Abstract
Introduction
Early childhood caries is a multifactorial disease. Saliva plays an important role in initiation and protection against caries, and its composition is greatly affected by nutritional status. This study was conducted to determine the impact of salivary lactoperoxidase and histatin-5 on the severity of ECC in relation to nutritional status.
Materials and methods
The sample consisted of 120 children aged 5 years, classified into eight groups: mild ECC in underweight children, mild ECC in normalweight children, moderate ECC in underweight children, moderate in ECC normal weight children, severe ECC in underweight children, severe ECC in normalweight, caries-free (control) underweight children and caries-free normalweight children. Each group consisted of 15 children. Stimulated saliva was collected. Salivary lactoperoxidase was analysed using Human LPO/ Lactoperoxidase ELISA Kit (CLIA)-LS-F29892, and salivary histatin-5 was analysed using Human Histatin-5 ELISA Kit MBS705083_48T.
Results
Lactoperoxidase and histatin-5 concentrations were significantly higher in caries-free children than in children with ECC, and they were higher in children with mild ECC than in children with moderate ECC or in children with severe ECC. They were significantly higher among children with normal weight than among those who were underweight (p < 0.01). ECC and nutritional status recorded non-significant interactions with both LPO and HST-5 (p > 0.01), but there was significant interaction between these two variables and LPO and HST-5 together (p < 0.01). The Pearson's correlation coefficient test recorded significant negative correlations between ECC severity and both salivary lactoperoxidase and histatin-5 among the eight study groups, whereas significant positive correlations were recorded between BMI values and both salivary lactoperoxidase and histatin-5 among the eight study groups.
Conclusion
Salivary lactoperoxidase and histatin-5 may be affected by nutritional status, and these two parameters may play an important role in caries prevention at high concentrations. There is interaction between these two parameters and ECC severity and nutrition.
Keywords: Early childhood caries severity, Nutritional status, Salivary lactoperoxidase, Salivary histatin-5
Abbreviations: ECC, early childhood caries; LPO, lactoperoxidase; HST-5, histatin-5
1. Introduction
Early childhood caries (ECC) is the presence of one or more decayed (non-cavitated or cavitated lesion), missing (due to caries), or filled primary teeth in children aged 71 months (5 years) or younger. Clinically, ECC has several unique characteristics, such as rapid development of caries that affects a number of teeth soon after they emerge in the oral cavity. It develops on tooth surfaces that are usually at low risk for caries, such as the labial surfaces of maxillary incisors and lingual and buccal surfaces of maxillary and mandibular molars. Several terminologies have been used to describe ECC as nursing bottle caries, nursing caries, rampant caries, baby bottle caries, baby bottle tooth decay, milk bottle syndrome, and prolonged nursing habit caries (Feldens et al., 2010, Anil and Anand, 2017).
ECC is a multifactorial disease that results from the interaction of various etiological factors, including cariogenic microorganisms, fermentable carbohydrates (substrate), and susceptible tooth surface/host. Local environmental factors, including the composition of teeth and saliva and the nutritional status, are the most important factors affecting early childhood caries (Ja’fer, 2011, Kadoum and Salih, 2014).
Nutritional status may act as an important risk factor in ECC as recorded by several studies (Slabsinskiene et al., 2010, Senesombath et al., 2010, Kadoum and Salih, 2014), and saliva is one of the important protective factors against dental caries development by its chemical composition and physiological properties (Palmer et al., 2010, Kadoum and Salih, 2014).
Salivary enzymes such as lysozyme and lactoperoxidase play an important role in the maintenance of oral health and are the first line of defence of the host against pathogens such as Streptococcus mutans, Streptococcus sobrinus and Streptococcus sanguinis. Salivary lactoperoxidase (LPO) is a protein with unique enzymatic activity that protects salivary proteins from bacterial degradation. It works in conjunction with thiocyanate and hydrogen peroxide. The products of this interaction inhibit glucose metabolism and oxidise bacterial sulfhydryl groups (Bafort et al., 2014, Gornowicz et al., 2014).
Salivary histatine-5 (HST-5) is one of the protective factors in saliva; it is a salivary peptide that consists of 24 amino acids produced in parotid and sublingual salivary glands. The HST group includes HST-1, −2, −3, −4, −5 and −6. From the HST group, salivary HST-5 was demonstrated to possess fungicidal and fungistatic properties against Candida albicans and antiviral activity against the human immunodeficiency virus (HIV), in addition to antibacterial properties against several types of oral bacteria (Lang et al., 2010, Krzyściak et al., 2015). This study was conducted to determine the impact of salivary LPO and HST-5 on the severity of ECC in relation to nutritional status.
2. Materials and methods
2.1. The sample
The study and the control groups consisted of 120 children aged 5 years old, classified into eight groups: mild ECC in underweight children, mild ECC in normal-weight children, moderate ECC in underweight children, moderate ECC normal-weight children, severe ECC in underweight children, severe ECC in normal-weight, caries-free (control) underweight children, and caries-free normal-weight children. Each group consisted of 15 children. ECC was diagnosed clinically according to the following criteria:
• Any smooth surface of an antero-posterior deciduous tooth that is decayed, missing (due to caries) or filled in children aged 5 years old.
• A decay-missing-filled teeth (DMFT) index equal to or greater than 6 at the age of 5 years old (American Academy of Pediatrics - Pediatric Dentistry, 2010).
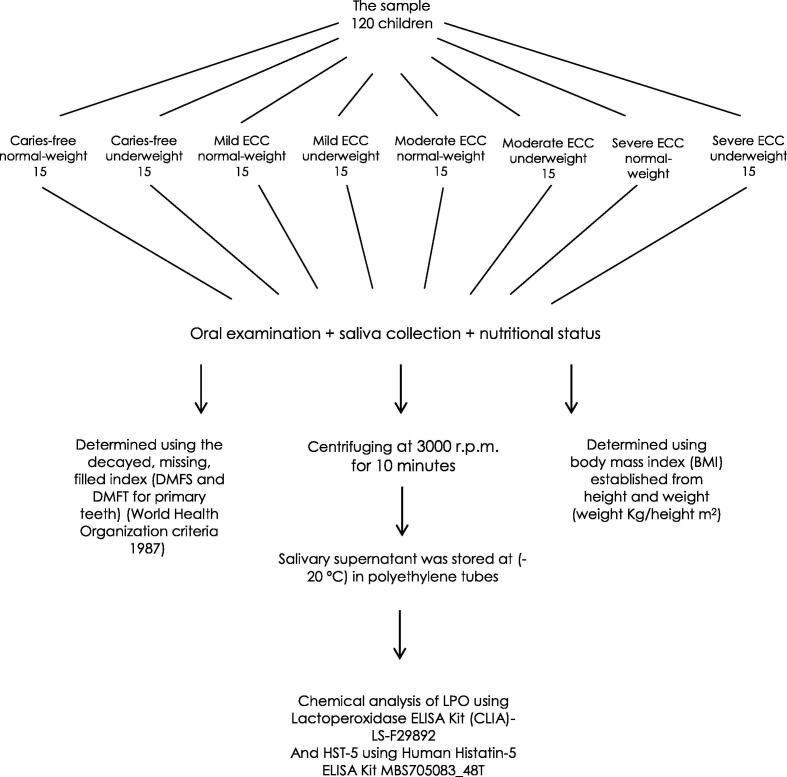
None of the participants were taking any food supplements or drugs, and they were free from any systemic diseases. The study design is illustrated in Fig. 1. The objectives of the study were explained to each child’s parents according to informed consent, and they agreed to participate. Involvement of human subjects in this work was in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Fig. 1.
Study design (illustrates the study group classifications and the steps of salivary sample preparation, oral examinations and recording of the nutritional status.
2.2. Assessment of dental caries, nutritional status and collections of saliva
Assessment and recording of caries experience was done by the application of the decayed, missing, filled (DMFS and DMFT for primary teeth) index (World Health Organization criteria, 2013). Patients were classified into three groups according to decay fraction (ds) of the DMFS index (mild with decayed surfaces <6, moderate with decayed fraction from 6 to-13 and severe with decayed fraction ˃13 [Chawd et al., 2011, Kadoum and Salih, 2014] each group consisted of 15 children and the same number of children was included in the control group. Nutritional status was determined by using the body mass index (BMI) produced from calculation of height and weight: weight kg/height m2; the reference population defined by the National Centre for Health Statistics, in collaboration with the National Centre for Chronic Disease Prevention and Health promotion was used. To determine the nutritional status of each child involved in this study, the BMI value was crossed against age on special charts one prepared for boys and the other prepared for girls. Stimulated saliva was collected between 9 AM and-11 AM. Each participant was asked not to eat or drink (except water) one hour before collection. They were seated in a relaxed position in a dental chair without any heavy physical stress and were asked to chew a piece of Arabic gum for one minute and then expectorate all saliva (Tenovuo and Lagerlöf, 1994). Each saliva sample was then centrifuged (Hettich Universal 16 A centrifuge) at 3000 revolutions per minute (r.p.m) for 10 min. The salivary supernatant was stored at −20 °C in polyethylene tubes for subsequent chemical analysis.
2.3. Analysis of saliva and data
After thawing the frozen salivary samples, salivary lactoperoxidase and salivary histatin-5 were analysed. Salivary lactoperoxidase was analysed using Human LPO/Lactoperoxidase enzyme-linked immunosorbent assay (ELISA) Kit (CLIA)-LS-F29892, designed for the quantitative measurement of lactoperoxidase in biological fluids. The procedure’s principle works on each well of the supplied microtiter plate that has been pre-coated with a target-specific capture antibody. Standards or samples are added to the wells and the target antigen binds to the capture antibody. A biotin-conjugated detection antibody is then added which binds to the captured antigen. Unbound detection antibody is washed away. An avidin-horseradish peroxidase (HRP) conjugate is then added which binds to the biotin. Unbound avidin-HRP conjugate is washed away. A chemiluminescent substrate is then added which reacts with the HRP enzyme, resulting in light development.
Salivary histatin-5 was analysed using Human Histatin-5 ELISA Kit MBS705083_48T, designed for the quantitative measurement of histatin-5 in serum, plasma and saliva. The procedure’s principle works on antibodies specific for histatin-5 that have been pre-coated on a microplate. Standards and samples are pipetted into the wells and any histatin-5 present is bound by the immobilised antibody. After removing any unbound substances, a biotin-conjugated antibody specific for histatin-5 is added to the wells. After washing, avidin-conjugated HRP is added to the wells. Following a wash to remove any unbound avidin-enzyme reagent, a substrate solution is added to the wells and colour develops in proportion to the amount of histatin-5 bound in the initial step. The colour development is stopped and the intensity of the colour is measured.
Each saliva sample was analysed using an ELISA reader device, HumaReader HS. Data were analysed using SPSS (Statistical Package for Social Sciences; IBM Company, USA) software, version 19, by application of both descriptive statistics, including number, mean and standard deviation and application of interferential statistics, including two-way univariate analysis of variance (two-way ANOVA), two-way multivariate analysis of variance (two-way MANOVA), and Pearson’s correlation coefficient. The confidence limit was accepted at 95% (P < 0.05).
3. Results
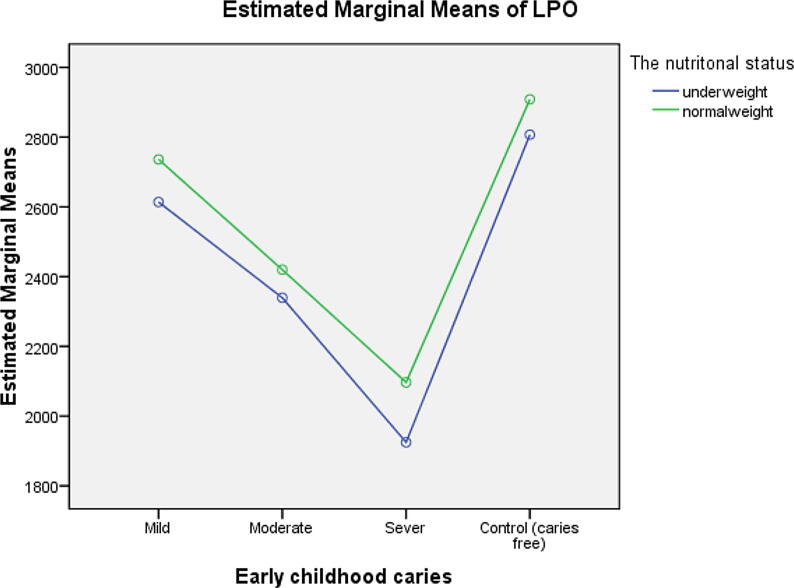
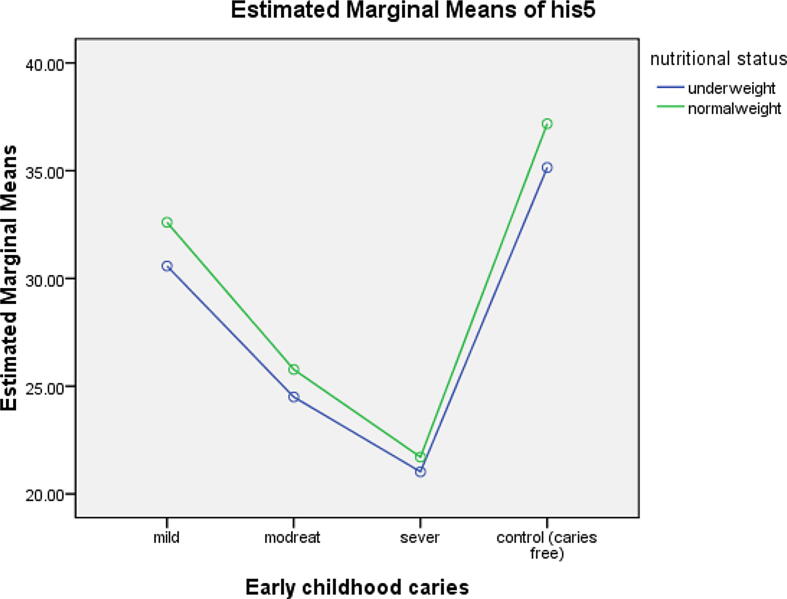
The LPO and HST-5 concentrations were higher among caries-free children than among ECC-affected children; in descending order, they were higher among children with mild ECC, followed by those with moderate ECC and, finally, by those with severe ECC. They were higher among children with normal weight than among those who were underweight. Caries-free normal-weight children had the highest concentrations of LPO and HST-5, followed in order by the caries-free underweight children, the normal-weight children with mild ECC, the underweight children with mild ECC, the normal-weight children with moderate ECC, the underweight children with moderate ECC, the normal-weight children with severe ECC and, finally, the underweight children with severe ECC as shown in Table 1. The profile plot estimating the marginal means of LPO and HST-5 among the control and study groups is illustrated in Fig. 2, Fig. 3, respectively.
Table 1.
Distribution of salivary lactoperoxidase (nmol/L) and histatin-5 (ng/ml) among the control and study groups.
| Salivary LPO | |||||
|---|---|---|---|---|---|
| Study populations | Control (caries free) Mean ± SD (n) | Mild ECC (ds < 6) Mean ± SD (n) | Moderate ECC (ds 6–13) Mean ± SD (n) | Sever ECC (ds˃13) Mean ± SD (n) | Total (n) |
| Underweight | 2807 ± 63 (15) | 2613 ± 85 (15) | 2339 ± 52 (15) | 1924 ± 34 (15) | 2421 ± 339 (60) |
| Normal-weight | 2908 ± 31 (15) | 2736 ± 109 (15) | 2419 ± 59 (15) | 2096 ± 196 (15) | 2540 ± 333 (60) |
| Total (n) | 2857 ± 71 (30) | 2674 ± 114 (30) | 2379 ± 68 (30) | 2010 ± 163 (30) | |
| Salivary histatin-5 | |||||
| Underweight | 35.1 ± 0.7 (15) | 30.5 ± 1.9 (15) | 24.4 ± 0.8 (15) | 21.0 ± 1.5 (15) | 27.8 ± 5.6 (60) |
| Normal-weight | 37.1 ± 0.4 (15) | 32.6 ± 2.3 (15) | 25.7 ± 0.6 (15) | 21.7 ± 1.3 (15) | 29.3 ± 0.1 (60) |
| Total (n) | 36.1 ± 1.2 (30) | 31.5 ± 2.3 (30) | 25.1 ± 1.0 (30) | 21.3 ± 1.4 (30) | |
SD: standard deviation; nmol/L: nanomoles per litre; ng/ml: nanogram per millilitre, ECC: early childhood caries; n: number.
Fig. 2.
Profile plot of the estimated marginal mean of LPO.
Fig. 3.
Profile plot of the estimated marginal mean of HST-5.
Both ECC severity and nutritional status had statistically significant interactions with LPO (p < 0.005). The differences in the mean of the LPO were statistically significant between the control and the ECC groups and between underweight and normal-weight children. There was no statistically significant interaction between ECC severity and nutritional status in LPO. Furthermore both ECC severity and nutritional status had statistically significant interactions with HST-5 (p < 0.005). The differences in the mean of HST-5 were statistically significant between the control and the ECC groups and between underweight and normal-weight children. There was no statistically significant interaction between ECC severity and nutritional status in HST-5. There was statistically significant interaction between ECC severities and nutritional status in both salivary LPO and HST-5 (p < 0.005), as shown in Table 2.
Table 2.
Statistical differences and interactions of both salivary lactoperoxidase and salivary histatin-5 among control and study groups.
| Two-way ANOVA test | |||
|---|---|---|---|
| Salivary lactoperoxidase | |||
| Variables | Two-way ANOVA test | ||
| F-test | P value | ||
| Early childhood caries | 468.64 | 0.000** | |
| Nutritional status | 48.46 | 0.000** | |
| Early childhood caries*nutritional status | 1.31 | 0.275 | |
| Salivary histatin-5 | |||
| Variable | Two-way ANOVA test | ||
| F-test | P value | ||
| Early childhood caries | 681.17 | 0.000** | |
| Nutritional status | 35.58 | 0.000** | |
| Early childhood caries*nutritional status | 1.67 | 0.17 | |
| Two-way MANOVA test | |||
| Variables | F-test | P value | Wilks, ^ |
| Early childhood caries | 184.62 | 0.000** | 0.028 |
| Nutritional status | 25.83 | 0.000** | 0.682 |
| Early childhood caries*nutritional status | 4.046 | 0.004** | 0.753 |
Highly significant.
Pearson’s correlation coefficient indicated significant negative correlation between both salivary LPO and HST-5 and the ECC severity among the six study groups, as shown in Table 3. Pearson’s correlation coefficient indicated significant positive correlations between both salivary LPO and HST-5 and the BMI value among the six study groups, as shown in Table 4.
Table 3.
Correlations between salivary lactoperoxidase and histatin-5 of the study groups and ECC severity.
| Salivary LPO correlations | ||||||
|---|---|---|---|---|---|---|
| Study groups | Mild ECC |
Moderate ECC |
Severe ECC |
|||
| r | P value | r | P value | r | P value | |
| Underweight | −0.70 | 0.003** | −0.87 | 0.000** | −0.77 | 0.001** |
| Normal-weight | −0.82 | 0.000** | −0.35 | 0.196 | −0.79 | 0.000** |
| Salivary histatin-5 correlations | ||||||
| Study groups | Mild ECC | Moderate ECC | Severe ECC | |||
| r | P value | r | P value | r | P value | |
| Underweight | − 0.94 | 0.000** | − 0.95 | 0.000** | − 0.96 | 0.000** |
| Normal-weight | − 0.87 | 0.000** | − 0.68 | 0.005** | − 0.95 | 0.000** |
Highly significant.
Table 4.
Correlations between salivary lactoperoxidase and histatin-5 of the study groups and BMI values.
| Salivary LPO correlations | ||||||
|---|---|---|---|---|---|---|
| Study groups | Mild ECC |
Moderate ECC |
Sever ECC |
|||
| r | P value | r | P value | r | P value | |
| Underweight | 0.69 | 0.003** | 0.86 | 0.000** | 0.74 | 0.001** |
| Normal-weight | 0.90 | 0.000** | 0.75 | 0.000** | 0.83 | 0.000** |
| Salivary histatin-5 correlations | ||||||
| Study groups | Mild ECC | Moderate ECC | Sever ECC | |||
| r | P value | r | P value | r | P value | |
| Underweight | 0.88 | 0.000** | 0.89 | 0.000** | 0.95 | 0.000** |
| Normal-weight | 0.92 | 0.000** | 0.73 | 0.005** | 0.84 | 0.000** |
Highly significant.
4. Discussion
Dental caries is the most common disease affecting children and adolescents worldwide. Saliva plays a vital role in immunologicale defence against carious lesions (Palmer et al., 2010). The major salivary components are enzymes such as lactoperoxidase and proteins such as histatin-5. Studies have been conducted to determine the effect of salivary lactoperoxidase and histatin-5 on caries severity among adolescents (Gornowicz et al., 2014) and to determine salivary histatin-5 among children with ECC (Jurczak et al., 2015). The relationship between saliva composition and nutritional status has been examined by several studies (Kadoum and Salih, 2014, Méjean et al., 2015a, Méjean et al., 2015b), but no previous study has analysed the interactions among these two salivary components, nutritional status and ECC. This study was conducted to determine the interactions between both salivary lactoperoxidase and histatin-5 on the severity of ECC and nutritional status.
According to the present study, LPO and histatin-5 were statistically significantly higher among caries-free children, followed by those with mild ECC and then those with moderate caries and, finally, those with severe caries, and significant interactions were recorded between ECC severity and both LPO and HST-5. These results were in accordance with the study by Krzyściak et al. (2015), but disagreed with the studies by Gornowicz et al., 2014, Jurczak et al., 2015.
However, HST-5 possessed killing and growth-inhibitory activity against several species of oral bacteria, including that of S. mutans, by destabilising the cellular membranes of the bacteria and assimilating their surfaces leading to cell damage. Furthermore, HST-5 demonstrated an anti-biofilm-forming activity against S. mutans by acting as a ligand, causing co-aggregation of S. mutans cells with other types of physiological flora microorganisms, thus inhibiting the adhesive properties of S. mutans on teeth surfaces and gums. Therefore, the pathogen may be removed from the oral cavity with saliva before the onset of the first stage of biofilm formation, thus inhibiting the process of cariogenesis (Lang et al., 2010, Krzyściak et al., 2015). LPO is a member of the mammalian heme peroxidase family that uses hydrogen peroxide (H2O2) to catalyse the oxidation of thiocyanate (SCN-) and convert it to hypothiocyanite (OSCN-). LPO and thiocyanate are components of the human salivary secretions, whereas hydrogen peroxide is excreted by oral bacteria. The OSCN- ion inhibits bacterial glyceraldehyde 3-P dehydrogenases and thereby stops the bacterial production of acids from sugars. The inhibition of bacterial acid production by OSCN- has been implicated in playing an important role in the prevention of dental caries (Fragoso et al., 2010, Bafort et al., 2014).
These results could be further supported by the significant negative correlations that were recorded between dental caries severity and LPO and HST-5 among the study groups, indicating that elevation of these two parameters in saliva may increase salivary protection against dental caries among children.
LPO and HST-5 were also statistically significantly higher among the normal-weight children than among the underweight children, and nutritional status had a significant effect in both LPO and HST-5. There are limitations in studies regarding the interactions between salivary composition and nutritional status. However, the results of the present study were in accordance with a study by Méjean et al., 2015a, Méjean et al., 2015b.
Pathological process and disease symptoms in the oral cavity are often associated with impaired saliva composition and secretion rate, and saliva composition and secretion are greatly influenced by nutrition. During childhood, nutrition plays an essential role in the growth and development of the child (Ghosh et al., 2015). Studies by Kadoum and Salih, 2014, Munther, 2016 found that under nutrition during childhood may be associated with reduction in the saliva secretion rate, in salivary elements and in protein secreted per minute. A study by Sheetal et al. (2013) demonstrated that malnutrition also interferes negatively with humoral and cellular immune responses and with tissue and reparative functions.
These results could be further supported by the significant negative correlations that were recorded between the nutritional status and LPO and HST-5 among the study groups, indicating that under nutrition may affect the protein composition of saliva and its immune and protective properties. However, other salivary factors such as flow rate, pH and viscosity may be estimated in addition to salivary composition in relation to nutritional status.
There was no significant interaction between ECC severity and nutritional status in either LPO or HST-5 separately, but there was significant interaction between ECC severity and nutritional status in LPO and HST-5 together. These results could be confusing, but they may indicate that nutrition can affect salivary composition and the immune system, whereas ECC severity is affected by salivary alterations associated with nutrition. Although interaction among salivary composition and ECC severity and nutrition has been found, the exact mechanism of both LPO and HST-5 in caries severity and progression is controversial among the studies (Gornowicz et al., 2014, Krzyściak et al., 2015, Jurczak et al., 2015). Further studies are recommended to determine the exact mechanisms of these two variables in ECC in relation to nutritional status.
5. Conclusion
Salivary lactoperoxidase and histatin-5 may be affected by nutritional status, and these two parameters may play an important role in caries prevention at high concentrations. There is interaction among these two parameters and ECC severity and nutrition. However, further studies in this field are recommended. Salivary flow rate, secretory rate and viscosity need to be conducted in future studies, in addition to salivary composition in relation to ECC and nutritional status.
Ethical statement
This work had been approved by the ethical committee in college of dentistry/University of Baghdad. Furthermore, for each volunteer the objectives of the study were explained to, and they approved to participate. Involvement of human subjects in this work was in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- American Academy of Pediatrics - Pediatric dentistry, 2010. 63rd Annual Session, Hilton Hotel and Towers Chicago, Illinois.
- Anil S., Anand P. Early Childhood Caries: Prevalence, Risk Factors, and Prevention. Front. Pediatr. 2017;5(157):PMC5514393. doi: 10.3389/fped.2017.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafort F., Parisi O., Perraudin J., Jijakli M. Mode of Action of Lactoperoxidase as Related to Its Antimicrobial Activity: A Review Article. Enzyme Res. 2014 doi: 10.1155/2014/517164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawd J., Chaduvula N., Patel H., Jain S. Salivary SIgA and dental caries activity. Indian Pediatric. 2011;48:719–721. doi: 10.1007/s13312-011-0113-y. [DOI] [PubMed] [Google Scholar]
- Feldens C.A., Giugliani E.R., Duncan B.B., Drachler Mde L., Vitolo M.R. Long-term effectiveness of a nutritional program in reducing early childhood caries: a randomized trial. Commun. Dent. Oral Epidemiol. 2010;38(4):324–332. doi: 10.1111/j.1600-0528.2010.00540.x. [DOI] [PubMed] [Google Scholar]
- Fragoso M., Torbati A., Fregien N., Conner G. Molecular heterogeneity and alternative splicing of human lactoperoxidase. Arch. Biochem. Biophy. 2010;482:52–57. doi: 10.1016/j.abb.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Pallavi S., Nagpal B., Hegde U. Nutrition and Oral Health: A Review. Indian J. Appl. Res. 2015;5(11):546–549. [Google Scholar]
- Gornowicz A., Tokajuk Q., Bielawska A., Maciorkowska E., Jabłoński R., Wójcicka A., Bielawski K. The assessment of sIgA, histatin-5, and lactoperoxidase levels in saliva of adolescents with dental caries. Med. Sci. Monit. 2014;20:1095–1100. doi: 10.12659/MSM.890468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja’fer Z. The level of streptococcus mutans and lactobacilli in the dental plaque among rampant, nursing and caries free children. J. Bagh. College Dentistry. 2011;23(4):158–162. [Google Scholar]
- Jurczak A., Kościelniak D., Papież M., Vyhouskaya P., Krzyściak W. A study on β-defensin-2 and histatin-5 as a diagnostic marker of early childhood caries progression. Biol. Res. 2015;48(61) doi: 10.1186/s40659-015-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoum N., Salih B. Selected salivary constituents, physical properties and nutritional status in relation to dental caries among 4–5 year’s old children (Comparative study) J. Bagh. College Dentistry. 2014;26(2):150–156. [Google Scholar]
- Krzyściak W., Jurczak A., Piątkowski J., Kościelniak D., Gregorczyk-Maga L., Kołodziej L., Monika A., Papież A., Olczak-Kowalczyk D. Effect of histatin-5 and lysozyme on the ability of Streptococcus mutans to form biofilms in in vitro conditions. Adv. Hygiene Exp. Med. 2015;69:1056–1066. [PubMed] [Google Scholar]
- Lang C., Böttner M., Holz C., Veen M., Ryser M., Reindl A., Pompejus M., Tanzer J. Specific Lactobacillus/Mutans Streptococcus co–aggregation. J. Dent. Res. 2010;89:175–179. doi: 10.1177/0022034509356246. [DOI] [PubMed] [Google Scholar]
- Méjean C., Neyraud M., Issanchou Y., Martin C., Bozonnet S., Urbano C., Schlich P., Hercberg S., Péneau S., Feron G. Salivary composition is associated with liking and usual nutrient intake. PLoS ONE. 2015;10(9):e0137473. doi: 10.1371/journal.pone.0137473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méjean C., Morzel M., Neyraud E., Issanchou S., Martin C., Bozonnet S., Urbano C., Schlich P., Hercberg S., Péneau S., Feron G. Salivary composition is associated with liking and usual nutrient intake. PLoS ONE. 2015;10(9):e0137473. doi: 10.1371/journal.pone.0137473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munther Sh. Nutritional status among a group of preschool children in relation to concentration of selected elements in saliva and caries severity (a comparative study) J. Bagh. College Dentistry. 2016;28(1):147–152. [Google Scholar]
- Palmer C.A., Kent R., Jr, Loo C.Y., Hughes C.V., Stutius E., Pradhan N. Diet and caries-associated bacteria in severe early childhood caries. J. Dent. Res. 2010;89(11):1224–1229. doi: 10.1177/0022034510376543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senesombath S., Nakornchai S., Banditsing P., Lexomboon D. Early childhood caries and related factors in Vientiane, Lao PDR. Southeast Asian J. Trop. Med. Public Health. 2010;41(3):717–725. [PubMed] [Google Scholar]
- Sheetal A., Hiremath V.K., Patil A.G., Sajjansetty S., Kumar S. Malnutrition and its Oral Outcome – A Review. J. Clin. Diagnostic Res. 2013;7(1):178–180. doi: 10.7860/JCDR/2012/5104.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabsinskiene E., Milciuviene S., Narbutaite J., Vasiliauskiene I., Andruskeviciene V., Bendoraitiene E.A. Severe early childhood caries and behavioral risk factors among 3-year-old children in Lithuania. Medicina (Kaunas) 2010;46(2):135–141. [PubMed] [Google Scholar]
- Tenovuo J., Lagerlöf F. Saliva. In: Thylstrup A., Fejerskov F., editors. Textbook of clinical cariology. Munksgaard; Copenhagen: 1994. [Google Scholar]
- WHO . 5th ed. World Health Organization; Geneva, Switzerland: 2013. Oral health surveys basic methods. [Google Scholar]