Abstract
Pathogen affects plant growth, host health and productivity. Endophytes, presumed to live inside the plant tissues, might be helpful in sustaining the future of agriculture. Although recent studies have proven that endophytes can be pathogenic, commensal, non-pathogenic, and/or beneficial, this review will focus on the beneficial category only. Beneficial endophytes produce a number of compounds which are useful for protecting plants from environmental conditions, enhancing plant growth and sustainability, while living conveniently inside the hosts. The population of endophytes is majorly controlled by location, and climatic conditions where the host plant grows. Often the most frequently isolated endophytes from the tissues of the plant are fungi, but sometimes greater numbers of bacteria are isolated. Beneficial endophytes stand a chance to replace the synthetic chemicals currently being used for plant growth promotion if carefully explored by researchers and embraced by policymakers. However, the roles of endophytes in plant growth improvement and their behavior in the host plant have not been fully understood. This review presents the current development of research into beneficial endophytes and their effect in improving plant growth.
Keywords: Environmental condition, Food availability, Plant productivity, Rhizosphere, Tissue
1. Introduction
It has been reported that the world’s population is likely to increase up to 9.1 billion by 2050 (Liu et al., 2017). For this reason, governments at all levels are trying their best to ensure a continuous increase in agricultural productivity. However, ways to meet up with this target are becoming difficult. Climate change, urban sprawl, poor land management and over-dependence on synthetic fertilizers are some of the factors posing threats to agricultural development (Smith et al., 2016). The adoption of plant growth-promoting (PGP) microorganisms (beneficial endophytes) as biofertilizers in agriculture has shown great promise in providing an effective and eco-friendly approach in ensuring food security (Glick, 2014). Endophytes are examples of microorganism with these prospects.
Endophytic microbes are microorganisms that successfully colonize the tissue of vascular plants and have been reported to be isolated in most plants in these group (Brader et al., 2017, Fadiji and Babalola, 2020a). They are initially known not to be harmless to the host plants and their association with plants can be obligate or facultative (Nair and Padmavathy, 2014). A recent study by Brader et al. (2017) showed that endophytes can also be defined in terms of their ecological niche and not only the function they perform in the host. The study further revealed that some species of endophytes can either pathogenic or beneficial. The majority of the endophyte do not show any harmful effects on a few plant species, however, when tested on other plants, they may be pathogenic. The pathogenicity attribute of endophytes can be based on a number of biotic interactions and environmental factors. For example, fluorescent Pseudomonads, known to be beneficial to most plants, can be pathogenic to the leatherleaf plant under special conditions (Kloepper et al., 2013).
Nevertheless, endophytes have been observed to be active in biological control of phytopathogens, plant growth enhancement, and in the production of compounds or metabolites of biotechnological or pharmaceutical importance (Sharma et al., 2017). Growth of endophytes is generally strongly restricted by plants, and in order to overcome this hindrance, endophytes make use of numerous mechanisms of action in adapting to new living environments (Dudeja et al., 2012).
Endophytic bacteria are classified as those bacteria that live inside or on the surface of disinfected plant tissues and coexist symbiotically (Patle et al., 2018). Endophytic fungi, on the other hand, are fungi that reside inside the tissues of a plant without having any harmful effect on the plant. Even though most of them are not host-specific, certain group of endophytic fungi possess a greater occurrence in some plants, indicating their preference for these plant families as their host (Fadiji and Babalola 2020a). There exists a wide diversity of endophytes, mostly with a great improvement in their ecological roles alongside the production of numerous amazing chemical secondary metabolites. Endophytes were reported to be naturally resident in many host plants (Suryanarayanan, 2013). Different endophytes can be found in different parts of a plant mainly in the stem, leaves or root (Fürnkranz et al., 2012). Most endophytes that are found in vascular plants were discovered to employ a plant-fungus interaction. This type of interaction is symbiotic; most endophytes compliment nutrients got from the host plants and also contribute significant benefits to the host plants. These endophytes live harmlessly within the tissues of the host they have colonized, thereby facilitating an indirect defense against herbivores (Bamisile et al., 2018).
Endophytes receive nutrition as well as protection from the host, while encouraging uptake of nutrients and protecting the host from abiotic and biotic stresses and pests. It has also been reported that the availability of endophytes affects the health of the plant, developments, growth, and the different types of the plant community, ecosystem functioning and population dynamic (Hardoim et al., 2015). Many endophytic microbes have been reported to have developed gradually finding their ways into the plant, and as this association continues, they devise new ways to inhabit, evolve, establish and improve the association they have established with the host (Goyal et al., 2016).
Different endophytic microbes exist mainly in roots of plants and decrease from the stem to the leaves. Different endophytic microbe species can be present in numerous plants, while some of the same species can be in a single plant. Some endophytes present in the host remain as latent, while other interactions may be pathogenic or non-pathogenic (Arora and Ramawat, 2014, Arora and Ramawat, 2017). In a bid to ensure stable symbiosis, endophytes produce many compounds which help in promoting host plant growth and improving environmental adaptation (Das and Varma, 2009).
One of the recent problems, agriculturists battle with is over-dependence on synthetic fertilizers for improving the growth of plants, which has several side effects on human health and is not eco-friendly. Efforts towards the improvement of endophytic resources could give us numerous benefits, such as the discovery of effective and novel metabolic compounds that might not easily be synthesized through chemical means. As a result of this, an urgent need for a proper understanding of the benefits of beneficial endophytes, the biology of plants and the ecology of the microbes are required. A number of experiments have been carried out, trying to evaluate how endophytes colonize the host vegetative tissues alongside their impact on growth promotion and health. This study proposed to give an outlook of beneficial endophytes (bacterial and fungal) and their potentials in improving plant growth with an emphasis on current trends in endophytic research.
2. Distribution pattern of endophytes in plant tissue
Endophytic microorganisms can be grouped into three main categories based on the approach they adopt while living inside plants. Obligate endophytes are microorganisms that cannot reproduce outside the plant tissue and most times are transferred through seed instead of developing in rhizospheric soil (Hardoim et al., 2015). Facultative endophytes are microorganisms that live freely in soil but colonize the plant roots at the slightest opportunity, using a systematic approach (Hardoim et al., 2015). The endophytic microbes that are helpful in enhancing plant growth and health belong to this category (Hardoim et al., 2015). Passive endophytes are microorganisms that do not originally intend to colonize the plant tissues but end up colonizing them due to events, such as wounds on the root hairs. Passive life may affect endophytes by making them less active, since the technical know-how required for cellular colonization of a plant is lacking (Hardoim et al., 2011), thus making them less appropriate as promoters of plant growth. However, a recent study showed that endophytes associate with plants in many forms, including fungi and bacteria (Mycoplasma or actinomycetes) that colonize plant tissues (Gouda et al., 2016).
The distribution of endophytes living inside plants depends strongly on a combination of the allocation of plant resources and the ability to colonize. Endophytes in the roots of plants often penetrate the site at which lateral roots emerge and help in colonizing the epidermis, in the root cracks and below the root hair zone (Zakria et al., 2007). Colonizers of this nature can effectively establish populations both intracellularly and intercellularly (Zakria et al., 2007). Once colonization is established, endophytes can relocate to other parts of the plant, through the vascular tissues from where they begin to proliferate systemically (Johnston-Monje and Raizada, 2011). Johnston-Monje and Raizada (2011) demonstrated the transport of the endophytes using green-fluorescent-protein (GFP) labeling, into roots and tissues, the results showed that endophytes introduced into stems proceed into the roots and rhizosphere, thus suggesting that there may be a continuous distribution of endophytic organisms in the microbiome of the root.
The second factor affecting the distribution of endophytes is the way resources are allocated in the whole plant. Many unique endophytic microbe communities reside freely in the tissues of most plants (Johnston-Monje and Raizada, 2011). To buttress our point, studies by Garbeva et al. (2001) discovered that Pseudomonas sp. were more prominent in the stems of potatoes (Solanum tuberosum) than in the roots which enhanced crop growth, after its growth was considered for one month. Surette et al. (2003) suspected that higher presence of endophyte within crowns of carrot as compared with the metaxylem tissues might be attributed to a higher level of photosynthate present in the crown regions, which probably supply more resources for a larger community to increase. When plant tissues are effectively colonized, endophytes can be freely distributed in the host plant, thereby enhancing plant growth promotion. However, discovering the mechanism behind this distribution is still an important focus.
3. Root colonization behavior of endophytes
Endophytes have the capacity to colonize any part of the plant including the embryo of seeds. The endophytes increase as the seedling germinates and during its early growth (Shade et al., 2017). As the seedlings continue to grow, the interactions between soil and roots microbiome start. The first step involved in the colonization process, especially for endophytic bacterial cells. is called attachment or adhesion (Kandel et al., 2017). Most bacterial endophytes in the surroundings of plant roots move towards the roots through chemotactic affinities for the exudates released by the roots of the plants. This is often followed by attachment to the surface of the plant root, which is very important in penetrating the entry sites at the lateral root emergence region or through other areas, as a result of wounds or mechanical injuries. The exopolysaccharides produced by bacterial cells may also help in enhancing the attachment of bacterial endophytes to the root surface and this is very important for endophytic colonization at early stages. The EPS secreted by bacterial endophyte Gluconacetobacter diazotrophicus Pal5 was reported as a vital factor for surface attachment and colonization of rice root (Meneses et al., 2011). Some structures of the bacterial cells such as cell surface polysaccharides, fimbriae and flagella can also aid the attachment of bacterial cells to plant roots. However, in a study carried on maize endophytes, it was observed that lipopolysaccharide (LPS) produced by bacterial endophyte is important for its attachment and endophytic colonization of the maize roots (Balsanelli et al., 2010). Also, it had been reported that binding of N-acetyl glucosamine of LPS with the lectins of the maize root is needed to enhance the attachment and subsequent colonization of the plant roots by the bacterial endophyte (Balsanelli et al., 2013). Microbial interactions in the rhizosphere are triggered by plant exudates which enhances the entrance of endophytes in the root of the plant. Endophytes eventually propel tissue colonization of plant and later continue by moving in the stem, leaves and the entire plant endosphere (Kandel et al., 2017).
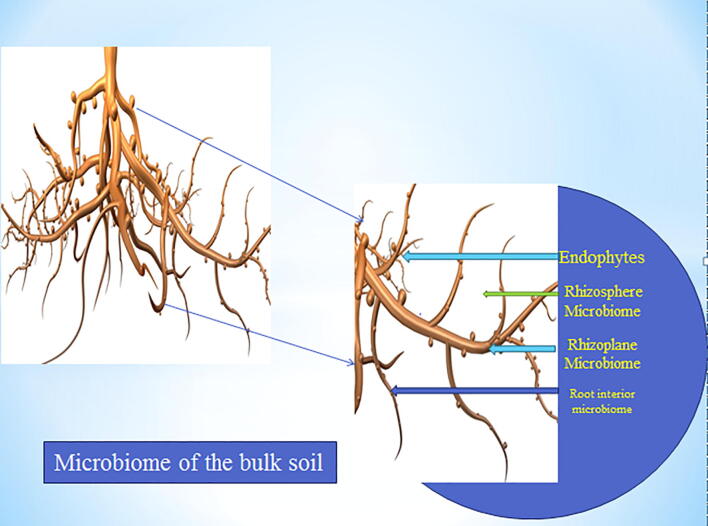
Endophytic microbes are ever-present in many species of plant, living actively or latently enhancing tissue colonization. Endophytic bacteria are numerous and they colonize many species of plant. The spread of endophytes starts from the root and decreases across the plant stem and leaves. Immunological labeling techniques with the aid of monospecific polyclonal antibodies were applied against two Herbaspirillum frisingense strains and green fluorescent protein (GFP)-fluorescence tagging, the result showed that H. frisingense successfully colonize the root of Miscanthus sinensis (Rothballer et al., 2008). The capability of endophytes to successfully colonize the inner tissues of the host plant has exposed their importance in agricultural practice. The differences among endophytes in the endosphere are governed by questionable events which influence colonization processes. Soil factors have a great influence on how differently the community of endophytes colonizes the plant. The initial steps which soil bacteria use in colonizing plant roots are still questionable, considering the fact that it depends solely on the interaction that exists between plant root and bacterium. Reports have it that the way plant roots are colonized rests greatly on the diversity, abundance, physiological status and distribution of the supposed endophytes in the soil (Van Overbeek and Van Elsas, 2008). Various factors determine the community structures of endosphere and endophytic colonization. The capability of soil bacteria to enter the root of the plant through induced chemotaxis movement and colonize it effectively through microcolony formation and attachment is the distinctive factor an organism must possess in order to become an endophyte. Endophytes show some signs of their interaction with the plants by colonization and the formation of structures which are similar to ectendomycorrhiza and ectomycorrhizal. Endophytes are present in the vascular tissues of the plants serving as hosts, making asymptomatic colonization intracellularly or intercellularly throughout the root. Genetically engineered derivatives and wild-type strain PsJN of Burkholderia sp. strain PsJN tagged with gfp (PsJN: gfp2x) or gusA (PsJN: gusA11) genes were inoculated in the rhizosphere of Vitis vinifera L. cv. Chardonnay plantlets. The results showed that Burkholderia sp. strain PsJN successfully colonized root surfaces, cell walls and the whole surface of some rhizodermal cells (Compant et al., 2005). An endophytic fungus identified as Hypocrea lixii isolate F3ST1 was able to colonize onion plants thereby propelling antixenotic repellence of T. tabaci (Muvea et al., 2015). Patel and Archana (2017) reported that Acinetobacter sp. and Achromobacter sp. isolated from Poaceae family (maize, wheat pearl millet, sorghum, and rice) colonized the root of wheat and enhanced growth improvement. Bacillus sp. from tomato plant improved the growth of the wheat by colonizing its root (Tian et al., 2017). Meneses et al. (2017) showed that Gluconacetobacter diazotrophicus isolated from sugarcane successfully colonized the root of the rice plant and enhanced its growth. Herbaspirillum seropedicae isolated from sorghum also colonized the root and leaf of maize plant changing the metabolic profile and nitrogen fixation (Brusamarello-Santos et al., 2017). Change in gene expression was reported when Herbaspirillum seropedicae colonized the root of wheat (Pankievicz et al., 2016). Pseudomonas fluorescence was able to colonize the tissues of the plant, thereby leading to growth enhancement, when exposed to phosphate deficient conditions (Otieno et al., 2015). A study by Patel and Archana (2017) showed that Ralstonia sp. isolated from the Poaceae family colonized the root of maize and enhanced its growth. Endophytic bacteria Consortium (Pseudomonas spp., Paenibacillus spp., Sphingomonas azotifigens) was also able to colonize the root, stem, and leaf of Ryegrass and aid its growth promotion (Castanheira et al., 2017). Fig. 1 shows the different microbiomes present in the root region of a plant. In summary, it is evident that endophytes can colonize the tissues of the plant both intracellularly and extracellularly. Despite the fact that endophytes can be found in almost all tissues of the plant, roots still have the closest contact with the soil and may function as the first channel through which endophyte penetrates the plant.
Fig. 1.
The model of the microbiomes present in the root.
4. Contributions of endophytes to plant growth promotion
Endophytes have been reported to confer many types of protection to their host plant, viz. deterring herbivores by the production of alkaloids that are toxic to grasses, endurance to thrive in hot springs, and protection from pests in dicots (Arora and Ramawat, 2017). Endophytes are said to share close similarities with pathogens residing in the host plant. More evidence shows that the interaction between pathogen and endophytes occurs in different dimensions in the different hosts, and apparently, the physiology of the plant that has been disturbed may inhibit the pathogen’s growth, modify the nutrient balance in a way that will favor endophyte, or trigger plant’s defense mechanism (Busby et al., 2016). The plant’s colonization by endophytic fungi proffers enhanced defense against some nematodes which affect plants. This is a complex occurrence, and the antagonistic mechanism exhibited by endophytes is yet to be fully understood (Busby et al., 2016). Thus, endophytes have great importance in the efficiency of the pathosystem and also in plant’s diversity, survival, and conservation (Arora and Ramawat, 2017). Endophytes influence plant activities in many dimensions and the actual functions of endophytes have not been clearly defined, but host plants generally benefit from the presence of endophytic microorganisms in their tissues. Promotion of plant growth can be passively or actively achieved by endophytes using different mechanisms, even as metabolites from endophytes confer different health to host plants by triggering plant survival in abiotic and biotic conditions, and also plant growth enhancement. The summary of the applications of endophytes is presented in Fig. 2.
Fig. 2.
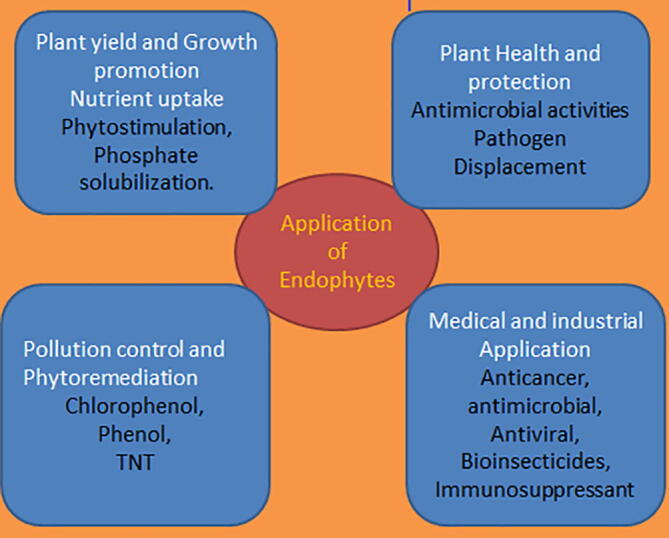
Applications of endophytic microbes in various research fields.
4.1. Phytostimulation or biofertilization
The application of endophytes in agricultural sustainability has increased crop growth and yield (Li et al., 2016, Kumar et al., 2017). For years now, researches have established that endophytes possess the capacity to colonize plant tissues, thereby creating a strong symbiotic association with their hosts (Kumar et al., 2017). The interaction results in enhancement of plant growth and improvement in the plant’s ability to survive under stress (Saravanakumar and Samiyappan, 2007). Biofertilizers are an eco-friendly, cheap, and renewable source of nutrients to plants which help in reducing our dependence on chemical fertilizers and play a significant role in increasing nutrient availability, thereby enhancing plant growth (Pal et al., 2015). Endophytes produce many phytohormones, some of which include cytokinins, auxins, and gibberellic acids. A study carried out on a wild cottonwood (Populus trichocarpa), shows that a diazotrophic endophytic bacterium, Burkholderia vietnamiensis was isolated, which supports plant growth promotion by secreting indole acetic acid (IAA) (Xin et al., 2009). The claim was established by comparing B. vietnamiensis inoculated plants with control plants, and it was found that more dry biomass weight and increased nitrogen content were gained by the inoculated plant. Increased amounts of bioactive compounds GA4, GA7, and GA4 were reported from a novel fungus strain, Cladosporium sphaerospermum which was discovered in Glycine max (L) Merr. roots, which helps in improving the growth of soybean and rice plants maximum (Hamayun et al., 2009). An endophytic fungus Porostereum spadiceum AGH786 was inoculated with Soya Bean seedling under NaCl stress in the greenhouse. The result showed that phytohormones such as GAs, JA and ABA, and isoflavones were secreted but GAs was secreted in higher quantity than in the control (Hamayun et al., 2017). Potshangbam et al. (2017) reported that some endophytic fungi such as Fusarium, Sarocladium, Aspergillus, and Penicillium isolated from maize and rice plants were determining factors in plant growth improvement. The organisms were observed to enhance disease suppression, stress tolerance and plant growth improvement. Some promising endophytic bacteria were isolated from Echinacea purpurea and Lonicera japonica in a study carried out by Gupta et al. (2016). The isolates were found effective in siderophores production, phosphate solubilization, hydrogen cyanide production, indole acetic acid production, and fixing of atmospheric nitrogen. Endophytic bacteria are becoming prominent in plant growth promotion because of their ability to increase the nitrogen present in the soil. Some endophytic bacteria such as Rhizobium spp. and non-nodulating strains such as Brevibacillus choshinensis, Microbacterium trichothecenolyticum, Micromonospora spp. and Endobacter medicaginis have been reported to be present in the root nodules of a plant (Igiehon and Babalola, 2018).
Fouda et al. (2015) studied endophytic fungi isolated from Asclepias sinaica and identified as Penicillium chrysogenum and Alternaria alternata. The results showed that the isolates enhanced root growth and root elongation, which was attributed to ammonia and IAA production. Abdallah et al. (2016) conducted research on endophytic bacteria isolated from Withania somnifera fruits to assess their ability to promote plant growth. The result showed that the most active isolate Alcaligenes faecalis was found to produce indole-3-acetic acid and enhance phosphate solubilization. Also, endophytic fungi associated with mangrove were assessed for their ability to promote the growth of Oryza sativa L. It was reported that all the endophytic fungi isolated enhanced the growth of O. sativa L. “Cempo Ireng” (Tumangger et al., 2018). Different phytohormones produced by endophytes will improve plant growth and reduce the dependence on synthetic fertilizers.
4.2. Antimicrobial activity
Many beneficial endophytes discovered in plants have been found to exhibit antimicrobial properties. They assist in the control of some pathogenic microorganisms in plants and/or animals. Most endophytes in medicinal plants exhibit broad-spectrum bioactivity towards pathogenic microorganisms (Devaraju and Satish, 2011). About 37 bacterial endophytes were isolated from Samanea saman Merr. and Tectona grandis L. plants, and results showed that eighteen isolates produced effective inhibitory compounds against Escherichia coli, Staphylococcus aureus, and Bacillus subtilis, and the growth of Candida albicans was inhibited by 3 isolates through in vitro method (Chareprasert et al., 2006). The antimicrobial potential of the endophytic fungi like Alternaria sp., Chaetomium sp., Alternaria tenuissima, Colletotrichum truncatum, Dothideomycetes sp., Thielavia subthermophila, Nigrospora oryzae, discovered in a medicinal plant known as Tylophora indica, were tested against Fusarium oxysporum and Sclerotinia sclerotiorum and found to strongly inhibit their growth (Kumar et al., 2011). A summary of other studies is presented in Table 1.
Table 1.
Summary of other studies on the antimicrobial activities and bioactive compounds produced by endophytes.
| Endophytes | Type of Endophytes | Host Plant | Pathogen active against | Compounds secreted. | References |
|---|---|---|---|---|---|
| Phomopsis sp. | Endophytic fungi | Plumeria acutifolia | Pseudomonas sp, Escherichia coli, Klebsiella sp, Bacillus subtilis, Staphylococcus aureus. | – | Nithya and Muthumary (2010) |
| Phomopsis sp. | Endophytic fungi | Allanmands cathartica | Pseudomonas sp, E. coli, Klebsiella sp., B. subtiis, S. aureus. | Terpene | Nithya and Muthumary (2011) |
| Fusarium solani | Endophytic fungi | Taxus baccata | Staphylococcus. epidermidis, S. aureus, S. flexneri, B. subtilis. | 1-tetradecene, 8-pentadacanone, 8-octadecanone, 10-nonadecanone,octylcyclohexane | Tayung et al. (2011) |
| Xylaria cubensis, Cyanodermella sp., Lasella sp. | Endophytic fungi | Citrus, Zanthoxylum of Rutaceae and Cinnamomum of Lauraceae | Erwinia carotovora, Xanthomonas campestries, Ralstonia solanceae. | – | Ho et al. (2012) |
| Alternaria sp, C. gloeosporoides, Fusarium sp, Pestatiopsis sp. | Endophytic fungi | Biota orientalis, Pinus excels and Thuja occidentalis | Streptococcus faecalis, Salmonella typhi, | – | Subbulakshmi et al. (2012) |
| Botrytis sp. | Endophytic fungi | Ficus benghalensis | Klebsiella sp, E.coli, | – | Senthilmurugan et al. (2013) |
| Aspergillus sp. | Endophytic fungi | Bauhinia guianensis | E.coli, P.aurigonosa, S.aureus, B.subtilis | Fumigaclavine C and Pseurtotin C | Pinheiro et al. (2013) |
| Pestalotiopsis mangiferae | Endophytic fungi | Mangifera indica Linn | E.coli, B.subtilis, K. pneumonia. | 4-(2,4,7-trioxa-bicyclo[4,10]-heptan-3-yl | Subban et al. (2013) |
| Alternaria alternata, A. citrimacularis, A.niger | Endophytic fungi | Aegle marmelos | S.typhi, Proteus mirabilis, S.epdermidis, S.aureus, Shigella Sp, Shigella sp., P. aeruginosa, E.coli, K.pneumoniae | – | Mani et al. (2015) |
| Bacillus atrophaeus, Bacillus mojavensis | Endophytic bacteria | Glycyrrhiza uralensis (Licorice) | F. oxysporim, Fulvia fulva, A. solani, C. goleosporoides, Verticillium dahlia | 1,2-bezenedicarboxyl acid, Methyl ester, Decanodioic acid, bis(2-ehtylhexyl)ester. | Mohamad et al. (2018) |
| Arthrinium sp. MFLUCC16-1053 | Endophytic fungi | Zingiber cussumunar | Staphylococcus aureus, E.coli, | Laurenan-2-one, 3E-cembrene A, Β-cyclocitral, sclareol, cembrene, farnesol, β- isocomene | Pansanit and Pripdeevech (2018) |
| Xylaria sp., Penicillium sp. | Endophytic fungi | Piper aduncum, Aliberta macrophylla | Cladosporium cladosporoides, C. sphaerospernum | Dihydroiso-1-caumarin, (3R,4R)-4,7-dihydrooxymellein, (R)-7-hydroxylmellein. | Oliveira et al. (2011) |
| Fusarium solani | Endophytic fungi | Taxa baccata | Candida albicans, C. tropicalis | Octylcyclohexane, 8-octadecanone, 1-tetradecane, 8-pentadecanone, 10-nonadecanone | Tayung et al. (2011) |
| Alternaria sp, C. gloesporoides, Fusarium sp., Pestalotropsis sp. | Endophytic fungi | Biota orientalis, Pinus excels, Thuja occidentalis | C. albicans | – | Subbulakshmi et al. (2012) |
| Phoma sp. | Cinnamomum mollissimum | Aspergillus niger | 5-hydroxyramulosin | Santiago et al. (2012) | |
| Lasmenia sp, Ophiceras tenuisporium, Xylaria cubensis, Cyanodermella sp. | Endophytic fungi | Citrus, Zanthoxylum of Ruatceae, Cinnamomum of Laureceae | Alternaria solani, B. cinera, Colletotrichum gloesporiodes, C. higginsianum, C. lageniformis, Fusariun oxysporium, Monacha fruticola, Penicillium digitatnum, Puccinia sidii, Pythium aphanidermatum | – | Ho et al. (2012) |
| Chaetomium globosum, Myrothecium verrucaria | Endophytic fungi | Caloptropis procera | Alternaria alternata, Botrytis cinera, F. oxysporum, Pythium ultimum | – | Gherbawy and Gashgari (2014) |
| Phomopsis sp. | Endophytic fungi | Aconitum carmichaeli | Clinical Isolates | Gavodermside D and Clavasterols | Wu et al. (2013) |
|
Pestalopsis mangiferae Meyerozima sp and Chaetomium globosum. |
Endophytic fungi |
Mangifera indica Linn Trattinnickia rhoifolia (Burseraceae) and Protium heptaphyllum |
C. albicans F. oxysporum |
- Cladosporin, chaetoviridin A and Chaetoatrosin A |
Subban et al., 2013, Fierro-Cruz et al., 2017 |
4.3. Source of bioactive compounds
Endophytes are able to synthesize some bioactive compounds that strengthen plant defense against pathogenic organisms, and some of these compounds have been used in the discovery of novel drugs. A recent report has it that many natural products originated from endophytes, some of which include terpenoids, flavonoids, alkaloids, and steroids. Antibiotics, anticancer, antidiabetic, immunosuppressants, antiviral and biological control agents, among others, are some of the characteristics attributed to bioactive metabolites present in endophytes (Joseph and Priya, 2011).
Geldanamycin and rifamycin are Maytansinoids, which belongs structurally to ansamycin family of polyketide macrolactams and most times are produced by three close families of the plant (Rhamnaceae, Celastraceae, and Euphorbiaceae) and some bacteria isolates such as Actinosynnema pretiosum and mosses. It has been speculated that rhizospheric microbes might also take part in the plant’s maytansinoids biosynthesis (Nair and Padmavathy, 2014).
Another group of biologically active compounds produced by endophytes is siderophores, which help in chelating microorganism iron ions for improved plant growth. They have been applied in the area of medicine and agriculture. They are also an important component of microorganisms which show a virulence trait, consequently affecting animals, people, and plants. Studies were conducted on five different strains of an endophytic fungus with dark septate identified as Phialocephala fortinii, and three siderophores were produced, namely ferrichrome C, ferricrocin, and ferrirubin, whose secretion depends greatly on the iron (III) concentration and pH of the growth medium (Nair and Padmavathy, 2014). However, P. fortinii shows promise for use in industrial manufacturing of siderophores. A plant Taxus chinensis produced an endophyte identified as Metarhizium anisopliae, which was discovered to be the source of taxol (Liu et al., 2009). Also, the leaves of a medicinal plant identified as Justicia gendarussa harbor an endophyte named Colletotrichum gloeosporioides, which is also notable for the production of taxol (Gangadevi and Muthumary, 2008). Table 1 gives a summary of some other studies where bioactive compounds were produced by endophytes.
4.4. Biocontrol activities
Endophytic microorganisms are often acknowledged as having some biocontrol activities, and are therefore a possible replacement for inorganic chemicals. Endophytes play a beneficial role not only for controlling conifers, but in insect herbivory too (Posada and Vega, 2006). A fungal endophyte identified as Beauveria bassiana has been reported to control pathogens of insects such as borer insects, which mostly attack seedlings of sorghum (Tefera and Vidal, 2009), and coffee (Posada and Vega, 2006). Botrytis cinerea is an organism that causes rot of tomato fruits and reduces their shelf life and postharvest quality. However, bacterial endophytes identified as Bacillus subtilis, which was found resident in the tissues of Speranskia tuberculata, gave a strong antagonistic effect through in vitro studies on B. cinerea (Wang et al., 2009). Pinellia ternate agglutinin (PtA) gene was expressed in Chaetomium globosum YY-11, an endophyte discovered in grape seedlings, alongside Enterobacter sp. and Bacillus subtilis which are endophytic bacteria got from the seedlings of maize (Zhao et al., 2010). These recombinant endophytic genes were active in controlling populations of pests such as in the seedlings of most crops. Also, in a related study, Enterobacter cloacae that harbors PtA gene was discovered as an active bio-insecticidal agent in controlling white-backed planthopper, Sogatella furcifera (Zhang et al., 2007). However, the application of recombinant endophytic organisms as biocontrol agents becomes essential, since they produce antipest proteins through a novel technique for controlling plant pests, these endophytes can successfully colonize crop plants. A summary of similar studies is presented in Table 2 below. This biocontrol activity by endophytes boosts plant resistance to diseases and reduces dependence on pesticides.
Table 2.
Summary of major findings on the biocontrol activities of endophytes
| Endophytes | Type of endophytes | Plant Source | Pathogens | Major Findings | References |
|---|---|---|---|---|---|
| Ulocladium, Penicillium, Cladosporium, Aspergillus, Fusarium Chaetomium, Alternaria, Paecilomyces, Bipolaris, Trichoderma, Diaporthe, Nigrospora and Phoma. | Endophytic fungi | Strawberry leaves | Third instar larvae of D. fovealis | The result showed that Paecilomyces isolates were found to induce the highest mortality rates on the pathogens | Amatuzzi et al. (2018) |
| Ochrobactrum sp. (CB361-80) and Pantoea sp (CC372-83) | Endophytic fungi | (Cucumis sativa L.) | Pseudomonas syringae pv. Lachrymans | The result showed that the isolates were able to control angular leaf spot disease in cucumber | Akbaba and Ozaktan (2018) |
| Serratia (B17B), Enterobacter (E), and Bacillus (IMC8, Y, Ps, Psl, and Prt) | Endophytic bacteria | Papaya, snap bean and flowering dogwood | Phytophthora capsici | Phytophthora blight, caused by Phytophthora capsici, which is the most destructive disease of bell pepper in the United States was successfully reduced invitro | Irabor and Mmbaga (2017) |
| Leptosphaeria sp., Penicillium simplicissimum, Acremonium sp., and Talaromyces flavus | Endophytic fungi | Cotton | Verticillium dahliae strain Vd080 | The Verticillium wilt of cotton was controlled and improvement in cottonseed yield in tested cotton fields was observed. | Yuan et al. (2017) |
| Endophyte A22F1 | Endophytic fungi | Flowering dogwood (Cornus florida) | Phytophthora capsici | The result showed the control of root rot pathogens in pepper. | Mmbaga and Gurung (2018) |
4.5. Nutrient cycling
One of the important processes of balancing existing nutrients and making present the nutrients for every component in an ecosystem is called the nutrient cycle. Biodegradation of biomasses that are dead is one of the numerous methods of bringing used minerals back into the ecosystem which consequently brings them to the level where they can be utilized by the organism. This then becomes a continuous chain process. Many saprophytic organisms perform an active role in the nutrient cycling process. Some studies have proved that endophytes showcase a vital function in the biodegradation of host plant litters (Promputtha et al., 2010). In plant litter biodegradation, endophytic microorganisms, first of all, colonize the plant and then trigger the saprophytic organisms to act on it through an antagonistic reaction, thereby giving an increase in the decomposition of litters (Nair and Padmavathy, 2014). He et al. (2012) reported that virtually all endophytes have the potential for organic matter decomposition, some of which include hemicellulose, lignin, and cellulose, which are desired in decomposing different groups of organic matters.
4.6. Biodegradation and bioremediation
Most endophytic microbes have the capacity to decompose complex organic compounds. Bioremediation is a way of removing waste and pollutants present in the environment by the activities of a microorganism. It is a bioprocess that depends greatly on microorganisms in the breaking down of waste products. This is achievable because of the numerous microorganisms which are available in nature. The impact of endophytic microbes in exhibiting bioremediation by Nicotiana tabaccum was studied by Mastretta et al. (2009). The inoculation of N. tabaccum alongside endophytic microbes showed an increase in the biomass number when exposed to cadmium (Cd) stress, and the number of noninoculated plants was lower when compared to inoculated plants. This finding, however, showed the beneficial roles of endophytes from the seeds of plants on the accumulation and toxicity of metals. Some fungal endophytes were assessed for their ability to degrade the plastic polymer polyester polyurethane (PUR) (Russell et al., 2011). Many organisms showed their capacity for the degradation of PUR effectively in liquid and solid media; however, genus Pestalotiopsis gave the best result. Two isolates of Pestalotiopsis microspora successfully used PUR as their only carbon source when exposed to anaerobic and aerobic conditions. An enzyme serine hydrolase was predicted to be responsible for this attribute when molecular characterization was carried out, this enzyme can boost the stress tolerance potentials of the plant (Russell et al., 2011).
Endophytic bacteria aid phytoextraction of most heavy metals. Many studies on how endophytic bacteria can remove heavy metals have been carried out, indicating endophytes can help enhance the stress tolerance potential of the plant (Rajkumar et al., 2010). Endophytes are also found to be active in the degradation of polyaromatic hydrocarbon (PAH) (Radwan, 2009). Many types of microorganism nowadays can produce strong surface bioactive biomolecules of biosurfactants with varying molecular size and chemical properties. The bioremediation ability of an endophytic bacteria identified as Pseudomonas fluorescence RE1 (GenBank: MF102882.1) was assessed on heavy metals such as Cr, Cd, Ni, and Zn. The study revealed that the endophyte was able to withstand heavy metals at high concentration and can be used for survival by plants in environments contaminated with heavy metal (Karnwal, 2018). This biodegradation and bioremediation activities attributed to endophyte could be helpful for the survival of the plant in extreme condition.
4.7. Cold and drought stress tolerance
Endophytes have been reported to enhance plant tolerance to cold stress. A study carried out by Subramanian et al. (2015) on tomato plants showed that inoculation with the psychrotolerant endophytic bacteria, Pseudomonas vancouverensis OB155 and P. frederiksbergensis OS261 enhances survival under cold stress (10–12◦C). Reduced membrane damage and elevated antioxidant activities were recorded when compared with the control plant. However, genes for cold acclimation (LeCBF1 and LeCBF3) were produced by the endophyte inoculated plants (Subramanian et al., 2015). Also, an endophyte, Burkholderia phytofirmans strain PsJN induced growth and also strengthened the cell wall of Arabidopsis which resulted in increased resistance to cold stress (Su et al., 2015). Endophytes were also reported to boost plant tolerance to drought. Through the transcriptomics method, it was observed that endophytic B. phytofirmans PsJN showed diverse functions when inoculated in potato plants (Sheibani-Tezerji et al., 2015). Transcript used in cellular homeostasis, transcriptional regulation and ROS detoxification were improved in potato inoculated with B. phytofirmans PsJN in a drought stress area. This indicates that endophytes can detect physiological changes in plants and regulate gene expression for adaptation to that environment. Bacterial endophytes therefore have the prospect of being used as a protective agent in agricultural practices under severe climatic conditions and they can affect physiological responses of the plant to stresses.
4.8. Secretion of volatile organic compounds
An endophytic fungus known as Hypoxylon sp. which was found resident in the tissues of Persea indica gave an array of volatile organic compounds (VOCs) notable among them were 1,8–1-methyl-1,4-cyclohexadiene, cineole, temporarily reported as alpha-methylene-alpha-fenchocamphorone, among others that are yet to be identified. It produced a strong VOC antimicrobial compound active in inhibiting Phytophthora cinnamomi, Botrytis cinerea, Cercospora beticola, Sclerotinia sclerotiorum, and Cercospora beticola. This may have a big impact in the interactions between the fungus and how it survives in the host tissue (Tomsheck et al., 2010). They undeniably showed that Hypoxylon sp. produced 1, 8-cineole (a monoterpene), which is a novel compound. This octane derivative can be used as a fuel additive just as many VOCs produced by Hypoxylon sp. The study suggests that sourcing for fungi that can produce VOCs like Hypoxylon sp. will increase their utilization in industries, medicine, and in the production of energy for improved agricultural practices.
Phomopsis sp. a fungal endophyte, which was unusually isolated from Odontoglossum sp., secreted a distinct number of VOCs which are benzene, ethanol, and 2- propanone, and a monoterpene having a peppery odor called sabinene (Singh et al., 2011). Gases from Phomopsis sp. have antifungal characteristics and mixtures of the VOCs have similar antibiotic activity against numerous plant pathogenic fungi. A natural thujospen was also revealed to be produced by Penicillium decumbens Thom C. (Polizzi et al., 2011). Suwannarach et al. (2013) showed that Nodulisporium sp. CMU-UPE34 was able to produce 31 VOCs. The GC–MS analysis of the results showed that numerous VOCs are produced, among which are acids, alcohols, esters, and monoterpenes. However, eucalyptol, also called 1, 8-cineole was the only volatile compound found to be produced in a large quantity. Many chemicals such as butyl, ethanol, and ethyl acetate which are VOCs spectrum have been reported to be produced naturally by Ceratocystis fimbriata, after thorough GC–MS analysis, and have biotechnological importance in plant growth promotion (Li et al., 2015, Kaddes et al., 2019). More studies need to be carried out and channeled towards VOCs that have antimicrobial properties which will help in improving plant growth.
4.9. Combined roles performed by some endophytes
A number of endophytic microbes are known to possess the ability to carry out different activities within their hosts. Some Endophytes were discovered to have both antimicrobial and herbicidal properties (Li et al., 2012). An endophytic bacterium, Bacillus sp. SLS18, common for plant growth-promotion, was also studied for its activity in biomass production when Solanum nigrum L. was exposed to manganese and cadmium l (Li et al., 2012). Results showed that it displayed great resistance against antibiotics and heavy metals. The strain was also found to produce siderophores, indole-3-acetic acid, and 1-aminocyclopropane-1-carboxylic acid deaminase.
5. The influence of environmental conditions on endophytic microbe population
Endophytes are numerous and they survive in different environments, and some may even grow at extreme conditions (Compant et al., 2010). The population of endophytes varies from species to species and plant to plant. In the same species, the endophytic population may not only be unique from one region to another but also differs with a change in climatic conditions in the same region. Some of the major factors affecting endophytes are temperature, elevation, latitude, and rainfall which can work together in influencing the composition of endophytes in plants. Climate change may result in an uncontrolled rainfall which could either occur in short supply or in excess (Enebe and Babalola, 2018). Excessive rainfall leads to flooding and erosion. These factors can sometimes affect the physiology of the plant, thus revamping plant and endophyte interactions. Chareprasert et al. (2006) studied temporal changes and the way they affect the total endophytic fungi population and observed that matured teak leaves (Tectona grandis L.) and the rain tree (Samanea saman Merr.) gave a greater number of species and genera, with higher frequency of colonization as compared to juvenile leaves, and their presence increased across the rainy season. Thongsandee et al. (2012) reported that the endophytic population and frequency in Gingko biloba L. shows considerable difference in sampling dates for all organs of plants studied, which are, petiole, young leaves, and twigs. They observed that Phyllosticta sp. was present in both petioles and leaves initially examined starting from August with its peak in October. Phomopsis sp. was also detected in all twigs examined throughout the planting year. These results infer that the abundance of the two dominant endophytes differed with seasons and are also organ-specific.
Dry environments may be helpful in selection and discovery of drought-tolerant endophytes (Yandigeri et al., 2012); studies focusing on an area in Namibia characterized with a prolonged dry season showed that many endophytic microbe strains that are desiccation-resistant were detected in maize, pearl millet, and sorghum (Grönemeyer et al., 2012). Similarly, environments that are cold help in the selection of endophytes that are psychrophilic (Nissinen et al., 2012).
However, recent studies carried out on the endophere microbiome of plants using high-throughput sequencing have showed that genotype (Rodríguez-Blanco et al., 2015), host plant species (Ding and Melcher, 2016), growing season (e.g., of trees) (Shen and Fulthorpe, 2015, Ding and Melcher, 2016), developmental stage (e.g., seedling or mature plant) (Yu et al., 2015, Ren et al., 2015a), geographical location (field conditions) (Edwards et al., 2015), host plant nutrient status (Hameed et al., 2015), fertilization (Rodríguez-Blanco et al., 2015) and cultivation practice (Edwards et al., 2015) are some of the factors reported to have significant influence on the plant endopshere microbiome.
Studies comparing the diversity and abundance of endophytic bacteria between transgenic glyphosate-resistant cultivars and wild-type of soybean plants observed a higher diversity and abundance in the culturable endophytes compared with the wild-type plants (de Almeida Lopes et al., 2016). They reported that the genotype of the plant influenced the functional diversity of bacterial endophytes and IAA-producing strains were isolated from one of the three genotypes of sweet potato studied.
Alongside host properties, variations in environmental temperature and CO2 regulate bacterial endophyte communities. Understanding how bacterial endophytes respond to climate change, especially in the case of high temperature and CO2, can help in terms of policies that involve environmental issues. A study by Ren et al. (2015b) showed that bacterial endophytes from the plant leaves are more influenced by climate than bacterial communities of the soil. The community structure of bacterial endophytes inhabiting leaves of rice was affected by high CO2 levels at the filling and tillering stages, but not at maturity, and this effect can be linked to the level of N fertilization levels (Ren et al., 2015a). Also, (Ren et al., 2015b) showed that endophytes community inhabiting leaves at different locations within the plant reacted differently to increase in CO2. Available oxygen also affected bacterial endophytes community inhabiting rice, especially the diazotrophs.
6. Challenges and advances in isolation and identification of endophytes
Most endophytes have been found to be culturable, although some are still not culturable. This has widespread effects in measuring and identifying endophyte community structure and diversity. Recent studies have proved the existence of endophytes through various cultivation-independent experiments and fluorescence in situ hybridization-confocal laser scanning microscopy studies (Berg et al., 2014). The use of modern molecular tools alongside complimenting culture-independent techniques is now widespread. These methods have their base as a polymerase chain reaction (PCR), useful for amplifying a DNA region, most times through16S rRNA, subsequently followed by purification methods for analyzing endophyte communities, some of which sometimes include community fingerprinting or cloning techniques (Gao and Tao, 2012). However, the biased results attributed to PCR present one of the major challenges faced in identifying these endophytes (Lu et al., 2018). Currently, researchers are considering ways of combining both culture-independent and dependent approaches because each has bases inherent to it (Reinhold-Hurek and Hurek, 2011).
The merging of culture-independent methods and culture-dependent approaches has helped in discovering numerous endophytes that are uncultured in most plant species (Pereira et al., 2011). These species might show some important functional roles in the plant (Sessitsch et al., 2012). New techniques will help scientists to further explore the world of these organisms despite reports of being uncultivable (Stewart, 2012). The use of Ribosomal DNA (rDNA) ITS has been established to be a valuable source in resolving phylogenetic relationships among genera or species staring from lower levels (Nair and Padmavathy, 2014). It was also recorded that the identification of nonsporulating fungi using ITS sequences analysis was effective in reducing the effect of the biased report often associated with fungi identification. Furthermore, ITS data and the Large Subunit (LSU) are strong tools to end the difficulty often associated with the taxonomy of endophytic microbes from Basidiomycetes (Rungjindamai et al., 2008). In addition, more recently, genomic and metagenomic studies have gained a lot of attention in endophytic research, as the approach can help to identify different microbes (culturable and nonculturable) present in an environment (Fadiji and Babalola, 2020b). This approach will also help to predict the functions of endophytes as regards whether it is beneficial, pathogenic or nonpathogenic and also identification of many uncharacterized taxa (Brader et al., 2017, Fadiji and Babalola, 2020b).
7. Limitations in the use of endophytes
There is a serious need to explore the world of endophytic microorganisms in a bid to identify competent ones that will perfect their function effectively under the influence of complex rhizospheric plant–microbe interactions, and different ecological situations. This is because numerous problems already exist which are associated with the applications of endophytes, some of which originate from microbial community-plant interaction complexity and exhibition of poor rhizospheric competence in the presence of endogenous microorganisms (Schulz et al., 2002). The population of endophytes is also disturbed by a persistent change in the condition of their environment and emerging soil biological, chemical and physical properties. Being affected with factors earlier mentioned, the effectiveness of an endophytic microbial population is not clear.
Apart from assessing the functionality of endophytes, marketing, proper formulation, and production methods are also some of the limitations in the use of these beneficial microbes for agricultural practices. Another concern with the use of endophytes from plants is that some of them are opportunistic pathogens for animal, plant, or human pathogens and the application of these microbes can cause mild to severe illness and sometimes outbreaks of disease.
8. Future outlook
Considering the importance of endophytes, it is strongly recommended that future studies should focus on the way the endophytes react with the plant host in order to ascertain the best way to make them effective for continuous crop production. Most endophytes known for their numerous functions were isolated through culture-dependent methods; there is still a need to explore culture-independent techniques such as genomics and metagenomics studies in order to be able to detect more novel functions and species. Also, the mechanisms of action of most endophytes are yet to be fully understood. Though some studies are ongoing in this regard, it is very important that the different underlying mechanisms of action of these endophytes should be urgently examined, especially in the way they interact with other microbes in the tissue of plants. Mechanisms backing up the ways of distribution are not clear because endophyte species differ from one plant to the other; they are still a novel field to be explored. A better understanding of functions encoded by endophytic genomes could help us to have insight to the mechanisms involved in plant–microbe interactions and establish genomic determinants of endophyte lifestyle. Experiments studying the transcriptome characterization dynamics of most endophytes and their host plants are promising methods in understanding some of the factors that drive plant–endophyte interactions. Further studies can also focus on the following:
-
i.
Plant-microbe interaction for adaptation and stress tolerance.
-
ii.
How host plant secondary metabolism is affected by symbiosis.
-
iii.
How microbial secondary metabolism is affected by symbiosis.
-
iv.
The use of metagenomics and bioinformatics tools for the determination of endophyte diversity, evolutionary relationship and prediction of the real functions of endophytes.
9. Conclusion
Attention has been shifted to the world of endophytes due to their ability to promote plant growth through different mechanisms and functions as shown in this study. Numerous species of endophytes isolated from many agricultural plants shows that they play a notable role in balancing plant physiology, restoration of available nutrients in the plant, and phytoremediation among others. The world of endophytes has attracted many researchers in the last couple of years, as shown by the over 32,000 articles published about their important attributes as seen on Google Scholar, in both review and research papers. It is a known fact that sustainable agriculture needs self-contained functioning and inputs that are cheap and ecofriendly. To combat the emerging increase in food demand, the use of biological dependent techniques is needed, of which this study has presented endophytes as a possible option. Still, the limitations facing endophytes are some of the hurdles affecting their usage in agriculture.
Author’s contribution
AEF and OOB conceived the ideas, collected the data and develop the manuscript. Authors have carefully read the final manuscript and have agreed that the manuscript be published.
Funding
The study was funded by the National Research Foundation, South Africa (UID123634).
Declaration of Competing Interest
There is no conflict of interest whatsoever from the author
Acknowledgements
AEF gratefully acknowledged National Research Foundation, South Africa/The World Academy of Science African Renaissance PhD scholarship (Ref: UID116107). OOB appreciates the National Research Foundation, South Africa for the research grant (Ref: UID123634) that has supported research in her laboratory.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdallah R.A.B., Mejdoub-Trabelsi B., Nefzi A., Jabnoun-Khiareddine H., Daami-Remadi M. Isolation of endophytic bacteria from Withania somnifera and assessment of their ability to suppress Fusarium wilt disease in tomato and to promote plant growth. J. Plant Pathol. Microbiol. 2016;7(352):2. [Google Scholar]
- Akbaba M., Ozaktan H. Biocontrol of angular leaf spot disease and colonization of cucumber (Cucumis sativus L.) by endophytic bacteria. Egyptian J. Biol. Pest Co. 2018;28(1):14. [Google Scholar]
- Amatuzzi R., Cardoso N., Poltronieri A., Poitevin C., Dalzoto P., Zawadeneak M., Pimentel I. Potential of endophytic fungi as biocontrol agents of Duponchelia fovealis (Zeller)(Lepidoptera: Crambidae) Braz. J. Biol. 2018;78(3):429–435. doi: 10.1590/1519-6984.166681. [DOI] [PubMed] [Google Scholar]
- Arora, J., Ramawat, K., 2014. Biology and biotechnology of gum yielding Indian trees. In: Ramawat, K.G, Mérillon, J.-M., huja, M.R. (Eds.). Tree Biotechnology.CRC Press, Boca Raton NY, pp. 125–150.
- Arora J., Ramawat K.G., 2017. An Introduction to Endophytes. In: Maheshwari D. (Eds). Endophytes: Biology and Biotechnology. Sustainable Development and Biodiversity, vol 15. Springer, Cham. http://doi.org/10.1007/978-3-319-66541-2_1.
- Balsanelli E., Serrato R.V., De Baura V.A., Sassaki G., Yates M.G., Rigo L.U., Monteiro R.A. Herbaspirillum seropedicae rfbB and rfbC genes are required for maize colonization. Environ. Microbiol. 2010;12(8):2233–2244. doi: 10.1111/j.1462-2920.2010.02187.x. [DOI] [PubMed] [Google Scholar]
- Balsanelli E., Tuleski T.R., de Baura V.A., Yates M.G., Chubatsu L.S., de Oliveira Pedrosa F., Monteiro R.A. Maize root lectins mediate the interaction with Herbaspirillum seropedicae via N-acetyl glucosamine residues of lipopolysaccharides. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0077001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamisile B.S., Dash C.K., Akutse K.S., Keppanan R., Wang L. Fungal endophytes: beyond herbivore management. Front. Microbiol. 2018;9:544. doi: 10.3389/fmicb.2018.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg G., Grube M., Schloter M., Smalla K. Unraveling the plant microbiome: looking back and future perspectives. Front. Microbiol. 2014;5:148. doi: 10.3389/fmicb.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brader G., Compant S., Vescio K., Mitter B., Trognitz F., Ma L.-J., Sessitsch A. Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Annu. Rev. Phytopathol. 2017;55:61–83. doi: 10.1146/annurev-phyto-080516-035641. [DOI] [PubMed] [Google Scholar]
- Brusamarello-Santos L.C., Gilard F., Brulé L., Quilleré I., Gourion B., Ratet P., Hirel B. Metabolic profiling of two maize (Zea mays L.) inbred lines inoculated with the nitrogen fixing plant-interacting bacteria Herbaspirillum seropedicae and Azospirillum brasilense. PLoS ONE. 2017;12(3) doi: 10.1371/journal.pone.0174576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby P.E., Ridout M., Newcombe G. Fungal endophytes: modifiers of plant disease. Plant Mol. Biol. 2016;90(6):645–655. doi: 10.1007/s11103-015-0412-0. [DOI] [PubMed] [Google Scholar]
- Castanheira N.L., Dourado A.C., Pais I., Semedo J., Scotti-Campos P., Borges N., Fareleira P. Colonization and beneficial effects on annual ryegrass by mixed inoculation with plant growth promoting bacteria. Microbiol. Res. 2017;198:47–55. doi: 10.1016/j.micres.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Chareprasert S., Piapukiew J., Thienhirun S., Whalley A.J., Sihanonth P. Endophytic fungi of teak leaves Tectona grandis L. and rain tree leaves Samanea saman Merr. World J. Microb. Biot. 2006;22(5):481–486. [Google Scholar]
- Compant S., Reiter B., Sessitsch A., Nowak J., Clément C., Barka E.A. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microbiol. 2005;71(4):1685–1693. doi: 10.1128/AEM.71.4.1685-1693.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S., Van Der Heijden M.G., Sessitsch A. Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol. Ecol. 2010;73(2):197–214. doi: 10.1111/j.1574-6941.2010.00900.x. [DOI] [PubMed] [Google Scholar]
- Das A., Varma A., 2009. Symbiosis: The Art of Living. In: Varma A., Kharkwal A.C. (Eds.). Symbiotic Fungi. Soil Biology, vol 18. Springer, Berlin, Heidelberg. http://doi.org/ 10.1007/978-3-540-95894-9_1.
- de Almeida Lopes K., Carpentieri-Pipolo V., Oro T., Stefani Pagliosa E., Degrassi G. Culturable endophytic bacterial communities associated with field-grown soybean. J. Appl. Microbiol. 2016;120(3):740–755. doi: 10.1111/jam.13046. [DOI] [PubMed] [Google Scholar]
- Devaraju R., Satish S. Endophytic Mycoflora of L. and Studies on Antimicrobial Activity of its Endophytic sp. Asian J. Exp. Biol. Sci. 2011;2:75–79. [Google Scholar]
- Ding T., Melcher U. Influences of plant species, season and location on leaf endophytic bacterial communities of non-cultivated plants. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0150895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeja S.S., Giri R., Saini R., Suneja-Madan P., Kothe E. Interaction of endophytic microbes with legumes. J. Basic Microbiol. 2012;52(3):248–260. doi: 10.1002/jobm.201100063. [DOI] [PubMed] [Google Scholar]
- Edwards J., Johnson C., Santos-Medellín C., Lurie E., Podishetty N.K., Bhatnagar S., Sundaresan V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. 2015;112(8):E911–E920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enebe M.C., Babalola O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: a survival strategy. Appl. Microbiol. Biotechnol. 2018;102(18):7821–7835. doi: 10.1007/s00253-018-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadiji A.E., Babalola O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020;8:467. doi: 10.3389/fbioe.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadiji A.E., Babalola O.O. Metagenomics methods for the study of plant-associated microbial communities: A review. J. Microbiol. Methods. 2020;170 doi: 10.1016/j.mimet.2020.105860. [DOI] [PubMed] [Google Scholar]
- Fierro-Cruz J.E., Jiménez P., Coy-Barrera E. Fungal endophytes isolated from Protium heptaphyllum and Trattinnickia rhoifolia as antagonists of Fusarium oxysporum. Rev. Argent. Microbiol. 2017;49(3):255–263. doi: 10.1016/j.ram.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Fouda A.H., Hassan S.E.-D., Eid A.M., Ewais E.E.D. Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.) Ann. Agric. Sci. 2015;60(1):95–104. [Google Scholar]
- Fürnkranz M., Lukesch B., Müller H., Huss H., Grube M., Berg G. Microbial diversity inside pumpkins: microhabitat-specific communities display a high antagonistic potential against phytopathogens. Microb. Ecol. 2012;63(2):418–428. doi: 10.1007/s00248-011-9942-4. [DOI] [PubMed] [Google Scholar]
- Gangadevi V., Muthumary J. Isolation of Colletotrichum gloeosporioides, a novel endophytic taxol-producing fungus from the leaves of a medicinal plant, Justicia gendarussa. Mycologia Balc. 2008;5:1–4. [Google Scholar]
- Gao D., Tao Y. Current molecular biologic techniques for characterizing environmental microbial community. Front. Env. Sci. Eng. 2012;6(1):82–97. [Google Scholar]
- Garbeva P., Van Overbeek L., Van Vuurde J., Van Elsas J. Analysis of endophytic bacterial communities of potato by plating and denaturing gradient gel electrophoresis (DGGE) of 16S rDNA based PCR fragments. Microb. Ecol. 2001;41(4):369–383. doi: 10.1007/s002480000096. [DOI] [PubMed] [Google Scholar]
- Gherbawy Y., Gashgari R. Molecular characterization of fungal endophytes from Calotropis procera plants in Taif region (Saudi Arabia) and their antifungal activities. Pl Biosystems. 2014;148(6):1085–1092. [Google Scholar]
- Glick B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014;169(1):30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Gouda S., Das G., Sen S.K., Shin H.-S., Patra J.K. Endophytes: a treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016;7:1538. doi: 10.3389/fmicb.2016.01538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S., Ramawat K.G., Mérillon J.M. Different shades of fungal metabolites: an overview. In: Mérillon J.-.M., Ramawat K.G., editors. Fungal Metabolites. Springer; Switzerland: 2016. pp. 1–29. [Google Scholar]
- Grönemeyer J.L., Burbano C.S., Hurek T., Reinhold-Hurek B. Isolation and characterization of root-associated bacteria from agricultural crops in the Kavango region of Namibia. Plant Soil. 2012;356(1–2):67–82. [Google Scholar]
- Gupta H., Saini R., Pagadala V., Kumar N., Sharma D., Saini A. Analysis of plant growth promoting potential of endophytes isolated from Echinacea purpurea and Lonicera japonica. J. Soil Sci. 2016;16(3):558–577. [Google Scholar]
- Hamayun M., Hussain A., Khan S.A., Kim H.-Y., Khan A.L., Waqas M., Jan S. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front. Microbiol. 2017;8:686. doi: 10.3389/fmicb.2017.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamayun M., Khan S.A., Ahmad N., Tang D.S., Kang S.-M., Na C.I., Lee B.H. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009;25(4):627–632. [Google Scholar]
- Hameed A., Yeh M.W., Hsieh Y.T., Chung W.C., Lo C.T., Young L.S. Diversity and functional characterization of bacterial endophytes dwelling in various rice (Oryza sativa L.) tissues, and their seed-borne dissemination into rhizosphere under gnotobiotic P-stress. Plant Soil. 2015;394(1–2):177–197. [Google Scholar]
- Hardoim P.R., Andreote F.D., Reinhold-Hurek B., Sessitsch A., van Overbeek L.S., van Elsas J.D. Rice root-associated bacteria: insights into community structures across 10 cultivars. FEMS Microbiol. Ecol. 2011;77(1):154–164. doi: 10.1111/j.1574-6941.2011.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardoim P.R., Van Overbeek L.S., Berg G., Pirttilä A.M., Compant S., Campisano A., Sessitsch A. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015;79(3):293–320. doi: 10.1128/MMBR.00050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Han G., Lin Y., Tian X., Xiang C., Tian Q., He Z. Diversity and decomposition potential of endophytes in leaves of a Cinnamomum camphora plantation in China. Ecol. Res. 2012;27(2):273–284. [Google Scholar]
- Ho M.-Y., Chung W.-C., Huang H.-C., Chung W.H., Chung W.H. Identification of endophytic fungi of medicinal herbs of Lauraceae and Rutaceae with antimicrobial property. Taiwania. 2012;57(3):229–241. [Google Scholar]
- Igiehon N.O., Babalola O.O. Rhizosphere microbiome modulators: contributions of nitrogen fixing bacteria towards sustainable agriculture. Inter. J. Environ. Res. Publ. Health. 2018;15(4):574. doi: 10.3390/ijerph15040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irabor A., Mmbaga M. Evaluation of Selected Bacterial Endophytes for Biocontrol Potential against Phytophthora Blight of Bell Pepper (Capsicum annuum L.) J. Plant Pathol. Microbiol. 2017;8(10):31–34. [Google Scholar]
- Johnston-Monje D., Raizada M.N. Conservation and diversity of seed associated endophytes in Zea across boundaries of evolution, ethnography and ecology. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B., Priya R.M. Bioactive Compounds from Endophytes and their Potential in. Am. J. Biochem. Mol. Biol. 2011;1(3):291–309. [Google Scholar]
- Kaddes A., Fauconnier M.L., Sassi K., Nasraoui B., Jijakli M.-H.J.M. Endophytic Fungal Volatile Compounds as Solution for Sustainable Agriculture. Molecules. 2019;24(6):1065. doi: 10.3390/molecules24061065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel S., Joubert P., Doty S. Bacterial endophyte colonization and distribution within plants. Microorganisms. 2017;5(4):77. doi: 10.3390/microorganisms5040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnwal A. Use of Bio-Chemical Surfactant Producing Endophytic Bacteria Isolated from Rice Root for Heavy Metal Bioremediation. Pertanika J. Trop. Agric. Sci. 2018;41(2):2. [Google Scholar]
- Kloepper J.W., McInroy J.A., Liu K., Hu C.H. Symptoms of Fern Distortion Syndrome resulting from inoculation with opportunistic endophytic fluorescent Pseudomonas spp. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0058531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Saxena R., Tomar R.S. 2017. Endophytic Microorganisms: Promising Candidate as Biofertilizer. In: Panpatte D., Jhala Y., Vyas R., Shelat H. (Eds.). Microorganisms for Sustainability, vol 6. Springer, Singapore. http://doi.org/10.1007/978-981-10-6241-4_4.
- Kumar S., Kaushik N., Edrada-Ebel R., Ebel R., Proksch P. Isolation, characterization, and bioactivity of endophytic fungi of Tylophora indica. World J. Microbiol. Biotechnol. 2011;27(3):571–577. [Google Scholar]
- Li J., Zhao G.Z., Huang H.Y., Qin S., Zhu W.Y., Zhao L.X., Strobel G. Isolation and characterization of culturable endophytic actinobacteria associated with Artemisia annua L. Antonie Van Leeuwenhoek. 2012;101(3):515–527. doi: 10.1007/s10482-011-9661-3. [DOI] [PubMed] [Google Scholar]
- Li Q., Wu L., Hao J., Luo L., Cao Y., Li J. Biofumigation on post-harvest diseases of fruits using a new volatile-producing fungus of Ceratocystis fimbriata. PLoS ONE. 2015;10(7) doi: 10.1371/journal.pone.0132009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Geng X., Xie R., Fu L., Jiang J., Gao L., Sun J. The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of Hybrid Pennisetum. Biotechnol. Biofuels. 2016;9(1):190. doi: 10.1186/s13068-016-0592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Carvalhais L.C., Crawford M., Singh E., Dennis P.G., Pieterse C.M., Schenk P.M. Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017;8:2552. doi: 10.3389/fmicb.2017.02552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Ding X., Deng B., Chen W. Isolation and characterization of endophytic taxol-producing fungi from Taxus chinensis. J. Ind. Microbiol. Biotechnol. 2009;36(9):1171. doi: 10.1007/s10295-009-0598-8. [DOI] [PubMed] [Google Scholar]
- Lu G.-H., Hua X.-M., Liang L., Wen Z.L., Du M.H., Meng F.F., Yang Y.H. Identification of major rhizobacterial taxa affected by a glyphosate-tolerant soybean line via shotgun metagenomic approach. Genes. 2018;9(4):214. doi: 10.3390/genes9040214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani V.M., Gnana Soundari A.P., Karthiyaini D., Preethi K. Bioprospecting endophytic fungi and their metabolites from medicinal tree Aegle marmelos in Western Ghats. India. Mycobiol. 2015;43(3):303–310. doi: 10.5941/MYCO.2015.43.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastretta C., Taghavi S., Van Der Lelie D., Mengoni A., Galardi F., Gonnelli C., Vangronsveld J. Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. Int. J. Phytoremed. 2009;11(3):251–267. [Google Scholar]
- Meneses C., Gonçalves T., Alquéres S., Rouws L., Serrato R., Vidal M., Baldani J. Gluconacetobacter diazotrophicus exopolysaccharide protects bacterial cells against oxidative stress in vitro and during rice plant colonization. Plant Soil. 2017;416(1–2):133–147. [Google Scholar]
- Meneses C.H., Rouws L.F., Simões-Araújo J.L., Vidal M.S., Baldani J.I. Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte Gluconacetobacter diazotrophicus. Mol. Plant Microbe Interact. 2011;24(12):1448–1458. doi: 10.1094/MPMI-05-11-0127. [DOI] [PubMed] [Google Scholar]
- Mmbaga M., Gurung M.A. Screening of Plant Endophytes as Biological Control Agents against Root Rot Pathogens of Pepper (Capsicum annum L.) J. Plant Pathol. Microbiol. 2018;9(435):2. [Google Scholar]
- Mohamad A., Abdalla O., Li L., Ma J., Hatab S.R., Xu L., Hedlund B.P. Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Front. Microbiol. 2018;9:924. doi: 10.3389/fmicb.2018.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muvea A., Meyhöfer R., Maniania N., Poehling H.-M., Ekesi S., Subramanian S. Behavioral responses of Thrips tabaci Lindeman to endophyte-inoculated onion plants. J. Pest. Sci. 2015;88(3):555–562. [Google Scholar]
- Nair D.N., Padmavathy S. Impact of endophytic microorganisms on plants, environment and humans. Sci. World J. Article ID. 2014;250693:1–11. doi: 10.1155/2014/250693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen R.M., Männistö M.K., van Elsas J.D. Endophytic bacterial communities in three arctic plants from low arctic fell tundra are cold-adapted and host-plant specific. FEMS Microbiol. Ecol. 2012;82(2):510–522. doi: 10.1111/j.1574-6941.2012.01464.x. [DOI] [PubMed] [Google Scholar]
- Nithya K., Muthumary Secondary metabolite from Phomopsis sp. isolated from Plumeria acutifolia Poiret. Recent Res Sci Technol. 2010;2(4):4. [Google Scholar]
- Nithya K., Muthumary J. Bioactive metabolite produced by Phomopsis sp., an endophytic fungus in Allamanda cathartica Linn. Recent Res Sci Technol. 2011;3(3):3. [Google Scholar]
- Oliveira C.M., Regasini L.O., Silva G.H., Pfenning L.H., Young M.C., Berlinck R.G., Araujo A.R. Dihydroisocoumarins produced by Xylaria sp. and Penicillium sp., endophytic fungi associated with Piper aduncum and Alibertia macrophylla. Phytochem. Lett. 2011;4(2):93–96. [Google Scholar]
- Otieno N., Lally R.D., Kiwanuka S., Lloyd A., Ryan D., Germaine K.J., Dowling D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Singh H., Farooqui A., Rakshit A. Fungal biofertilizers in Indian agriculture: perception, demand and promotion. J. Eco-friendly Agri. 2015;10(2):101–113. [Google Scholar]
- Pankievicz V., Camilios-Neto D., Bonato P., Balsanelli E., Tadra-Sfeir M., Faoro H., Passetti F. RNA-seq transcriptional profiling of Herbaspirillum seropedicae colonizing wheat (Triticum aestivum) roots. Plant Mol. Biol. 2016;90(6):589–603. doi: 10.1007/s11103-016-0430-6. [DOI] [PubMed] [Google Scholar]
- Pansanit A., Pripdeevech P. Antibacterial secondary metabolites from an endophytic fungus, Arthrinium sp. MFLUCC16-1053 isolated from Zingiber cassumunar. Mycology. 2018;9(4):264–272. doi: 10.1080/21501203.2018.1481154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J.K., Archana G. Diverse culturable diazotrophic endophytic bacteria from Poaceae plants show cross-colonization and plant growth promotion in wheat. Plant Soil. 2017;417(1–2):99–116. [Google Scholar]
- Patle P., Navnage N., Ramteke P. Endophytes in plant system: roles in growth promotion, mechanism and their potentiality in achieving agriculture sustainability. Int. J. Chem. 2018;6:270–274. [Google Scholar]
- Pereira, P., Ibáñez, F., Rosenblueth, M., Etcheverry, M., Martínez-Romero, E., 2011. Analysis of the bacterial diversity associated with the roots of maize (Zea mays L.) through culture-dependent and culture-independent methods. ISRN Ecol Article ID 938546, 1–10.
- Pinheiro, E.A.A., Carvalho, J.M., dos Santos, D.C.P., Feitosa, A.D.O., Marinho, P.S.B., Guilhon, G.M.S.P., Marinho, A.M.D.R., 2013. Antibacterial activity of alkaloids produced by endophytic fungus Aspergillus sp. EJC08 isolated from medical plant Bauhinia guianensis. Nat. Prod. Res. 27(18), 1633–1638. [DOI] [PubMed]
- Polizzi V., Fazzini L., Adams A., Picco A.M., De Saeger S., Van Peteghem C., De Kimpe N. Autoregulatory properties of (+)-thujopsene and influence of environmental conditions on its production by Penicillium decumbens. Microb. Ecol. 2011;62(4):838. doi: 10.1007/s00248-011-9905-9. [DOI] [PubMed] [Google Scholar]
- Posada F., Vega F.E. Inoculation and colonization of coffee seedlings (Coffea arabica L.) with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) Mycoscience. 2006;47(5):284–289. [Google Scholar]
- Potshangbam M., Devi S.I., Sahoo D., Strobel G.A. Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017;8:325. doi: 10.3389/fmicb.2017.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promputtha I., Hyde K.D., McKenzie E.H., Peberdy J.F., Lumyong S. Can leaf degrading enzymes provide evidence that endophytic fungi becoming saprobes? Fungal Divers. 2010;41(1):89–99. [Google Scholar]
- Radwan S., 2009. Phytoremediation for oily desert soils. In: Singh A., Kuhad R., Ward O. (Eds.). Advances in applied bioremediation. Soil Biology, vol 17. Springer, Berlin, Heidelberg. http://doi.org/10.1007/978-3-540-89621-0_15.
- Rajkumar, M., A,N., Prasad, M.N.V., Freitas, H., 2010. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 28(3), 142–149. [DOI] [PubMed]
- Reinhold-Hurek B., Hurek T. Living inside plants: bacterial endophytes. Curr. Opin. Plant Biol. 2011;14(4):435–443. doi: 10.1016/j.pbi.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Ren G., Zhang H., Lin X., Zhu J., Jia Z. Response of leaf endophytic bacterial community to elevated CO2 at different growth stages of rice plant. Front. Microbiol. 2015;6:855. doi: 10.3389/fmicb.2015.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Zhu C., Alam M.S., Tokida T., Sakai H., Nakamura H., Jia Z. Response of soil, leaf endosphere and phyllosphere bacterial communities to elevated CO2 and soil temperature in a rice paddy. Plant Soil. 2015;392(1–2):27–44. [Google Scholar]
- Rodríguez-Blanco A., Sicardi M., Frioni L.J.B. Plant genotype and nitrogen fertilization effects on abundance and diversity of diazotrophic bacteria associated with maize (Zea mays L.) Biol. Fert. Soils. 2015;51(3):391–402. [Google Scholar]
- Rothballer M., Eckert B., Schmid M., Fekete A., Schloter M., Lehner A., Hartmann A. Endophytic root colonization of gramineous plants by Herbaspirillum frisingense. FEMS Microbiol. Ecol. 2008;66(1):85–95. doi: 10.1111/j.1574-6941.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- Rungjindamai N., Pinruan U., Choeyklin R., Hattori T., Jones E. Molecular characterization of basidiomycetous endophytes isolated from leaves, rachis and petioles of the oil palm, Elaeis guineensis. Fungal Divers. 2008;33:139–161. [Google Scholar]
- Russell J.R., Huang J., Anand P., Kucera K., Sandoval A.G., Dantzler K.W., Koppstein D. Biodegradation of polyester polyurethane by endophytic fungi. Appl. Environ. Microbiol.. 2011;77(17):6076–6084. doi: 10.1128/AEM.00521-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago, C., Fitchett, C., Munro, M. H., Jalil, J., Santhanam, J. 2012. Cytotoxic and antifungal activities of 5-hydroxyramulosin, a compound produced by an endophytic fungus isolated from Cinnamomum mollisimum. Evid Based Complement Alternat. Med. 2012, Article ID 689310, pages, 1–6. [DOI] [PMC free article] [PubMed]
- Saravanakumar D., Samiyappan R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J. Appl. Microbiol. 2007;102(5):1283–1292. doi: 10.1111/j.1365-2672.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- Schulz B., Boyle C., Draeger S., Römmert A.-K., Krohn K. Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol. Res. 2002;106(9):996–1004. [Google Scholar]
- Senthilmurugan V., Sekar R., Kuru S., Balamurugan S. Phytochemical screening, enzyme and antibacterial activity analysis of endophytic fungi Botrytis sp. isolated from Ficus benghalensis (L.) Int. J. Pharm. Res. Biol. Sci. 2013;2(4):264–273. [Google Scholar]
- Sessitsch A., Hardoim P., Döring J., Weilharter A., Krause A., Woyke T., Rahalkar M. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant Microbe Interact. 2012;25(1):28–36. doi: 10.1094/MPMI-08-11-0204. [DOI] [PubMed] [Google Scholar]
- Shade A., Jacques M.-A., Barret M. Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin. Microbiol. 2017;37:15–22. doi: 10.1016/j.mib.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Sharma I.P., Chandra S., Kumar N., Chandra D. PGPR: Heart of Soil and Their Role in Soil Fertility. In: Meena V., Mishra P., Bisht J., Pattanayak A., editors. Agriculturally Important Microbes for Sustainable Agriculture. Springer; Singapore: 2017. [DOI] [Google Scholar]
- Sheibani-Tezerji R., Rattei T., Sessitsch A., Trognitz F., Mitter B. Transcriptome profiling of the endophyte Burkholderia phytofirmans PsJN indicates sensing of the plant environment and drought stress. MBio. 2015;6(5):e00621–00615. doi: 10.1128/mBio.00621-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S.Y., Fulthorpe R.J. Seasonal variation of bacterial endophytes in urban trees. Front. Microbiol. 2015;6:427. doi: 10.3389/fmicb.2015.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.K., Strobel G.A., Knighton B., Geary B., Sears J., Ezra D. An endophytic Phomopsis sp. possessing bioactivity and fuel potential with its volatile organic compounds. Microb. Ecol. 2011;61(4):729–739. doi: 10.1007/s00248-011-9818-7. [DOI] [PubMed] [Google Scholar]
- Smith P., House J.I., Bustamante M., Sobocká J., Harper R., Pan G., Rumpel C. Global change pressures on soils from land use and management. Glob. Change Biol. 2016;22(3):1008–1028. doi: 10.1111/gcb.13068. [DOI] [PubMed] [Google Scholar]
- Stewart E.J. Growing unculturable bacteria. J. Bacteriol. 2012;194(16):4151–4160. doi: 10.1128/JB.00345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F., Jacquard C., Villaume S., Michel J., Rabenoelina F., Clément C., Vaillant-Gaveau N. Burkholderia phytofirmans PsJN reduces impact of freezing temperatures on photosynthesis in Arabidopsis thaliana. Front. Plant Sci. 2015;6:810. doi: 10.3389/fpls.2015.00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subban K., Subramani R., Johnpaul M. A novel antibacterial and antifungal phenolic compound from the endophytic fungus Pestalotiopsis mangiferae. Nat. Prod. Res. 2013;27(16):1445–1449. doi: 10.1080/14786419.2012.722091. [DOI] [PubMed] [Google Scholar]
- Subbulakshmi G., Thalavaipandian A., Ramesh V. Bioactive endophytic fungal isolates of Biota orientalis (L) Endl., Pinus excelsa Wall. and Thuja occidentalis L. Int. J. Adv. Life Sci. 2012;4:9–15. [Google Scholar]
- Subramanian P., Mageswari A., Kim K., Lee Y., Sa T. Psychrotolerant endophytic Pseudomonas sp. strains OB155 and OS261 induced chilling resistance in tomato plants (Solanum lycopersicum Mill.) by activation of their antioxidant capacity. Mol. Plant Microbe Interact. 2015;28(10):1073–1081. doi: 10.1094/MPMI-01-15-0021-R. [DOI] [PubMed] [Google Scholar]
- Surette M.A., Sturz A.V., Lada R.R., Nowak J. Bacterial endophytes in processing carrots (Daucus carota L. var. sativus): their localization, population density, biodiversity and their effects on plant growth. Plant Soil. 2003;253(2):381–390. [Google Scholar]
- Suryanarayanan T.S. Endophyte research: going beyond isolation and metabolite documentation. Fungal Ecol. 2013;6(6):561–568. [Google Scholar]
- Suwannarach N., Kumla J., Bussaban B., Nuangmek W., Matsui K., Lumyong S. Biofumigation with the endophytic fungus Nodulisporium spp. CMU-UPE34 to control postharvest decay of citrus fruit. Crop Prot. 2013;45:63–70. [Google Scholar]
- Tayung K., Barik B., Jha D., Deka D. Identification and characterization of antimicrobial metabolite from an endophytic fungus, Fusarium solani isolated from bark of Himalayan yew. Mycosphere. 2011;2(3):203–213. [Google Scholar]
- Tefera T., Vidal S. Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. Biocontrol. 2009;54(5):663–669. [Google Scholar]
- Thongsandee W., Matsuda Y., Ito S. Temporal variations in endophytic fungal assemblages of Ginkgo biloba L. J. For. Res. 2012;17(2):213–218. [Google Scholar]
- Tian B., Zhang C., Ye Y., Wen J., Wu Y., Wang H., Cheng Z. Beneficial traits of bacterial endophytes belonging to the core communities of the tomato root microbiome. Agric. Ecosyst. Environ. 2017;247:149–156. [Google Scholar]
- Tomsheck A.R., Strobel G.A., Booth E., Geary B., Spakowicz D., Knighton B., Ezra D. Hypoxylon sp., an endophyte of Persea indica, producing 1, 8-cineole and other bioactive volatiles with fuel potential. Microb. Ecol. 2010;60(4):903–914. doi: 10.1007/s00248-010-9759-6. [DOI] [PubMed] [Google Scholar]
- Tumangger, B.S., Nadilla, F., Baiduri, N., Mardina, V., 2018. In vitro screening of endophytic fungi associated with mangroveas biofertilizer on the growth of black rice (Oryza sativa L.“ Cempo Ireng”). Paper presented at the IOP Conference Ser.: Mater. Sci. Eng.. 420(1), 012080.
- Van Overbeek L., Van Elsas J.D. Effects of plant genotype and growth stage on the structure of bacterial communities associated with potato (Solanum tuberosum L.) FEMS Microbiol. Ecol. 2008;64(2):283–296. doi: 10.1111/j.1574-6941.2008.00469.x. [DOI] [PubMed] [Google Scholar]
- Wang S., Hu T., Jiao Y., Wei J., Cao K. Isolation and characterization of Bacillus subtilis EB-28, an endophytic bacterium strain displaying biocontrol activity against Botrytis cinerea Pers. Front. Agric. China. 2009;3(3):247–252. [Google Scholar]
- Wu S.H., Huang R., Miao C.P., Chen Y.W. Two new steroids from an endophytic fungus Phomopsis sp. Chem. Biodivers. 2013;10(7):1276–1283. doi: 10.1002/cbdv.201200415. [DOI] [PubMed] [Google Scholar]
- Xin G., Zhang G., Kang J.W., Staley J.T., Doty S.L. A diazotrophic, indole-3-acetic acid-producing endophyte from wild cottonwood. Biol. Fert. Soils. 2009;45(6):669–674. [Google Scholar]
- Yandigeri M.S., Meena K.K., Singh D., Malviya N., Singh D.P., Solanki M.K., Arora D.K. Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul. 2012;68(3):411–420. [Google Scholar]
- Yu X., Yang J., Wang E., Li B., Yuan H. Effects of growth stage and fulvic acid on the diversity and dynamics of endophytic bacterial community in Stevia rebaudiana Bertoni leaves. Front. Microbiol. 2015;6:867. doi: 10.3389/fmicb.2015.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Feng H., Wang L., Li Z., Shi Y., Zhao L., Zhu H. Potential of endophytic fungi isolated from cotton roots for biological control against verticillium wilt disease. PLoS ONE. 2017;12(1) doi: 10.1371/journal.pone.0170557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakria M., Njoloma J., Saeki Y., Akao S. Colonization and nitrogen-fixing ability of Herbaspirillum sp. strain B501 gfp1 and assessment of its growth-promoting ability in cultivated rice. Microbes. Environ. 2007;22(3):197–206. [Google Scholar]
- Zhang J., Subramanian S., Zhang Y., Yu O. Flavone synthases from Medicago truncatula are flavanone-2-hydroxylases and are important for nodulation. Plant Physiol. 2007;144(2):741–751. doi: 10.1104/pp.106.095018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Qi G., Zhang X., Lan N., Ma X. Controlling sap-sucking insect pests with recombinant endophytes expressing plant lectin. Nature Precedings. 2010;21:article 21. doi: 10.1038/npre.2010.4862.1. [DOI] [Google Scholar]