) – experimental group, AL and AH
3.2.1. Calcium analysis
In low dose (100 IU/kg bw) there is an increase in Ca level and reached the peak at 5th day up to 11.45 ± 0.4 mg/dl and again started decreasing till 60th day (Table 1). At the same time for high dose (500 IU/kg bw) the uptake of Ca is higher than low dose and reached maximum on 7th day at a range of 13.74 ± 0 mg/dl. and again it reduced till 60th day (Fig. 1).
3.2.2. Inorganic phosphate analysis
In low dose (100 IU/kg bw) there is an increase in Pi level and reaching the peak at 5th day up to 6.23 ± 0.5 mmol/l and again started decreasing till 60th day (Table 2). Similarly for high dose (500 IU/kg bw) the uptake of Pi was higher than the low dose and reached maximum on 7th day at a range of 7.04 ± 0.2 mmol/l and again it reduced till 60th day (Fig. 3).
Table 2.
Serum Inorganic phosphorus concentration (mmol/l) of L.rohita injected with graded level of Vitamin D3 and reared in group A and B.
| Sr. No. | Day | Control (A) 20 ppm Ca |
Exp.(AL) 100 IU/kg b.w. |
Exp.(AH) 500 IU/kg b.w. |
Control (B) 40 ppm Ca |
Exp.(BL) 100 IU/kg b.w. |
Exp.(BH) 500 IU/kg b.w. |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 4.37 ± 0.2 | 4.34a ± 0.1 | 4.41a ± 0.2 | 4.24 ± 0.3 | 4.31a ± 0.2 | 4.24a ± 0.4 |
| 2 | 1 | 4.23 ± 0.1 | 4.56a ± 0.3 | 4.62a ± 0.2 | 4.29 ± 0.2 | 4.52a ± 0.2 | 4.55a ± 0.1 |
| 3 | 2 | 4.21 ± 0.2 | 5.35b ± 0.2 | 6.75b ± 0.1 | 4.28 ± 0.1 | 5.88b ± 0.3 | 6.87b ± 0.1 |
| 4 | 3 | 4.33 ± 0.4 | 5.88b ± 0.2 | 6.89b ± 0.3 | 4.31 ± 0.2 | 5.96b ± 0.1 | 6.92b ± 0.2 |
| 5 | 5 | 4.19 ± 0.2 | 6.23c ± 0.5 | 6.92b ± 0.4 | 4.34 ± 0.3 | 6.89c ± 0.2 | 7.42b ± 0.3 |
| 6 | 7 | 4.26 ± 0.1 | 5.11b ± 0.3 | 7.04c ± 0.2 | 4.29 ± 0.3 | 6.72b ± 0.5 | 7.66c ± 0.2 |
| 7 | 9 | 4.27 ± 0.2 | 4.80a ± 0.4 | 6.18b ± 0.1 | 4.22 ± 0.2 | 5.48a ± 0.1 | 6.53b ± 0.1 |
| 8 | 11 | 4.32 ± 0.3 | 4.12a ± 0.2 | 5.94a ± 0.1 | 4.39 ± 0.1 | 4.14a ± 0.3 | 5.47a ± 0.2 |
| 9 | 15 | 4.39 ± 0.1 | 4.41a ± 0.2 | 4.19a ± 0.2 | 4.41 ± 0.2 | 4.22a ± 0.3 | 4.42a ± 0.1 |
| 10 | 30 | 4.40 ± 0.2 | 4.29a ± 0.3 | 4.27a ± 0.4 | 4.28 ± 0.3 | 4.20a ± 0.2 | 4.24a ± 0.2 |
| 11 | 60 | 4.28 ± 0.4 | 4.30a ± 0.1 | 4.34a ± 0.2 | 4.20 ± 0.3 | 4.41a ± 0.3 | 4.17a ± 0.3 |
Different superscript in a column differ significantly at 95% confidence limit (P < 0.05) A = Control group (20 ppm Ca) with 0.0 IU Vitamin D3/kg bw (only solvent); AL = Group with low dose IP, 100 IU Vitamin D3/kg bw; AH = Group with high dose IP, 500 IU Vitamin D3/kg bw; B = Control group (40 ppm Ca) with 0.0 IU Vitamin D3/kg bw (only solvent); BL = Group with low dose IP, 100 IU Vitamin D3/kg bw; BH = Group with high dose IP, 500 IU Vitamin D3/kg bw.
3.2.3. Histological analysis
Cellular structures are shown in Fig. 7, Fig. 8 on day- 5 showing the nuclear volume of cells records an increase and they become partially de-granulated as is evident by their weak staining response. There was an increased dilatation of sinusoids (Fig. 8) and these changes get exaggerated. The results of cellular activities are in correspondence with the serum levels of Ca and inorganic phosphate and demonstrating that there was the hypocalcemic response of CS gland.
Fig. 7.
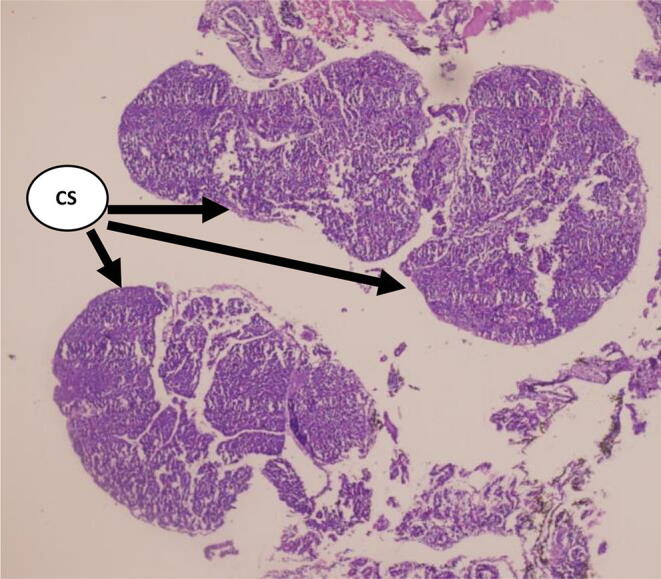
Section of Corpuscles of Stannius in the fish, Labeo rohita in Experimental group AL (H and E 4X) Day-5 (Ca 20 ppm and 100 IU D3).
Fig. 8.
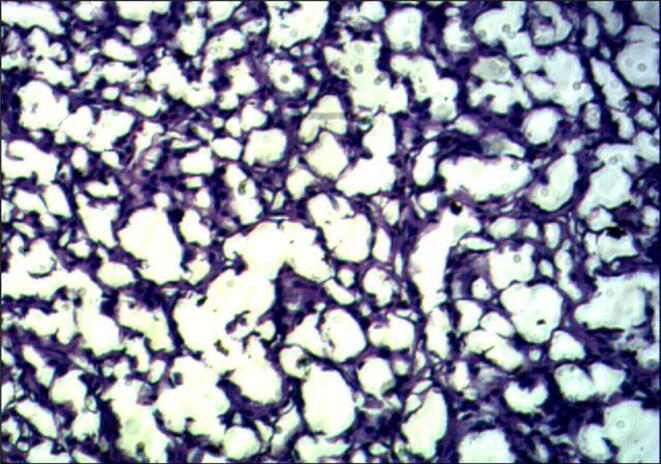
Section of Corpuscles of Stannius in the fish, Labeo rohita in Experimental group AL (H and E 60X) Day-5 (Ca 20 ppm and 100 IU D3).
3.3. Group B (40 ppm Ca) – experimental group, BL and BH
3.3.1. Calcium analysis
In low dose (100 IU/kg bw) there was an increase in Ca level and reaching peak at 5th day up to 12.48 ± 0.5 mg/dl and started decreasing till 60th day (Table 1, Fig. 2). At the same time for high dose (500 IU/kg bw) the uptake of Ca was higher than the low dose and reached maximum on 5th day at a range of 13.87 ± 0.3 mg/dl and it reduced till 60th day (Fig. 2).
3.3.2. Inorganic phosphate analysis
In low dose (100 IU/kg bw) there was an increase in Pi level and reaching peak at 5th day up to 6.89 ± 0.2 mmol/l and started decreasing till 60th day (Table 2, Fig. 4). At the same time for high dose (500 IU/kg bw) the uptake of Pi was higher than the low dose and reached maximum on 7th day at a range of 7.66 ± 0.2 mmol/l and it reduced to normal condition as in case of control till 60th day (Fig. 4).
3.3.3. Histological analysis
The cellular structures are shown in Fig. 9, Fig. 10 on day-7 showing the nuclear volume of cells records an increase and they become partially de-granulated as is evident by their weak staining response. Also, there is an increased dilatation of sinusoids (Fig. 10) and these changes get further exaggerated and complete exhaustion of the gland was recorded.
Fig. 9.
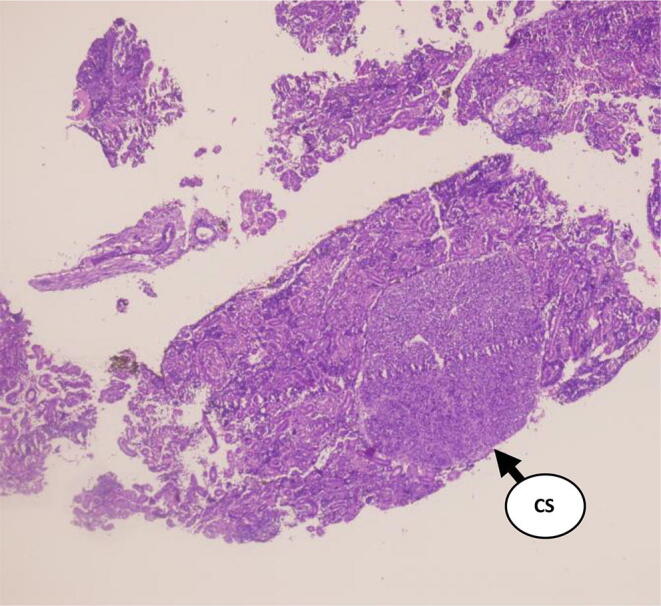
Section of Corpuscles of Stannius in the fish, Labeo rohita in Experimental group BH (H and E 4X) Day-7 (Ca 40 ppm and 500 IU D3).
Fig. 10.
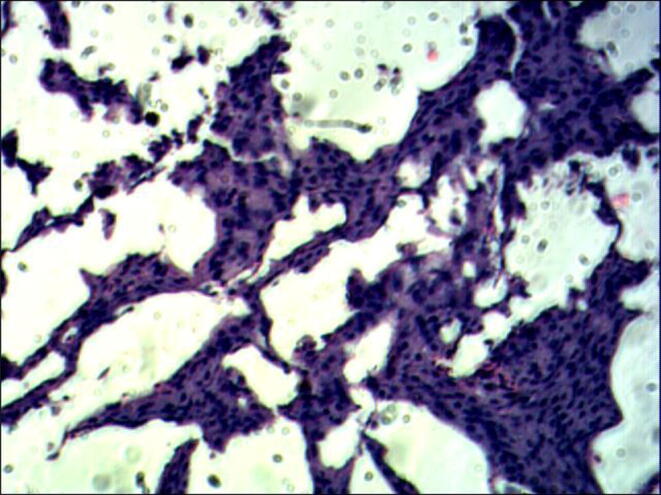
Section of Corpuscles of Stannius in the fish, Labeo rohita in Experimental group BH (H and E 60X) Day – 7 (Ca 40 ppm and 500 IU D3).
4. Discussion
4.1. Serum Ca concentration
Serum Ca concentration level in Labeo rohita is calculated and it is found to be higher on the 5th day of fish, which is reared in group A with a high dose (500 IU/kg bw) of vitamin D3. At the same time, it is found to be higher on the 7th day of fish which is reared in group B with high dose (500 IU/kg bw) of vitamin D3. Srivastav et al (1997a), who also observed vitamin D metabolites affect the serum Ca level in freshwater catfish Heteropneustes fossilis, in which there is an increase in serum Ca level at the day of 3 and 5, which were injected with intraperitoneal Vitamin D3 and Srivastav et al. (1997b) also done a similar experiment in freshwater mud eel Amphipnous cuchia and reported that there is an increase in serum Ca level at day 10 when it is reared in Ca-rich environment and injected with 100 ng of vitamin D3 for 100 g bw/day. Bansal et al. (1979) showed increased serum Ca levels in Labeo rohita due to chronic chlordane exposure. This result shows similarity to the experiment done by Srivastava et al. (2012) in which the fish Notopterus notopterus treated with three level of vitamin D3 dosage like 100, 500, 1000 IU/kg bw. In this, there is a peak levels of serum Ca is found on day 5 on the three levels. But the amount or level of Ca intake varies with the dosage. The dosage with 1000 IU/kg bw shown higher absorption of Ca from the environment which is followed by 500 and 100 IU. A number of authors studied hypercalcemic effects of vitamin D3 metabolites (Singh and Srivastav, 1996, Swarup and Srivastav, 1982, Srivastav et al., 1985, Srivastav et al., 1998, Hayes et al., 1986, Srivastav and Singh, 1992), and showed that hypercalcemia depends on exposure time as well as on the type and concentration of the vitamin D3 metabolite used (Swarup et al., 1984, Srivastav et al., 1993). Responses to vitamin D treatments vary not only within but also among species. For example, injecting 1,25(OH)2D3 in emerald rock cod (Pagothenia bernacchii) reduced free plasma Ca but left total plasma Ca levels unchanged, suggesting an increased fractional binding of Ca to plasma proteins (Fenwick et al., 1984).
In contrast, Sundell and Norman (1993) repeatedly injected 1,25(OH)2D3 in the Atlantic cod and observed an increase of free Ca while total Ca levels remained unchanged. In male Mozambique tilapia (O. mossambicus) IP injections of 1,25(OH)2D3 increased total plasma Ca without altering the free Ca levels (Srivastav et al., 1998). Vitamin D3 injected male catfish (Clarias batrachus) acclimated to low Ca water increased their serum total Ca levels as compared with control fish. However, this increase doubled in vitamin D3 injected catfish from water supplemented with extra Ca when compared to controls from the same water, although the duration of this increase was shorter than the one in fish from low Ca water (Swarup and Srivastav, 1982). A similar observation was performed in freshwater mud eel, Amphipnous cuchia (Srivastav, 1983). Magnitude and duration of the increase of plasma Ca in response to vitamin D3 are dependent on the Ca concentration in the water (Srivastav and Srivastava, 1997). If Ca is not sufficiently available from the water or the diet, then fish can supplement plasma Ca from internal sources. Intra-peritoneal injections of unfed common carp with physiological doses of either vitamin D3 or 1,25(OH)2D3 resulted in hypercalcemia and hyperphosphatemia (Swarup et al., 1991), which suggests that the minerals must have been derived from internal sources. Similarly, daily injections with vitamin D3 or 1, 25(OH)2D3 in fed American eel (A. rostrata), increased plasma Ca and phosphorus, while this effect was absent in unfed eels (Fenwick et al., 1984). The plasma Ca levels in the fish are actually not controlled and/or regulated by the endocrine system only; other hormones like stanniocalcin (Pierson et al., 2004), parathyroid hormone and related protein (Guerreiro et al., 2007, Abbink and Flik, 2007), and the prolactin (Flik et al., 1984, Flik et al., 1989, Seale et al., 2006) are involved in the control mechanism as well.
4.2. Serum inorganic phosphate (Pi) concentration
In this experiment serum phosphorus concentration level in Labeo rohita is calculated and it is found to be higher on the 7th day of fish which is reared in group A with a high dose (500 IU/kg bw) of vitamin D3. At the same time, it is found to be higher on the 7th day of fish which is reared in group B with a high dose (500 IU/kg bw) of vitamin D3. Srivastav et al (1997a) who also observed vitamin D metabolites affect the serum phosphorus level in freshwater catfish (Heteropneustes fossilis), in which there is an increase in serum phosphorus level from day 3 to 10, and showing a hyperphophatemic reaction which was injected with intraperitoneal Vitamin D3. Srivastav et al. (1997b) done a similar experiment in freshwater mud eel Amphipnous cuchia and reported that there is an increase in serum phosphorus level at 5th, when it is reared in Ca-rich environment and injected with 100 ng of vitamin D3 for 100 g bw/day.
In contrast to Ca, fish must obtain phosphate via the diet as water phosphate levels are normally very low, and direct uptake of phosphate from the water is likely insignificant in fishes. Little information on the involvement of vitamin D3 in phosphate metabolism in fish exists. Responses to vitamin D3 metabolites on plasma phosphate vary between species. Daily intraperitoneal injection with vitamin D3 or 1, 25(OH)2D3 increase plasma phosphate in catfish (C. batrachus) (Swarup et al., 1984), American eel (Fenwick et al., 1984) and C. carpio (Swarup et al., 1991), but not in Mozambique tilapia (Rao and Raghuramulu, 1995). In unfed American eel (Fenwick et al., 1984) and freshwater mud eel, A. cuchia (Srivastav, 1983) plasma phosphate increased after intra-peritoneal vitamin D3 injection. Apparently, in addition to phosphate reabsorption in the kidney (Fenwick and Vermette, 1989), it can be mobilized by vitamin D3 metabolites from a non-dietary source, presumably bone or soft tissues (Lopez et al., 1977).
4.3. Histology of Corpuscles of Stannius (CS)
In the present study, Labeo rohita injected with vitamin D3 and kept in two different calcium environment exhibits degranulation of the CS cells by increase in the volume of the cells and sinusoidal dilatation. Earlier workers have considered hypertrophy of CS cells as an indication of the activity of CS in response to hypercalcemia (Olivereau and Olivereau, 1978, Srivastav et al., 1985, Srivastav and Srivastav, 1988). The volume and density of CS cells increase as a result of external Ca concentration (Urasa and Wendelaar Bonga, 1987). Suryawanshi and Mahajan, 1976, Ahmad and Swarup, 1979 have also reported that the activity of CS increases in the fishes kept in calcium-rich freshwater. In Labeo rohita hypercalcemia results in degranulation of AF-positive cells. Aida et al. (1980) have suggested that the secretory activity of cells of CS may be directly affected by plasma ion levels, especially Ca ions. The present study supports this suggestion and also it agrees with the observations of Wendelaar Bonga et al (1980). According to Wendelaar Bonga et al (1980) the type-1 cells of CS are more active in the fish adapted to diluted or full-strength seawater than in freshwater specimens. For this response they have stated that the high activity of this cell in seawater is apparently due to the high Ca concentration of seawater. The degranulation of the cells of CS of Labeo rohita can be attributed to the increased release of hypocalcemic factor (Stanniocalcin) from CS to encounter the elevated level of Ca caused by vitamin D3 treatment. The sinusoidal dilatations in response to hypercalcemia in Labeo rohita is similar to the observations on Clarias batrachus (Srivastav et al., 1985, Srivastav and Srivastav, 1988). The degeneration among few corpuscular cells observed in Labeo rohita in response to prolonged hypercalcemia is in agreement with the results obtained by Hiroi (1970) on Oncorhynchus sp, Srivastav et al., 1985, Srivastav and Srivastav, 1988 on Clarias batrachus and histological and ultrastructure studies of CS in African catfish, Clarias gariepinus (Karkit et al., 2019). Advanced evidence that Ca2+ influx in branchial, intestinal (and likely in renal Ca2+ transporting cells) is controlled by stanniocalcin (STC) (Buttler, 1993, Flik et al., 1993, Wendelaar Bonga and Pang, 1986a).
The degeneration is due to the exhaustion of corpuscular cells. In the CS of Labeo rohita kept in low Ca freshwater there is an increased storage of granules. This can be attributed to the observed decrease in the serum Ca and inorganic phosphate levels. Hypoactive cells have been noticed in the fish expose to low-Ca seawater (Wendelaar Bonga et al., 1980). Storage of secretory granules within calcitonin cells (which secrete a hypocalcemic factor in mammals) in response to hypocalcemia has also been reported earlier by Gittes et al., 1968, Srivastav and Swarup, 1982. In mammals, it has been suggested by Hirsch and Munson (1969) that the heavy accumulation of secretory granules in calcitonin cells during hypocalcemia results due to little or no calcitonin secretion and continuance of its biosynthesis. In the present study, the same principle seems to be involved. In a freshwater medium, there is no change in the cells of CS after vehicle-injected fish. This may be due to the non-involvement of this cell type in Ca homeostasis as according to Wendelaar Bonga et al., 1976, Wendelaar Bonga et al., 1980. In Ca-rich medium the cells of CS of fish and Ca/Pi levels after vehicle or vitamin D3-treatment exhibit a decrease in the volume. This conforms with the reports of Wendelaar Bonga et al., 1976, Wendelaar Bonga et al., 1980, Meats et al., 1978 who have reported indications for a reduction of secretory activity of CS from fish transferred from freshwater to seawater. Buttler, 1993, Flik et al., 1993, and Wendelaar Bonga and Pang, 1986a, Wendelaar Bonga and Pang, 1986b had reported Ca2+ influx in branchial, intestinal, renal Ca2+ transporting cells is controlled by stanniocalcin in marine fishes.
5. Conclusion
The hypercalcemic and hyperphophatemic responses were more elevated in a higher doses of vitamin D3 when fishes are reared in both the concentrations of calcium levels. Further, it is observed that the normocalcemic and normophosphatemic responses were faster in a lower dose of D3 in both rearing conditions. It is of interest to note in the present study that CS cells exhibit an increased nuclear volume in Ca-rich when compared to those observed in low Ca freshwater. This increased activity of CS cells in Ca-rich freshwater may be attributed to the possible increase in the serum Ca and inorganic phosphate levels. Thus, this study helps in understanding the Ca and Pi regulation in commercially important freshwater Indian major carp fish, Labeo rohita reared in different calcemic environments of water available for aquaculture. A future study is needed to establish the mechanism of action and regulation of calcium homeostasis in important culturable food fish species reared in different water/soil conditions with special reference to saline and/or sodic soil water areas that are rising day by day in many countries including India.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors are thankful to the Director and Vice-Chancellor, ICAR–Central Institute of Fisheries Education, Mumbai; for providing all the facilities required for the present work. The funds provided by Indian Council of Agricultural Research, New Delhi, for the research work are also acknowledged.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbink W., Flik G. Parathyroid hormone-related protein in teleost fish. Gen. Comp. Endocrinol. 2007;152(2–3):243–251. doi: 10.1016/j.ygcen.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Ahmad N., Swarup K. Corpuscles of Stannius of Mystus vittatus in relation to calcium and sodium rich environments. Arch. Biol. (Bruxelles) 1979;90(1):1–22. [PubMed] [Google Scholar]
- Aida K., Nishioka R.S., Bern H.A. Degranulation of the Stannius corpuscles of coho salmon (Oncorhynchus kisutch) in response to ionic changes in vitro. Gen. Comp. Endocrinol. 1980;41:305–313. doi: 10.1016/0016-6480(80)90073-8. [DOI] [PubMed] [Google Scholar]
- APHA, 1998. Standard Methods for thr Examination of Water and Wastewater, Washington DC, 20th ed., Method 3500 – Ca-D.
- Bansal S.K., Verma S.R., Gupta A.K., Dalela R.C. Physiological dysfunction of the haemopoietic system in a freshwater teleost, Labeo rohita following chronic chlordane exposure. Bullet. Environ. Contaminat. Toxicol. 1979;22:674–680. doi: 10.1007/BF02027006. [DOI] [PubMed] [Google Scholar]
- Buttler D.G. Stanniectomy increases renal magnesium and calcium excretion in freshwater Norht American eels (Anguilla rostrata) J. Exp. Biol. 1993;181:107–118. [Google Scholar]
- Fenwick J.C. Effect of stanniectomy on calcium activated adinosinetriphosphatase activity in the gill of freshwater adapted North American eel, Anguilla rostrate Le Sueur. General Comparat. Endocrinol. 1976;29:383–387. doi: 10.1016/0016-6480(76)90052-6. [DOI] [PubMed] [Google Scholar]
- Fenwick J.C., Forster M.E. Effect of stanniectomy and hypophysectomy on total plasma cortisol level in the eel (Anguilla Anguilla L.) Gen. Comparat. Endocrinol. 1972;19:184–191. doi: 10.1016/0016-6480(72)90019-6. [DOI] [PubMed] [Google Scholar]
- Fenwick J.C., Smith K., Smith J., Flik G. Effect of various vitamin D analogs on plasma Ca and phosphorus and intestinal Ca absorption in fed and unfed American eels, Anguilla rostrata. Gen. Comparat. Endocrinol. 1984;55:398–404. doi: 10.1016/0016-6480(84)90010-8. [DOI] [PubMed] [Google Scholar]
- Fenwick J.C., Vermette M.G. Vitamin D3 and the renal handling of phosphate in American eels. Fish Physiol. Biochem. 1989;7:351–358. doi: 10.1007/BF00004728. [DOI] [PubMed] [Google Scholar]
- Fiske C.H., Subbarow Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925;66:375–400. [Google Scholar]
- Fontaine M. Corpuscles de Stannius et regulation ionique (Ca, K, Na) du milieu interieur de l’ Anguille (Anguilla Anguilla L.) CR. Acad. Sci. Paris. 1964;259:875–878. [PubMed] [Google Scholar]
- Flik G., Bonga S.E.W., Fenwick J.C. Ca2+- dependent phosphatase and Ca2+-dependent ATPase activities in plasma membranes of eel gill epithelium—III. Stimulation of branchial high-affinity Ca2+-ATPase activity during prolactin-induced hypercalcemia in American eels. Comp. Biochem. Physiol. 1984;79:521–524. doi: 10.1016/0305-0491(84)90359-6. [DOI] [PubMed] [Google Scholar]
- Flik G., Fenwick J.C., Bonga S.E.W. Calcitropic actions of prolactin in freshwater North American eel (Anguilla rostrata Le Sueur) Am. J. Physiol. Regul. Integrat. Comparat. Physiol. 1989;257:74–79. doi: 10.1152/ajpregu.1989.257.1.R74. [DOI] [PubMed] [Google Scholar]
- Flik G., Vander Veldan J.A., Dechering K.J., Verbost P.M., Shoenmakers Th J.M., Kolar Z., Wendelaar Bonga S.E. Ca2+ and Mg2+ transport in gills and guts of tilapia, Orechromis mossambicus; a review. J. Exp. Zool. 1993;265:356–366. [Google Scholar]
- Flik, G., Verbost, P.M., Wendelaar Bonga, S.E., 1995. Calcium transport process in fishes. In: Wood, C.M., Shuttleworth, T.J., (Eds.), Fish Physiology vol. 14 Cellular and Molecular approaches to fish ionic regulation. New York: Academic Press; pp. 317–343.
- Flik, G., Verbost, P.M., 1996. Calcium homeostasis in fish. In: Dacke, C., Danks, J., Caple, I , Flik, G., (Eds), The Comparative Endocrinology of calcium regulation. Journal of Endocrinology Ltd., Bristol. pp. 43–53.
- Gittes R.F., Toverud S.V., Cooper C.W. Effects of hypercalcemia and hypocalcemia on the thyrocalcitonin content of rat thyroid glands. Endocrinology. 1968;82:83–90. doi: 10.1210/endo-82-1-83. [DOI] [PubMed] [Google Scholar]
- Gu Jie, Li Jing-Woei, William Ka-Fai Tse, Chan Ting-Fung, Lai Keng-Po, Wong Chris Kong-Chu. Transcriptomic responses of corpuscle of Stannius gland of Japanese eels (Anguilla japonica) to Changes in Water Salinity. Sci. Rep. 2015;5(9836):1–8. doi: 10.1038/srep09836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro P.M., Renfro J.L., Power D.M., Canario A.V.M. The parathyroid hormone family of peptides: structure, tissue distribution, regulation, and potential functional roles in Ca and phosphate balance in fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:679–696. doi: 10.1152/ajpregu.00480.2006. [DOI] [PubMed] [Google Scholar]
- Hayes M.E., Guilland-Cumming D.F., Russell R.G.G., Henderson I.W. Metabolism of 25-hydroxycholecalciferol in a teleost fish, the rainbow trout (Salmo gairdneri) Gen. Comp. Endocrinol. 1986;64(1):143–150. doi: 10.1016/0016-6480(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Hiroi Morphological changes in the Corpuscles of Stannius of the chum salmon, Oncorynchus keta, during the migration and maturation. Bull. Fac. Fish. Hokkaido Univ. 1970;21:179–192. [Google Scholar]
- Hirsch P.F., Munson P.L. Thyrocalcitonin. Physiol. Rev. 1969;49:548–622. doi: 10.1152/physrev.1969.49.3.548. [DOI] [PubMed] [Google Scholar]
- ICLARM, 2001. Naga, the ICLARM quarterly. July to December, vol. 24. Nos. 3 and 384p.
- Ishibashi K., Imai M. Prospect of a stanniocalcin endocrine/paracrine system in mammals. Am. J. Physiol. Renal Physiol. 2002;282:F367–F375. doi: 10.1152/ajprenal.00364.2000. [DOI] [PubMed] [Google Scholar]
- Johnson D.W. Variations in the inter-renal and Corpuscles of Stannius of Mugil cephalus from Colarado river and its estuary. Gen. Comp. Endocrinol. 1972;19:7–25. doi: 10.1016/0016-6480(72)90002-0. [DOI] [PubMed] [Google Scholar]
- Joshi A.D. New insights In to physiological and pathophysiological functions of stanniocalcin-2. Front. Endocrinol. 2020;11(172):1–8. doi: 10.3389/fendo.2020.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkit M.W., Elewa Y.H.A., Salem H.F., Bareedy M.H. Stannius corpuscles in African catfish (Clarias gariepinus): histological and ultrastructure studies. Adv. Anim. Vet. Sci. 2019;7(2):6–11. [Google Scholar]
- Lopez E., Peignoux-Deville J., Lallier F., Colston K.W., MacIntyre I. Response of bone metabolism in the eel Anguilla anguilla to injections of 1,25-di-hydroxyvitamin D. Calcif. Tissue Res. 1977;22:19–23. doi: 10.1007/BF02064034. [DOI] [PubMed] [Google Scholar]
- Luo C.W., Pisarska M.D., Aaron J.W.H. Identification of a stanniocalcin paralog, stanniocalcin-2, in fish and the paracrine actions of stanniocalcin-2 in the mammalian ovary. Endocrinology. 2005;146(1):469–476. doi: 10.1210/en.2004-1197. [DOI] [PubMed] [Google Scholar]
- McCormic S.D. Methods for non-lethal gill biopsy and measurements of Na+, K+-ATPase activity. Can. J. Fish. Aquat. Sci. 1993;50:656–658. [Google Scholar]
- Meats M., Ingleton P.M., Chester Jones I., Garland H.O., Kenyon C.I. Fine structure of the Corpuscles of Stannius of the trout, Salmo gairdneri, and structural changes in response to increased environmental salinity and Ca ions. Gen. Comp. Endocrinol. 1978;36:451–461. doi: 10.1016/0016-6480(78)90084-9. [DOI] [PubMed] [Google Scholar]
- Olivereau M. Corpuscles of Stannius in seawater eels. Am. Zool. 1964;4:415. doi: 10.1007/BF00219656. [DOI] [PubMed] [Google Scholar]
- Olivereau M., Olivereau J. Hypercalcemia and Corpuscles of Stannius in seawater eels. Cell Tissue Res. 1978;186:81–96. doi: 10.1007/BF00219656. [DOI] [PubMed] [Google Scholar]
- Palma P.F.S., Bock C., Silva T.S., Guerreiro P.M., Power D.M., Pörtner Hans-Otto, Canário A.V.M. STC1 and PTHrP modify carbohydrate and lipid metabolism in liver of a teleost fish. Sci. Rep. 2019;9(723):1–12. doi: 10.1038/s41598-018-36821-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang P.K.T., Pang R.K., Griffith R.W. Corpuscles of Stannius: Lack of direct involvement in regulation of serum sodium, potassium, and chloride in the teleost, Fundulus heteroclitus. Gen. Comp. Endocrinol. 1975;26:179–185. doi: 10.1016/0016-6480(75)90133-1. [DOI] [PubMed] [Google Scholar]
- Perry S.F., Goss C.G., Fenwick J.C. Inter-relationship between gill chloride cell morphology and calcium uptake in freshwater teleost. Fish Physiol. Biochem. 1992;10:327–337. doi: 10.1007/BF00004482. [DOI] [PubMed] [Google Scholar]
- Pierson P.M., Lamers A., Flik G. The stress axis, stanniocalcin, and ion balance in rainbow trout. Gen. Comp. Endocrinol. 2004;137(3):263–271. doi: 10.1016/j.ygcen.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Prasad M., Kumar A., Srivastav S.K., Srivastav A.K. Alterations in the corpuscles of stannius of Euphorbia royleana treated catfish, Heteropneustes fossilis. Iran. J. Toxic. 2017;11(3):27–32. [Google Scholar]
- Rao D.S., Raghuramulu N. Vitamin D and its related parameters in freshwater wild fishes. Comp. Biochem. Physiol. 1995;111A:191–198. [Google Scholar]
- Richards T.D.J., Fenton A.L., Syed R., Wagner G.F. Characterization of stanniocalcin-1 receptors in the rainbow trout. ISRN Endocrinol. 2012;2012:1–11. doi: 10.5402/2012/257841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale A.P., Fiess J.C., Hirano T., Cooke I.M., Grau E.G. Disparate release of prolactin and growth hormone from the tilapia pituitary in response to osmotic stimulation. Gen. Comp. Endocrinol. 2006;145:222–231. doi: 10.1016/j.ygcen.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Singh S., Srivastav A.K. Vitamin D3-induced histological changes in the corpuscles of Stannius of a freshwater catfish, Heteropneustes fossilis Kept Either in Artificial Freshwater, Calcium-Rich Freshwater or Calcium-Deficient Freshwater. Okajimas Folia Anat. Jpn. 1996;73(2–3):75–81. doi: 10.2535/ofaj1936.73.2-3_75. [DOI] [PubMed] [Google Scholar]
- Srivastav A.K., Swarup K. Effects of calcitonin on calcitonin cells, parathyroid glands and serum electrolytes in the house shrew Suncus murinus. Acta Anat. 1982;114:81–87. doi: 10.1159/000145581. [DOI] [PubMed] [Google Scholar]
- Srivastav A.K., Srivastava S.K. Impact of deltamethrin on serum Ca and inorganic phosphate of freshwater catfish, Heteropneustes fossilis. Bull. Environ. Contam. Toxicol. 1997;59(5):841–846. doi: 10.1007/s001289900558. [DOI] [PubMed] [Google Scholar]
- Srivastav S.P., Swarup K., Srivastav A.K. Structure and behaviour of Stannius corpuscles in relation to vitamin D3 induced hypercalcemia in male Clarias batrachus. Cell. Mol. Biol. 1985;31:1–5. [PubMed] [Google Scholar]
- Srivastav A.J., Flik G., Bonga S.E.W. Plasma Ca and stanniocalcin levels of male tilapia, Oreochromis mossambicus, fed Ca-deficient food and treated with 1,25 dihydroxyvitamin D3. Gen. Comp. Endocrinol. 1998;110:290–294. doi: 10.1006/gcen.1998.7074. [DOI] [PubMed] [Google Scholar]
- Srivastav A.K. Calcaemic responses in the freshwater mud eel, Amphipnous cuchia, to vitamin D3 administration. J. Fish Biol. 1983;23:301–303. [Google Scholar]
- Srivastav A.K., Srivastav S.K., Sasayama Y., Suzuki N., Norman A.W. Vitamin D metabolites affect serum calcium and phosphate in freshwater catfish, Heteropneustes fossilis. Zool. Sci. 1997;14:743–746. [Google Scholar]
- Srivastav A.K., Tiwari P.R., Srivastav S.K., Sasayama Y., Suzuki N. Vitamin D3-induced calcemic and phosphatemic responses in the freshwater mud eel Amphipnous cuchia maintained in different Ca environments. Braz. J. Med. Biol. Res. 1997;30:1343–1348. doi: 10.1590/s0100-879x1997001100014. [DOI] [PubMed] [Google Scholar]
- Srivastav S.K., Jaiswal R., Srivastav A.K. Response of serum Ca to administration of 1,25-dihydroxyvitamin D3 in the freshwater carp Cyprinus carpio maintained either in artificial freshwater, Ca-rich freshwater or Ca-deficient freshwater. Acta Physiol. Hung. 1993;81:269–275. [PubMed] [Google Scholar]
- Srivastav A.K., Srivastav S.P. Corpuscles of Stannius of Clarias batrachus in Response to 1, 25 Dihydroxyvitamin D3 Administration. Zoolog. Sci. 1988;5:197–200. [Google Scholar]
- Srivastav A.K., Singh S. Effect of Vitamin D3 administration on the serum Ca and inorganic phosphate levels of the freshwater catfish, Heteropneustes fossilis, maintained in artificial fresh water, Ca-rich fresh water, and Ca-deficient fresh water. Gen. Comp. Endocrinol. 1992;87(1):63–70. doi: 10.1016/0016-6480(92)90150-i. [DOI] [PubMed] [Google Scholar]
- Srivastava S.M., Srivastava P.P., Dayal R., Chowdhary S., Lakra W.S., Singh S.P., Pandey A.K. Vitamin D3 induced hypercalcemic response in threatened bronze feather back (Notopterus notopterus, pallas). O. J. Anim. Feed Res. 2012;2(2):162–165. [Google Scholar]
- Sundell K., Norman A.W. 1,25(OH)2 vitamin D3 increases ionized plasma Ca concentrations in the immature Atlantic cod Gadus morhua. Gen. Comp. Endocrinol. 1993;91:344–351. doi: 10.1006/gcen.1993.1135. [DOI] [PubMed] [Google Scholar]
- Suryawanshi S.A., Mahajan S.M. Role of ultimobranchial body and Corpuscles of Stannius in regulation of the plasma calcium and phosphorus levels in the teleost, Heteropneustes fossilis. Acta. Biol. Acta. Sci. Hung. 1976;27:269–274. [PubMed] [Google Scholar]
- Swarup K., Das V.K., Norman A.W. Dose-dependent vitamin D3 and 1,25-dihydroxyvitamin D3-induced hypercalcaemia and hyperphosphataemia in male cyprinoid Cyprinus carpio. Comp. Biochem. Physiol. 1991;100:445–447. [Google Scholar]
- Swarup K., Srivastav S.P. Vitamin D3 induced hypercalcemia in male catfish, Clarias batrachus. Gen. Comp. Endocrinol. 1982;46:271–274. doi: 10.1016/0016-6480(82)90209-x. [DOI] [PubMed] [Google Scholar]
- Swarup K., Norman A.W. Depletion of Ca and Pi in scales, bones and muscles associated with vitamin D3 induced hypercalcemia hyperphosphatemia in Cyprinus carpio. Proc. Natl. Acad. Sci. India. 1996;66:243–252. [Google Scholar]
- Swarup K., Norman A.W., Srivastav A.K., Srivastav S.P. Dose-dependent vitamin D, and 1,25-dihydroxyvitamin D,-induced hypercalcemia and hyperphosphatemia in male catfish Clarias batrachus. Comp. Biochem. Physiol. 1984;78B:553–555. [Google Scholar]
- Tanega C., Radman D.P., Flowers B., Sterba T., Wagner G.F. Evidence for stanniocalcin and a related receptor in annelids. Peptides. 2004;25(10):1671–1679. doi: 10.1016/j.peptides.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Trinder P. Colorimetric microdetermination of calcium in serum. Analyst. 1960;85:889–894. [Google Scholar]
- Urasa F., Wendelaar Bonga S.E. Effects of Ca and phosphate on the Corpuscles of Stannius of the teleost fish, Oreochromis mossambicus. Cell Tissue Res. 1987;249:681–690. doi: 10.1007/BF00214644. [DOI] [PubMed] [Google Scholar]
- Verbost P.M., Flik G., Fenwick J.C., Greco A.M., Pang P.K.T., Wendelaar Bonga S.E. Branchial calcium uptake: possible mechanism of control by stanniocalcin. Fish Physiol. Biochem. 1993;11:205–215. doi: 10.1007/BF00004568. [DOI] [PubMed] [Google Scholar]
- Wendelaar Bonga S.E.W., Greven J.A.A., Veenhuis M. The relationship between ionic composition of the environment and secretory activity of the endocrine cell types of Stannius in the teleost Gasterosteus aculeatus. Cell Tissue Res. 1976;175:297–312. doi: 10.1007/BF00218708. [DOI] [PubMed] [Google Scholar]
- Wendelaar Bonga S.E., Greven J.A.A. The relationship between prolactin cell activity, environmental calcium, and plasma calcium in the teleost, Gasterosteus aculeatus. Observation on fish stanniectomized fish. Gen. Comp. Endocrinol. 1978;36:90–101. doi: 10.1016/0016-6480(78)90054-0. [DOI] [PubMed] [Google Scholar]
- Wendelaar Bonga S.E.W., Van der Meij J.C.A., Pang P.K.T. Evidence for two secretory cell types in the Stannius bodies of the teleosts Fundulus heteroclitus and Carassius auratus. Cell Tissue Res. 1980;212:295–306. doi: 10.1007/BF00233962. [DOI] [PubMed] [Google Scholar]
- Wendelaar Bonga S.E., Pang P.K.T. Stannius corpuscle. In: Pang P.K.T., Schreinman M.P., editors. Vertebrate Endocrinology: Fundamentals and Biomedical implications. Academic Press; New York: 1986. pp. 105–137. [Google Scholar]
- Wendelaar Bonga S.E., Pang P.K.T. Stannius corpuscle. In: Pang P.K.T., Schreinman M.P., editors. Vertebrate Endocrinology: Fundamentals and Biomedical implications. Academic Press; New York: 1986. pp. 436–464. [Google Scholar]
- Yoshiko Y., Aubin J.E. Stanniocalcin 1 as a pleiotropic factor in mammals. Peptides. 2004;25(10):1663–1669. doi: 10.1016/j.peptides.2004.04.015. [DOI] [PubMed] [Google Scholar]