Abstract
Beneficial effects of silicon (Si) on growth have been observed in some plant species, reportedly due to stoichiometric changes of C, N, and P. However, little is known about the effects on the stoichiometric relationships between C, N, and P when silicon is supplied via different modes in sorghum and sunflower plants under salt stress conditions. Therefore, the current study was performed to investigate the impact of differing modes of Si supply on shoot biomass production and C:N:P stoichiometry in sorghum and sunflower plants under salt stress. Two experiments were performed in a glass greenhouse using the strong Si-accumulator plant sorghum, as well as the intermediate type Si-accumulator sunflower, both of which were grown in pots filled with washed sand. Plant species were cultivated for 30 days in the absence or presence of salt stress (0 or 100 mM) and supplemented with one of four Si treatments: control plants (without Si), 28.6 mmol Si L−1 via foliar application, 2.0 mmol Si L−1 via nutrient solution, and combined application of foliar and nutrient solution, each group with five replications. The results revealed that supplied Si modified the C, N, and P concentrations, thereby enhancing the C:N:P stoichiometry and shoot dry matter of sorghum and sunflower plants under salt stress. Both application of Si via nutrient solution, as well as combined application via foliar and nutrient solution, increased the C:N ratio in both plant species under salt stress, but in sorghum plants decreased the C:P and N:P ratios and increased the shoot biomass production by 39%, while in sunflower plants increased the C:P and N:P ratios and increased the shoot biomass production by 24%. Our findings suggest that salt stress alleviation by Si impacts C:N:P stoichiometric relationships in a variable manner depending on the ability of the species to accumulate Si, as well as the route of Si administration.
Keywords: Carbon, Ecological stoichiometry, Helianthus annuus, Macronutrients, Salinity, Sorghum bicolor
Abbreviations: −Si, Control no added Si; F, foliar application of Si; R, root application of Si; RF, and combined foliar and root applications of Si; −Si −Na, non-Si treatment under non-NaCl stress; −Si +Na, non-Si treatment under NaCl stress; F −Na, foliar Si treatment under non-NaCl stress; F +Na, foliar Si treatment under NaCl stress; R −Na, root Si treatment under non-NaCl stress; R +Na, root Si treatment under NaCl stress; RF −Na, combined Si treatment under non-NaCl stress; RF +Na, combined Si treatment under NaCl stress; HCl, Hydrochloric acid; LDM, leaves dry matter; SDM, stem dry matter; SDM, shoot dry matter; Si × NaCl, Si–NaCl interaction; S, Scheffe; Si, Silicon; Na+, sodium; SiNaKE, Stabilized sodium and potassium silicate
1. Introduction
Salinity is the main abiotic stress that severely inhibits plant growth and consequently decreases their productivity (Hussain et al., 2016). The restrictive effects of salinity on plant life are mainly due to the osmotic and toxic effects of sodium (Na+) and chloride (Cl−) ions, reduced osmotic potential, and changes in nutrient uptake, leading to large disturbances in carbon photosynthesis and leaf growth rate (Munns, 2011). Excess salts slow down plant growth, mainly due to the increased energy expenditure required to absorb additional soil water and make biochemical adjustments (Hussain et al., 2016). Excess salts also decrease stomatal opening, limiting CO2 assimilation (Asrar et al., 2017). Reduced availability of CO2 alters the ratios of ATP/ADP and NADPH/NADP+ (i.e. electron and proton acceptors), causing over-reduction of photosystems and producing reactive oxygen species (ROS) which subsequently damage photosynthetic machinery (Pinheiro and Chaves, 2011). This effect of salt stress on reducing the photosynthetic rate has been previously reported in both sorghum plants (Bafeel, 2014; Zhou et al., 2018) and sunflower plants (Kaya et al., 2019; Taher et al., 2018).
In plants, NaCl stress has a strong influence on the metabolism of nitrogen (N) and carbon (C), which is reflected in various physiological and biochemical processes (Anjum et al., 2017), such as the N assimilation steps, thereby decreasing the amount of these nutrients within the plant (Naliwajski and Skłodowska, 2018). Silicon (Si) may be employed to minimize the adverse effects of salinity (Coskun et al., 2019; Etesami and Jeong, 2018; Rios et al., 2017), and the beneficial effects of Si on alleviating salt stress have previously been demonstrated in both sorghum (Liu et al., 2015; Yin et al., 2016) and sunflower (Ashraf et al., 2015; Conceição et al., 2019) plants. Si may be immobilized in cell walls as plant opals, called phytoliths (Epstein, 1999), which requires less energy when compared to enzymatic synthesis of structural carbon compounds (Raven, 1983). This implies that Si incorporation in plant tissue may, to some extent, substitute for the production of C compounds such as cellulose, whereas lignin appears to be unaffected by Si availability (Schoelynck et al., 2010).
Coordination between C and N metabolism and regulation of the C:N ratio occur in physiological processes, such as photosynthesis and amino acid synthesis (Huarancca et al., 2018). Stoichiometric analysis of C, N, and phosphorous (P) can be useful for evaluating the variation of N and P in plant tissues subjected to abiotic stresses, but may be influenced by specific plant physiology (Wang et al., 2019). However, until now, studies on the effects of Si on stoichiometry have been restricted to grass plants under water stress (Eneji et al., 2008) and wheat plants under winter conditions (Neu et al., 2017). Little is known so far concerning the effects of Si availability on biomass production and nutrient stoichiometry in sorghum and sunflower plants gown under salt stress conditions.
A formation of organic compost including Si could induce modifications in the structural nutrient stoichiometry of C:N:P, benefiting biomass production in plants under saline stress. Thus, we hypothesized that supplied Si may alleviate salt stress by altering the C, N, and P concentrations in vegetal tissues, thereby modifying the C:N:P stoichiometric relationships favoring shoot biomass production. Furthermore, these beneficial effects of Si may differ between high Si-accumulator species, such as sorghum, and intermediate Si-accumulators, such as sunflower, and could also vary depending on the Si application mode. Therefore, the present study was performed to investigate the impact of Si applied either by foliar or root application on the C:N:P stoichiometric relationships, as well as biomass production, in sorghum and sunflower plants grown under salt stress conditions.
2. Materials and methods
2.1. Plant material and experimental conditions
Seeds of sorghum (Sorghum bicolor L. ‘Moench’ Dekalb 540) and the yellow dwarf sunflower (Helianthus annuus L. ‘Double Sungold’) were germinated in vermiculite mixture (3:1) in trays placed in a growth inside a glass greenhouse under specific conditions (natural light conditions, temperature 26/16 °C, 10/14 h day/night photoperiod, and 65–75% relative humidity). The seeds and seedlings of both plant species were irrigated using demineralized water three times per day for 16 days. After this time, seedlings of both plant species were transplanted to 4-dm3 polyethylene pots that were filled with washed sand. The sand was carefully washed a twice with 100 mL of a 0.05 M solution of sulfuric acid (H2SO4). After washings with acid, each sand sample was wash with enough tap water to remove the acid and remaining substrate, and finally was wash with demineralized water. Individual pots contained either two sorghum plants or two sunflower plants.
The nutrient solution (NS) used was Hoagland and Arnon (1950) with some modifications. The iron source changed from FeEDTA to Fe-EDDHMA and the order of NS preparation was as follows, first 2 mmol Si L−1 was added and the pH was adjusted to 5.0 using 1 N hydrochloric acid (HCl), second the NaCl was added or not, according to the treatment, and finally the other nutrients were added and then pH was adjusted and maintained at 5.0 ± 0.8, using HCl before applying to the plants. After transplanting, NS was quickly added, starting at 25% ionic strength until plant acclimatization per 1 week (50 mL NS per pot), after which the ionic strength was increased to 50% over 2 weeks (100 mL NS per pot), and finally to 75% by the end of the experiment (150 mL NS per pot). The amount of water lost by evapotranspiration was replaced by NS in all the experimental periods.
2.2. Experimental design and Si and salt treatments
After transplanting, plants of both species were arranged in a randomized block design, factorial scheme 4 × 2. A total of 80 pots, 40 for each plant species were moved and rearranged within each block daily to give a random distribution of growth conditions in the glasshouse during the experimental period We evaluated the application of four different Si treatments: control/no added Si (-Si), 28.6 mmol Si L−1 via foliar spraying (F); 2.0 mmol Si L−1 via root (R) and the combined application via root and foliar spraying (RF). Plants were grown in either the absence (-NaCl) or presence (+NaCl) of NaCl (0 or 100 mM of NaCl, respectively), with five replications, obtaining the following combination treatments: −Si −Na, non-Si treatment under non-NaCl stress; −Si +Na, non-Si treatment under NaCl stress; F −Na, foliar Si treatment under non-NaCl stress; F +Na, foliar Si treatment under NaCl stress; R −Na, root Si treatment under non-NaCl stress; R +Na, root Si treatment under NaCl stress; RF −Na, combined Si treatment under non-NaCl stress; and RF +Na, combined Si treatment under NaCl stress. Stabilized sodium and potassium silicate (SiNaKE) were used to maintain the concentration of Si treatments.
The SiNaKE source, used for applying the Si treatments, was applied via NS and/or foliar sprayings. K+ introduced by SiNaKE were balanced among all treatments, including the control groups, by calculating additional K+ and then adding K+ balanced by potassium chloride (KCl).
For plants of both species, following one week of acclimatization foliar applications of Si were initiated and 100 mM NaCl (10 dS m−1 electrical conductivity) was added in the NS and kept until the end of the experiment. The initial pH of foliar solution after the addition of Si was 10.0–11.5, which was adjusted to 7.0–7.5 using HCl before applying to the plants. Foliar sprays were performed on the fully developed leaves at S2–S4 phenological stages for sorghum plants (Vanderlip and Reeves, 1972) and the V4-V8 phenological stages for sunflower plants (Schneiter and Miller, 1981). Foliar spraying of Si was carried out around 10 days (four application during the experimental period) for both plant species (Calero et al., 2019). We considered the following conditions for performing the foliar application of Si: the relative humidity was >60% and the ambient temperature was between 20 °C and 27 °C. The initial pH of NS after the addition of 2.0 mmol L−1 of Si was 8.0–9.5, which was adjusted to 5.5–6.0 using HCl before applying to the plants (Hurtado et al., 2020). Si treatments (applicable to both the R and RF treatment groups) was incorporated in Hoagland NS and applied in the rooting medium throughout the entire experimental period (Olivera et al., 2019).
2.3. Determination of carbon, nitrogen, and phosphorus concentrations
Forty days after grown the plants were harvested in the S4 stage for sorghum and V8 stage for sunflowers. After harvesting the plants, they were sequentially washed in distilled water, followed by detergent solution (0.2%), then HCl solution (0.1%), and finally twice with demineralized water (Calero et al., 2019). The leaves and stems were then transferred on to paper sacks and dried to a constant weight using a forced ventilation oven (TE 394-3, Tecnal, Piracicaba, São Paulo, Brazil) at 60 °C and weighed to obtain the shoot biomass production (SDM). The shoots were then pulverized with a Wiley mill fitted with a stainless-steel chamber and blades (IKA-WERKE, GMBH & CO. KG, Germany). Finely pulverized shoot samples were used to measure the total C and N concentration by dry combustion (1000 °C) using an elemental analyzer (LECO Truspec CHNS) calibrated with the pattern LECO 502–278 of wheat (C = 45.00% and N = 2.68%) (Olivera et al., 2019b). The dried SDM was digested with a diacid mixture of nitric acid (HNO3) and perchloric acid (HClO4) in a 3:1 ratio for P analysis. Total P concentrations were measured using the molybdenum antimony colorimetric method and an ultraviolet spectrophotometer subsystem (model SP-1105, Ningbo Hinotek Technology, Shanghai, China) after digestion following the methods described by Bataglia et al. (1983). Thus, the C:N, C:P, and N:P ratios were calculated on a C, N, and P concentrations basis.
2.4. Statistical analysis
All values are presented as mean ± standard error of the mean (S.E.) of five replicates. This experiment was repeated twice and samples for nutrients estimations were collected in 5 replicates and each replicate/sample was assayed twice. The data presented in this paper were subjected to statistical analysis by factorial analysis to test the main effects of the four levels of Si applied and the two levels of NaCl and their interactions (Si × NaCl). Data obtained for the applied treatments were analyzed assuming normality by the Shapiro–Wilk test and the unequal variances by the Fisher’s test (p < 0.05). The data were then subjected to a two-way analysis of variance (ANOVA) using the software R (R Core Team, 2018). The Scheffe test (p < 0.05) was carried out to determine if significant differences existed among means. All figures were created using GraphPad Prism v8.0 (GraphPad Inc., San Diego, CA, USA).
3. Results
3.1. Effects of salt stress and different Si supply on leaf, stem, and shoot biomass
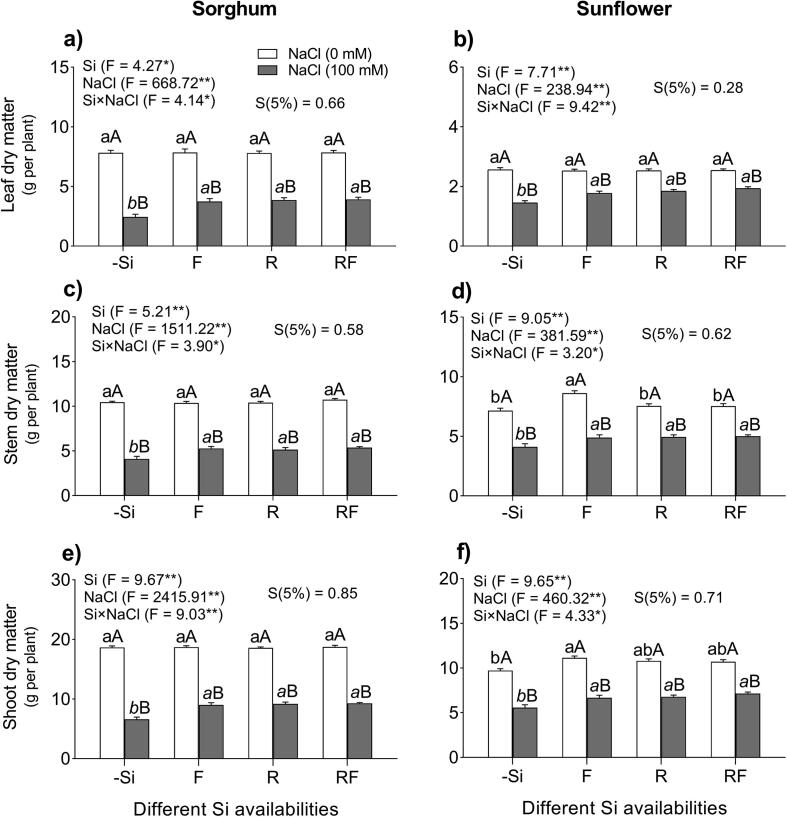
The results indicate that there was an interaction (p < 0.0001) between NaCl and Si on the leaf, stem, and SDM accumulation in both plant species (Fig. 1a–f). The applied NaCl stress (100 mM) decreased the leaf dry matter (LDM) by 69%, stem dry matter (StDM) by 61%, and SDM by 65% in sorghum plants, while in sunflower plants the reduction was 43, 42 and 43% respectively (Fig. 1a–f). However, in salt-stressed sorghum plants, the three methods of Si application (F, R and RF) increased the LDM by 57%, StDM by 28%, and SDM by 39% compared to the plants grown under −Si +Na treatment (p < 0.0001) (Fig. 1a). On the other hand, in salt-stressed sunflower plants, all of the Si (F, R and RF) treatments increased the LDM by 28%, StDM by 20%, and SDM by 25% than the plants grown under −Si +Na conditions (Fig. 1b).
Fig. 1.
Effect of different methods of Si application on leaf, stem, and shoot dry matter in sorghum (a, c, e) and sunflowers plants (b, d, f) plants grown under the absence and presence of NaCl in the nutrient solution, and different Si supplementation: (0) control treatment (-Si), (F) foliar Si application (28.6 mmol L−1), (R) nutrient solution Si application (2 mmol L−1), (RF) combined foliar and nutrient solution applications of Si. Data are means of 5 replications ± standard error (SE). Different normal lowercase letters (e.g., a, b) indicate significant differences among Si treatments under non-salt stress; different italic lowercase letters (e.g., a, b, c) indicate significant differences among Si treatments under salt stress and uppercase letters (e.g., A, B) indicate significant differences between non-salt and salt treatments at the same method of Si application, according to the Scheffe test. ANOVA, *p < 0.05 and **p < 0.01; Si × NaCl, silicon–sodium chloride interaction, S, Scheffe values (p < 0.05). Stack bars show the S.D., based on the average of five replicates.
3.2. Effects of salt stress and different Si supply on the concentration of C, N, and P in shoots
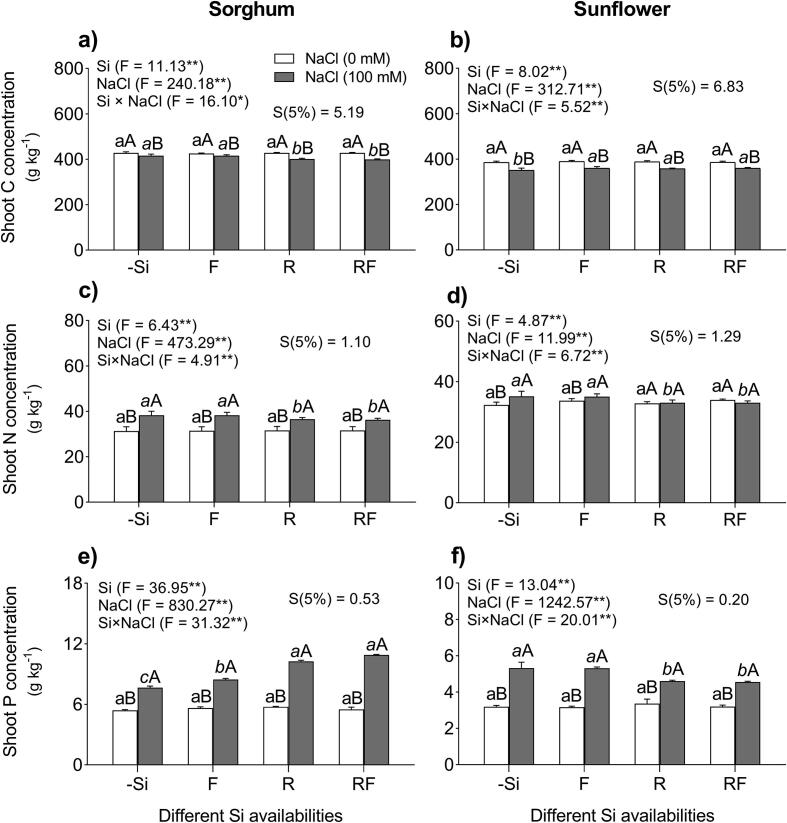
The ANOVA revealed a significant interaction (p < 0.0001) between NaCl and Si after 30 d of the treatments. Significant effects of both NaCl and Si on C, N, and P concentrations in shoots of sorghum and sunflower plants were also observed (Fig. 2a–f). Under non-salt stress conditions, the C concentrations [C] were not affected in shoots of sorghum plant, regardless of the Si treatments (Fig. 2a, b). In contrast, under salt stress conditions, the [C] decreased in shoot of sorghum by 6% and sunflower by 9% compare to the plants under −Si −Na conditions (Fig. 2a, b). In salt-stressed sorghum plants, the [C] was 7% higher in the F and RF treatments and showed a significant difference (p < 0.0001) compared to the other Si treatments (Fig. 2a). However, in salt-stressed sunflower plants, the [C] increased by 8% in all Si treatments (F, R, and RF) compared to the plants grown under −Si +Na treatment (p < 0.005) (Fig. 2b).
Fig. 2.
Effect of different methods of Si application on elemental concentrations of carbon, nitrogen and phosphorous in shoots of sorghum (a, c, e) and sunflower (b, d, f) plants grown under the absence and presence of NaCl in the nutrient solution, and different Si supplementation: (0) control treatment (no Si), (F) foliar Si application (28.6 mmol L−1), (R) nutrient solution Si application (2 mmol L−1), (RF) combined foliar and nutrient solution applications of Si. Data are means of 5 replications ± S.E. Different normal lowercase letters (e.g., a, b) indicate significant differences among Si treatments under non-salt stress; different italic lowercase letters (e.g., a, b, c) indicate significant differences among Si treatments under salt stress and uppercase letters (e.g., A, B) indicate significant differences between non-salt and salt treatments at the same method of Si application, according to the Scheffe test. ANOVA, *p < 0.05 and **p < 0.01. Si × NaCl, silicon–sodium chloride interaction, S, Scheffe values (p < 0.05). Stack bars show the standard deviation (S.D.) based on the average of five replicates.
On the other hand, in sorghum plants exposed to NaCl stress, the N concentrations [N] in plant shoots increased by 22%, while in salt-stressed sunflower plants the shoot [N] was increased by 11% in comparison with the non-salt-stressed plants (Fig. 1c, d). In salt-stressed sorghum plants, the shoot [N]decreased by 9% under Si R and RF treatments compared to the others Si treatments (p < 0.031) (Fig. 2c). Similarly, in salt-stressed sunflower plants, both the Si R and RF treatments decreased the shoot [N] by 10% and showed a significant difference (p < 0.019) compared to the others Si treatments (Fig. 2d).
In both plant species cultivated under non-salt stress conditions the addition of Si did not affect the shoot P concentrations [P] (Fig. 1e, f). However, salt stress (100 mM) increased the shoot [P] by 36% in sorghum plants and by 66% in sunflower plants compared to the plants under −Si −Na treatment (Fig. 2e, f). In salt-stressed sorghum plants, the shoot [P] increased under all Si treatments (F, R, and RF) and showed significant difference (p < 0.0001) compared to the non-Si treated plants, especially in the R and RF treatments (38% increase), while the Si (F) treatment increased the [P] by 10% in comparison with the plants under −Si +Na treatment (Fig. 2e). In salt-stressed sunflower plants, the shoot [P] decreased by 10% in the Si (R) and Si (RF) treatments, compared to the others Si treatments (p < 0.0005) (Fig. 2f).
3.3. Effects of salt stress and different Si supply on the stoichiometric ratios of C:N, C:P, and N:P
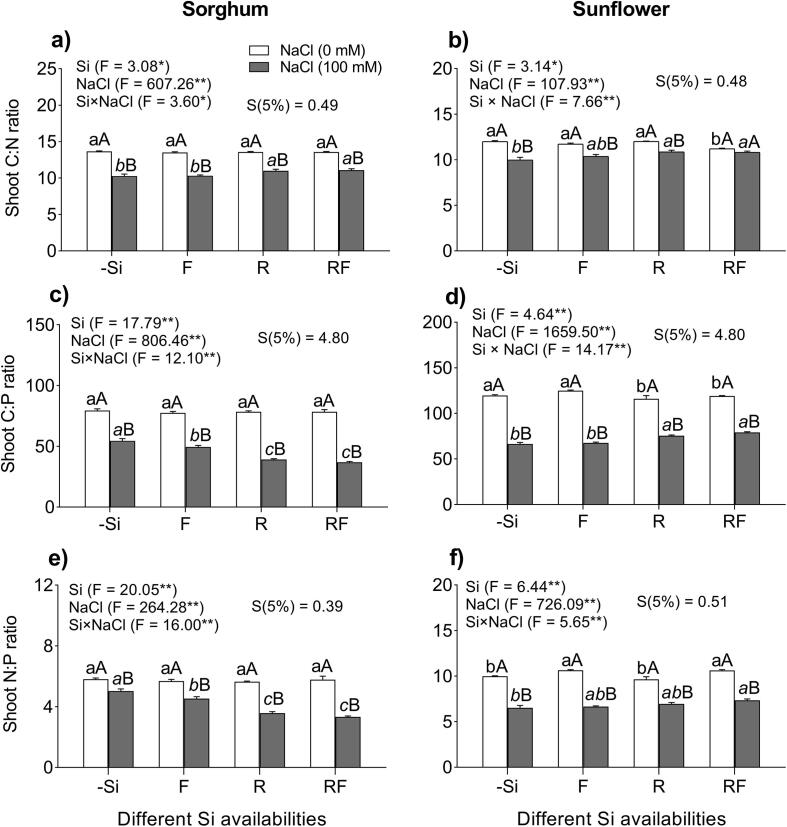
ANOVA revealed an interaction (p < 0.0001) between NaCl and Si in the stoichiometric ratios of C:N, C:P, and N:P in the shoots of sorghum and sunflower plants (Fig. 3a–f). Salt stress (100 mM) markedly decreased the shoots C:N ratios in sorghum plants by 25% and in sunflower plants by 17% compared to the plants grown under −Si −Na treatments (Fig. 3a, b). However, in salt-stressed sorghum and sunflower plants, the shoot C:N ratios increased by 8 and 9% respectively, in the S and RF treatments, compared to the others Si treatments (Fig. 3a, b).
Fig. 3.
Effect of different methods of Si application on the C:N, C:P, and N:P ratios in shoots of sorghum (a, c, e) and sunflowers plants (b, d, f) plants grown under the absence and presence of NaCl in the nutrient solution, and different Si supplementation: (0) control treatment (no Si), (F) foliar Si application (28.6 mmol L−1), (R) nutrient solution Si application (2 mmol L−1), (RF) combined foliar and nutrient solution applications of Si. Data are means of 5 replications ± S.E. Different normal lowercase letters (e.g., a, b) indicate significant differences among Si treatments under non-salt stress; different italic lowercase letters (e.g., a, b, c) indicate significant differences among Si treatments under salt stress and uppercase letters (e.g., A, B) indicate significant differences between non-salt and salt treatments at the same method of Si application, according to the Scheffe test. ANOVA, *p < 0.05, **p < 0.01. Si × NaCl, silicon–sodium chloride interaction, S, Scheffe values (p < 0.05). Stack bars show the S.D., based on the average of five replicates.
On the other hand, the applied NaCl stress (100 mM) decreased the C:P ratio in the shoots of sorghum plants by 31%, and in the salt-stressed sunflower plants by 44% compared to the plants under −Si −Na treatment (Fig. 3c, d). Similarly, in salt stressed sunflower plants all the Si treatments (F, S, and RF) decreased the C:P ratio and showed significance difference (p < 0.0001) compared to the plants under −Si +Na treatment, but the highest decreases (33%) were obtained with the application of the Si R and RF treatments. The C:P ratio decreased by 10% in the Si (F) treatment than the plants grown under −Si +Na treatment (Fig. 3c). In contrast, in salt-stressed sunflower plants, the N:P ratio increased by 15% under the S and RF treatments compared to the other Si treatments (p < 0.009) (Fig. 3d).
Salt stress (100 mM NaCl) decreased the N:P ratio in the shoots of sorghum plants by 14% and sunflower plants by 33% compared to the −Si −Na treatment (Fig. 3e, f). In salt stressed sorghum plants, the N:P ratio decreased by 32% in the Si R and RF treatments than the plants under −Si +Na treatment (p < 0.0001). In addition, foliar application of Si (F) decreased the N:P ratio by 11% compared to the control non-Si treated plants (Fig. 3e). In contrast, in salt-stressed sunflower plants, the combined application of Si (RF) increased the N:P ratio by 12% and showed significance difference (p < 0.0019) compared to the plants under −Si +Na treatment (Fig. 3f).
4. Discussion
Our study shows for the first time the effects of different methods of Si application on the C, N, and P stoichiometry of sorghum and sunflower plants grown under salinity conditions. Several authors suggested various potential mechanisms by which Si can stimulate plant tolerance against salt stress (Al-Huqail et al., 2017; Alzahrani et al., 2018; Olivera Viciedo et al., 2020). From this study, it was evident that Si alleviated salt stress effects in sorghum and sunflower plants. Plant growth characteristics were increased with the different methods of Si application of both plant species. These improvements in both plant species growth under salt stress may be attributed to that Si is enhanced of antioxidative defense mechanism (Alzahrani et al., 2018).
In the present study, we observed that the salt stress decreased stoichiometric homeostasis (H) in both plant species and was partly reversed by all methods of Si application mediated a beneficial effect on SDM production in both plant species grown under salt stress, which may help clarify the increasing of SDM (39%) in sorghum plants (Fig. 1a, c, e), whereas in sunflower the increases the SDM (23%) (Fig. 1b, d, f). These results suggest that Si plays a key role in attenuating salt stress, resulting in a higher production of SDM. Similar trends were observed by Si application to the growth medium in salt-stressed sorghum (Hurtado et al., 2020; Liu et al., 2015; Yin et al., 2016, Yin et al., 2013) and sunflower plants (Ashraf et al., 2015; Calero et al., 2019). In addition, Si helps plants to maintain the SDM of the upper parts of plants by decreasing oxidative damage and ionic toxicity under salinity conditions by enhancing the mineral nutrients status (Calero et al., 2019). The higher SDM recorded in salt-stressed plants treated with Si suggests that the Si could maintain higher levels of H. Similar observations have been made in other Si-accumulator plants under drought stress (Eneji et al., 2008). Hence, the findings reported in this work could facilitate a deeper understanding of the potential mechanisms adopted by sorghum and sunflower plants due to exogenous Si supplementation during saline stress.
Salinity stress decreased the [C] (Fig. 2a, b), presumably by the lower activity of photosynthesis, caused by the osmotic stress, nutritional imbalance and toxicity (Rios et al., 2017). This is considered as one of the major reasons for a lowered C gain and growth under salt stress (Netondo et al., 2004). The present study showed contradictory effects of different methods of Si application on C concentration in the both plant species under salinity conditions. In salt-stressed sorghum plants the Si R and Si RF treatments showed lower [C], whereas all Si treatments increased the [C] in salt-stressed sunflower plants (Fig. 2a, b). These results suggest that the Si application method plays an important role in the modifying [C] and vary among plant species. Thus, the dilution effect on the [C] in the salt-stressed sorghum plant tissues appeared due to the higher shoot growth of the plants (Sardans et al., 2012; Zhang et al., 2017). One possible reason for this marked reduction in the [C] might be attributed to the deposition of Si in the growth medium, may be due to a partial replacement of organic compounds of C (such as cellulose) by Si in plant tissues, consistent with previous findings reported on Phragmites australis (Schaller et al., 2012). This would be advantageous to the plant due to the deposition of Si in the form of phytoliths, formed in the cell wall at low energy cost (Raven, 1983) and able to confer similar defenses to the refractory polymers such as lignin (Schoelynck et al., 2010). The proposed explanations for these findings included that root Si application may increase salt stress tolerance in sorghum plants by decreasing the proline concentrations in shoots (Lee et al., 2010; Pei et al., 2010), which could benefit the plant by reserving more energy for coping with stresses (Yin et al., 2013). Another probable mechanism of Si decreasing the [C] is decreased rate of transpiration, which help to the stomata closure for longer periods during the day. This, in turn, leads to the decreased C assimilation rate and water loss, resulting in maintenance of the C assimilation at low water availability (Khan et al., 2017). These results suggested that the sorghum plants respond very well to the application of Si (R) and combined Si (RF) and the adverse effects of salinity (water and nutrient limitations), a finding that was corroborated by higher SDM (Fig. 1a–f). This suggests that the observed decline of the [C] in sorghum plants under salinity conditions can reflect change for a more viable defense mechanism when Si is sufficient and available under salt stress conditions.
On the other hand, in salt-stressed sunflower, all methods of Si application (F, R, and RF) increasing the shoot [C]. These increases in shoot [C] by Si indicates that there was an enrichment of C. This phenomenon involves the increment of C-rich secondary compounds, like the phenolics and lignin, that are reduced to N-rich metabolites (Kasurinen et al., 2006). One possible explanation for this is that the high [C] by Si application was ascribed to the enhanced SDM. The underlying mechanisms of this response are probably associated with an improved photosynthesis rate with Si application under salinity conditions (Asrar et al., 2017). Another probable mechanism of Si increasing shoot [C] is enhanced the proline concentration, which can be degraded, and used as a source of C in plants recovering from salt stress as well as a membrane stabilizer and a free radical scavenger (Al-Huqail et al., 2017; Li et al., 2018; Mansour and Ali, 2017). Increasing the photosynthesis rate is another mechanism whereby Si maintains the C concentration in salt-stressed plants (Asrar et al., 2017; Liu et al., 2019). Thus, it was deduced that Si play an important role in the increased [C] in intermediate-Si accumulating plant (sunflower) grown under salinity conditions.
Our results indicated that in shoots both plant species cultivated under salinity conditions, in general, there was an increase in the [N] and [P] (Fig. 2c–f). These effects caused by salinity occurred due to the dilution effects of these nutrient on plant tissue from the decrease in SDM (Fig. 1a–f), and do not mean that there was an increase in uptake of these nutrients. However, the effect of Si on increasing the N uptake in Si-accumulating plants under saline conditions is poorly documented (Haddad et al., 2018). In agreement with the present results, augmentation with foliar spraying of Si increased the [N] and [P] in the non-Si accumulating moringa (Moringa oleifera L.) plants (Hussein and Abou-baker, 2014), although in Si-accumulating plants this is poorly studied and documented.
In the current study, there was a decrease in the shoot [N] following treatment with either Si (R) or Si (RF) in salt-stressed sorghum plants (Fig. 2c). This effect is probably due to the dilutional effect of these nutrients, given the higher shoot growth of plants (Sardans et al., 2012; Zhang et al., 2019). These results suggest that Si application can increase the nutrient use efficiency (NUtE) and the amount of C fixed per N uptake unit (Neu et al., 2017), which the Si application in salt stressed plants can maintain higher growth rates at lower [N] and these findings also consistent with previous studies in this plant species (Calero et al., 2019). Therefore, lower [N] may be invested in other functions, such as reproduction (Sterner and Elser, 2002). Similar results were reported previously with Si addition in the growth medium in rice (Abdel-Haliem et al., 2017). This result was partly consistent with the report of Sardans et al. (2012) who found that the low [N] is more closely associated with C-assimilation capacity.
However, in salt-stressed sunflower all methods of Si application increased the shoot [N] (Fig. 1d). These increasing in the [N] in the cell could facilitate various fundamental processes, such as photosynthesis, stomatal development, cell division, pigment production, and net primary production of plants (Minden and Olde Venterink, 2019; Tian et al., 2019). In addition, the application of Si increased the [N] in the shoots of other eudicots (previously referred to as dicots) grown under saline stress, such as canola (Brassica napus L.) (Haddad et al., 2018), chili (Capsicum annumn L.) (Tantawy et al., 2015), zinnia (Zinnia elegans Jacq) (Manivannan et al., 2015), sunflower (Conceição et al., 2019) and cucumber plants (Alsaeedi et al., 2019). This effect could be explained by the fact that salt-stressed plants produce more proline and glycine betaine in leaves, as N source compounds, to protect themselves against the adverse effects of salinity (Calero Hurtado et al., 2020).
In the present study, all Si treatments increased the shoot [P] in salt-stressed sorghum plants, especially the Si R and combined Si RF treatments (Fig. 1e). The variation by Si in the increasing [P] are probably due to a dilution effect (Zhang et al., 2020, Zhang et al., 2017). These results are in agreement with the recent findings about interference between Si and P metabolism of gramineous plants (Eneji et al., 2008; Schaller et al., 2012). These results indicate that the use of Si in the growth medium modifies the P uptake rates under salt stress, and also this varying of this nutrient could be due to the differential use of P by the plants (Elser, 2006), with high [P] is more closely associated with growth rate capacity (Sardans et al., 2012). Si application was reported to increase [P] under salinity stress in Spartina densiflora plants (Mateos-Naranjo et al., 2013). On the other hand, in the current study, shoot [P] was also decreased by Si (R and RF) treatments in salt-stressed sunflower plants (Fig. 1f). These results indicate that there was a well-adjusted effect in intermediate Si-accumulating plants between the combination of salt stress and the Si application in the growth medium and combined (R and RF) treatments. This decline in shoot [P] by Si addition in the growth medium, helped to increase the P uptake and use efficiency under salt stress conditions (Calero et al., 2019). Our results suggested that the growth of sunflower plants under salinity conditions was limited by P. Contrarily, other studies also revealed that Si application maintained higher [P] under salt stress conditions, such as Egyptian clover (Abdalla, 2011), canola (Haddad et al., 2018), and moringa (Hussein and Abou-baker, 2014). The results suggested that the effects of Si in the uptake and translocation of mineral nutrients in salt stressed plants, vary among plant species and the methods of application of Si, which strongly suggests that Si functions in plants grown under salinity conditions are complex.
Our results reveal that the responses of plant nutrient ratios to a combination of NaCl and Si were remarkably different between the species of plants, as well as between the different modes of Si application (Fig. 2a–f). To our knowledge, this is the first study describing experimental evidence of the C:N, C:P, and N:P stoichiometric relationships in high Si-accumulator (sorghum) and intermediate-type Si-accumulator (sunflower) plants grown under moderate salt stress conditions. We observed that the supplementation of Si (S and RF) increased the C:N ratio in both plant species grown under salt stress, presumably as result of decreased [N] (Fig. 2c–d), and increasing shoots biomass accumulation (Fig. 1a, c, e). Our results suggested that the growth of sorghum plants under salinity conditions was limited by N. This indicates that Si applications, provide a high stoichiometric homeostasis in both salt-stressed plants. These results suggest that Si-induced changes in shoots C:N ratios, which may an important mechanism to predict how plant productivity will respond to different scenarios, including salinity conditions (Siefert and Ritchie, 2016; Yue et al., 2017). Chen et al. (2015) reported that C:N ratio is also related to the senescence process in leaves. These changes in C:N by Si application could be an important mechanism delay the leaves senescence caused by the salt stress. These findings indicate that Si addition in the growth medium have a tendency to increase C:N ratio in Si-accumulating and intermediate-Si-accumulating plant under salinity conditions.
In the present study, we also observed a different response by sorghum and sunflower plants in the C:P and N:P ratios to Si supply under saline conditions. Our results showed that not find no regularity of C:P and N:P ratios under salt stress conditions, resulting from different availability of Si (Fig. 3c–f). In this study, we found that C:P and N:P ratios decreased in al Si treatments in salt-stressed sorghum plants (Fig. 2c, e). As noted above, these effects may be associated with the decreasing of [N] and increasing [P] under salinity conditions with the application of Si (S and RF), converting more SDM (Fig. 1a–f). These results indicate a balancing effect between salinity and the application of Si. Therefore, application of Si (R or RF) under moderate salt stress would have a more obvious effect on P uptake than on the assimilation of C and N in the Si-accumulating plants (sorghum). However, studies have shown that Si application minimizes the water deficit by increasing the N:P ratio (Eneji et al., 2008). One explanation for the differences observed in the C:P and N:P ratios between the studied species may be the availability of Si, as well as the amount absorbed under saline conditions. However, it is still unclear how plant response to change of C:P and N:P ratios in saline environment. In concordance with our results, contradictory effects of Si on the C:P and N:P ratios has been previously observed in wheat plants (Neu et al., 2017). These findings indicate that Si application in the growth medium have a tendency to decrease C:P and N:P ratios in Si-accumulating plant (sorghum) grown under salinity conditions.
On the other hand, we observed that the intermediate Si-accumulating species (sunflower) was increased the C:P and N:P ratios under Si (R or RF) treatments (Fig. 3d, f) and responds very well to the combination of salt stress and Si application via the roots, corroborated by a higher production of SDM (Fig. 1a–f). These results suggest that supplementation with Si in the growth medium plays a stronger role in C assimilation than N and P uptake, given the decreases in the [N] and [P] of the plant (Fig. 2d, f). These results are in agreement with a study conducted in wheat plants, where Si addition in the growth medium increased C:N and C:P ratios (Neu et al., 2017). However, under Si applications, there was a high shoot of H in in salt-stressed sunflower plants. The significantly higher C:P and N:P ratios under Si R and RF treatments suggested that Si induced an increase in P use efficiency.
Thus, our hypotheses were proven, indicating the results confirm improved crop performance in plants responding to salt stress following Si application, as a result of homeostatic stoichiometric changes to the C:N:P ratios that influence plant growth. Furthermore, these results suggest that Si-accumulator plants, such as sorghum, may respond better to salt stress conditions if Si is provided in the root, and that intermediate-type Si-accumulator plants, such as sunflower, may benefit more from combined treatment with Si via foliar and root applications.
5. Conclusions
Our results reveal that Si supply increased the aerial biomass production and modified the stoichiometry of both plant species cultivated under saline conditions. This increase can be attributed to the subsequent effects on the stoichiometry of these nutrients in sorghum and sunflower plants. The supplementation of Si via NS, and the combined application of foliar and NS Si, increased the C:N ratios in both plant species under saline conditions, but the C:P and N:P ratios changed, decreasing in sorghum plants while increasing in sunflower plants. However, the foliar spraying of Si also changed the stoichiometry, decreasing the C:N ratio in both crops, while the C:P and N:P ratios were increased in sorghum plants and decreased in sunflower plants. Our findings suggest that the benefits of Si in alleviating the effects of salinity has impact on the C:N, C:P and N:P stoichiometric relationships, which vary with the ability of the species to accumulate Si as well as the method of Si application.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We wish to thank the São Paulo State University (UNESP), for providing the necessary facilities for this study, and also the GENPLANT research for their help and assistance during the experiments. The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) [Finance Code 001, 2017-0]; and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [142490, 2018-0] for providing the PhD fellowship for the first author.
Author Contributions
R.M.P. and D.A.C. initialized and designed the experiment. D.A.C., A.C.H., G.S.S.J., and D.O.V. conducted the experiments and analyzed samples and data. M.C.P., A.C.H, and Y.P.D., performed the C, N, and P concentrations analysis. A.C.H. wrote the manuscript with contributions of R.M.P., D.A.C., and M.C.P. All authors have read and approved the final manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdalla M.M. Impact of diatomite nutrition on two Trifolium alexandrinum cultivars differing in salinity tolerance. Int. J. Plant Physiol. Biochem. 2011;3:233–246. doi: 10.5897/IJPPB11.040. [DOI] [Google Scholar]
- Abdel-Haliem M.E.F., Hegazy H.S., Hassan N.S., Naguib D.M. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017;99:282–289. doi: 10.1016/j.ecoleng.2016.11.060. [DOI] [Google Scholar]
- Al-Huqail A.A., Alqarawi A.A., Hashem A., Ahmad Malik J., Abd_Allah E.F. Silicon supplementation modulates antioxidant system and osmolyte accumulation to balance salt stress in Acacia gerrardii Benth. Saudi J. Biol. Sci. 2017;30:1–9. doi: 10.1016/j.sjbs.2017.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaeedi A., El-Ramady H., Alshaal T., El-Garawany M., Elhawat N., Al-Otaibi A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019;139:1–10. doi: 10.1016/J.PLAPHY.2019.03.008. [DOI] [PubMed] [Google Scholar]
- Alzahrani Y., Kuşvuran A., Alharby H.F., Kuşvuran S., Rady M.M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018;154:187–196. doi: 10.1016/j.ecoenv.2018.02.057. [DOI] [PubMed] [Google Scholar]
- Anjum S.A., Ashraf U., Tanveer M., Khan I., Hussain S., Shahzad B., Zohaib A., Abbas F., Saleem M.F., Ali I., Wang L.C. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017;8:1–12. doi: 10.3389/fpls.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M., Abid M., Teixeira da Silva J.A., Shahzad S.M., Hussain A., Imtiaz M. Silicon and potassium nutrition enhances salt adaptation capability of sunflower by improving plant water status and membrane stability. Commun. Soil Sci. Plant Anal. 2015;46:991–1005. doi: 10.1080/00103624.2015.1018527. [DOI] [Google Scholar]
- Asrar H., Hussain T., Hadi S.M.S., Gul B., Nielsen B.L., Khan M.A. Salinity induced changes in light harvesting and carbon assimilating complexes of Desmostachya bipinnata (L.) Staph. Environ. Exp. Bot. 2017;135:86–95. doi: 10.1016/j.envexpbot.2016.12.008. [DOI] [Google Scholar]
- Bafeel S.O. Physiological parameters of salt tolerance during germination and seedling growth of Sorghum bicolor cultivars of the same subtropical origin. Saudi J. Biol. Sci. 2014;21:300–304. doi: 10.1016/j.sjbs.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataglia O.C., Teixeira J.P.F., Furlani P.R., Furlani A.M.C., Gallo J.R. first ed. Instituto Agronômico de Campinas; Campinas: 1983. Métodos de análise química de plantas. (In portuguese) [Google Scholar]
- Calero A., Aparecida D., Prado R., Sousa Junior G., Felisberto G. Silicon attenuates sodium toxicity by improving nutritional efficiency in sorghum and sunflower plants. Plant Physiol. Biochem. 2019;142:224–233. doi: 10.1016/j.plaphy.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Calero Hurtado A., Chiconato D.A., Prado R. de M., Sousa G. da S., Junior, Gratão P.L., Felisberto G., Olivera Viciedo D., Mathias dos Santos D.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotoxicol. Environ. Saf. 2020;203 doi: 10.1016/j.ecoenv.2020.110964. [DOI] [PubMed] [Google Scholar]
- Chen D., Wang S., Xiong B., Cao B., Deng X. Carbon/Nitrogen imbalance associated with drought-induced leaf senescence in Sorghum bicolor. PLoS ONE. 2015;10:e0137026. doi: 10.1371/journal.pone.0137026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conceição S., Neto C., Marques E., Barbosa A., Galvão J., de Oliveira T., Okumura R., Martins J., Costa T., Gomes-Filho E. Silicon modulates the activity of antioxidant enzymes and nitrogen compounds in sunflower plants under salt stress. Arch. Agron. Soil Sci. 2019;65:1237–1247. doi: 10.1080/03650340.2018.1562272. [DOI] [Google Scholar]
- Coskun D., Deshmukh R., Sonah H., Menzies J.G., Reynolds O., Ma J.F., Kronzucker H.J., Bélanger R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019;221:67–85. doi: 10.1111/nph.15343. [DOI] [PubMed] [Google Scholar]
- Elser, J., 2006. Biological stoichiometry: A chemical bridge between ecosystem ecology and evolutionary biology. In: American Naturalist. https://doi.org/10.1086/509048. [DOI] [PubMed]
- Eneji A.E., Inanaga S., Muranaka S., Li J., Hattori T., An P., Tsuji W. Growth and nutrient use in four grasses under drought stress as mediated by silicon fertilizers. J. Plant Nutr. 2008;31:355–365. doi: 10.1080/01904160801894913. [DOI] [Google Scholar]
- Epstein E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:641–664. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- Etesami H., Jeong B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018;147:881–896. doi: 10.1016/j.ecoenv.2017.09.063. [DOI] [PubMed] [Google Scholar]
- Haddad C., Arkoun M., Jamois F., Schwarzenberg A., Yvin J.C., Etienne P., Laîné P. Silicon promotes growth of Brassica napus L., and delays leaf senescence induced by nitrogen starvation. Front. Plant Sci. 2018;9:1–13. doi: 10.3389/fpls.2018.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland D.R., Arnon D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950;347:1–32. [Google Scholar]
- Huarancca T., Scartazza A., Pompeiano A., Ciurli A., Lu Y., Guglielminetti L., Yamaguchi J. Nitrate reductase modulation in response to changes in c/n balance and nitrogen source in Arabidopsis. Plant Cell Physiol. 2018;59:1248–1254. doi: 10.1093/pcp/pcy065. [DOI] [PubMed] [Google Scholar]
- Hurtado, A.C., Chiconato, D.A., de Mello Prado, R., Sousa Junior, G. da S., Viciedo, D.O., Díaz, Y.P., Peña Calzada, K., Gratão, P.L., 2020. Silicon alleviates sodium toxicity in sorghum and sunflower plants by enhancing ionic homeostasis in roots and shoots and increasing dry matter accumulation. Silicon 12 (in press). https://doi.org/10.1007/s12633-020-00449-7.
- Hussain M.I., Lyra D.A., Farooq M., Nikoloudakis N., Khalid N. Salt and drought stresses in safflower: a review. Agron. Sustain. Dev. 2016;36:1–31. doi: 10.1007/s13593-015-0344-8. [DOI] [Google Scholar]
- Hussein M.M., Abou-baker N.H. Growth and mineral status of moringa plants as affected by silicate and salicylic acid under salt stress. Int. J. Plant Soil Sci. 2014;3:163–177. [Google Scholar]
- Kasurinen A., Riikonen J., Oksanen E., Vapaavuori E., Holopainen T. Chemical composition and decomposition of silver birch leaf litter produced under elevated CO2 and O3. Plant Soil. 2006;282:261–280. doi: 10.1007/s11104-005-6026-6. [DOI] [Google Scholar]
- Kaya M.D., Akdoğan G., Kulan E.G., Dağhan H., Sari A. Salinity tolerance classification of sunflower (Helianthus annuus L.) and safflower (Carthamus tinctorius L.) by cluster and principal component analysis. Appl. Ecol. Environ. Res. 2019;17:3849–3857. doi: 10.15666/aeer/1702_38493857. [DOI] [Google Scholar]
- Khan W., Aziz T., Hussain I., Ramzani P.M.A., Reichenauer T.G. Silicon: a beneficial nutrient for maize crop to enhance photochemical efficiency of photosystem II under salt stress. Arch. Agron. Soil Sci. 2017;63:599–611. doi: 10.1080/03650340.2016.1233322. [DOI] [Google Scholar]
- Lee S.K., Sohn E.Y., Hamayun M., Yoon J.Y., Lee I.J. Effect of silicon on growth and salinity stress of soybean plant grown under hydroponic system. Agrofor. Syst. 2010;80:333–340. doi: 10.1007/s10457-010-9299-6. [DOI] [Google Scholar]
- Li Z., Song Z., Yan Z., Hao Q., Song A., Liu L., Yang X., Xia S., Liang Y. Silicon enhancement of estimated plant biomass carbon accumulation under abiotic and biotic stresses. A meta-analysis. Agron. Sustain. Dev. 2018 doi: 10.1007/s13593-018-0496-4. [DOI] [Google Scholar]
- Liu B., Soundararajan P., Manivannan A. Mechanisms of silicon-mediated amelioration of salt stress in plants. Plants. 2019;8:307. doi: 10.3390/plants8090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Yin L., Wang S., Zhang M., Deng X., Zhang S., Tanaka K. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Exp. Bot. 2015;111:42–51. doi: 10.1016/j.envexpbot.2014.10.006. [DOI] [Google Scholar]
- Manivannan A., Soundararajan P., Arum L.S., Ko C.H., Muneer S., Jeong B.R. Silicon-mediated enhancement of physiological and biochemical characteristics of Zinnia elegans ‘Dreamland Yellow’ grown under salinity stress. Hortic. Environ. Biotechnol. 2015;56:721–731. doi: 10.1007/s13580-015-1081-2. [DOI] [Google Scholar]
- Mansour M.M.F., Ali E.F. Evaluation of proline functions in saline conditions. Phytochemistry. 2017;140:52–68. doi: 10.1016/J.PHYTOCHEM.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Mateos-Naranjo E., Andrades-Moreno L., Davy A.J. Silicon alleviates deleterious effects of high salinity on the halophytic grass Spartina densiflora. Plant Physiol. Biochem. 2013;63:115–121. doi: 10.1016/j.plaphy.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Minden V., Olde Venterink H. Plant traits and species interactions along gradients of N, P and K availabilities. Funct. Ecol. 2019;33:1611–1626. doi: 10.1111/1365-2435.13387. [DOI] [Google Scholar]
- Munns R. Plant adaptations to salt and water stress. Differences and commonalities. In: Turkan I., editor. Advances in Botanical Research. Elsevier Ltd; Amsterdam, The Netherlands: 2011. pp. 1–32. [DOI] [Google Scholar]
- Naliwajski M.R., Skłodowska M. The relationship between carbon and nitrogen metabolism in cucumber leaves acclimated to salt stress. PeerJ. 2018;2018 doi: 10.7717/peerj.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netondo G.W., Onyango J.C., Beck E. Sorghum and salinity. Crop Sci. 2004;44:806–811. doi: 10.2135/cropsci2004.8060. [DOI] [Google Scholar]
- Neu S., Schaller J., Dudel E.G. Silicon availability modifies nutrient use efficiency and content, C:N: P stoichiometry, and productivity of winter wheat (Triticum aestivum L.) Sci. Rep. 2017;7:1–8. doi: 10.1038/srep40829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera D., Prado R., Lizcano R., Santos L.C., Calero A., Nedd L.L., Castellanos L. Silicon supplementation alleviates ammonium toxicity in sugar beet (Beta vulgaris L.) J. Soil Sci. Plant Nutr. 2019;19:413–419. doi: 10.1007/s42729-019-00043-w. [DOI] [Google Scholar]
- Olivera D., Prado R., Martínez C.A., Habermann E., Piccolo M. Short-term warming and water stress affectPanicum maximumJacq. stoichiometric homeostasis and biomass production. Sci. Total Environ. 2019;681:267–274. doi: 10.1016/j.scitotenv.2019.05.108. [DOI] [PubMed] [Google Scholar]
- Olivera Viciedo, D., Mello Prado, R., Lizcano Toledo, R., Salas Aguilar, D., Santos, L.C.N., Calero Hurtado, A., Peña Calzada, K., Betancourt Aguilar, C., 2020. Physiological role of silicon in radish seedlings under ammonium toxicity. J. Sci. Food Agric. 100 (in press). https://doi.org/10.1002/jsfa.10587. [DOI] [PubMed]
- Pei Z.F., Ming D.F., Liu D., Wan G.L., Geng X.X., Gong H.J., Zhou W.J. Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J. Plant Growth Regul. 2010;29:106–115. doi: 10.1007/s00344-009-9120-9. [DOI] [Google Scholar]
- Pinheiro C., Chaves M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011;62:869–882. doi: 10.1093/jxb/erq340. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2018. R: A language and environment for statistical computing; 2015.
- Raven J.A. The transport and function of silicon in plants. Biol. Rev. 1983;58:179–207. doi: 10.1111/j.1469-185x.1983.tb00385.x. [DOI] [Google Scholar]
- Rios J.J., Martínez-Ballesta M.C., Ruiz J.M., Blasco B.B., Carvajal M., Martinez-Ballesta M.C., Ruiz J.M., Blasco B.B., Carvajal M. Silicon-mediated improvement in plant salinity tolerance: the role of aquaporins. Front. Plant Sci. 2017;8:1–10. doi: 10.3389/fpls.2017.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardans J., Rivas-Ubach A., Peñuelas J. The C:N: P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012 doi: 10.1016/j.ppees.2011.08.002. [DOI] [Google Scholar]
- Schaller J., Brackhage C., Gessner M.O., Bäuker E., Gert Dudel E. Silicon supply modifies C:N: P stoichiometry and growth of Phragmites australis. Plant Biol. 2012;14:392–396. doi: 10.1111/j.1438-8677.2011.00537.x. [DOI] [PubMed] [Google Scholar]
- Schneiter A.A., Miller J.F. Description of sunflower growth stages. Crop Prot. 1981;21:901–903. doi: 10.2135/cropsci1981.0011183X002100060024x. [DOI] [Google Scholar]
- Schoelynck J., Bal K., Backx H., Okruszko T., Meire P., Struyf E. Silica uptake in aquatic and wetland macrophytes: a strategic choice between silica, lignin and cellulose? New Phytol. 2010;186:385–391. doi: 10.1111/j.1469-8137.2009.03176.x. [DOI] [PubMed] [Google Scholar]
- Siefert A., Ritchie M.E. Intraspecific trait variation drives functional responses of old-field plant communities to nutrient enrichment. Oecologia. 2016;181:245–255. doi: 10.1007/s00442-016-3563-z. [DOI] [PubMed] [Google Scholar]
- Sterner R.W., Elser J.J. first ed. Princeton University Press; 2002. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. [Google Scholar]
- Taher M., Beyaz R., Javani M., Gürsoy M., Yıldız M. Morphological and biochemical changes in response to salinity in sunflower (Helianthus annus L.) cultivars. Ital. J. Agron. 2018;11:141–147. doi: 10.4081/ija.2018.1096. [DOI] [Google Scholar]
- Tantawy A.S., Salama Y., El-Nemr M.A., Abdel-Mawgoud A. Nano silicon application improves salinity tolerance of sweet pepper plants. Int. J. ChemTech Res. 2015;8:11–17. [Google Scholar]
- Tian D., Kattge J., Chen Y., Han W., Luo Y., He J., Hu H., Tang Z., Ma S., Yan Z., Lin Q., Schmid B., Fang J. A global database of paired leaf nitrogen and phosphorus concentrations of terrestrial plants. Ecology. 2019;100:e02812. doi: 10.1002/ecy.2812. [DOI] [PubMed] [Google Scholar]
- Vanderlip R.L., Reeves H.E. Growth stages of sorghum [Sorghum bicolor (L) Moench] Crop Sci. 1972;64:13–16. doi: 10.2134/agronj1972.00021962006400010005x. [DOI] [Google Scholar]
- Wang J., Liu X., Zhang X., Li L., Lam S.K., Pan G. Changes in plant C, N and P ratios under elevated [CO2] and canopy warming in a rice-winter wheat rotation system. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-41944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Wang S., Li J., Tanaka K., Oka M. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiol. Plant. 2013;35:3099–3107. doi: 10.1007/s11738-013-1343-5. [DOI] [Google Scholar]
- Yin L., Wang S., Tanaka K., Fujihara S., Itai A., Den X., Zhang S. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 2016;39:245–258. doi: 10.1111/pce.12521. [DOI] [PubMed] [Google Scholar]
- Yue K., Fornara D.A., Yang W., Peng Y., Li Z., Wu F., Peng C. Effects of three global change drivers on terrestrial C:N: P stoichiometry: a global synthesis. Glob. Chang. Biol. 2017;23:2450–2463. doi: 10.1111/gcb.13569. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang Y., Cai C. Multielemental stoichiometry in plant organs: a case study with the alpine herb gentiana rigescens across southwest China. Front. Plant Sci. 2020;11:1–12. doi: 10.3389/fpls.2020.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Xie J., Lyu M., Xiong D., Wang J., Chen Y., Li Y., Wang M., Yang Y. Short-term effects of soil warming and nitrogen addition on the N: P stoichiometry of Cunninghamia lanceolata in subtropical regions. Plant Soil. 2017;411:395–407. doi: 10.1007/s11104-016-3037-4. [DOI] [Google Scholar]
- Zhang Q., Zhou J., Li X., Yang Z., Zheng Y., Wang J., Lin W., Xie J., Chen Y., Yang Y. Are the combined effects of warming and drought on foliar C:N:P: K stoichiometry in a subtropical forest greater than their individual effects? For. Ecol. Manage. 2019;448:256–266. doi: 10.1016/j.foreco.2019.06.021. [DOI] [Google Scholar]
- Zhou T., Meng L., Ma Y., Liu Q., Zhang Y., Yang Z., Yang D., Bian M. Overexpression of sweet sorghum cryptochrome 1a confers hypersensitivity to blue light, abscisic acid and salinity in Arabidopsis. Plant Cell Rep. 2018;37:251–264. doi: 10.1007/s00299-017-2227-8. [DOI] [PubMed] [Google Scholar]