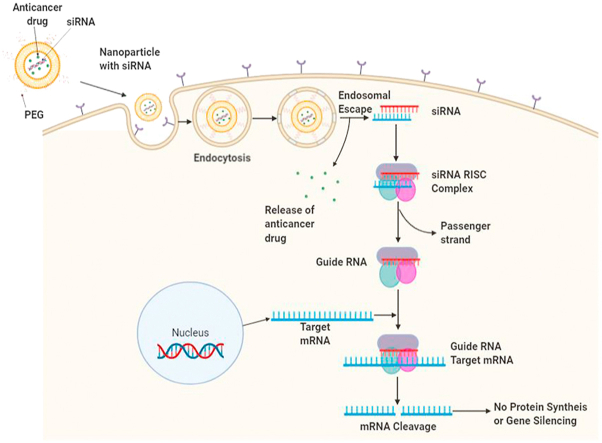
Abstract
In many ways, cancer cells are different from healthy cells. A lot of tactical nano-based drug delivery systems are based on the difference between cancer and healthy cells. Currently, nanotechnology-based delivery systems are the most promising tool to deliver DNA-based products to cancer cells. This review aims to highlight the latest development in the lipids and polymeric nanocarrier for siRNA delivery to the cancer cells. It also provides the necessary information about siRNA development and its mechanism of action. Overall, this review gives us a clear picture of lipid and polymer-based drug delivery systems, which in the future could form the base to translate the basic siRNA biology into siRNA-based cancer therapies.
KEY WORDS: Small interfering RNA (siRNA), Nanomedicine, Liposomes, Micelles, Cancer, Polymer
Abbreviations: APOB, apolipoprotein B; AQP-5, aquaporin-5; Atufect01, β-l-arginyl-2,3-l-diaminopropionicacid-N-palmityl-N-oleyl-amide trihydrochloride; AuNPs, gold nanoparticles; AZEMA, azidoethyl methacrylate; BMA, butyl methacrylate; B-PEI, branched polyethlenimine; CFTR, cystic fibrosis transmembrane conductance regulator gene; CHEMS, cholesteryl hemisuccinate; CHOL, cholesterol; CMC, critical micelles concentration; DC-Chol, 3β-[N-(N′,N′-dimethylaminoethane)carbamoyl]cholesterol; DMAEMA, 2-dimethylaminoethyl methacrylate; DNA, deoxyribonucleic acid; DOPC, dioleylphosphatidyl choline; DOPE, dioleylphosphatidyl ethanolamine; DOTAP, N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate; DOTMA, N-[1-(2,3-dioleyloxy)propy]-N,N,N-trimethylammoniumchloride; DOX, doxorubicin; DSGLA, N,N-dis-tearyl-N-methyl-N-2[N′-(N2-guanidino-l-lysinyl)] aminoethylammonium chloride; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; DSPE, 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine; DSPE-MPEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt); DSPE-PEG-Mal: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (mmmonium salt), EPR; enhanced permeability and retention, Galnac; N-acetylgalactosamine, HIF-1α; hypoxia-inducible factor-1α, KSP; kinesin spindle protein, LDI; lysine ethyl ester diisocyanate, LPD/LPH; lipid-protamine-DNA/hyaluronic acid, MDR; multiple drug resistance, MiRNA; micro RNA, MPEG; methoxypoly(ethylene glycol), MPEG-PCL; methoxy polyethylene glycol-polycaprolactone, mRNA; messenger RNA, MTX; methotrexate, NIR; near-infrared, NP; nanoparticle, NRP-1; neuropilin-1, PAA; 2-propylacrylicacid, PAH-b-PDMAPMA-b-PAH; poly(acrylhydrazine)-block-poly(3-dimethylaminopropyl methacrylamide)-block-poly(acrylhydrazine), PCL; poly(Ε-caprolactone), PCL-PEG; polycaprolactone-polyethyleneglycol, PCL-PEG-PHIS; poly(Ε-caprolactone)-polyethyleneglycol-poly(l-histidine), PCL-PEI; polycaprolactone-polyethylenimine, PDMA; poly(N,N-dimethylacrylamide), PDO; 1,3-propanediol, PEG-b-PDMAEMA-b-Ppy; poly(ethylene glycol)-block-poly(2-dimethylaminoethyl methacrylate)-block poly(pyrenylmethyl methacrylate), PEG-b-PLL; poly(ethylene glycol)-block-poly(l-lysine), PEI; polyethylenimine, PEO-b-P(DEA-Stat-MEMA; poly(ethylene oxide)-block-poly(2-(diethylamino)ethyl methacrylate)-stat-poly(methoxyethyl methacrylate), PEO-b-PCL; poly(ethylene oxide)-block-poly(Ε-caprolactone), PE-PCL-b-PNIPAM; poly(N-isopropyl acrylamide), pentaerythritol polycaprolactone-block-poly(N-isopropylacrylamide); PE-PCL-b-PNVCL, pentaerythritol polycaprolactone-block-poly(N-vinylcaprolactam); PiRNA, piwi-interacting RNA; PLA, poly-l-arginine; PLGA, poly lactic-co-glycolic acid; PLK-1, polo-like kinase 1; PLL, poly-l-lysine; PPES-b-PEO-b-PPES, poly(4-(phenylethynyl)styrene)-block-PEO-block-poly(4-(phenylethynyl)styrene); PTX, paclitaxel; RES, reticuloendothelial system; RGD, Arg-Gly-Asp peptide; RNA, ribonucleic acid; RNAi, RNA interference; RISC, RNA-induced silencing complex; RNAse III, ribonuclease III enzyme; S–Au, thio‒gold; SEM, scanning electron microscope; SiRNA, short interfering rNA; SNALP, stable nucleic acid-lipid particles; TCC, transitional cell carcinoma; TEM, transmission electron microscopy; Tf, transferrin; Trka, tropomyosin receptor kinase A; USPIO, ultra-small superparamagnetic iron oxide nanoparticles; UV, ultraviolet; VEGF, vascular endothelial growth factor; ZEBOV, Zaire ebola virus
Graphical abstract
The latest development in the lipids and polymeric nanocarrier for siRNA delivery to the cancer cells were highlighted. It also provides the necessary information about siRNA development and its mechanism of action.
1. Introduction
In the recent past, one of the most transformed fields of science is molecular biology. This transformation has occurred on several fronts; one of them is the small non-coding RNA, which regulates gene expression. Based on their biological roles and structures, small non-coding RNAs are classified into three main categories: miRNAs, siRNAs, and piRNAs1.
siRNA, also known as short interfering RNA, is a type of non-coding double-stranded RNA of 20–23 nucleotide base pairs in length. As the name suggests, it acts by interfering with the expression of the specific gene having a complementary sequence. The siRNA is similar to microRNA in terms of functions, except that the microRNA can regulate the expression of hundreds of genes via imperfect base pairing. In contrast, siRNA binds more specifically to the single gene at a particular location1. Although siRNA and miRNA are noncoding RNAs that share a common role in gene silencing and regulation, their mode of action and clinical potential are different. One of the significant differences between these two is that the miRNA has multiple targets, whereas siRNA has only one mRNA target. The clinical application of these two is thus, different from each other. The therapeutic potential of siRNAs and miRNAs is verified in the treatment of cancer and certain other diseases and infections2.
SiRNA is produced from the long dsRNAs and small hairpin RNAs with the help of enzyme dicer. It prevents the process of translation by degrading mRNA. This function of the siRNA is seen as one of the most critical therapeutic tools for the treatment of various genetic disorders, including cancer.
2. SiRNA production and interference mechanism
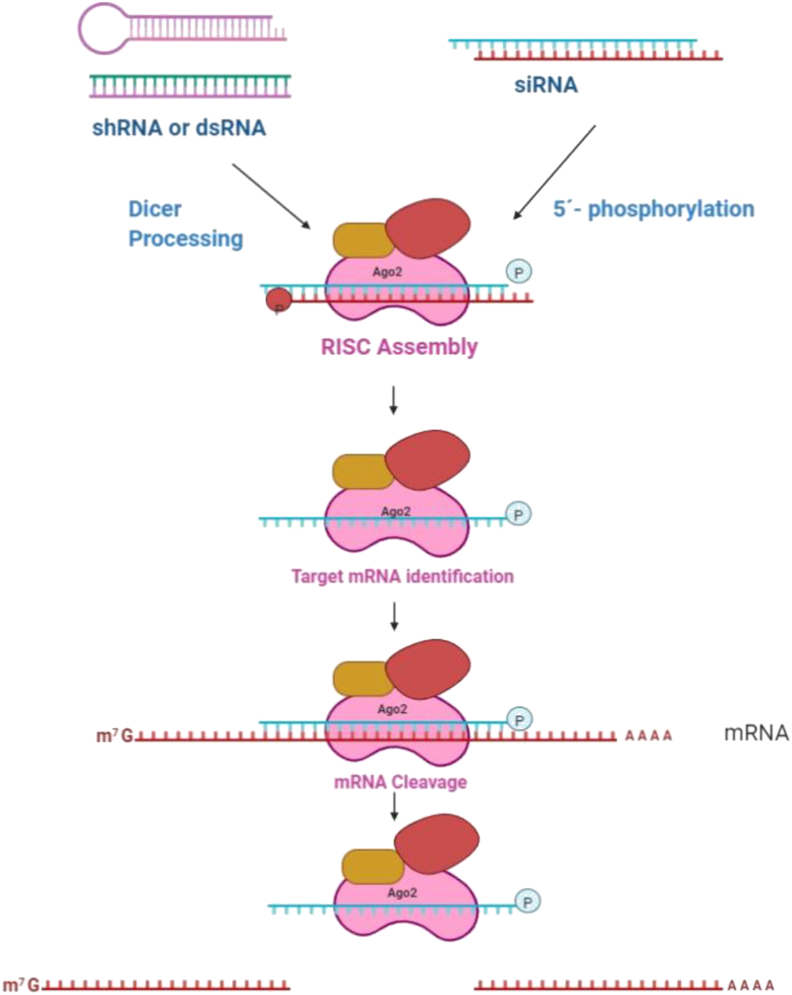
Sense and antisense strands of siRNA are transcribed from the same loci of the DNA template. This is the endogenous source of the small RNA molecules1. RNA molecules can also be introduced exogenously, which has already become a vital tool in laboratory medicine and research. Due to transcription from the same loci of DNA, RNA strands have the complementary sequence, which leads to the formation of the double-strand RNA molecules. Once formed, double-strand RNA, along with the associated proteins, moves in the cytosol through the nuclear pores where it cleaved to create the single strand siRNA. The enzyme responsible for the cleavage is the dicer, an RNase III type enzyme (RNA specific endonuclease). This cleavage leads to the overhang of two nucleotides at the 3′ ends and monophosphate at 5′ ends. siRNA thus formed, in association with ith ARGONAUTE and other proteins, create the silencing effector complex, which binds to the target mRNA via Watson–Crick base pairing. In most cases, silencing is the direct effect of this interaction. In short, after cleavage by dicer, the small RNA molecules of around 21 nucleotides are loaded on to the multiprotein complex (ribonucleoprotein), called RISC3. The loading efficiency of different siRNAs into the RISC varies considerably. Several studies revealed that one of the key features which affect the loading efficacy is the structure of RNA. Due to the variation of the loading efficacy, potency of the downstream effect of siRNA on gene silencing also varies. α-form helix is supposed to have the perfect and stable fit than the β-form helix to trigger the RNA interference4. In the case of the exogenous pathway (externally introduced siRNA), siRNA of the same length could directly load into the RISC without prior processing by the dicer enzyme4. Once loaded, one of the two strands (having the same nucleotide sequence with that of mRNA) separates from the RISC complex and degrades. This strand, which degrades, is called a passenger strand, and the strand having the complementary sequence to that of target mRNA is known as guide RNA. The guide strand remains attached to the RISC and guides the complex to the target mRNA. After proper recognition of the mRNA nucleotide sequence, complementary to that of guide RNA, the cleavage process starts4. The silencing of the target gene takes place by cleaving the mRNA around 10 to 11 nucleotides upstream of 5′ monophosphate end of the guide RNA. This process is catalysed with the help of enzyme Ago2, which is one of the most important components of RISCs. RISCs once cleave the target mRNA, undergoes recyclisation to carry out a similar event (Fig. 1)4. This model of target mRNA cleavage is supported by the in vitro studies carried out by the Nykanen et al5. They confirmed the formation of siRNA from dsRNA is ATP-dependent, loading of siRNA to the RISC is ATP-independent, unwinding of the siRNA complex to generate reactant complex is ATP-dependent and identification and cleavage of the target site of the mRNA are ATP-independent process. The group also confirmed the cleavage of the target mRNA at a single site precisely in the region complementary to the nucleotide sequence of guide siRNA5. Further, the Hutvagner et al.6 established that the RISC is recycled to be used multiple times, confirming its catalytic nature.
Figure 1.
Steps in RISC formation and function. Reprinted with the permission from Ref. 4. Copyright © 2012, ACS Publications.
3. SiRNA for cancer treatment
Current research in oncology is focused on understanding and targeting the genetic changes in the cancer cells. Recent knowledge of the genetic mutations in the cancer cells has allowed us to use classical chemotherapeutic agents in a better way. This knowledge is also helping us to develop advanced non-classical gene-based therapeutic agents7. Among the non-classical, siRNA is a useful therapeutic tool to knock-down the genes which are directly or indirectly responsible for the abnormal proliferation of cancerous cells. This possibility has fueled optimism in gene-based cancer therapy. In the near future, personalised treatment based on the genetic mutations will be possible, and siRNA is the front runner among the therapeutic interventions. The incredible gene silencing ability of siRNA has proven to be the crucial tool in understanding the genetic functions in plants and animals. Elbashir et al.8 first demonstrated the gene silencing ability of 21- and 22-nucleotide siRNA produced by the enzymatic action of ribonuclease III on dsRNA. They confirmed the inhibition of genes in various mammalian cell lines, including HeLa and human HEK. Following this demonstration, it was realized that this function of siRNA could be developed into a non-conventional new drug class that could directly inhibit the disease-causing or promoting genes. siRNA-based gene silencing is crucial for the targets which are not druggable or accessible to the small molecules, antibodies, or proteins9. Several in-vivo and in-vitro studies have confirmed that the abnormal cancerous cell proliferation could be significantly inhibited by siRNA-mediated silencing10. Moreover, siRNA has shown great promise in potentiating chemotherapy by sensitizing the drug-resistant cancer cells11,12. Present comprehensive research is also focused on the identification of the genes that, when silenced, boost the sensitivity towards chemotherapy. Therapeutic agents developed to target these mutated genes not only have the potential to target the cancerous cells but, rescue the healthy cells from the collateral damage13. In the present scenario, RNA interference is a widely used tool to identify and target them. Numerous studies reporting the use of siRNA on increasing the sensitivity towards chemotherapy via silencing are available; the detail is summarized in Table 114, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42.
Table 1.
Chemotherapy-based sensitization using siRNA.
| siRNA | Target gene | Target protein | Target drug | Cancer | Observation | Ref. |
|---|---|---|---|---|---|---|
| Anti-MDR1 silencing RNA | ABCB1 | P-gp 1 also known as MDR1 | DOX or MTX | Cancer known to overexpress the MDR1 gene to develop drug resistance | siRNA downregulated MDR1 mRNA expression by 50% in breast carcinoma and osteosarcoma cell lines. It inhibited tumor cell proliferation up to 90% (P < 0.01), when co-administered with DOX or methotrexate, despite the known chemoresistance of the cell lines. siRNAs reduced the IC₅₀ of DOX and methotrexate by more than 10-fold (P < 0.01) | 14 |
| EK-specific siRNA | DEK gene (involved in chromatin reconstruction) | DEK nuclear protein | Mitoxantrone or piroxicam carboplatin | TCC in canine | This study confirmed that DEK mRNA knock-down in canine TCC cell lines could inhibit proliferation, decrease cell viability, and enhance sensitivity. The outcome suggests that DEK inhibition may support cell survival and represent a valid target for novel therapeutics or combination therapies with classical anti-cancer drugs | 15 |
| siRNA against survivin | Survivin (BIRC5) | Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5 | Gemcitabine | Human pancreatic cancer cell lines of Panc-1 and BxPC3 | It was observed that the suppression of survivin could enhance the chemosensitivity of pancreatic cancer cells to gemcitabine | 16 |
| Dual siRNA-mediated silencing of Mcl-1 and Survivin in U-937 AML cells | Myeloid cell leukaemia-1 gene (MCL-1) and survivin (BIRC5) | Myeloid cell leukaemia-1 | Etoposide | U-937 AML cells | The results confirmed that MCL-1 and survivin have a crucial role in cell survival and sensitivity of U-937 cells to etoposide | 17 |
| siRNA against RRM2 | RRM2 gene coding for the M2 subunit of ribonucleotide reductase | Ribonucleotide reductase | Gemcitabine | Pancreatic ductal adenocarcinoma cell lines PANC1, MIAPaCa2, BxPC3, and Capan2 | Simultaneous action of RRM2 silencing and gemcitabine resulted in suppressed proliferation, enhanced apoptosis and reduced metastasis. The RRM2 silencing induced gemcitabine chemoresistance in pancreatic adenocarcinoma | 18 |
| siRNA against TS1058 | TYMS | Thymidylate synthase | DOX | Human colon cancer RKO | TS1058 siRNAs were found to be effective inhibitors of TS expression and could chemosensitise colon cancer cells to DOX | 19 |
| siRNA against VEGF | VEGFA | VEGF | DOX | Hep3B cells | VEGF gene silencing was found to enhance the chemosensitivity of Hep3B cells towards DOX | 20 |
| RBFOX3-specific siRNA | RBFOX3 | RNA binding protein, FOX-1 homolog | 5-FU | Human hepatocellular carcinoma cells (SNU-449, Hep3B, Bel-7402, SNU-387, and HepG2) and human immortalized hepatic cell line MIHA | RBFOX3 gene silencing induced the cell apoptosis, inhibited migration and invasion mediated by 5-FU | 21 |
| siRNA against c-Src | SRC | c-Src tyrosine kinase also known as proto-oncogene tyrosine-protein kinase Src | Gemcitabine | PANC1, MIAPaCa2, BxPC3, and Capan2 pancreatic adenocarcinoma cell lines | c-Src played a crucial role in pancreatic adenocarcinoma chemoresistance and could be a possible target for therapeutic agents | 22 |
| EGFR siRNA | EGFR | Epidermal growth factor receptor | Cisplatin, 5-FU, and docetaxel | Human head and neck squamous carcinoma cell lines HSC-2 (JCRB0622) and SAS (JCRB0260) | EGFR gene silencing in combination with cisplatin, 5-FU, and docetaxel increased chemosensitivity of all the drugs with an increase in apoptosis | 23 |
| siRNA targeting stathmin | STMN1 | Stathmin, also known as metablastin and oncoprotein 18 | Taxanes | Human osteosarcoma cell lines (Saos-2 and MG63) | Stathmin downregulation along with Taxanes showed potent anti-cancer activity in human osteosarcomas | 24 |
| DR1-targeting siRNA (siMDR1) | MDR1 | MDR1 | PTX | Human colon cancer cell line HT-29 | MDR1 gene silencing significantly reduced the MDR1 expression in human colon CSCs, and enhanced chemosensitivity to PTX | 25 |
| Survivin-targeted siRNA | Survivin (BIRC5) | Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5 | Chemosensitivity | Androgen-independent prostate cancer cell lines PC-3, PC-3M, and DU145, and androgen-dependent prostate cancer cell lines LNCaP and 22RV1 | Silencing of survivin by RNAi inhibited cell proliferation and enhanced chemosensitivity of prostate cancer cells | 26 |
| Bmi1 siRNA | BMI1 | Polycomb complex protein BMI-1 | Cisplatin | Human endometrial cancer cell line HEC1A and Ishikawa cell lines | Duel treatment with cisplatin and BMI1 silencing resulted in a synergistic anti-cancer effect, which was higher than that was shown by cisplatin alone | 27 |
| SaOS−2/NRP-1-siRNA | NRP1 | NRP-1 | DOX | Human osteosarcoma cell SaOS-2 | NRP-1 gene silencing significantly enhanced chemosensitivity to DOX | 28 |
| siRNAs targeting Girdin | CCDC88A | Girdin | Oxaliplatin | CACO-2, D2, DLD1, HCT15, HCT116, HUTU80, SW48, SW480, SW620, SW837, CX-1, COLO205, GP2D, GP5D, HCT15, LS174T and LS180 | Girdin silencing enhances chemosensitivity of colorectal cancer cells to oxaliplatin via TOP2B down-regulation | 29 |
| siRNAs targeting RRM2 | RRM2 Gene which codes for the M2 subunit of ribonucleotide reductase | Ribonucleotide reductase | DOX | PANC-1, a pancreatic carcinoma cell line, HEK293A, a human embryonic kidney cell line | SiRRM2 was found to significantly inhibit pancreatic tumor growth alone or in combinations with DOX | 30 |
| siRNA against HIF-1α | HIF1A | HIF-1α | Gemcitabine | MIA PaCa-2 cells | The HIF-1α silencing resulted in decreased cell proliferation and enhanced chemosensitivity towards gemicitabine | 31 |
| DNMT1 siRNA | DNMT | DNA methyl transferase | Taxol | Human brain cell line GOS-3 (grade II/III oligo-dendroglioma, DMSZ, Germany) and U87-MG (grade IV glioblastoma) | siRNA mediated silencing followed by Taxol after 48 h or a combination of siRNA followed by TMZ after 24 h was found to be an effective glioma therapy | 32 |
| siRNA against TRK | NTRK1 | TrkA | PTX | Human breast cancer cell line MCF-7 | Results indicate that TrkA signalling plays a vital role in breast cancer chemo-resistance and metastasis. TrkA is an important therapeutic target | 33 |
| siRNA against TGF-β1 | TGFB1 | TGF-β1 | Temozolomide | SKOV3 cells | Results indicate that TGF-β1 silencing inhibits cancer cell growth and enhances chemosensitivity by induction of BRCA1/Smad3 signaling. | 34 |
| Plk-1-specific siRNA | PLK1 | PLK-1 | Gemcitabine | Human pancreatic adenocarcinoma cell lines AsPC-1, PANC1, and BxPC3, and the normal pancreas cell line HPDE6c7 | Duel action of Plk-1 silencing and gemcitabine chemotherapy has synergistic anti-cancer activity against pancreatic carcinoma | 35 |
| NRF2-siRNA | NFE2L2 | Nuclear factor erythroid 2-related factor 2 (NRF2) | DOX, cisplatin, and sorafenib | Human osteosarcoma cell lines 143B (CRL-8303) and MG63 (CRL-1543) | Recombinant NRF2-siRNA was effective to sensitize both 143B and MG63 cells to DOX, cisplatin, and sorafenib, which was associated with significant downregulation of NRF2-targeted ATP-binding cassette (ABC) efflux transporters (ABCC3, ABCC4, and ABCG2) | 36 |
| Survivin siRNA | Survivin (BIRC5) | Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5 | Cisplatin | HepG2 and SMMC-7721 hepatocellular carcinoma cells | Suppression of survivin expression by RNAi attenuated the malignant phenotype of hepatocellular carcinoma cells. Cells also showed decreased proliferation, increased apoptosis, and caspase-3 activity, and increased chemosensitivity to cisplatin | 37 |
| AQP-5 siRNA | AQP5 | AQP-5 | DOX | DOX Resistant breast cancer cell line MCF-7 (MCF-7/ADR) | Inhibition of AQP-5 expression may reverse the drug resistance and enhance the chemosensitivity of breast cancer cells | 38 |
| Survivin siRNA | Survivin (BIRC5) | Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5 | Cisplatin | Human pancreatic carcinoma cell line Panc-1 | The knock-down of the survivin gene expression in Panc-1 cells effectively induced apoptosis with the simultaneous increase in the cisplatin sensitivity | 39 |
| Micelle/siRNA against ABCB1 complex | ABCB1 | P-gp 1 also known as MDR1 | DOX | CF-7/ADR cell lines | siRNA and DOX-loaded micelles were found to induce apoptosis and inhibit the growth of MDR tumors | 40 |
| siRNAs against survivin | Survivin (BIRC5) | Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5 | PTX | MDR lung cancer cell line (H460/cDDP) | siRNA targeting survivin has the potential to enhance the sensitivity of drug-resistant lung cancer cells to paclitaxel | 41 |
| siRNA for DPYD or TYMS | DPYD and TYMS | Dihydropyrimidine dehydrogenase, thymidylate synthase | 5-FU | Urothelial carcinoma | Thymidylate synthase was found to play an essential role in the prognosis of upper tract urothelial carcinoma, and siRNA may be a principal-agent for urothelial carcinoma treatment | 42 |
Several genetic mutations in tumor suppressor and oncogene lead to the transformation of a normal to the cancerous cells. Numerous classical drugs target the critical signaling molecules and inhibit the proteins and enzymes which directly or indirectly alter the gene functions. In-depth knowledge of the loss of functions and gain of functions may help to use conventional medicines or investigate the new ones for better therapeutic outcomes. Loss of functions and gain of functions of onco- or tumor-suppressor genes could affect tumor growth, apoptosis, sensitivity to the chemo and radiotherapy, and development of resistance towards chemotherapy. Identification of the gene which enhances or inhibits the sensitivity towards the radiation or chemotherapy could be the attractive target for cancer treatment. Drugs identified to selectively target such genes have the potential to enhance the cytotoxic effect of therapy.
Nowadays, gene silencing by siRNA is a crucial tool to pinpoint the gene responsible for the specific pathological condition. With extensive siRNA libraries available, it's easy to identify the targets for selective and specific drug development. Such target identification also helps in exploring the role of the particular set of genes in tumorigenesis43. Presently, the RNA interference tools like siRNA are widely used in studying the mammalian cellular signalling pathways. An in-depth exploration of cellular cell signalling pathways, especially in cancer cells, could help in the identification of the responsible genes. One very crucial example is the identification of the AKTcooperating kinases to enhance the action of Akt inhibitor. Morgan-Lappe et al.44 identified AKT cooperating kinases by screening a library of kinase-specific siRNA to enhance the cytotoxic effect of AKT inhibitor A-443654. There are a few other crucial signalling molecules responsible for cancer identified using RNAi, such as 1) Aza-Blanc et al.45 identified modulators of TRAIL-induced apoptosis. 2) MacKeigan et al.46 identified phosphatases and kinases enzymes responsible for apoptosis and chemoresistance. 3) Futami et al.47 identified molecules involved in Thapsigargin-induced apoptosis.4) Brummelkamp et al.48 confirmed that the loss of cylindromatosis activates NF-κB and inhibits apoptosis. 5) Berns et al.49 in a large scale screening study carried out on human cells, identified several new components of the P53 cell signalling pathways. 6) Kittler et al.50 identified several genes in HeLa cells, which are essential for cell division.
Several in-vitro, animal, preclinical, and some clinical trials have confirmed the sequence-specific binding of siRNA to the mRNA, and its site-specific cleavage results in the downregulation or inhibition of the genes responsible for cancer or other pathological conditions51. Irrespective of site-specificity, recent clinical trials have identified several hurdles in its clinical translation, which include degradation by the ribonucleases enzymes, stability of siRNA molecules in physiological conditions, inflammation reactions, site-specific and controlled release of siRNA, and efficient delivery vehicle. All these barriers must be overcome for the success of the siRNA in cancer treatment. Chemical modification may be required to improve the stability and reduce the immune activation of siRNA molecules52. The carrier system, which could not only deliver the siRNA molecules to the site of action but also protect it from the ribonucleases, is needed. PEGylated or tumour-targeting ligand conjugated nanoparticles composed of the lipids and other stimuli-sensitive polymers might improve the specificity and effectiveness of siRNA53. Although the siRNA has open new doors for the cancer treatment, it required fine-tuning to impart stability and delivery vehicle to carry it safely at the site of action. In the following section of the review, we have discussed the significant hurdles in siRNA delivery and the approaches which are under investigation for its safe and efficient delivery.
4. Recent advances in siRNA delivery to cancer cells
Highly charged molecules like RNA have several unfavorable characteristics, like rapid nucleases base destruction, enhanced clearance by the kidney, immune activation, and inefficient delivery to the cancer cells, which hindered its development. One major problem is its physiochemical characters; they are hydrophilic, negatively charged, and have a high molecular weight, which makes it impossible to cross the lipid membrane of the cell. Moreover, if siRNA enters via endocytosis, they could be subjected to a rapid degradation process during endosome lysosome trafficking and could not pass through the nuclear membrane54. The therapeutic success of siRNAs in cancer not only depends on its delivery to the tumor site, but for the highest clinical benefit, it must be administered systemically or orally. For systemic delivery of the siRNA, the foremost hurdle clinical scientists facing are: 1) getting siRNA delivered to the specific gene site without affecting the healthy cells, 2) maintaining the optimum level of siRNA at the site of action, 3) enhancing its efficiency by increasing cellular uptake, and 4) monitor efficiencies. One of the approaches to overcome these challenges is the development of novel delivery systems. The ideal delivery system for the siRNA to the cancer cells should: 1) prevent the nuclease-based degradation, 2) promote targeted site delivery, 3) facilitate cellular internalization, 4) avoid endosomal pathway, and 5) release siRNA at the site of action54.
Several siRNA delivery platforms are under clinical investigation. Non-viral systems include lipid-based vectors (e.g., liposomes, PEGylated liposomes, lipidoids, etc.), organic and inorganic nano-vectors, nanogels, peptide carriers (e.g., cell-penetrating peptides), etc. Non-viral delivery systems could deliver the siRNA with lesser safety concerns. They are easy to prepare, highly stable, non-mutagenic with excellent transfection efficiencies.
4.1. Lipid-based vectors to transport siRNA
Since the 1960s, liposomes underwent several changes that range from unilamellar vesicles composed of amphiphilic molecules to targeted liposomes for site-specific drug delivery55. Liposomes can entrap hydrophilic molecules in their aqueous core, whereas the hydrophobic molecules get trapped inside the lipid layer. One essential advancement is its upgrade to the stealth liposomes. Stealth liposomes contain lipids complexed with polymers, mostly PEG, in such a way that polymers are directed outwards from the liposomes. Such modification prevents the identification of the liposomes by the immune system and reduces the hepatic clearance. In targeted liposomes, functional lipids groups like –COOH or–NH2 are present at the distal terminal of polymers, which help them to link with the targeting ligands (proteins, like peptides or antibodies). Functionalization at the distal terminal end could also help to introduce pH-sensitive or hydrolysable groups to develop the pH- or chemical-sensitive liposomes. Cationic liposomes are one of the promising variations of the liposomes composed/of the cationic lipids and zwitterionic lipids, also called as the helper lipids. When such positively charged liposomes encounter the DNA molecule, they form the complex called lipoplex due to the electrostatic attraction between positively charged liposomes and negatively charged DNA molecules. Such complex on binding with the cell surface undergoes fusion and introduces DNA molecules inside the cells56.
Felgner et al.57 were the first who used the cationic lipids to transfect the cells with DNA. They used synthetic cationic lipid DOTMA for the preparation of liposomes. DOTMA facilitated the fusion of the liposomes with the lipid membrane of the cells. Fusion with the cell membrane has helped to achieve a high rate of DNA transfection57. During the last 30 years, different cationic lipids were developed to deliver the DNA and its products to the cells. Liposomes were the first delivery system developed from the cationic lipids. Most of the lipoplexes are not solely made up of the cationic lipids but are composed of the combination of lipids, such as DOPC or DOPE, CHOL, and some other natural lipids58, 59, 60.
Liposomes composed of the combination of helper lipids like DOPC, DOPE, and DSPC, are found to have a better fusion character than the liposomes made up of only the cationic lipids61. Overall, the loading of siRNA into the liposomes occurs because of the electrostatic charges; and sometimes, chances of nonspecific interactions with the serum or plasma proteins increased. Such non-specific interactions could lead to the activation of the immunogenic response and rapid clearance from the circulation system62.
4.1.1. Advanced cationic lipid-based siRNA delivery system
Cholesterol or DOPE is added to the cationic formulation not only to enhance the stability of the liposomes but also to enhance its cellular uptake63. Helper cationic polymers were introduced in the formulation to increase the siRNA entrapment inside the liposome core. For example, protamine was added in the formulation (DOTAP/Chol) to increased siRNA entrapment64. To improve the siRNA loading capacity, cationic liposomes were formulated using AtuFECT01, neutral/helper lipid phospholipidDPhyPE, and DSPE-PEG65. The loading capacity of siRNA is also found to increase when it is modified chemically to conjugated to 2′-O-methyl, and 2′-fluoro and CHOL9. SiRNA modified with 4′-C-guanidinocarbohydrazidomethyl-5-methyl uridine was found to have better silencing efficiency. Other notable modified siRNAs are GalNAc-conjugated siRNA, 2-OMe-phosphorodithioate-modified siRNA (higher loading capacity in RISC), CHOL-conjugated siRNAs (having better pharmacokinetic characters), hydroxyethylglycine PNA (hegPNA)-capped 3′ and 5′ siRNAs (protection against serum nucleases) and hydrophobically-modified siRNAs (improved stability and higher internalisation)66,67.
To improve the blood stability and pharmacokinetic characters, PEG was added to the cationic liposome formulation, which enhanced the blood circulation time68. A higher ratio of PEG enhances the circulation times but, at the same time, hampers the cellular uptake and endosomal escape, which means that the optimum ratio of the PEGylated lipids is essential69. Wrapsomes were proposed by Yagi et al.70 where siRNA/DOTAP forms the core, and neutral lipid bilayer composed of egg phosphatidylcholine and PEG lipid forms the wrap. Wrapsomes were found to have improved circulation time along with higher stability70.
The drawback of PEGylation, i.e., decreasing cellular uptake and endosomal escape, could be overcame by the approach of Carmona et al.71 The group coupled PEG-2000 dialdehyde to the cationic liposome composed of cholesteryl polyamine–N1-cholesteryloxycarbonyl-3,7-diazanonane-1,9-diamine, neutral lipids (DOPE) and CHOL–PEG350 aminoxy lipid via oxime linkage. This linkage is stable at pH 7 but decomposes at pH 5, releasing the PEG but, at the same time, offers the advantage of PEGylation71. Such cationic liposomes linked with PEG via an oxime bridge could become an important delivery system for siRNA delivery in the acidic microenvironment of a tumor. Nanoparticles having PEG linked with lipids susceptible to the proteins like matrix metalloproteinase was also developed72.
Some biogenic materials like hyaluronic acid were also added in the cationic liposome formulation to reduce immune identification. Such nanoparticles were found to enhance the siRNA delivery-mediated silencing of luciferase in B16F10 tumor cells73. To take advantage of lipid-polymer-based nanoparticles, cationic lipid‒polymer hybrid nanoparticles were prepared by a single-step nanoprecipitation of a cationic lipid (N,N-bis(2-hydroxyethyl)-N-methyl-N-(2-cholesteryloxycarbonyl aminoethyl) ammonium bromide, BHEM-Chol) and amphiphilic polymers for systemic delivery of siRNA. The lipid polymeric nanoparticles were found to efficiently deliver the siRNA to BT474 cells and, at the same time, escape the loaded siRNA from the endosome into the cytoplasm74.
To induce and enhance the cellular uptake and release of siRNA (endosomal escape), helper lipids like DOPE and 1,2-distear-oyl-sn-glycero-3-phosphocholine were added to the formulation of the cationic liposome. Similarly, 3-β-(N-[N″,N″-dimethylaminoethane] carbamoyl) cholesterol (DC-Chol) and dioleoylphosphatidylethanolamine (DOPE)-based lipoplexes were found to enhance the transfection efficiency of the siRNA75.
4.1.1.1. SNALP®
One of the critical developments in the cationic siRNA delivery system is the introduction of SNALP76. In general, SNALPs consist of modified siRNA, which is enclosed inside the bilayer membrane made up of cationic‒zwitterionic lipids with an outermost shield of PEG. It is primarily made up of three distinct lipids: a cationic ionisable lipid (1,2-dilinoleyloxy-3-dimethylaminopropane), a helper lipid (Chol or fusogenic lipids), and a PEG lipid. The electrostatic force of attraction between the positive charge of the SNALP membrane and the negative charge of the cell membrane assists the process of cellular uptake76.
In a study reported by Morrissey et al.76, two siRNA, namely HBV263 and HBV1583, targeted to the hepatitis B virus were chemically modified to protect it from nucleases. The efficiency of these modified siRNAs was studied in the mouse model of hepatitis B virus by delivering it using SNALP system. Better efficacy of modified siRNA delivery via SNALP was observed when compared to the same but unmodified siRNA. At the same time, improved half-life in plasma was also noted. In 2006, Zimmermann et al.77 have reported the first study of gene silencing in non-human primates. They described the silencing of the APOB gene, which is a target for heart disorders. APOB-specific siRNA entrapped inside the SNALP was administered via IV injection to the cynomolgus monkeys. SiRNA was found to cleave the mRNA at the site reported in the RNAi mechanism. Within the first 24 h, a reduction in the APOB protein and serum cholesterol was observed, and the effect persists for 11 days, indicating the importance of the SNALP system77. In a preclinical study, Judge et al.78 delivered the siRNA targeting the PLK1 and KSP in mice using SNALP. This report suggests the usefulness of the SNALP in delivering the siRNA load to the cancerous cells. Similarly, SNALP was used to deliver the microRNA (miR)-199b-5p. Delivery of miR-199b-5p was found to downregulate the HES1, and CSC levels in the colon (HT-29, CaCo-2, and SW480), breast (MDA-MB231T, and MCF-7), prostate (PC-3), glioblastoma (U-87), and MB (Daoy, ONS-76, and UW-228) cells79. In another antiviral study, Geisbert et al.80 silence the Zaire Ebola virus (ZEBOV) RNA polymerase by delivering siRNA using SNALP in guinea pigs model. miR-199b-5p administered using SNALP was found to hamper the proliferation with no sign of apoptosis. The effect of SNALP delivery system in the leukemia cell suspension was first reported by He et al.81 In an attempt to improve the liver fibrosis treatment SNALP surface modified with polypeptide, PPB was successfully used to deliver siRNAs against heat shock protein82. This data indicates that a system like SNALP is very critical in delivering the siRNA to the cancer cells without side effects79.
Recently, protein AXL which is involved in metastasis in both ovarian and uterine cancer was silenced by anti-AXL-siRNA using the novel delivery platform called p5RHH. P5RHH is composed of the cationic peptide (Melittin). When p5RHH enters the cell, it releases the siRNA upon protonation of histidine residue inside the acidic environment of endosomes83.
4.1.1.2. Atuplex®
In 2011 a German-based biotech company developed a chemically modified siRNA, called AtuRNAi, and a delivery system (Atuple) for in-vivo application. The modified siRNA has the added advantage of better resistance towards the nucleases enzymes and higher stability in blood. As it has better stability, it is required in less quantity for the same therapeutic effects with a better half-life. SiRNA in this modified approach was kept as much natural/non-synthetic as possible by modifying the natural building blocks at only 2′ sugar backbone position. No in-vitro and in-vivo induction in the genes associated with the inflammatory cytokines, including interferon, was observed. AtuRNAi products are available at a lower cost as compared with classical siRNA molecules. In addition, “silence therapeutics” has also developed a novel lipid-based delivery platform for AtuRNAi known as AtuPLEX. This proprietary owned complex is made to deliver AtuRNAi to the target cells in vivo. Atuplex composition involves the use of fusogenic lipids, which enhance the cellular uptake and assist the endosomal escape. This formulation was found very suitable for the delivery of therapeutic siRNA to inhibit the genes involved in the angiogenesis process. For specific requirements, “silence therapeutic” also included a PEG coating to prevent the interaction with blood protein and hide it from the macrophages. The company has around 50 patent applications covering different Atuplex compositions and uses. In one of the studies, “silence therapeutics” has reported the preclinical data of their AtuRNAi product called Atu027 for the treatment of solid tumors84,85.
4.1.1.3. Rondel®
Rondel is another important nanotechnology-based delivery system for the siRNA. This system uses the electrostatic force of attraction between the negatively charges DNA or RNA molecules and the positively charged linear polymer with alternate cyclodextrin molecules85. Adamantane, which is highly water-soluble, is another essential component of the Rondel system present in the cyclodextrin cavity to form the inclusion complex. PEG chains, linked to the inclusion complex on the outside of the nanoparticles, acts as a stabilizing agent and prevent its aggregation. A variety of the targeting ligands could be conjugated to the distal end of the PEG‒adamantane‒cyclodextrin inclusion complex to enable them to selectively link with the cells expressing the protein identified by the ligand. Chitosan is another low molecular weight carbohydrate used for the formation of nanoparticles to deliver siRNA. The rationale behind the use of such sugar type molecules was to impart the biocompatible character to the delivery system, to make them more stable in the biological fluids, and to enhance their transfection capabilities85.
As the name suggests, cyclodextrins are the linked glucose-α (1→4) molecules to form the circular basket shape. Hydroxy group of the sugar molecules in this basket topology are directed outwards, engulfing the upper and lower rim of the basket. In this configuration, the methine protons (H-5 and H-3) are directed towards the inner cavity of the basket. These structural features impart the amphiphilic characters, enabling them to charge with the drugs of different physiochemical characters. This characteristic is utilized by pharmaceutical companies to develop an efficient drug delivery system for the poorly water-soluble, pH liable, or biodegradable drugs86, 87, 88.
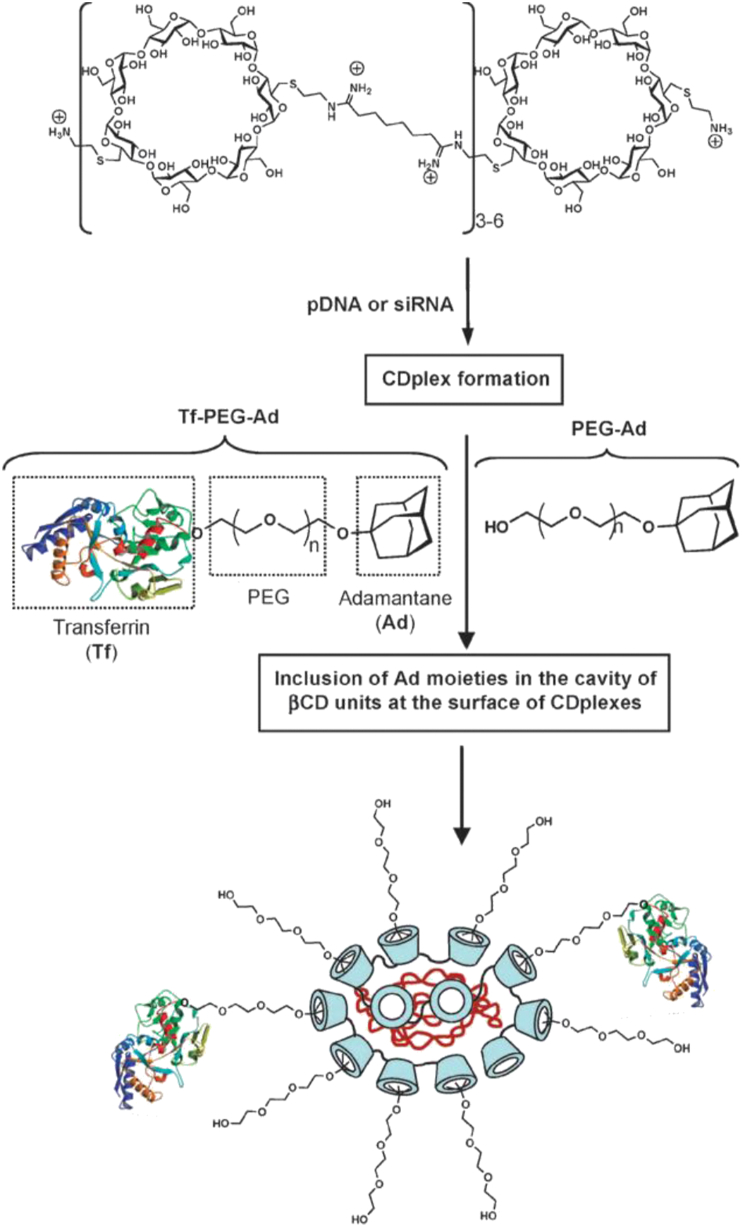
Cyclodextrin molecule was selected for the RONDEL complex because of its low immunogenic character and toxicity, and its ability to acts as a basket and to form the non-covalent interactions with the hydrophobic molecules. In 1999, the first case of the cationic polymer cyclodextrin complex formation, characterisation, transfection efficiency, and successful delivery of plasmid DNA was reported89. To overcome the aggregation of cyclodextrin polymer and pDNA nanoparticles, neutral stabilising polymer, PEG was linked with the hydrophobic adamantane to form the stable complex85. Suzie Pun et al.90 proposed the new method for polyplex modification, which utilized the ability of cyclodextrin polymer and adamantane to form the inclusion complexes. Non-PEGlyated polyplexes were found to aggregate in the salt solution, whereas PEGylated polyplexes remained stable at the physiological salt solution. Linking of the targeting ligand to the PEG‒adamantane conjugate further facilitated the site-specific receptor-mediated delivery of the complex. Galactosylated PEG adamantane inclusion complex was found to have a 10-fold higher efficiency than the un-galactosylated complex90. The first in-vivo proof of concept was proposed soon after the introduction of the murine model of Ewing's sarcoma91. The delivery system was composed of the cyclodextrin-containing polycation specifically used to bind and simultaneously protect the siRNA (siRNA for EWS/Fli1 fusion oncogene). The transferrin protein was used as the targeting ligand to target the transferrin (Tf) receptor. The control, i.e., without the transferrin conjugated to the polyplexes, has no antitumor effect91. PEGylated inclusion complex linked with transferrin complexed with the luciferase encoding gene when transfected to K562 leukaemia cells, resulting in better anti-cancer activity as compared to the inclusion complex with the linked transferrin protein (Fig. 2)92,93. Soon after the in-vivo success of the siRNA delivery, this concept was first commercialised by the pharmaceutical company (Calando Pharmaceuticals) in 2008. Human Tf was used at the targeting agent to deliver the siRNA (siRNA targeting the M2 subunit of ribonucleotide reductase) using the cyclodextrin polycation delivery system (RONDEL) in the non-human primates93. The trade name of the product was CALAA-01. This siRNA delivery via transferrin-linked RONALD inhibits tumor growth via RNA interference to reduce expression of the M2 subunit of ribonucleotide reductase (R2). Dose-dependent study of siRNA revealed the safety profile of the delivery system after the multiple systemic injections94.
Figure 2.
Schematic representation of the elaboration of the transferrin targeted pDNA- or siRNA-CDP nanoparticles (RONDEL). Reprinted with the permission from Ref. 93. Copyright © 2011, Royal Society of Chemistry.
4.1.2. DC-Chol/DOPE cationic liposomes
Cationic liposomes, composed of DC-Chol and DOPE (DC-Chol/DOPE liposome), were used to deliver recombinant genes into established tumors. They are considered as the most efficient vector for the transfection of DNA into cells. Nabel et al.95 had effectively delivered the human HLA-B7 gene into subcutaneous melanoma in clinical trials using DC-Chol/DOPE liposomes. The findings suggested that the transferred HLA-B7 gene was expressed and localized to the site of injection, and no apparent toxicity or anti-DNA antibodies was formed, which indicated the successful delivery of these cationic liposomes DC-Chol/DOPE95. In another study, a double-blinded, placebo-controlled trial assessing the safety and efficacy of liposome-mediated DNA transfer to the nasal epithelia of cystic fibrosis patients using cationic liposomes DC-Chol/DOPE and an expression plasmid containing a human CFTR cDNA was conducted96.
DC-Chol/DOPE transfection system works well only when the lipids are present in the right proportion. A ratio of 3:2 or 1:1 of DC-Chol/DOPE in liposomes was found to have maximum transfection efficiency97. It was observed that DOPE is a crucial component of the transport system for the optimum function98. This system also has a similar issue of stability due to aggregation and immune identification, which could be overcame by PEGylation99. Although PEGylation enhances the transfection efficiency of the cationic liposomes, a very long chain and high density could affect the transfection efficiency of the liposomes100. Various other factors could influence transfection efficiency75.
Maitani et al.98 have prepared three formulations of liposomes in the ratio of 1:0, 3:2, and 1:2 (DC-Chol: DOPE) to evaluate the effect of chloroquine on endosomal escape. Chloroquine is known to increase the endosomal pH, and hence its impact on the formulation was studied. Pretreatment with the pH raising agent has shown no effect on formulation having the composition of 1:0 and 3:2, but has a profound effect on the formulation with 1:2 ratios in terms of reduced transfection efficiency98. As of today, DC-Chol/DOPE cationic liposomes are one of the best carrier systems available for the siRNA delivery to the cancer cells. But this has its own drawbacks, like not suitable for systematic delivery because of the aggregate's formation with blood protein. To overcome this issue, Lee et al.101 have PEGylated the DC-Chol/DOPE cationic liposomes for kinesin spindle protein siRNA delivery to the cancer cells and to check its fate on systemic delivery. PEGylated composition was found to have a longer half-life in blood and enhance tumor accumulation as compared to non-PEGyated lipoplexes. PEGylated siRNA delivery has better silencing effects than the non-PEGylated siRNA and at the same time they remained hidden from the immune system of mice. These results indicated that in the coming days, DC-Chol/DOPE is better placed to deliver the siRNA via systemic delivery101. Liu et al.102 have used DC-Chol/DOPE cationic liposomes system to deliver the siRNA against the ferritin to check its effects on iron homeostasis in glioma cells and chemosensitivity. On intratumoral injections of liposomes-containing ferritin siRNA, around 80% of ferritin protein inhibition was observed in two days. This decrease in the ferritin level was positively correlated with the enhanced chemosensitivity towards the carmustine102. To overcome the short-term gene silencing effects of siRNA, Seraj et al.103 have designed Eg5shRNA-expressing plasmids to produce Eg5 hairpin RNA. To deliver this RNA, they used PEGylated DC-Chol/DOPE cationic liposomes and observed that the single systemic dose of Eg5 hairpin RNA expressing plasmid had long term Eg5 gene silencing effect in tumor-bearing mice. This system was also found to have no immunogenicity103. A study was reported by Tseng et al.104 and found out the impact of disaccharides on the internalisation of plasmid on different vectors. Increased cellular delivery was observed when co-formulated with disaccharides104. The ability of DC-Chol/cholesterol liposomes to carry pDNA into 293T cells was investigated. A formulation containing cholesterol was found to have not only uniform particle size and lower turbidity, but also better transfection efficiency105,106. Among stimuli-sensitive cationic liposomes, pH-sensitive has very low transfection efficiency. To improve the transfection efficiency of pH-sensitive liposomes, Chen et al.107 prepared complexes containing DC-Chol and DOPE liposomes and pH-sensitive liposomes composed of CHEMS and DOPE, and evaluated the influence of various factors on pDNA transfection efficiency. All DC-Chol/DOPE liposome/pDNA and pH-sensitive liposome complexes showed similar pH sensitivity107. DC-Chol/DOPE cationic liposomes are optimised for transfection in the absence of serum. Further understanding of the difference between such compositions will lead to the better designing of the DC-Chol-DOPE liposomes. Transfection efficiency was further found to increase with the addition of protamine in the formulation108. Kisoon et al.109 in one of their report, described the synthesis of the CHOL derivative 3β[N-(N′N′,N′-trimethylamino-propane)-carbamoyl] cholesterol, in which a propylamidooxy spacer separated the cationic trimethylamino head group from the hydrophobic and rigid cholesteryl ring system, and used them to make liposomes with an equimolar ratio of DOPE. The CHOL derivative, in combination with DOPE was found to provide better protection to the pDNA against the nuclease digestion and has better transfection efficiency109. To study the effect of PEGylation on gene silencing, Hattori et al.110 used four types of cationic CHOL derivatives and three types of dialkyl or trialkyl cationic lipids and prepared seven types of PEGylated cationic lipoplexes that contained 1% (mol/mol) PEG2000-DSPE. The PEGylation helped to reduce the aggregation with the blood components on intravenous injection. PEGylated cationic lipoplexes with N,N-dimethyl-N-octadecyloctadecan-1-aminium bromide has shown significant gene silencing effects in the lungs110. Overall, this study also revealed that 1% (mol/mol) of PEG and variation in cationic lipids severely affected the gene silencing effects of siRNA. The selection of cationic lipids is critical for the success of the PEGylated cationic liposomes110.
Despite the success of cationic lipids, hurdles like endosomal escape, cytosolic delivery, and lipid toxicity are still restricting the exploration of its full potential. To address this critical challenge, Lechanteur et al.111,112 prepared four different cationic liposomal formulations using DOTAP and DC-CHOL, and a different ratio of CHOL and DOPE. SiRNA was complexed with liposomes at six different siRNA/lipid molar ratios. The group confirmed that the nature of the lipid and lipid/siRNA ratio severely affected the cytotoxicity. It was observed that the cell–cell viability was reduced by 70% with liposomes composed of DOTAP/CHOL/DOPE (1/0.75/0.5) at a lipid/siRNA ratio of ten, whereas, at the molar ratio (Lipid/siRNA) of 2.5, the same formulation was found to be safe. For all the formulation, the transfection efficiency was found to be almost the same111,112.
Overall, for the successful development of CHOL/DOPE lipoplexes, it is not only essential to select the proper cationic lipids, but the ratio of lipids to siRNA plays a crucial role. Another critical point to be noted is that the acidity of exosomes plays a vital role in the exosomal escape. Hence it is essential to stress the proper selection of pH-sensitive lipids.
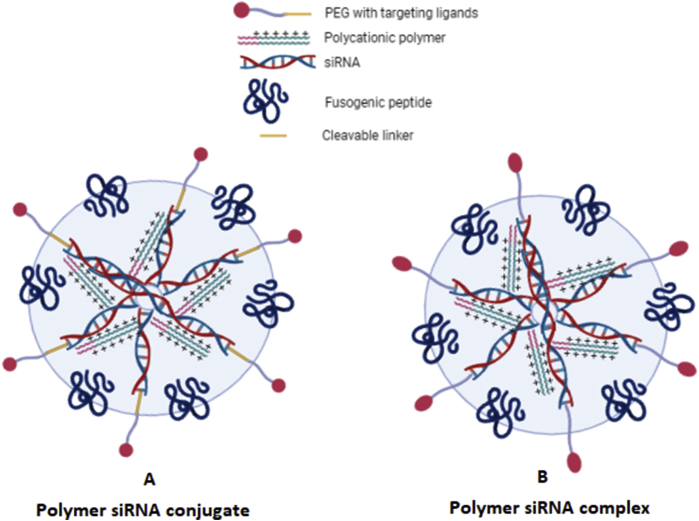
5. Lipid-protamine‒DNA/hyaluronic acid (LPD/LPH) nanoparticles
The effort to improve the transfection efficacy of the cationic liposomes is focused on the development of the new cationic lipids and polymers. Theoretically, cationic lipids or polymers having multiple positive charges shall have better transfection efficiency as compared to the monovalent cationic lipids. So the increase in the overall negative charges on the RNA or DNA molecules with the simultaneous increase in the positive charges on the cationic lipids could have better electrostatic charges and transfection efficiency113. Research in this area is mostly based on the trail, and hence enhancing the transfection efficiency of the existing cationic polymers is desirable.
One of the significant disadvantages of the DC-CHOL liposomes is its low transfection efficiency because of the larger nucleic acid/liposome complexes. The size of this complex at optimum nucleic acid to liposome ratio varies between 0.6 and 1 μm95. Liposomal complex aggregates to form the larger particles. However, several measured are under consideration to prevent aggregation. For example, in a clinical trial for malignancy treatment, DMRIE/DOPE, a cationic liposome was prepared, which does not aggregate to form the larger particles114. DC-CHOL/DOP liposome was prepared to transfect the CFTR gene and restores its activity in cystic fibrosis patients. This liposome has shown no sign of aggregation and achieved maximum transfection with altered transfection protocol. DNA/Liposome complex was prepared at high pH 8 to prevent the aggregation96.
Wagner et al.115 have shown that the shape of the DNA-Liposome complex plays a crucial role in the receptor-mediated endocytosis of the targeted ligand linked liposomes. In this transfection system, transferrin conjugated to the liposome acted as a targeting ligand, and polycation part acted as the counterpart for the electrostatic link with the negatively charged transferrin molecule. Polycation also squeezed the DNA molecules to form the doughnut-shaped delivery system. The degree of DNA condensation was found to be directly linked with the transfection efficiency. In this study, it was revealed that replacement of the large portion of the transferrin polylysine with free polylysine, improved the transfection efficacy of the delivery system. The addition of free polycation could further enhance transfection efficiency. It was also observed that protamine and histone could also be replaced with the polycationic part to get condensation of the DNA115.
Gao et al.116 tested some high molecular weight cationic polymers to check their effects on the transfection efficiency of the cationic liposomes. Poly(l-lysine), poly(l-ornithine), and poly(d-lysine) and polybrene were found to be equally effective in potentiating the transfection efficiency. However, the treatment of cationic liposomes with poly(d-lysine) or polybrene has led to deleterious effects on the cell, indicating that poly(l-lysine) or protamine are the safer alternatives to enhance the transfecting effect of the cationic liposomes. Gao et al.117 again in 2013 developed liposome‒polycation‒DNA complex functionalised with anti-epidermal growth factor receptor Fab’ antibody to target the epidermal growth factor receptor of the hepatocyte.
Clinical application of LPD to deliver siRNA to target C-MYC, MDM2, and VEGF by LPD was investigated. Silencing of these genes using LPD delivered siRNA lead to the reduction in the metastasis events of B16F10 melanoma cells in vivo. This study indicates that the LDH could be the most desirable tool to deliver the siRNAs to the cancer cells and could be the base for the future drug delivery system118.
The concept was proved valid with the development of PEGylated LPD (LPD‒PEG‒anisamide) nanoparticles, which boosted the siRNA delivery to cancer cells and simultaneous silencing of the associated gene, leading to the cancer cell growth inhibition119. SiRNA against the survivin, delivered by LPD‒PEG‒AA was not only found to induce the apoptosis process but also sensitize the cancer cells towards the cisplatin64.
Similarly, siRNA against EGFR delivered by LPD‒PEG‒AA was found to inhibit the EGFR expression in the cancer cells along with enhancing apoptosis120. These studies indicate that the targeted liposomes could be a powerful tool to deliver siRNA to the cancer cells. One of the most critical characters of the efficient delivery system is its inertness towards the immune system. On this front LPD system has little toxicity and inertness as confirmed by Chono et al73. The group has developed an LPD nanosystem to deliver siRNA systematically to the cancer cells. Cationic liposomes formed by mixing protamine, hyaluronic acid, and siRNA were coated with the cationic polymers. The complex thus formed again modified by adding lipids DSPE‒PEG or by adding targeted PEGylated lipids like DSPE‒PEG‒anisamide. Anisamide is the ligand for the receptor expressed on the B16F10 melanoma cells. The liposome system developed had higher loading and transfection efficacy along with the low immunotoxicity in the dose range of 0.15–1.2 mg siRNA/kg73.
Two important issues of the siRNA therapy and delivery system are the 1) non-specific delivery, including poor uptake by the cancer cells, and 2) unfavorable pharmacokinetics, including nucleases degradation and rapid clearance from the systematic circulation. Both issues were tried to be resolved by Chen et al.121 by delivering siRNA and DOX together. The group developed LPD nanoparticles for the site-specific delivery of the siRNA to the cancer cell of the mice by modifying LPD system with the NGR (asparagine–glycine–arginine) peptide. NGR is a ligand for the aminopeptidase N (CD13), mostly overexpressed in the tumor cells. The system was found to be efficient in delivering the siRNA to the cells and successfully down-regulate the target gene in HT-1080 cells121.
Chen et al.122 confirmed that the c-MYC siRNA could sensitize the cancer cells towards the paclitaxel. PEGlyation plays a vital role in the stability of the nanoparticles; hence, to determine the efficiency of the PEG linker, Deng et al.123 compared the siRNA delivery efficiency of DSPE-PEG-COOH or DSPE-PEG-MAL derivatives linked with the anti-EGFR Fab’ via a post-insertion approach. Immuno LPD, where anti-EGFR Fab’ linked through the DSPE-PEG-MAL conjugation, was found to be more efficient in delivering the siRNA to the target cell than the nanoparticles where anti-EGFR Fab’ was linked via DSPE-PEG-COOH linkage123.
Overall, siRNA is the crucial tool in gene therapy, and its delivery to the target cell is a critical barrier to overcome. A combination of siRNA and chemotherapeutic agents has recently achieved tremendous attention because of their synergistic action, better anti-cancer activity, low side effects, and fewer incidence of the drug resistance emergence. In combination therapy, siRNA and chemotherapeutic agents must have the synergistic action and should not have the antagonistic impact. To make cancer therapy more specific and safer, liposomal delivery using the targeting ligand significantly improves the efficiency of chemo and gene therapy. LPD not only offers the opportunity of delivering the siRNA/chemotherapeutic agents, but can also deliver the siRNA/therapeutic agent specifically to cancer cells if modified to link them with the targeting ligand, thereby avoiding the side effects. The following Table 2124, 125, 126, 127, 128, 129 represents the various valuable work carried out in the chemotherapeutic delivery to the cancer cells using LPDs.
Table 2.
Recent application of LPD for gene therapy.
| Formulation | Composition | Target gene | siRNA/drug | Remark | Ref. |
|---|---|---|---|---|---|
| Cationic liposome‒polycation‒DNA and anionic liposome‒polycation‒DNA | Guanidinium-containing cationic lipid, i.e., N,N-distearyl-N-methyl-N-2-(N′-arginyl) aminoethyl ammonium chloride, DOPA, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, cholesterol, protamine sulfate (fraction X from salmon) and calf thymus DNA | MDR transporter | DOX and siRNA against MDR tumors | Enhanced DOX uptake was noted when VEGF siRNA (in LPD-I nanoparticles) and c-Myc siRNA (LPD-II nanoparticles) combined in nanoparticles. LPD-I, which was made up of DSAA has shown more toxicity then LPD-II | 124 |
| Lipid−polycation−DNA nanoparticles containing DOTAP and targeted with PEG conjugated with anisamide | Non-glycerol-based cationic lipid which includes guanidinium and a lysine residue as the cationic headgroup (DSGLA); two liposome formulation were prepared, one with DSGLA and other with DOTAP | EGFR of H460 tumor cells | EGFR siRNA | LPD‒PEG‒AA developed with DSGLA delivered siRNA to the H460 cells. Although the siRNA delivered by LPD‒PEG‒AA containing either DOTAP or DSGLA could silence EGFR expression, a synergistic cell killing was only observed with DSGLA. The formulation containing DSGLA could induce more cellular apoptosis | 124 |
| PEGylated 17β-HSD1-siRNA/LPD | 17β-HSD1-siRNA modified with RGD peptide, 1,2-dioleoyl-3-trimethylammonium-propane, CHOL, distearoylphosphatidylethanolaminepoly(ethylene glycol) and calf thymus DNA | HSD17B1 | 17β-HSD1-siRNA | Significant suppression of tumor growth in 17β-HSD1-siRNA/LPD -treated group when HSD17B1 gene expression was knocked down. The untreated group has not shown significant growth inhibition | 125 |
| Targeted LPD-shRNA delivery system | shRNA-luc/protamine complexes coated with cationic liposomes consisting of DOTAP and cholesterol. PEGylated lipid (DSPE-PEG5000) was introduced post-production of nanoparticles | Gene for brachyury protein | shRNA | The transfection efficiency of LPD-shRNA was higher than naked shRNA. shRNA delivered by LPD inhibited brachyury expression, enhanced apoptosis and downregulated mesenchymal biomarker and suppressed cell proliferation | 126 |
| LPD nanoparticles of multi-epitope peptides developed from the rat HER2/neu (rHER2/neu) oncogene | LPD NPs, including DOTAP/CHOL liposomes, protamine, and CpG oligonucleotides | Peptides produced from rat HER2/neu (rHER2/neu) oncogene to induce IFn-γ and CTL responses | Multi-epitope peptides from the rat HER2/neu (rHER2/neu) oncogene | Results demonstrate that rHER2/neu-peptides (p5 and p435) and their encapsulation can induce an antigen-specific immunity. This study also presented the first attempt to evaluate the effectiveness of natural rHER2/neu-peptides containing CTL multi-epitope and encapsulated in LPD NPs | 127 |
| Lipid-polycation-hyaluronic acid | Polymer metformin, Hyaluronic acid (for condensation), cationic 1,2-dioleoyl-3-trimethylammonium-propane chloride salt (DOTAP) for liposome. DOTAP/CHOL (1:1, mol/mol) cationic liposome | VEGF siRNA for human lung cancer xenograft | VEGF siRNA, metformin | PolyMet successfully combined the intrinsic anti-cancer efficacy of metformin with the capacity to carry siRNA to enhance the therapeutic activity of anti-cancer gene therapy | 128 |
| Targeted LPD conjugated with anti-EGFR antibody | DOTAP (chloride salt), CHOL, DSPE-mPEG and DSPE-PEG-Mal | EGFR in breast cancer cell lines MDA-MB-231, SK-BR3 and MCF-7 | siRNA | The in-vivo accumulation of targeted LPD was higher than that of non-targeted LPD in MDA-MB-231 tumor 24 h post intravenous injection. | 129 |
5.1. Lipid/phosphate/calcium nanoparticles (LCP)
Despite the LPD success in delivering therapeutic siRNA to the cancer cells, improvement in terms of cellular uptake and bioavailability is required. LPC in terms of assembly is similar to the LPD except that the core of LPD is substituted with siRNA trapped nano-size calcium phosphate precipitate prepared by water-in-oil micro-emulsions130. This particular system was first reported by Li et al.130 in 2010, describing its utility in siRNA delivery. It was hypothesised that the inorganic ion would degrade inside the acidic pH of the exosome, leading to swelling and bursting, and ultimately release siRNA trapped inside it. PEGylation was further carried out, and anisamide, which is a ligand for the sigma-1 receptor, was conjugated. In their study, siRNA against luciferase was used as a model to predict the gene silencing effect of this new carrier in H-460 cells. Nanoparticles conjugated with the targeting agent, anisamide, have shown better gene silencing effects than the unconjugated. This formulation was also found better when compared with LPD.
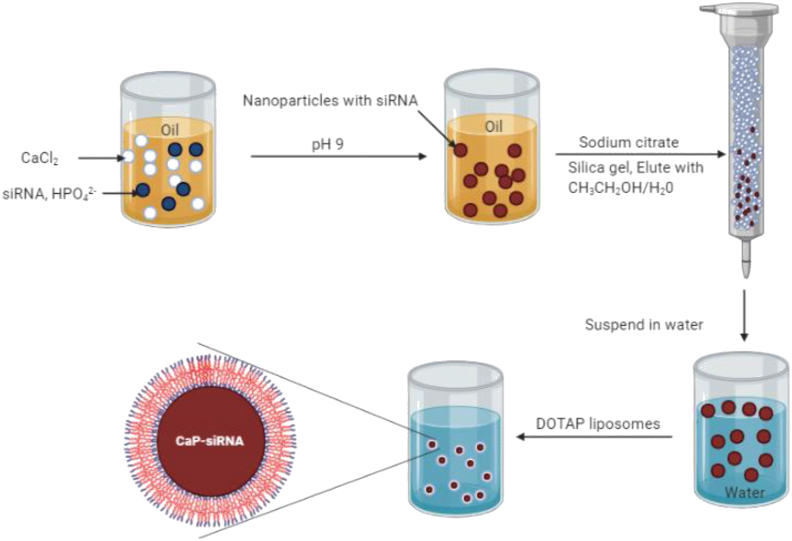
In LPD, which was reported by the same group, the DNA protamine complex was wrapped by the cationic liposome to form the positively charged particles. The positively charged particle then was further modified to include PEG and target ligands to impart site-specific delivery and better circulation time. This system, though successful, needed improvement in terms of endosomal escape. To overcome this issue, LCP nanoparticles were proposed. The 1st generation LCP (LCP-I) was made up of citrate-stabilized calcium phosphate core wrapped by cationic liposomes. The particle thus formed are suitable for the post-insertion of PEG and ligand conjugation131. The process of purification of LCP-I was tedious; hence, second generation LCP-II was proposed by Huang et al.131 (Fig. 3). In LCP-II, a lipid called DOPA was introduced inside the core to stabilize the nanocarriers. A similar reverse microemulsion method was used for their preparation. However, the sodium citrate was replaced with DOPA. In both LCP-I and LCP-II, the entrapment of siRNA or DNA occurred at the precipitation step. Other than siRNA, DNA chemotherapeutic agents having phosphate groups are the good candidates for LCP encapsulation131.
Figure 3.
The formation process of liposome/phosphate/calcium (LPC) nanoparticles. Reprinted with the permission from Ref. 131. Copyright © 2010, Taylor & Francis Group.
LCP is now the best-known nanocarriers for its efficiency in delivering the siRNA to the cancer cells. This efficiency is related to the fact that calcium and phosphate rapidly dissolve at the acidic pH of endosomes. This causes endosomal degradation, releasing the siRNA into the cytoplasm. Several modifications are still underway to modify this system for better delivery property. Maitra et al.132 have reported the preparation of calcium phosphate nanoparticles using a reverse microemulsion environment. The calcium phosphate colloidal system undergoes aggregation over the period of time, creating a severe stability issue. To overcome this issue, Sokolova et al.133 have developed a rapid precipitation method of calcium phosphate nanocarrier formation. This colloidal system has shown better stability over a period of time. To further explore the efficacy of the calcium phosphate nanoparticle, Liu et al.134 have developed the nanocarriers of 23.5–34.5 nm in diameters. This calcium phosphate system was found to be very efficient in delivering the DNA molecules with a very high transfection rate at the same time protecting DNA from degradation134. This system was also used to deliver DNA molecules by transfecting plasmid DNA135. Polyacrylic acid/calcium phosphate nanoparticles were reported by Wang et al.136 for delivering drug to the cancer cells. Radionuclide-like 177Lu and 111In were also successfully encapsulated inside the LCP along with the chemotherapeutic agents137.
Over the years, the LCP has shown success not only in delivering the siRNA molecules but also other treatment and imaging agents. In the future, there are many opportunities to combine the efficiency of LCP with other carriers for the simultaneous delivery of therapeutic and imaging agents.
5.2. Inorganic nanoparticles
Inorganic nanoparticles are most extensively used as an imaging probe because of their unique chemical and physical features that arise from their nanoscale size138. Several nanoparticle probes for imaging were developed using their magnetic, optical, and X-ray attenuation properties. Elements like gold, bismuth, and silver have been successfully used to contrast images of CT scans because of their high X-ray attenuation properties139, 140, 141. Similarly, inorganic elements and compounds like silver, gold, iron oxide, and silica were studied to analyze their utilization in drug delivery systems142,143. Only a few of these nanoparticle has reached to the advance stage, and most of them are in the initial phase of clinical development.
Moreover, silver and gold nanoparticles possess the peculiar optical property of surface plasmon resonance, which makes them different for the liposomes, micelles, and dendrimers144. Surface plasmon resonance is the basic principle behind several color-based biosensor techniques. It is an oscillation of conduction electrons at the interface between negative and positive permittivity material stimulated by incident light144. Surface plasmon resonance helps to measure the adsorption of materials on the planer surface of metal like gold and silver. Furthermore, because of their biocompatibility, they are now being explored to deliver DNA base product to the cytosol.
Drugs can be conjugated with the gold nanoparticles via covalent or electrostatic bonding and could be released inside the cells by external or biological stimuli145. Silver has reported antibacterial activity; however, few reports have been published confirming the use of silver nanoparticles for drug delivery. Prusty et al.146 developed stimuli-responsive polyacrylamide/dextran nanohydrogels composites material by in situ polymerization technique with incorporated reduced nanosilver. Jain et al.147 prepared iron oxide nanoparticles to target the antracyclinic antibiotic violamycine B1 to breast cancer. Cytotoxicity and the anti-proliferation effects of nanoparticles were tested in vitro on the breast adenocarcinoma cell line (MCF-7)147. Ngamcherdtrakul et al.148 developed the 47 nm mesoporous silica nanoparticle core coated with a crosslinked polyethyleneimine–polyethylene-glycol copolymer, conjugated with anti-human epidermal growth factor receptor type 2 siRNA and trastuzumab (a monoclonal antibody against human epidermal growth factor receptor). This nanoparticle was developed to enhance the half-life of siRNA in blood and DNA silencing effect of siRNA by explicitly targeting the cancer cells148.
Overall, the inorganic particles provide a useful medium for the development of the delivery vehicle for the siRNA149. They offer a high surface area to volume ratio, which ensures the high conjugation of siRNA and hence better loading. A crucial key to the success of the inorganic nanoparticle delivery system is the flexible surface chemistry, which provides the means to overcome the hurdles of safe siRNA delivery150. Additionally, it is easy to track the siRNA delivery to the cells because of their unique physical and chemical properties151.
Among the inorganic nanoparticles, because of their inertness, nontoxicity, and biocompatibility properties, gold nanoparticles are the most widely studied for siRNA delivery151. Strong interaction between sulfur and gold (S–Au bond) elements helps to conjugate the biological and synthetic compounds on to the surface of gold nanoparticles152. S–Au bond is composed of around 35% of partial covalent and 64% electrostatic characters153. An energy decomposition analysis indicated that gold had a greater covalent character with sulfur ligands relative to Cu and Ag154. Covalent linking to the gold nanoparticles did not affect and inhibit siRNA's biological activity155.
In recent times the research interest is grown significantly in polyvalent oligonucleotide nanoparticle conjugates, which consist of the core of the 2–250 nm, and several strands of oligonucleotide covalently conjugated to it156. The polyvalent oligonucleotide nanoparticle conjugates possess unique properties like cooperative binding, higher complementary strand binding, catalytic properties, easy intracellular entry without the use of additional transfection agents, and higher intracellular stability and resistance toward the nuclease enzymes, which makes them the potential candidate for gene silencing157, 158, 159, 160, 161. Seferos et al.156 examined the polyvalent oligonucleotide nanoparticle conjugates and explained the enzymatic resistance and intracellular stability. For stability study, they prepared 1 nm gold nanoparticles and functionalized them with the 20 base pair long oligonucleotides linked via 10 base pair linker DNA and propylthiol anchor156. The thick coat of oligonucleotides on the surface of nanoparticles was found to protect them against the enzymatic degradation of nucleases enzyme.
For conjugation, thiolate oligonucleotide reacted with the citrated-capped gold nanoparticles. During the reaction, oligonucleotide ligands displaced the citrate group of the gold nanoparticles and formed the gold thiol bond. Sodium chloride could be used to stabilize the charge repulsion, thereby allowing the more oligonucleotides to conjugate on the surface to create the dense monolayer coat. Around 250 nucleotides could be comfortably conjugate on the surface area of the gold nanoparticle of 15 nm size to give rise to polyvalent complex161. This conjugation method was successfully used to conjugate the oligonucleotide to the nanoparticles of the size between 2 and 250 nm162. Irrespective of the high negative charge, which could prevent the cellular uptake, polyvalent oligonucleotide gold nanoparticles have remarkable uptake, as seen in more than 50 different cell lines163,164. Cellular uptake was found to be the function of the oligonucleotide density on the nanoparticles; higher density was found to support the more efficient delivery157,160. The uptake of anionic nanoparticles (oligonucleotide conjugated) is attributed to the strong binding with the scavenger receptor, which is an essential protein involved in the receptor-mediated membrane transport system. The superfamily of the scavenger receptor proteins could bind different types of ligands, including polyanionic compounds like lipoproteins, apoptotic cells, cholesterol ester, phospholipids, proteoglycans, ferritin, and carbohydrates165. This recognition of the wide range of compounds allows the scavenger proteins to play a crucial role in pathology and homeostasis. Scavenger receptor protein undergoes endocytosis after binding to the ligands. This mechanism provides the universal mechanism of delivery to the healthy and disease cells. Targeted delivery to the cells over-expressing the surface proteins is also possible by conjugating the antibody against such protein to the polyvalent oligonucleotide gold nanoparticles166.
Hao et al.167 used synthetic tumor suppressor microRNA (miR-205) to conjugate with the oligonucleotide gold nanoparticles. These miRNA-conjugated polyvalent oligonucleotide gold nanoparticles were found to successfully inhibited the expression of the target protein167. Oishi et al.168 developed the delivery system for siRNA by complexing the thiolated siRNA with the gold nanoparticle to which poly(ethylene glycol)-bpoly(2-(N,N-dimethylamino)ethyl methacrylate) was conjugated. siRNA was found to significantly suppress the expression of luciferase expression in HuH cell line. Giljohann et al.169 reported the RNase-free polyvalent siRNA gold nanoparticles to silence the gene in HeLa cells. This siRNA-conjugated gold nanoparticle was found to have the six-time longer shelf life in the serum than their RNA duplex counterparts. The functionalization with siRNA leads to the development of nanoparticle with better cellular uptake without the need for chemical modifications or the use of other transfection medium169.
Local suppression of the genes in the skin presents the unique challenge of negative charge of large molecules like siRNA delivery. Zheng et al.170 reported the spherical nucleic acid nanoparticle conjugates, gold cores with conjugated siRNA. The siRNA conjugated nanoparticles were found to freely pass through 100% of skin cells in vitro, mouse and human epidermis within hours after application170. When siRNA against EGFR was delivered using this system locally to the skin of a hairless mouse, complete inhibition of EGFR expression and downstream ERK phosphorylation were observed170.
The success of siRNA delivery system depends on the endosomal escape, and this is also true in the case of the siRNA-conjugated inorganic nanoparticles. Massich et al.171 successfully demonstrated the endosomal escape of the siRNA after 4 h from the polyvalent nucleotide gold nanoparticles by tagging them with cyanine 5.
As discussed earlier, various cationic materials like lipids and polymers were used to condense the siRNA to form the nanoparticles. Additionally, various functionalized nanomaterials, like carbon nanotubes, iron oxide nanoparticles and gold nanoparticles, were also used to condense the siRNA for delivery172, 173, 174. These materials were also found to reduce toxicity as compared to the polymers175.
Compared to the plasmid size DNA, siRNA usually has less efficient interactions with the cationic materials because of the small size. Hence, siRNA required a high concentration of such materials for efficient compression or a material with high cationic characters. To overcome this issue, gold nanoparticles with conjugated cationic ligands were used for better interactions. Kim et al.175 reported the gold nanoparticles conjugated with dendritic PEI-like ligands to enhance the cationic characters. The siRNA-conjugated superamolecule developed using this protocol was found to have good gene inhibition activity with low toxicity175. Similarly, for the treatment of protest cancer, Fitzgerald et al.176 developed coated gold nanoparticles with poly(ethylenimine) to produce the poly(ethylenimine)‒gold nanoparticles complex. The complex was further conjugated with the targeting ligand anisamide to produce a cancer cell-targeted siRNA delivery system. Anisamids is the ligand for the sigma receptor, which is overexpressed on prostate cancer cells176. To silence the ROR1 oncogene, which is overexpressed in different cancers, Ahwazi et al.177 immobilized HIV-1 TAT peptide on gold nanoparticles and conjugated the particles with the ROR1‒siRNA for the potential breast cancer treatment. In an alternative approach Shirazi et al.178 synthesized and conjugated homochiral l-cyclic peptide to the gold nanoparticle to deliver the siRNA in HeLa cells. For the Dengue treatment, Paul et al.179 conjugated anti-DENV siRNAs with gold nanoparticles (AuNPs) and tested them in vitro.
Overall, among inorganic materials, gold nanoparticles have shown the potential to be the preferential delivery agent for the siRNA. Reports of various ligands, like cell-penetrating peptides, protein, antibody covalently linked to the gold nanoparticles, are available. These ligands have shown promising results for siRNA delivery7,180. The siRNA conjugated to the gold nanoparticles has shown resistance towards the nuclease-based degradation and promoted the timely endosomal release. However, such ligand‒gold nanoparticles lack serum stability and have a very short self-life, which prevents its long-lasting gene silencing effect. S–Au covalent chemistry is the most direct method to conjugate siRNA to gold nanoparticles. Oishi et al168. used S–Au chemistry to conjugate the siRNA to gold nanoparticles. They prepared 15 nm gold nanoparticles and conjugated it with S–PEG5000–PAMA7500 polymer followed by linking them with the thiolated siRNA. Jensen et al.181 extended this concept to produce spherical nucleic acid-linked gold nanoparticles. They used this system to knockdown the Bcl2L12 mRNA using the siRNA against it in glioma cells181. Gold nanoparticles covalently linked with the siRNA was further coated with streptavidin layer to attach cell-penetrating peptides through biotin-streptavidin ligation182. Gold nanoparticles are also developed into the advanced platform for targeted delivery. For example, thiol–siRNA and Arg-Gly-Asp were simultaneously conjugated to the gold nanoparticles to carry the siRNA to the lung tumor cells in the murine model.
The more convenient conjugation method to conjugate the siRNA is the non-covalent linking. Non-covalent linking is facilitated by the electrostatic attraction between the negatively charged siRNA and positively charged nanoparticles. For example, Kim et al.175 reported the gold nanoparticles conjugated with dendritic PEI-like ligands to enhance the cationic characters, which were letter conjugated with the negatively charged siRNA. For instance, cationic polymer like polylysine was also used to functionalize the gold nanoparticles, which was later used to entrap the siRNA for delivery183. One advanced method of siRNA conjugations is layer by layer coating of gold nanoparticles with alternate layers of cationic polymer and siRNA to give rise to the coat of gold nanoparticle‒cationic polymer‒siRNA‒cationic polymer. Elbakry et al.184 developed the gold nanoparticle using layer by layer approach and further investigated its usefulness in siRNA delivery. Similarly, Lee et al.186 for hyaluronic receptor-mediated siRNA delivery have developed the cysteamine-modified gold nanoparticles layered with siRNA‒polyethyleneimine‒hyaluronic acid. For extended gene silencing and lower toxicity, Lee et al.186 have used protease degradable polylysine as a biodegradable biopolymer. They conjugated the gold nanoparticles with the siRNA, which were then coated with the polylysine. The layer of poly-lysin was then degraded by the lysosomal cathepsin B enzyme ensuring the extended-release of siRNA185,186.
Inorganic nanoparticles have provided the unique stage for the effective delivery of siRNA to the cancer cells. Delivery of siRNA using organic nanoparticles can be fine-tuned by modifying the nanoparticle surface. As discussed, inorganic nanoparticles possess the unique physical and optical properties which could be used to track the fate of such particles inside the body. The potential of organic potential is not only limited to the siRNA delivery but could be used for diagnostic purpose. Despite the several advantages, several hurdles needed to be overcome to translate the lab research to the patients on the bed. For clinical translation, precise information about the safest route of administration, toxicity, immune response, long and sustained release of the siRNA is required. Overcoming these hurdles will need a better understanding of the central aspects of inorganic nanoparticle relation with living systems. Research on such interaction will ensure the faster translation of lab research to the clinical trials.
5.3. Micelles for siRNA delivery
Polymeric micelles are another nanocarrier system which has attracted remarkable attention as a potential carrier to deliver siRNA to the cancer cells. Micelles are made up of the blocks of the two or more polymers having an opposite affinity towards the same solvent. Thus, polymers of the micelles have amphiphilic characters. These amphiphilic block polymers, when suspended in a solvent, organized themselves to form the micelles depending upon the block affinity towards the solvent. If the diblock polymer suspends in the aqueous phase, the hydrophobic end of the polymer attempts to stay away from the aqueous phase forming the core of the micelles. In contrast, the hydrophilic part will face the aqueous phase forming the micelle shell. The formation of micelles only occurs at the concentration above the critical micelles concentration (CMC). Generally, micelles forming at the lower CMC are more stable and better to deliver the siRNA to the cancer cells. Polymers with high hydrophobicity characters exhibit better stability due to the lower CMC. In aqueous solution, less water-soluble compounds get trapped inside the hydrophobic core of the micelles, whereas the compounds with the higher hydrophilic characters remain in the intermediate layer187.
Micelles could be divided into two broad categories (Fig.4): 1) formed from the direct linking of the PEG through non-degradable linkages to siRNA to form the PEG‒siRNA complex; 2) formed from the direct condensation of the siRNA with the block amphiphilic polymers containing the polycations followed by micellization of block copolymer/siRNA complex188.
Figure 4.
Models of different polymeric micellar structures for siRNA delivery. (A) Polymer-siRNA conjugates complexed with polycation. (B) Block copolymers complexed with siRNA. Reprinted with the permission from Ref. 188. Copyright © 2012, Elsevier.
The advantage of the polymeric micelles is their ability to solubilize the water-insoluble compounds inside its core. This system helps to enhance the bioavailability of the drugs, the full potential of which is difficult to explore because of unfavorable pharmaceutical characters. Due to the low water solubility, it is sometimes challenging to achieve the complete therapeutic outcome of the compound. Most of the anticancer drugs are polycyclic compounds, hence has to face the same pharmaceutical challenges. If such drugs are administered via the parenteral route, the chances of building aggregates large enough to block small capillaries are very high189. Polymeric micelles could not only enhance bioavailability by inhibiting rapid extraction and solubilizing compounds at the core but also deliver them at the site of action if they are conjugated with the targeting ligands. Another distinct advantage of the micelles is its size (10–100 nm), which is small enough to remain in the circulatory system by avoiding the mononuclear phagocytic system and large enough to prevent fast renal clearance190. Further, leaky vasculature of the tumor helps the higher accumulation of the micelles via EPR effect191. Polymeric micelles formed at the lower CMC offer higher stability even if diluted in the higher volume of the body fluids, which allows the hydrophobic compounds to remain inside the core being protected for a longer time.
5.3.1. Passive and active micelles targeting
Polymeric micelles are 10–100 nm diameter nanocarriers. The hydrophobic core of the micelles carries the water-insoluble drugs, whereas the hydrophilic component helps to hide the assembly from RES and enhances its blood circulation time. This property allows micelles to accumulate passively (passive targeting) in the tumor having leaky vasculature (hypoxic tumors). Furthermore, the flexibility of the block copolymer chemistry permits the easy alteration of micelles structure according to the physical and chemical properties of the drug, ligand conjugation, tumor environment, and sensitiveness to external and internal stimuli. The features and functions of active and passive micelles targeting are summarized in Table 3192, 193, 194, 195, 196, 197, 198.
Table 3.
Passive and active micelles targeting.
| Passive targeting of the micelles | Active targeting of the micelles | Ref. |
|---|---|---|
| Depends on the permeability of the rapidly forming vasculature. Pathological conditions like inflammation support permeation of micelles into the solid tumors | Depends on the ligands linked to the micelles and the expression of the receptor proteins on the cancer cells. Accumulation is supported by pathological conditions like inflammation | 192 |
| Accumulation inside the tumor preferably depends on the EPR effect | Accumulation inside the cells depends on the targeting ligand, receptor protein interaction, abundance of the receptor protein and EPR | 193 |
| Passive targeting via micelles takes advantage of the poorly developed vasculature. Vasculature with large fenestrations formto keep in pace with higher demand of the nutrients and oxygen, which leaves the endothelial cells poorly aligned with a large opening between them | Tumor cells for survival express several proteins at a higher quantity than the normal cells. This feature allows selective accumulation of such micelles | 194 |
| No need to modify the surface of the micelle with the targeting agent. PEGylation is required to reduce rapid excretion and enhance stability | Polymeric micelles can be functionalized for active targeting by chemically modifying their surface with targeting ligands that show a strong specificity for antigens or receptors over-expressed on cancer cells | 195 |
| Preferential binding to the cancer cell is not required. PEGylation helps it to accumulate in the tumor via enhancing EPF effect | Actively targeted polymeric micelles decrease side-effects of drugs by allowing preferential accumulation in diseased cells and facilitate cellular uptake by receptor-mediated endocytosis | 196 |
| Does not guarantee the safe delivery of the DNA or siRNA to the cancer cells | Benefits the intracellular delivery of macromolecules like DNA, siRNA, and proteins | 197 |
| NA | Commonly used targeting, such as ligands including antibodies and their fragments, proteins, small molecules, peptides, aptamers, and sugar molecules | 198 |
5.3.2. Stimuli-responsive polymeric micelles
Distinct characters of the cancer cells or tumor microenvironment act as a stimulus of the drug release. In general, micelles made for such a stimulus carry the drug in one environment (extracellular or in normal tissue environment) and release it when such micelles enter in the distinct environment of the tumor and cancerous cells199. Polymeric diblocks could be used to prepare such micelles to respond to the intrinsic (redox potential, enzymes, cofactors, enzymatic products, and pH) or extrinsic stimuli (ultrasound, external magnetic field, temperature, and light). Stimulus-sensitive micelles release the drug load after the structural change/destruction in response to the external or internal stimuli. These stimuli generally lead to the destruction of the micelles via polymerization, aggregation, disintegration and isomerization, etc.199. The most common stimuli-responsive micelles are discussed in Table 4187,200, 201, 202, 203, 204.
Table 4.
Stimuli responsive siRNA delivery system.
| pH-Sensitive | Redox sensitive | Enzyme-sensitive micelles | Ultrasound | Magnetic field | Temperature-sensitive | Light-sensitive micelles |
|---|---|---|---|---|---|---|
| Drug delivery from micelles depends upon the pH the tumor | Drug delivery from micelles depends upon the change in the redox potential of the tumor microenvironment | Drug delivery from micelles depends upon altered expression of certain enzymes in cancerous cells | Drug delivery from micelles relies on the application of the high-pressure wave of a frequency of 20 kHz | Drug delivery from micelles depends upon the application of the external magnetic field | Drug delivery from micelles depends on the effects of the temperature on the heat-sensitive polymers | Drug delivery from the micelles depends on the UV–Visible or NIR light to trigger drug release |
| The pH of the tumor is 6.5 due to high lactic acid production, whereas the pH of the healthy tissues is around 7.4200. The pH of the internal cellular organelles drops between 4 and 6, depending upon the organelles201. | Cancer cells have higher redox potential (100–1000 fold higher) as compared to the outside of the cells | Polymers used to make these micelles has the groups which are recognized by the enzyme or by the products of the enzymatic reaction causing morphological changes or destruction of the micelles | Ultrasound frequencies in the range of 20–100 kHz can penetrate deep inside the body tissue crossing the various body fluids. | Such micelles are composed of paramagnetic responsive materials like Fe3O4 or Fe2O3. These materials respond to the externally applied magnetic field. | These micelles composed of the heat-responsive polymeric block, which upon exposure to the different temperatures, undergoes the phase change. Hydrophilic to hydrophobic changes are more common. | In these micelles, light-sensitive groups are included inside the block polymers, in the core, or on the shell. Generally, photosensitive groups or chromophores undergo the stereochemical conversion, e.g., cis to trans conversion or vice versa |
| These pH-gradients have been exploited successfully to design pH-sensitive polymeric micelles which can release their therapeutic payloads when they encounter a change in the pH of their microenvironment | Higher intracellular redox potential is due to the high concentration of the glutathione tripeptide (γ-glutamyl-cysteinyl-glycine). Polymeric micelles designed using the disulfide linkage, which could hold the drug at its core under normal redox potential,l but release it upon destabilization of disulphite bridge in higher redox potential. | These micelles could hold drugs in the absence of enzymes or enzymatic products. The most common enzymes exploited for such release are the proteases, lipases, and glycosidases, including the enzyme engaged in glycolysis, angiogenesis, fatty acid synthesis, and matrix metalloproteinase. | Such micelles could hold the drugs in the absence of ultrasonic wave stimulus but release it when they are disturbed upon exposure to the low-frequency ultrasonic waves | These micelles hold the drugs in the absence of the externally applied magnetic field but release it upon destruction by externally applied magnetic field. | These micelles hold the drugs at one phase of the polymeric block but release it upon phase change after exposure to the temperature change | These micelles carry the drugs in one confirmation of the photosensitive materials but release it after conversion to its alternate form upon exposure to the light source |
| Most commonly used pH-sensitive polymers202 are acrylic acid, methacrylic acid, propionic acid, 2-acrylmido-2-methylpropylsulfonic acid, 2-methacryloxyethylsulfonic acid, 3-methacryloxy-2-hydroxypropylsulfonic acid, ethylenesulfonic acid, sulfoxyethyl methacrylate hyaluronic acid, alginic acid, acrylamide, aminoethyl methacrylate, polylysine, polyhistidine, chitosan, etc. | The basic principle of redox-responsive polymeric drug delivery systems is to utilize the differences in redox potentials between tumors and normal tissues. It has been demonstrated that GSH/glutathione disulfide is the most abundant redox couple in animal cells. In the cytosol and nuclei, the concentration of GSH reaches 10, while outside the cell, the concentration drops to about 2–20 mmol/L. The tumor has 4-fold higher GSH concentrations than that of healthy tissue. Disulfide linkages have been applied broadly in reduction-responsive polymeric drug delivery systems187 | Azobenzene linkage is established at the copolymer junction of an amphiphilic diblock copolymer. Treatment with the enzyme azoreductase, in the presence of coenzyme NADPH, results in the cleavage of the azo-based copolymer junction, which disrupts the micellar assembly | The most common copolymers used in acoustically activated drug delivery belong to the Pluronic family of triblock copolymers, e.g., P105, F127, P85, L61, etc. | Jiang et al.203 prepared a series of alkyne functionalized PPES-b-PEO-b-PPES triblock copolymers by RAFT polymerization and introduced cobalt by the reaction of the alkyne functionalities with Co2(CO)8. The metallated copolymers were cross-linked and formed magnetic cobalt nanoparticles after heating at 120 °C | The most commonly used polymers to make thermosensitive micelles are PE-PCL-b-PNIPAM and PE-PCL-b-PNVCL204 | The most commonly used photosensitive materials are the azobenzenes and their derivatives. Other examples of the light-sensitive materials include 11 O-nitrobenzyl esters, coumarinyl esters and spiropyrans spirooxazines |
5.3.3. Recent multifunctional micelles delivery of siRNA to cancer cells
Recent approaches allow the integration of the best of different physical and chemical characters into single multifunctional micelles. When different functionalities were combining in a new hybrid micelle where each component is working in complete harmony and coordination with the other to give the simultaneous or sequential drugs/siRNA/diagnostic agents, such hybrid micelles are termed as multifunctional micelles. Thus, the ideal multifunction micelles could not only deliver the therapeutic agents but, if required, should also be able to deliver the diagnostic agents. Cancer, which is a multifactorial disease, is not only difficult to treat, but the perfect diagnosis is challenging. Recent advances in molecular therapies have developed very selective treatments. However, some of the cancer cells remain undetected, develop resistance over the period of time, and lead to therapy failure. Cancer cells and tumor environment have several distinct characters which differentiate them from the normal cells and tissue microenvironment205, which includes several deregulated protein expression, pH, distinct vasculature development, etc. Hence cancer requires a multi-faceted therapeutic and diagnostic approach. Considering all the distinct characters of the cancer cells and the versatility of the micelles to carry the different load, it becomes imperative to use multifunctional micelles for the treatment. Although incorporating all the ideal characters of drug delivery into one single vehicle is difficult, a blending of two or more characters is possible and is necessary for the cancer treatment. In recent times the research focused on the development of the multifunctional micelles to enhance the delivery efficiency, minimized the side effects, and simultaneous delivery of diagnostic agents. The recently reported multifunctional approaches are discussed in Table 5206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228.
Table 5.
Multifunctional micelles delivery of siRNA to cancer cells.
| Function | Block polymer | Targeted/non-targeted | Cancer type | Ref. |
|---|---|---|---|---|
| Micelles combined DOX and PTX delivery | PLGA-PEG | Yes: TAT peptide (cell-penetrating peptide) | Human carcinoma KB cell line | 206 |
| Polymeric micelles for siRNA (siRNAs against VEGF) delivery through the bloodstream to tumor models in mice | PEG-b-PLL | Yes: cyclo-arginine‒glycine‒glutamic acid (cRGD) peptide | H2BGFP-HeLa cells mice model | 207 |
| Micelles for anti-VEGF siRNA delivery | MPEG/PCL diblock copolymer | Yes: TAT peptide (cell penetrating peptide) | S-180 sarcoma cells | 218 |
| Polymeric micelles for macrophage-specific siRNA delivery | Oly(BMA-co-PAA-co-DMAEMA)-b-poly-(DMAEMA)-b-poly(AzEMA) triblock copolymer | Yes: mannosylated to achieve CD206 (mannose receptor)-targeted siRNA deliver | Immortalized human macrophages (THP-1) or human breast cancer cell lines (MDA-MB-231 and MDA-MB-468) | 222 |
| Multifunctional hybrid micelles with shell embedded magnetic nanoparticles for theranostic applications (magnetic oxide and oxorubicin) | Pluronic F127 and peptide-amphiphile(PA) pal-AAAAHHHD | Controlled drug release using magnetic field stimuli | HeLa cells | 223 |
| Tumor-cleavable nanomicelles (DOX) | Polyurethane was synthesized from biodegradable PCL and LDI | Yes: folic acid | L929 and HeLa cells | 224 |
| Multi-functional multiblock polyurethane micelles (PTX) | PDO and PCL-bearing pH-responsive hydrazone bonds | pH-sensitive drug release | 3T3 mouse fibroblasts and A431 cells and A431 tumor-bearing mice | 225 |
| MMP2-sensitive PTX-containing micellar | Self-assembling PEG2000-peptide-PTX conjugate, which contains the MMP2-cleavable octapeptide between PEG and PTX | Yes: TAT peptide (cell-penetrating peptide) | NSCLC xenograft mouse model | 226 |
| Hybrid micelle for co-delivery of PD-L1 siRNA and paclitaxel | 98% PCL-PEG and 2% PCL-PEI | Non targeted | B16F10 or DC2.4 cells and B16F10 melanoma tumor-bearing mice (C57BL/6) | 227 |
| Micelles for the combination therapy with siRNA (siMDR-1) and chemotherapeutics (DOX) | 4-Polyamidoamine conjugated with PEG-phospholipid | A2780 ADR, MCF7 ADR and MCF7 | MDR cancer cells: human ovarian carcinoma (A2780 ADR) and breast cancer (MCF7 ADR) | 228 |
| Light and pH dual sensitive micelles for siRNA delivery | Light and pH-sensitive triblock copolymer of PEG-b-PDMAEMA-b-PPy | Light and pH-sensitive micelles | MDA-MB-231 cells | 208 |
| Micelles for the co-delivery of MTX and survivin siRNA | Polyethlenimine and mPEG | Yes: linolenic acid | HeLa cells | 209 |
| Hybrid micelles for glypican-3 siRNA | PLGA‒PDPH (3-(2-pyridyldithio) propionyl hydrazide) | No | OV2944-HM-1 cells (HM-1), derived from the C57BL/6 × C3H/HeNF1 (B6C3F1) mouse | 210 |
| Tunable polymeric hybrid micelles to deliver survivine siRNA | PEI and mPEG amphiphilic polymers (PEI‒LA and mPEG‒LA) | Linoleic acid linked amphiphilic polymers | A549 cells | 211 |
| Facile hydrophobization micelles for siRNA (Plk1 siRNA)delivery | PEG-b-PLA micelles | No | DA-MB-231 cells | 212 |
| Micelles for EZH2 siRNA delivery | MPEG-PCL and DOTAP | Micelles protected siRNA delivery | U87 cells and GL261 | 213 |
| Duel function targeted micelles for programmed cell death ligand 1 (PD-L1) small interfering RNA (siRNA) delivery and indoleamine 2,3-dioxygenase inhibitor | Cholesterol conjugated cell-penetrating peptide lin TT1 (Chol–HHHHHHH–AKRGARST) | Yes: cell-penetrating peptide conjugated | 4T1 cells and 4T1 mouse breast cancer allograft tumor model | 214 |
| Multi-functional polymer micelle for the sequential delivery of VEGF siRNA and paclitaxel | Triblock copolymer of PCL‒PEG‒PHIS | Yes: folate conjugated | MCF-7 cells and HUVEC cells and MCF-7 tumor-bearing female nude mice | 215 |
| pH/Reduction dual-responsive micelles for targeted intracellular co-delivery of DOX and Bcl-2 siRNA | PEGylated cationic triblock copolymer of PAH-b-PDMAPMA-b-PAH | Yes: folate conjugated | MCF-7 cells | 216 |
| Micelles as nanocarriers for 7-ethyl-10-hydroxycamptothecin, USPIO, and siRNA against VEGF | PDMA-b-PCL | No | Human colon adenocarcinoma cell line LS174T and LS174T grafted mice model | 217s |
| Micelles for co-delivery of P-gp siRNA and DOX | Triblock polymers of PEG-b-(PCL-g-PEI)-b-PCL | Yes: folic acid conjugated | MCF-7 and MCF-7/ADR cells and tumor-bearing mice | 219 |
| Multifunctional polymeric micelles for the co-delivery of ZEB1 siRNA and DOX | Copolymer: PEG–PLL–PLLeu | No | H460 cells and subcutaneous H460 mice model | 220 |
| Multifunctional Polymeric Micelles P-gp siRNA and DOX | β-Cyclodextrin-poly-ethyl-enimine | No | MCF-7 andMCF-7/ADR cel | 40 |
| Multifunctional micellar nanocarriers for targeted co-delivery of MDR-1 siRNA and DOX | PEO-b-PCL | Integrin αvβ3-specific ligand (RGD4C) for active cancer targeting and the cell-penetrating peptide TAT for membrane activity | The P-gp overexpressing human melanoma cell line MDA-MB-435/LCC6MDR1 and female athymic nude mice (NCRNU-F) model of the MDA-MB-435/LCC6MDR1 cells | 221 |
Based on the literature review, the two common strategies employed to make the polymeric micelles involves: 1) direct conjugation of siRNA to hydrophilic (PEG) or hydrophobic (lipids) via non-degradable or degradable linkages. This is followed by their exposure to the polycations to form the micellar structures called polyion complex micelles(PIC). Poly(aspartic acid) or poly(l-lysine) or PEI is the most commonly used polyion segment229. 2) SiRNAs are complexed with an amphiphilic block copolymer having polycations segments followed by the micellization of the complex230. Nanocarriers, including micelles, cross the cell membrane by the endocytosis process. One of the significant causes of concern is the endosomal escape after endocytosis. SiRNA inside the endosomes are nothing better than outside the cells. They need to escape out of the endosomes to avoid the lysosome's lower pH (pH ∼4.5) and potential degradation. Endosomal escape makes the siRNA available in the cytosol to form the silencing complex. Cationic polymers are hence incorporated in the polymeric micelles, which release siRNA by disturbing the endosomes. Some polymeric micelles also included pH-sensitive polymers, which, when exposed to the endosomal pH, disrupt the endosomes and release siRNAs in the cytosol. Some micelles formulations utilized polymers, which, when exposed to the external stimuli like light or magnetic fields, destabilized the endosomes to release the siRNAs. Considering the current literature discussed in this section, we can assume that the multifunctional polymeric micelles are one of the front runners to establish itself as the best delivery option to carry siRNA inside the cancer cells. Multifunctional micelles are a combination of the different functionalities bought together to develop the ideal delivery platform. However, the basic architecture of modern micelles is getting more complex, which not only could create the reproducibility problem, but also high siRNA entrapment and better cellular uptake will be an issue. Such complexity could also become the hurdle in real-life clinical utilization, and finally, the approvals from the various regulatory bodies will be the challenge231.
5.4. Polymer-based siRNA delivery
Until now, the unavailability of the proper delivery vehicle has restricted the clinical application of siRNA. siRNAs, which are the double-stranded negative charge molecules with hydrophilic characters, are relatively impermeable to the cell membrane. Chemical modifications to change the characteristics of the siRNA are required to cargo them to the cytosol. Such modifications could adversely affect the binding properties, and in some cases, siRNA could even irreversibly change to an inactive molecule. As discussed, various lipids and polymer-based delivery vehicles systems based on the nanotechnology platform were developed. Among them, the polymer-based delivery system received wider acceptance. Different gene-based products like protein, nucleic acids, peptides were delivered to the cells using polymer-based delivery systems.
We have already discussed the lipid-based strategies and the impressive research that took place to develop them into a potential delivery candidate. Polymers also have a potential role to play in the cytosolic delivery of proteins, DNA, and siRNA232, 233, 234, 235. Various polymer compositions with different topology could be synthesized using techniques like atom transfer radical polymerization, reversible addition–fragmentation chain transfer polymerization, and ring-opening metathesis polymerization. At the same time, they can be readily derivatized by adding different functional groups to suit the applications. Moreover, the polymers with positive charge have developed a special interest in the delivery of siRNA. They not only form the reversible complex with the negatively charged RNA molecules but, but also facilitate the higher cellular uptake and endosomal escape. The stable reversible complex formation of siRNA with polymers is the challenge that needs to meet on a priority basis. Cationic polymers are already explored for their capability to carry the gene to the cell236, 237, 238. Due to the high negative charges on the siRNA, the cationic polymers bind and condense into the nanoparticles via ionic interactions. Even with the advancement in the polymers science, its use is restricted because of the limited number of binding sites available on the siRNA molecules. The limited interactions between the polymers and siRNA molecules lead to the formation of relatively unstable nanoparticles. This problem could be overcome with the utilization of hyperbranched polymers with large molecular weight and dendritic topology. Such polymers significantly enhance the transfection efficiency but at the cost of toxicity. For example, Yang et al.239 reported a nanoparticle system composed of high molecular weight linear PEI condensed with DNA and coated by a shell of polyethyleneglycol-modified (PEGylated) low molecular weight linear PEI. Compared with the commercial delivery system, a 16,000-fold increase in the transfection efficiency was observed. Although the nanoparticles offer substantial advantages of higher transfection efficiency, linear PEI toxicity was observed. siRNA delivery using high molecular weight polymers is still in the initial phase of development. Several attempts have been made to develop polymer with high transfection efficiency and lower toxicity240, 241, 242.
As discussed, intracellular delivery of the siRNA to the normal and cancer cell requires assistance from vectors. Vectors should not only be able to deliver the siRNA to the cytosol but also protect it from host nucleases. Recently, due to the vast interest in the siRNA therapeutic potential, a strong interest in the development of the non-toxic non-viral polymer-based vectors to improve the transfection efficiency was generated. Juanes et al.243 very recently explored the potential of amphiphilic polyhydrazones and the degree of polymerization for the intracellular delivery of siRNA. They also demonstrated that this system could also be adopted for the complexation of mRNA.
PEI, which played a crucial role in the plasmid DNA delivery, is not considered very efficient for siRNA delivery. The lower efficiency of PEI to transfect siRNA is because of the shorter length of siRNA as compared with the DNA molecules. The electrostatic force of attraction between the negatively charged siRNA and PEI is also not sufficient to hold the complex together, which dissociates at the anionic cell surface244. Another major concern with the use of PEI is its toxicity which severely limited its use in nucleic acid delivery245. However, recently due to the availability of the linear and branched derivatives of PEI in a wide range of molecular weight, interest in its delivery potential has been reestablished. Such derivatives are the less toxic variation of PEI with better protection for siRNA from the nucleases. The toxicity associated with PEI has been controlled by incorporating low molecular weight PEI into other polymeric constructs246. Chemical modification of the PEI also helps to introduce the functionalities like targeted delivery, higher resistance against the nucleases, better endosomal escape, prolonged systemic circulation, and external and internal stimuli-responsive release of siRNA13,247, 248, 249. The most feasible approach to mitigating the PEI toxicity is through the introduction of the hydrophobic characters. This approach, however, is associated with the reduction in the overall positive charges on the PEI, which could affect the polyplexes formation with siRNA. The optimum balance between the hydrophobic characters and overall positive charges is essential for the success of PEI in siRNA delivery. The addition of the alkylcarboxyl groups to the branched PEI is found to impart the hydrophobicity, and carboxylation up to 20% was found to be associated with the better endosomal escape250. An increased in the length of the alkyl chain, which is used to enhance the hydrophobic character, has also been associated with the improved stability of the siRNA complex and reduced toxicity as compared with the PEI having a molecular weight of 25 kDa251,252.
Low molecular weight PEI, along with the low toxicity, also has low transfection efficiency. This issue was overcome by the introduction of disulfide bonds in the cross-linked PEI253, 254, 255. The optimized equilibrium between branch density and cleavable disulfide bond within PEI is found to be the crucial factor in achieving better siRNA delivery256. Kim et al.257 combined low toxicity and better transfection efficiency by conjugating the hydrophobic lipid anchor, cholesterol chloroformate to the cationic head of low molecular weight branched PEI257. This complex was used to transfect the siRNA designed to inhibit the vascular endothelial growth factor in human prostate adenocarcinomas257. For targeted delivery of siRNA to inhibit dihydrofolate reductase enzyme, Biswal et al.258 developed folate-conjugated PEI. The complex was used to inhibit the folate reductase in human epidermal carcinoma258.
Similarly, Yamaoka et al.259 conjugated pullulan with PEI for liver targeting. siRNA was complexed with pullulan-containing PEI. Pullulan has a very high affinity towards the asialoglycoprotein receptor, highly expressed on the liver cells, ensuring the targeted delivery of siRNA. Similarly, galactose has a high affinity towards the asialoglycoprotein receptors, which was also used for the targeted delivery of siRNA molecules260,261. PEI derivatives were also studied for the stimuli-responsive release of siRNA. For example, Lee et al.262 conjugated PEI with embedded magnetite nanocrystals to develop stimuli-responsive release of siRNA.
PLGA is another polymer that is widely used for siRNA delivery. It is a copolymer composed of glycolic acid and lactic acid linked through an ester bond. The ester bond undergoes hydrolysis to form the monomers back. The rate of hydrolysis depends upon and could vary with the ratio of monomers, total molecular weight, structure, and shape of the polymer. PLGAs having different proportions of monomers, molecular weight, structures, and shapes are developed commercially for various biomedical applications. Nanoparticles composed of PLGA have been widely screened for the delivery of drugs and gene-based products. PLGA has created huge interest as an alternative to viral-based delivery of siRNA263. They offer the advantage of small particle size, relatively non-toxic, and sustained release profile264. In general, nucleic acids are loaded into PLGA-based nanoparticles by encapsulating it inside the core or by adsorption via electrostatic force of attraction between the modified positively charged surface of PLGA and negatively charged siRNA molecules. PLGA nanoparticles have created huge interest in the delivery of therapeutics because of their high stability, higher endocytosis rate, targeting ability by conjugating them with the targeting ligands, and biodegradability. PLGA matrix can entrap siRNA to provide resistance against RNase activity, and it also imparts favorable colloidal stability to the delivery formulation which facilitates safe and sustained, release profiles. For sustain release variation, degradation time of PLGA could be managed from days to years by varying the molecular weight and the ratio of its monomers256. The initial challenge in the loading of the negatively charged siRNA to the negatively charged PLGA nanoparticles is because there is no electrostatic interaction. Conjugation of the cationic moiety to the PLGA matrix could impart the stability and toxicity issues265. Such modification could also sometimes negatively affect the activity of siRNA. To overcome these flaws, Cun et al.266 used a double emulsion solvent evaporation method to incorporate the siRNA in PLGA nanoparticles. This research group successfully incorporated the siRNA inside the PLGA nanoparticles without affecting its stability and activity266. Although this approach is successful for loading siRNA in PLGA nanoparticles without the use of cationic conjugated, it has limited application because of the low loading and encapsulation efficiency266. The low loading is attributed to the repulsion force between the phosphate backbone of siRNA and the anionic acid groups in PLGA polymers. Using a similar method, Cun et al.267 also proposed various formulations of PLGA to load the siRNA molecules for therapeutic delivery. They optimized the formulations of PLGA by varying the siRNA load, PLGA concentration, ratio of monomers, water, and oil phase of the emulsion, and the amount of bovine serum albumin added to stabilize the emulsion. PLGA concentration was found to be critical in achieving the encapsulation efficiency of more than 70%. However, bovine serum albumin addition was found to enhance the encapsulation efficiency at lower PLGA concentration267. Despite of this outstanding character, with the lower electrostatic force of attraction with siRNA, and lack of endosomal escape, PLGA nanoparticles could not be efficiently used for the siRNA delivery. One of the most versatile strategies to overcome this issue is the use of polycations into PLGA-based nanoparticles. This strategy successfully enhanced the loading capacity of PLGA nanoparticles. To date, cationic compounds like DOTAP, PEI, or polyamine are conjugated with the PLGA nanoparticles at the cost of toxicity and high siRNA retardation. It is, therefore, essential to make less use of the cationic conjugated and employ the optimal formulation methods to achieve higher siRNA encapsulation and better release. Wang et al.268 developed different nanoparticles for hepatitis B treatment with siRNA. The nanoparticles were composed of PLGA, methoxy poly (ethylene glycol)–poly (lactide) (mPEG–PLA), and chitosan and PEI for surface coating269. For the optimized transfection of siRNA, Andersen et al.270 developed a method to conjugate the PLGA nanoparticle surface with polyethyleneimine by using a cetyl derivative. The formulation was used to silence the TNFα in J774.1 cells. In this method, sub-micron size particles were produced by employing the emulsion‒diffusion method using benzyl alcohol. The silencing of STAT3 in the dendritic cells is the crucial approach for cancer immunotherapy. Alshamsan et al.271 have successfully shown that STAT3 inhibition in B16 murine melanoma by siRNA polyplexes of PEI-linked PGLA promotes B16 cell death. Incorporation of the siRNA in the PEI-linked PGLA nanoparticles reduces the toxicity associated with PEI and also enhances the cellular uptake272. Different in vitro transfection efficiency study showed that the ability of PEI-linked PGLA nanoparticles to transfects depends on its ratio and the cell types273. Risnayanti et al.274 recently proposed PLGA nanoparticles as a delivery vehicle for MDR1 and BCL2 siRNA to MDR ovarian cancer cells. In this formulation, poly-l-lysine was used as a complexing agent for siRNA, which successfully inhibited the efflux of drugs from the ovarian cancer cells274. Patil et al.275 similarly developed PLGA nanoparticles for siRNA delivery, where they used PEI to enhance the electrostatic attraction between the siRNA molecules and PLGA matrix. The nanoparticles were prepared by using the double emulsion solvent evaporation method.
Hasan et al.276 developed a new method called “particle replication in nonwetting templates” for the preparation of PLGA nanoparticles coated with the lipids to deliver siRNA to the prostate cancer cells. This method was found to have the high encapsulation efficiency of siRNA in PLGA nanoparticles. Wang et al.277 also developed PLGA‒PEG cationic lipid nanoparticles by using the commonly employed double emulsion method. These nanoparticles have shown the very high encapsulation efficiency of around 90%, which effectively inhibits the target gene.
Chitosan, which is a linear polysaccharide made up of β-(1→4)-linked d-glucosamine (deacetylated unit) and N-acetyl-d-glucosamine (acetylated unit), has gained immense interest in the pharmaceutical industry. The characters like natural origin, abundance, non-immunogenicity, biocompatibility, and biodegradability have contributed immensely to its popularity for biological applications. It also possesses positive charges, which help to form the complex with the negatively charged siRNA. This character of chitosan makes it a useful non-viral vehicle for the siRNA delivery. d-Glucosamine residue, which has a pKa value of about 6.2–7.0, gives weak basic character to the chitosan. At the pH below its pKa value, protonation of the primary amines imparted cationic character to it. Such positively charge polymer forms polyplexes with DNA and siRNA, which pose a negatively charged phosphate backbone. Chitosan-mediated delivery of DNA is widely studied; however, its application for siRNA delivery is getting momentum in recent times278. Since the last decade, various formulations of chitosan are under the development for the siRNA delivery. For example, chitosan aspartate, chitosan glutamate, chitosan acetate, chitosan hydroxybenzotriazole, and chitosan hydrochloride were used to deliver siRNA to green fluorescent protein-expressing HeLa cells279. Chitosan water solubility was found to be increased when a water-soluble vitamin called thiamine pyrophosphate conjugated with the amine group of chitosan through its phosphate group. Also, the other amine groups of thiamine pyrophosphate, particularly the thaizolium moiety, remain protonated at physiological pH, allowing electrostatic binding with the negatively charged siRNA molecules. This enhanced attraction with siRNA and water solubility helps to increase transfection efficiency280. In another approach to introduce the secondary and tertiary amines, imidazole acetic acid was conjugated to the chitosan. This modification substantially helped to enhance the water solubility and endosomal escape of siRNA281. Interpolyelectrolyte siRNA/chitosan complexes developed by Howard et al.282 successfully overcame the issue of lower uptake and higher degradation of siRNA. A significant decrease in the enhanced green fluorescence protein-expressing epithelial cells in the bronchiole of mice via daily nasal administration of interpolyelectrolyte siRNA/chitosan complexes was observed282. As discussed earlier, calcium phosphate is an efficient siRNA delivery system but suffers due to the inconsistent particle size formation and transfection efficiencies. To overcome the issue, Choi et al.283 developed the CaP nanocarrier system by adding cationic glutamine-conjugated oligochitosan, which significantly enhanced the transfection efficiency. For pulmonary gene therapy, Ni et al.284 developed a pH-sensitive system composed of guanidinylated O-carboxymethyl chitosan and N-2-hydroxypropyltimehyl ammonium chloride chitosan for the successful delivery of siRNA to the lungs. The siRNA against the survivin delivered using this system, was found to inhibit cell growth by 30% and induced cell apoptosis by around 20%284. Sun et al.285 for efficient siRNA delivery developed poly(ethylene glycol)-modified chitosan carrier system. Improvement in the stability of siRNA, and better transfection efficiency of siRNA-loaded in poly(ethylene glycol)-modified chitosan nanoparticles in cancer cell line was observed285.
As discussed before, synthetic cationic polypeptides like PLL and PLA have also been widely studied for their gene delivery capabilities. However, these polypeptides are cytotoxic when used alone due to the very high cationic charges on them286. Several attempts were made to resolve this issue by combining them with hyaluronic acid and chitosan287,288. Plianwong et al.289 reported an efficient and easy-to-prepare method to combine chitosan with PLA to deliver siRNA to the cancer cell (HeLa cells expressing enhanced green fluorescent protein). Low solubility in the physiological condition is another severe issue which has restricted widespread use of chitosan in the siRNA delivery. Hydroxybutyl chitosan, a derivative, soluble under the neutral condition, was used by Wan et al.290 to target the tissue factor. The group used tissue factor targeting siRNA as a therapeutic tool for cardiovascular diseases. Hydroxybutyl chitosan, in the future, has the potential to deliver the siRNA to target the cancer cells290. Arami et al.291 reported the magnetic nanoparticles composed of polyethyleneglycol‒lactate polymer, chitosan, and polyethyleneimine. The biocompatible nanoparticle was successfully used to deliver the siRNA to human breast cancer MCF-7 and leukemia K562 cells291. Shen at al.292 reported a polymer-coated nanoparticle fabrication method for the siRNA delivery. The natural polyphenol (−)-epigallocatechin-3-O-gallate, which is a major compound found in green tea, was used to form the siRNA nanoparticles292. The polyphenol (−)-epigallocatechin-3-O-gallate could form strong electrostatic bonds with negatively charged compounds like DNA, RNA and proteins via hydrogen bonds293. The polyphenol (−)-epigallocatechin-3-O-gallate-siRNA complex once formed, was coated with the low-molecular-weight cationic polymer to develop the shell294. Shen et al.294 also conjugated the polyphenols like phenol, catechol and pyrogallol with low molecular weight polymers to efficiently deliver the siRNA molecules to the cytosol.
Polymer-mediated siRNA delivery to the cancer cells has several benefits, for example, it offers chemical modifications to make soluble derivatives, ligand conjugation for targeted delivery, biocompatibility, conjugation with inorganic materials, which can address various barriers related with efficient siRNA delivery. The integration of multiple functionalities into polymeric siRNA delivery systems could have intense influences on biomedical research and the ability to transform the spectrum of the therapeutic field in curing cancer.
6. Challenges, prospects and future plans in the delivery of siRNA
6.1. Challenges
In the coming time, siRNA holds the massive potential to be used as a therapeutic tool for genetic disorders. It provides a high degree of gene selectivity, which is difficult to achieve with the current treatment options. Thus, with the RNA-based therapeutics, the targets which were previously inaccessible can now be targeted selectively. For example, protein-coding and non-coding mRNAs and premRNAs, which were initially thought to be undruggable, can now be targeted with the siRNAs.
Several approaches are under development for the siRNA delivery to cells, and few of them are under clinical investigation295. As the siRNAs carry the negative charge, they are hindered from entering through the hydrophobic cell membrane. The effective strategy to administer the siRNAs inside the cells is through the endocytosis process. To be effective, siRNAs must exit out of the endosomes; otherwise, it could leave the cells via exocytosis process or may degrade by ribonuclease enzymes. Due to the off-site targeting, siRNA must be administered in the low dose, which prevents its optimum use. One of the most critical non-intended side effects is the innate immune system activation because of the immune motif in the siRNA sequence. Immune system activation motifs of siRNA could be identified by Toll-like receptors triggering the immune response along with the production of interferons (α or β) and inflammatory cytokines296.
Another major hurdle is the displacement of the natural siRNA from RISC meant for the normal physiological functions with the externally administered siRNA. In such cases, because of the partial binding of the siRNA, mRNA cleavage may not occur, but the cell could not carry out the normal cellular function297. Other factors that affect the effectiveness of the siRNA treatment include glomerular filtration, degradation by serum ribonucleases, endothelial barrier, and attack by immune cells297.
6.2. Prospects
The crucial aim of the research is the delivery of siRNA to the cancer cells after systemic administration. Leaky underdeveloped vasculature of growing tumors helps to uptake more nanoparticles inside the tumor via EPR. Still, only around 15% of the administered dose can accumulate in the tumor. Most of the lost treatment is linked to the nonspecific reticular endothelial system. For the uncharged compounds of molecular weight, less than 5000 Da is the uphill task. There are around 25 charged phosphodiester linkages forming the backbone of siRNA, which hinders the cellular uptake. On the other hand, nuclease enzymes of the blood cause the rapid degradation of siRNA, which further limits its bioavailability. Active transport via encapsulated ligand targeted nanocarriers offers the solution to this issue. PEGylated ligand targeted liposomal, or micelles are aimed to avoid the nonspecific reticular endothelial system clearance. These targeted nanocarriers exhibit better cellular uptake than the untargeted and, at the same time, protect the siRNA from the nucleases. Although siRNA has one specific mRNA target, reports of unintended silencing of the other genes having partial complementary regions causing severe side effects are available. Sometimes siRNA could also trigger the innate immune system to release pro-inflammatory cytokines. A variety of chemical modifications has been applied to the siRNA, which have tremendously improved the stability of siRNA in the blood and reduce off-site deposition. The chemical modification also hides the siRNA from the immune surveillance. Two of the most versatile modification of siRNA is the fluorination and methylation at the 2′-position298. Both these modifications are found to be well tolerated throughout sense and antisense strands. Primarily these modifications are aimed at enhancing the half-life of siRNA in plasma by protecting it from the nucleases. At present, we do not need to enhance the potency of the siRNA through chemical modifications. Preserving the existing potency is sufficient for the therapeutic purpose. However, when 2′-O-Me and 2′F-RNA modification was done in the same siRNA, 500-fold increase in the potency was observed when compared with the unmodified siRNA299. A report of enhanced degradation of mRNA is available when enoxacin is linked with the siRNA300. In addition to this, immune activation is another severe issue related to siRNAs. Nonspecific immune response to the therapeutic siRNA may initiate the unwanted side effects. This issue could be resolved by downregulating the immunogenic characters of siRNA. Immune response towards the siRNA is a complex phenomenon, the details of which are available elsewhere. In short, siRNAs are identified by the toll-like receptors, protein kinase, and helicases, which lead to the induced secretion of the pro-inflammatory cytokines. Few nucleotide sequences have been linked to the immune activation; for example, 5′-GUCCUUCAA-3′ is the immune stimulatory sequence. In fact, the RNA sequence rich in U nucleotide is more easily identified by the immune system via TLR7 receptors301. Immunogenic stimulation could be substantially reduced by chemical modifications; for example, siRNA modified with 2′F-RNA, 2′-O-Me, and DNA residues have shown to have no effects on cytokines76.
Chemical modification of siRNA is a rapidly evolving field. Although considerable progress has been made in imparting the stability to the siRNA via chemical modifications, still they are sequence-dependent. With significant development in the lipid and polymer bases nanocarriers and simultaneous advancement in the chemical modification, the possibility of translation of siRNA from the lab to the clinics is very high.
7. Future plans
We have achieved tremendous success in siRNA studies; however, to make it a successful therapeutic agent, improvement in safety, delivery, pharmacokinetics, and pharmacodynamics is required. The future success of siRNA will depend on the successful development of the nanocarrier with better loading, transfusion, and safety profile. The following section has discussed some of the immediate improvements for future applications.
7.1. Enhanced endosomal escape
siRNA inside the endosomes are the same as that of the outside of the cell. They have no therapeutic value unless they break the endosome and enter inside the cytosol. Chances of degradation at pH 4.5 of the lysosome are high if they do not escape from the endosome. Overall, endosomal escape is the major hurdle in the therapeutic application of siRNA. The present situation demands better external and internal stimulus-responsive polymers, which could release the drugs on exposure. Wei et al.302 have developed an ultrasound-responsive polymersome based on PEO-b-poly(DEA-stat-MEMA) block copolymer to evaluate its intracellular anticancer drug delivery pathway and in-vivo systematic antitumor effect. This polymersome showed a favourable endosomal escape ability302. Puri et al.303 recently studied sulfonated PEIs covalently linked to pyropheophorbide-α for photoactivation and modified amines (sulfo-pyro-PEI) for controlled endosomal escape. The results confirmed the on-demand release of the siRNA on photostimulation. Multivalent peptide-functionalized bioreducible polymers were developed for enhancing endosomal escape. It has been noted that the optimum number of hydrophobic side chains is essential for the micelle's assembly and cellular uptake, but an excess of it could lead to the less endosomal escape. Hence, in the future proper structural optimization of the polymers and construction of the nanoparticles are essentials to overcome the issue of the endosomal escape.
7.2. Conjugation with proteins and antibodies
Endosomal escape is an important event for the siRNA activity. The potency of the siRNA could be enhanced by increasing its serum half-life by conjugating with antibodies and proteins. One of them is the IgE (antibody), which is synthesized by the plasma cells. IgE, once synthesizes, remains in the blood and tissues for weeks. IgE-siRNA complex is hypothesized to have a similar self-life in the blood304. However, the potency of siRNA under investigation was found to be lower than the unconjugated form. Another option for the conjugation could be albumin, which could not only assist the delivery to the cancer cells but also enhance the pharmacokinetic properties of the siRNA. Lower potency of the IgE-siRNA complex does not ensure that the future work could have a similar impact. We have to keep investigating the other options until we could considerably increase the efficiency of the siRNA along with pharmacokinetic characters305.
7.3. Tissue targeting and cellular uptake
One of the hurdles of cancer therapy is the off-site targeting. siRNA engulfed inside the nanoparticles are not only protected from the nucleases and rapid clearance from the body but, when targeting ligands linked to such nanoparticles, the targeted release of siRNA is possible. When siRNA is entrapped inside the nanoparticle, the particle of less than 150 nm can easily reach the hepatocytes, but the similar fenestrations are not available in the other tissues, thereby restricting the entry. Several ligands targeted nanocarriers like galactose-linked liposomes were proven to be useful in enhancing the drug activity at the liver site. PEG conjugation is another method to avoid macrophage identification. Tumor targeting based only on PEGylation and passive diffusion through EPR is not always suitable for different types of cancer. Therefore, to reduce the off-site accumulation and to deliver siRNA inside the cancer cells, facilitated or active diffusion is the better choice. Ligand-conjugated endocytosis mediating delivery of the siRNA to the target cells could eliminate the possibility of the side effects and, at the same time, increase the efficiency of the siRNA. In the future, facilitated and active transportation, external stimuli mediation (e.g., magnetic field, ultrasonic waves, laser lights, sound, and light, etc.), and on-demand release of the siRNA needs to be explored further. For such approaches to be successful, smart stimuli responding polymers are required.
7.4. Multifunctional approach
Several hurdles need to be overcome to achieve the maximum potential of the therapeutic siRNA. The hurdles are endosomal escape, lower cellular uptake, rapid excretion, degradation by nucleases, and immune stimulation306. Combining all the solutions in one delivery system could lead to the development of the ideal carrier, which, however, is a difficult task. A multifunctional system that could connect most of the characters of the ideal delivery system could potentially replace the existing systems. The inclusion of the endosomal escape motifs in a multifunctional platform without altering the cellular uptake and potency of targeting ligand could enhance the overall performance of the system307. The addition of the targeting ligand could reduce the non-specific accumulation and, at the same time, could enhance the site-specific delivery. Chemical modification at the 2′ positon of RNA could protect the RNA molecules from nuclease degradation, and fusing lipids could enhance the cellular uptake. Combining all the motifs to get in one single device would increase the complexity of the delivery system. Hence, it is indispensable to study the overall physiochemical aspects of each component, how they complement each other's activity and, at the same time, perform their function independently308.
7.5. Novel targets and ligands
As discussed earlier, one of the biggest challenges in the therapeutic translation of the siRNA is the successful delivery to the cancer cells. This required the appropriate size of the nanoparticles conjugated with the ligands. Receptors for the targeting ligands are generally expressed on diseased cells. Such receptors assist in the endocytosis of the nanoparticles to which this ligand is linked. Several surface protein expression is enhanced in the disease condition, and the ligands for such proteins are identified. The ligands could be the antibodies, aptamers, cell-penetrating peptides, etc. Other than targeting the different types of cells, ligands for the endothelial cells of the different organs could be a useful tool to target the cancer of various organs. Identification of the ligand for the leucocyte, which in general is difficult to target, would be very useful in case of blood cancer and certain viral infections. In the future, transfection to a subset of leucocyte will be the challenge to meet. Very recently, CpG oligodeoxynucleotides, which binds to the TLR9 to initiate the innate immune response towards the foreign invention, was linked to the siRNA to target the B cells309. Similarly, an antibody against CD7 protein was used to target the siRNA to the T cells310. Similar to leucocytes, it is very difficult to deliver the siRNA using nanocarrier to the neurons. Identification of ligands for the neurons could be beneficial for the treatment of brain cancer, Alzheimer's, Parkinsonism, and infections like encephalitis. Rungta et al.311 have developed the siRNA–lipid nanoparticle systems to deliver siRNA to the neurons. Recently, polyelectrolyte‒gold nano assemblies were successfully used by Chaudhary et al.312 to deliver the siRNA to the neuronal cells. Solanki et al.313 in 2013 have developed a delivery platform known as nanotopography-mediated reverse uptake to deliver siRNA to the neural stem cells. A major breakthrough was achieved in crossing the BBB when Rabies virus glycoprotein was used as a targeting ligand to deliver the siRNA to the brain314. Even after the progress in the siRNA biology and delivery system, we have to keep looking for new targets for leukocytes and other cancers. The use and expansion of the protein database, and peptide and aptamer libraries could be useful for the siRNA delivery in the future.
7.6. Reducing toxicity of lipid and polymer-carriers
Lipid carriers offer several advantages to carry the drugs and gene products like siRNA. The distinct advantages include protection from nuclease-based degradation, site-specific targeting using targeting ligands, lower side effects, and better half-life of the drugs315. Liposomes prepared from lipids also have their disadvantages, which include a) large scaleup problems, b) low drug/siRNA entrapment efficiency, c) very poor long term storage stability, d) aggregation to form the bigger aggregates, e) licking of water-soluble drugs in the blood, and f) toxicity of the lipid components. Toxicity could occur because of toxic lipids or its metabolites, particle size large enough to block the small blood capillaries, interaction with the blood components, etc. Toxicity due to the lipids-based carriers is mostly because of the charges they carry. To minimize the side effect, it is necessary to select the lipids favoring the small particle size and total compositions, which support fewer overall charges on the surface. For example, the most commonly used lipid-based carriers are cationic liposomes, which can interact with several proteins, lipoproteins, and collagen leading to the formation of the aggregates or premature release of the drugs leading to the systematic toxicity316. Cationic lipids have proven to have hepatic toxicity; they inhibit the protein kinase c activity and could induce lung inflammations317. Cationic liposomes were also found to have a toxic effect on macrophages on short term exposure318. Conjugating the lipid carrier to the targeting ligand could help to reduce the side effects of the lipids. In the end, to overcome the issue of nanoparticle toxicity, it is advisable to access the key characters which contribute to the toxicity adequately, which include: a) proper physiochemical characterization, b) surface property characterization, c) proper assessment of in vitro toxicity studies, d) reactive oxygen species assays, e) toxicity studies in proper animal models, and f) genotoxicity studies. In the future, masking of the nanoparticle surface with various biocompatible and hydrophilic polymers would be the focus of the research to reduce the adverse effects. Agents like PEG, polyethylene oxide, polyoxamer, poloxamine, and polysorbate 80 were already under investigation for their role in offering the biocompatibility to the lipid nanocarriers319. Nowadays, the need for a more efficient surface masking agent is very high, and there could not be better agents than the natural polymers. Going forward, the future of lipid-based carriers in siRNA delivery to the cancer cells is bright.
8. Conclusions
Once inside the cell, siRNAs form the RISC and subsequently destruct the mRNA. However, due to the polyanionic charges, siRNAs are unable to cross the lipid membrane, making the suitable delivery vehicle an urgent requirement for siRNA-based therapies. Cationic lipid base nanoparticles containing ionizable amino lipids are the promising vehicle for the negatively charged nucleic acids. Interaction of amino lipids with the endosomal membrane allows the better endosomal escape and bioavailability. Despite being the favourable candidate for drug delivery, serious side effects have restricted their use. Along with the development of novel delivery vehicles, the development of the new lipids with no side effects is inevitable. Scientists are paying more attention to the hard-to-transfect leucocytes by developing specialized lipids.
So far, many different types of cationic and polymeric nanocarrier delivery systems are developed. In this review, we have discussed the popular siRNA delivery systems and their potential in cancer treatment. But we still have a lot of challenges to deal with before they can become the trusted delivery systems. The journey of cationic nanocarriers from untargeted to target to LPD and LCP nanocarriers with distinct advantages of better exosomal escape and cellular uptakes is phenomenal. The literature studies revealed that the PEGylation is crucial as it helps the nanoparticle to hide from the macrophages and enhances the blood circulation time. Modification of the siRNA is also critical to protect the siRNA from the nuclease-based degradation. Despite the creation of several nanocarriers with different functionalities, the final availability of siRNA for mRNA destruction is very less. This indicates that there is a vast scope in enhancing the ability of the nanocarriers. Strategies for the endosomal escape, cell and tissue targeting, and development of the novel biomaterials are crucial for the translation of siRNA from the lab to the patients on the bed.
Acknowledgments
Nitin Bharat Charbe is the recipient of “CONICYT FONDECYT POSTDOCTORADO FELLOWSHIP 2018, PROYECTO N° 3180250”.
Authors contributions
Nitin Bharat Charbe and Flavia C Zocconi conceptualized and wrote the manuscript. Nikhil D Amnerkar, Murtaza Tambuwala, Alaa A A Aljabali, and Saurabh Satija designed the figures. Kamal Dua, Saurabh C Khadse, Dinesh Kumar Chellappan, Rajendran Satheeshkumar and B Ramesh edited the manuscript. Meenu Metha, Poonam Negi, Garima Shrivastava, Hamid A Bakshi, Gaurav Gupta checked the figures and formatted the tables. All authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Nitin Bharat Charbe, Email: Nitinunimi@gmail.com.
Flavia C. Zacconi, Email: fzacconi@uc.cl.
References
- 1.Rao D.D., Vorhies J.S., Senzer N., Nemunaitis J. siRNA vs. shRNA: similarities and differences. Adv Drug Deliv Rev. 2009;61:746–759. doi: 10.1016/j.addr.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Tabernero J., Shapiro G.I., LoRusso P.M., Cervantes A., Schwartz G.K., Weiss G.J. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Canc Discov. 2013;3:406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 3.Tang G. siRNA and miRNA: an insight into RISCs. Trends Biochem Sci. 2005;30:106–114. doi: 10.1016/j.tibs.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Li Z., Rana T.M. Molecular mechanisms of RNA-triggered gene silencing machineries. Acc Chem Res. 2012;45:1122–1131. doi: 10.1021/ar200253u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nykänen A., Haley B., Zamore P.D. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell. 2001;107:309–321. doi: 10.1016/s0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 6.Hutvágner G., Zamore P.D. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 7.Oh Y.K., Park T.G. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev. 2009;61:850–862. doi: 10.1016/j.addr.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 9.Soutschek J., Akinc A., Bramlage B., Charisse K., Constien R., Donoghue M. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 10.de Fougerolles A., Vornlocher H.P., Maraganore J., Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye Q.F., Zhang Y.C., Peng X.Q., Long Z., Ming Y.Z., He L.Y. Silencing Notch-1 induces apoptosis and increases the chemosensitivity of prostate cancer cells to docetaxel through Bcl-2 and Bax. Oncol Lett. 2012;3:879–884. doi: 10.3892/ol.2012.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai Z., Zhang Z., Qu X., Han W., Ma X. Sensitization of breast cancer cells to taxol by inhibition of taxol resistance gene 1. Oncol Lett. 2012;3:135–140. doi: 10.3892/ol.2011.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiffelers R.M., Ansari A., Xu J., Zhou Q., Tang Q., Storm G. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:1–10. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez J., Bardin C., Rigal C., Anthony B., Rousseau R., Dutour A. Anti-MDR1 siRNA restores chemosensitivity in chemoresistant breast carcinoma and osteosarcoma cell lines. Anticancer Res. 2011;31:2813–2820. [PubMed] [Google Scholar]
- 15.Yamazaki H., Iwano T., Otsuka S., Kagawa Y., Hoshino Y., Hosoya K. SiRNA knockdown of the DEK nuclear protein mRNA enhances apoptosis and chemosensitivity of canine transitional cell carcinoma cells. Vet J. 2015;204:60–65. doi: 10.1016/j.tvjl.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Liu W.S., Yan H.J., Qin R.Y., Tian R., Wang M., Jiang J.X. SiRNA directed against survivin enhances pancreatic cancer cell gemcitabine chemosensitivity. Dig Dis Sci. 2009;54:89–96. doi: 10.1007/s10620-008-0329-4. [DOI] [PubMed] [Google Scholar]
- 17.Jafarlou M., Shanehbandi D., Dehghan P., Mansoori B., Othman F., Baradaran B. Enhancement of chemosensitivity by simultaneously silencing of Mcl-1 and Survivin genes using small interfering RNA in human myelomonocytic leukaemia. Artif Cells, Nanomed Biotechnol. 2018;46:1792–1798. doi: 10.1080/21691401.2017.1392969. [DOI] [PubMed] [Google Scholar]
- 18.Duxbury M.S., Ito H., Zinner M.J., Ashley S.W., Whang E.E. RNA interference targeting the M2 subunit of ribonucleotide reductase enhances pancreatic adenocarcinoma chemosensitivity to gemcitabine. Oncogene. 2004;23:1539–1548. doi: 10.1038/sj.onc.1207272. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz J.C., Chen T.M., Chu E. Small interfering double-stranded RNAs as therapeutic molecules to restore chemosensitivity to thymidylate synthase inhibitor compounds. Canc Res. 2004;64:1431–1435. doi: 10.1158/0008-5472.can-03-1203. [DOI] [PubMed] [Google Scholar]
- 20.Chung D.C., Long L.T., Son H.N., Bao L.T., Si D.M., Van Dong L. Downregulation of vascular endothelial growth factor enhances chemosensitivity by induction of apoptosis in hepatocellular carcinoma cells. Cell J. 2015;17:273–287. doi: 10.22074/cellj.2016.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T., Wu X., Li Y., Lu W., Zheng F., Zhang C. RBFOX3 regulates the chemosensitivity of cancer cells to 5-fluorouracil via the PI3K/AKT, EMT and cytochrome-c/caspase pathways. Cell Physiol Biochem. 2018;46:1365–1380. doi: 10.1159/000489153. [DOI] [PubMed] [Google Scholar]
- 22.Duxbury M.S., Ito H., Zinner M.J., Ashley S.W., Whang E.E. siRNA directed against c-Src enhances pancreatic adenocarcinoma cell gemcitabine chemosensitivity. J Am Coll Surg. 2004;198:953–959. doi: 10.1016/j.jamcollsurg.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 23.Nozawa H., Tadakuma T., Ono T., Sato M., Hiroi S., Masumoto K. Small interfering RNA targeting epidermal growth factor receptor enhances chemosensitivity to cisplatin, 5-fluorouracil and docetaxel in head and neck squamous cell carcinoma. Canc Sci. 2006;97:1115–1124. doi: 10.1111/j.1349-7006.2006.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R., Dong K., Lin F., Wang X., Gao P., Wei S.H. Inhibiting proliferation and enhancing chemosensitivity to taxanes in osteosarcoma cells by RNA interference-mediated downregulation of stathmin expression. Mol Med. 2007;13:567–575. doi: 10.2119/2007-00046.Wang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C., Zhao G., Liu J., Ma N., Chivukula P., Perelman L. Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J Control Release. 2009;140:277–283. doi: 10.1016/j.jconrel.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Shen J., Liu J., Long Y., Miao Y., Su M., Zhang Q. Knockdown of survivin expression by siRNAs enhances chemosensitivity of prostate cancer cells and attenuates its tumorigenicity. Acta Biochim Biophys Sin (Shanghai) 2009;41:223–230. doi: 10.1093/abbs/gmp005. [DOI] [PubMed] [Google Scholar]
- 27.Kim M., Lee S., Park W.H., Suh D.H., Kim K., Kim Y.B. Silencing Bmi1 expression suppresses cancer stemness and enhances chemosensitivity in endometrial cancer cells. Biomed Pharmacother. 2018;108:584–589. doi: 10.1016/j.biopha.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 28.Yue B., Ma J.F., Yao G., Yang M.D., Cheng H., Liu G.Y. Knockdown of neuropilin-1 suppresses invasion, angiogenesis, and increases the chemosensitivity to doxorubicin in osteosarcoma cells—an in vitro study. Eur Rev Med Pharmacol Sci. 2014;18:1735–1741. [PubMed] [Google Scholar]
- 29.Zhang Y.J., Li A.J., Han Y., Yin L., Lin M. Bin. Inhibition of Girdin enhances chemosensitivity of colorectal cancer cells to oxaliplatin. World J Gastroenterol. 2014;20:8229–8236. doi: 10.3748/wjg.v20.i25.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng S., Wang X., Weng Y.H., Jin X., Ji J.L., Guo L. siRNA knockdown of RRM2 effectively suppressed pancreatic tumor growth alone or synergistically with doxorubicin. Mol Ther Nucleic Acids. 2018;12:805–816. doi: 10.1016/j.omtn.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L., Kang W.K. The effect of HIF-1α siRNA on growth and chemosensitivity of Mia-paca cell line. Yonsei Med J. 2008;49:295–300. doi: 10.3349/ymj.2008.49.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shervington A., Patel R. Silencing DNA methyltransferase (DNMT) enhances glioma chemosensitivity. Oligonucleotides. 2008;18:365–374. doi: 10.1089/oli.2008.0128. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J., Wang L.S., Ye S.L., Luo P., Wang B.L. Blockage of tropomyosin receptor kinase a (TrkA) enhances chemo-sensitivity in breast cancer cells and inhibits metastasis in vivo. Int J Clin Exp Med. 2015;8:634–641. [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Xiang J., Wang J., Ji Y. Downregulation of TGF-β1 suppressed proliferation and increased chemosensitivity of ovarian cancer cells by promoting BRCA1/Smad3 signaling. Biol Res. 2018;51:1–7. doi: 10.1186/s40659-018-0205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu C., Zhang X., Sun G., Guo X., Li H., You Y. RNA interference-mediated silencing of the polo-like kinase 1 gene enhances chemosensitivity to gemcitabine in pancreatic adenocarcinoma cells. J Cell Mol Med. 2008;12:2334–2349. doi: 10.1111/j.1582-4934.2008.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li P.C., Tu M.J., Ho P.Y., Jilek J.L., Duan Z., Zhang Q.Y. Bioengineered NRF2‒siRNA is effective to interfere with NRF2 pathways and improve chemosensitivity of human cancer cells. Drug Metab Dispos. 2018;46:2–10. doi: 10.1124/dmd.117.078741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W., Zhu F., Jiang Y., Sun D., Yang B., Yan H. siRNA targeting survivin inhibits the growth and enhances the chemosensitivity of hepatocellular carcinoma cells. Oncol Rep. 2013;29:1183–1188. doi: 10.3892/or.2012.2196. [DOI] [PubMed] [Google Scholar]
- 38.Li X., Pei B., Wang H., Tang C., Zhu W., Jin F. Effect of AQP-5 silencing by siRNA interference on chemosensitivity of breast cancer cells. OncoTargets Ther. 2018;11:3359–3368. doi: 10.2147/OTT.S160313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo K., Song W., Gong Y., Hu S., Zhong W., Qiu W. Proceedings-2015 7th international conference on measuring technology and mechatronics automation. ICMTMA; Nanchang, China: 2015. Down-regulation of survivin expression by siRNA suppresses proliferation and enhances chemosensitivity in human pancreatic cancer cell line Panc-1; pp. 400–402. 2015, bl. [Google Scholar]
- 40.Shen J., Wang Q., Hu Q., Li Y., Tang G., Chu P.K. Restoration of chemosensitivity by multifunctional micelles mediated by P-gp siRNA to reverse MDR. Biomaterials. 2014;35:8621–8634. doi: 10.1016/j.biomaterials.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 41.Yang H., Fu J.H., Hu Y., Huang W.Z., Zheng B., Wang G. Influence of SiRNA targeting survivin on chemosensitivity of H460/cDDP lung cancer cells. J Int Med Res. 2008;36:734–747. doi: 10.1177/147323000803600416. [DOI] [PubMed] [Google Scholar]
- 42.Ide H., Kikuchi E., Hasegawa M., Kozakai N., Kosaka T., Miyajima A. Prognostic significance of 5-fluorouracil metabolism-relating enzymes and enhanced chemosensitivity to 5-fluorouracil by 5-chloro 2,4-dihydroxy-pyridine in urothelial carcinoma. BMC Canc. 2012;12:420. doi: 10.1186/1471-2407-12-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radia A.M., Yaser A.M., Ma X., Zhang J., Yang C., Dong Q. Specific siRNA targeting receptor for advanced glycation end products (RAGE) decreases proliferation in human breast cancer cell lines. Int J Mol Sci. 2013;14:7959–7978. doi: 10.3390/ijms14047959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan-Lappe S., Woods K.W., Li Q., Anderson M.G., Schurdak M.E., Luo Y. RNAi-based screening of the human kinome identifies Akt-cooperating kinases: a new approach to designing efficacious multitargeted kinase inhibitors. Oncogene. 2006;25:1340–1348. doi: 10.1038/sj.onc.1209169. [DOI] [PubMed] [Google Scholar]
- 45.Aza-Blanc P., Cooper C.L., Wagner K., Batalov S., Deveraux Q.L., Cooke M.P. Identification of modulators of TRAIL-induced apoptosis via RNAi-based phenotypic screening. Mol Cell. 2003;12:627–637. doi: 10.1016/s1097-2765(03)00348-4. [DOI] [PubMed] [Google Scholar]
- 46.MacKeigan J.P., Murphy L.O., Blenis J. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat Cell Biol. 2005;7:591–600. doi: 10.1038/ncb1258. [DOI] [PubMed] [Google Scholar]
- 47.Futami T., Miyagishi M., Taira K. Identification of a network involved in thapsigargin-induced apoptosis using a library of small interfering RNA expression vectors. J Biol Chem. 2005;280:826–831. doi: 10.1074/jbc.M409948200. [DOI] [PubMed] [Google Scholar]
- 48.Brummelkamp T.R., Nijman S.M.B., Dirac A.M.G., Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 49.Berns K., Hijmans E.M., Mullenders J., Brummelkamp T.R., Velds A., Heimerikx M. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature. 2004;428:431–437. doi: 10.1038/nature02371. [DOI] [PubMed] [Google Scholar]
- 50.Kittler R., Putz G., Pelletier L., Poser I., Heninger A.K., Drechsel D. An endoribonuclease-prepared siRNA screen in human cells identifies genes essential for cell division. Nature. 2004;432:1036–1040. doi: 10.1038/nature03159. [DOI] [PubMed] [Google Scholar]
- 51.Song J.J., Smith S.K., Hannon G.J., Joshua-Tor L. Crystal structure of argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 52.Chiu Y.L., Rana T.M. siRNA function in RNAi: a chemical modification analysis. RNA. 2003;9:1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L., Hou J., Liu X., Guo Y., Wu Y., Zhang L. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials. 2014;35:3840–3850. doi: 10.1016/j.biomaterials.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 54.Xu C.-fei., Wang J. Delivery systems for siRNA drug development in cancer therapy. Asian J Pharm Sci. 2015;10:1–12. [Google Scholar]
- 55.Bangham A.D., Horne R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 56.Tagami T., Nakamura K., Shimizu T., Ishida T., Kiwada H. Effect of siRNA in PEG-coated siRNA-lipoplex on anti-PEG IgM production. J Control Release. 2009;137:234–240. doi: 10.1016/j.jconrel.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Felgner P.L., Gadek T.R., Holm M., Roman R., Chan H.W., Wenz M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omidi Y., Hollins A.J., Benboubetra M., Drayton R., Benter I.F., Akhtari S. Toxicogenomics of non-viral vectors for gene therapy: a microarray study of lipofectin- and oligofectamine-induced gene expression changes in human epithelial cells. J Drug Target. 2003;11:311–323. doi: 10.1080/10611860310001636908. [DOI] [PubMed] [Google Scholar]
- 59.Yu W., Pirollo K.F., Yu B., Rait A., Xiang L., Huang W. Enhanced transfection efficiency of a systemically delivered tumor-targeting immunolipoplex by inclusion of a pH-sensitive histidylated oligolysine peptide. Nucleic Acids Res. 2004;32:1–10. doi: 10.1093/nar/gnh049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pirollo K.F., Zon G., Rait A., Zhou Q., Yu W., Hogrefe R. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Hum Gene Ther. 2006;17:117–124. doi: 10.1089/hum.2006.17.117. [DOI] [PubMed] [Google Scholar]
- 61.Lin Q., Chen J., Zhang Z., Zheng G. Lipid-based nanoparticles in the systemic delivery of siRNA. Nanomedicine. 2014;9:105–120. doi: 10.2217/nnm.13.192. [DOI] [PubMed] [Google Scholar]
- 62.Kedmi R., Ben-Arie N., Peer D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials. 2010;31:6867–6875. doi: 10.1016/j.biomaterials.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 63.Wasungu L., Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J Control Release. 2006;116:255–264. doi: 10.1016/j.jconrel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 64.Li S.D., Chen Y.C., Hackett M.J., Huang L. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol Ther. 2008;16:163–169. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santel A., Aleku M., Keil O., Endruschat J., Esche V., Fisch G. A novel siRNA–lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006;13:1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 66.Dana H., Chalbatani G.M., Mahmoodzadeh H., Karimloo R., Rezaiean O., Moradzadeh A. Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci. 2017;13:48–57. [PMC free article] [PubMed] [Google Scholar]
- 67.Wu S.Y., Yang X., Gharpure K.M., Hatakeyama H., Egli M., McGuire M.H. 2′-OMe-phosphorodithioate-modified siRNAs show increased loading into the RISC complex and enhanced anti-tumour activity. Nat Commun. 2014;5:1–12. doi: 10.1038/ncomms4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomes-Da-Silva L.C., Fonseca N.A., Moura V., Pedroso De Lima M.C., Simões S., Moreira J.N. Lipid-based nanoparticles for siRNA delivery in cancer therapy: paradigms and challenges. Acc Chem Res. 2012;45:1163–1171. doi: 10.1021/ar300048p. [DOI] [PubMed] [Google Scholar]
- 69.Buyens K., De Smedt S.C., Braeckmans K., Demeester J., Peeters L., Van Grunsven L.A. Liposome based systems for systemic siRNA delivery: stability in blood sets the requirements for optimal carrier design. J Control Release. 2012;158:362–370. doi: 10.1016/j.jconrel.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 70.Yagi N., Manabe I., Tottori T., Ishihara A., Ogata F., Jong H.K. A nanoparticle system specifically designed to deliver short interfering RNA inhibits tumor growth in vivo. Canc Res. 2009;69:6531–6538. doi: 10.1158/0008-5472.CAN-08-3945. [DOI] [PubMed] [Google Scholar]
- 71.Carmona S., Jorgensen M.R., Kolli S., Crowther C., Salazar F.H., Marion P.L. Controlling HBV replication in vivo by intravenous administration of triggered PEGylated siRNA-nanoparticles. Mol Pharm. 2009;6:706–717. doi: 10.1021/mp800157x. [DOI] [PubMed] [Google Scholar]
- 72.Hatakeyama H., Akita H., Ito E., Hayashi Y., Oishi M., Nagasaki Y. Systemic delivery of siRNA to tumors using a lipid nanoparticle containing a tumor-specific cleavable PEG–lipid. Biomaterials. 2011;32:4306–4316. doi: 10.1016/j.biomaterials.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 73.Chono S., Li S.D., Conwell C.C., Huang L. An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor. J Control Release. 2008;131:64–69. doi: 10.1016/j.jconrel.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang X.Z., Dou S., Wang Y.C., Long H.Y., Xiong M.H., Mao C.Q. Single-step assembly of cationic lipid–polymer hybrid nanoparticles for systemic delivery of siRNA. ACS Nano. 2012;6:4955–4965. doi: 10.1021/nn300500u. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y., Li H., Sun J., Gao W., Liu W., Li B. DC-Chol/DOPE cationic liposomes: a comparative study of the influence factors on plasmid pDNA and siRNA gene delivery. Int J Pharm. 2010;390:198–207. doi: 10.1016/j.ijpharm.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 76.Morrissey D.V., Lockridge J.A., Shaw L., Blanchard K., Jensen K., Breen W. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 77.Zimmermann T.S., Lee A.C.H., Akinc A., Bramlage B., Bumcrot D., Fedoruk M.N. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 78.Judge A.D., Robbins M., Tavakoli I., Levi J., Hu L., Fronda A. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009;119:661–673. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Antonellis P., Liguori L., Falanga A., Carotenuto M., Ferrucci V., Andolfo I. MicroRNA 199b-5p delivery through stable nucleic acid lipid particles (SNALPs) in tumorigenic cell lines. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:287–302. doi: 10.1007/s00210-013-0837-4. [DOI] [PubMed] [Google Scholar]
- 80.Geisbert T.W., Lee A.C., Robbins M., Geisbert J.B., Honko A.N., Sood V. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375:1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He W., Bennett M.J., Luistro L., Carvajal D., Nevins T., Smith M. Discovery of siRNA lipid nanoparticles to transfect suspension leukemia cells and provide in vivo delivery capability. Mol Ther. 2014;22:359–370. doi: 10.1038/mt.2013.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jia Z., Gong Y., Pi Y., Liu X., Gao L., Kang L. PPB Peptide-mediated siRNA-loaded stable nucleic acid lipid nanoparticles on targeting therapy of hepatic fibrosis. Mol Pharm. 2018;15:53–62. doi: 10.1021/acs.molpharmaceut.7b00709. [DOI] [PubMed] [Google Scholar]
- 83.Mills K.A., Quinn J.M., Roach S.T., Palisoul M., Nguyen M., Noia H. p5RHH nanoparticle-mediated delivery of AXL siRNA inhibits metastasis of ovarian and uterine cancer cells in mouse xenografts. Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-019-41122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aleku M., Schulz P., Keil O., Santel A., Schaeper U., Dieckhoff B. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Canc Res. 2008;68:9788–9798. doi: 10.1158/0008-5472.CAN-08-2428. [DOI] [PubMed] [Google Scholar]
- 85.Heidel J.D., Schluep T. Cyclodextrin-containing polymers: versatile platforms of drug delivery materials. J Drug Deliv. 2012;2012:1–17. doi: 10.1155/2012/262731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davis M.E., Brewster M.E. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 87.Stella V.J., Rao V.M., Zannou E.A., Zia V. Mechanisms of drug release from cyclodextrin complexes. Adv Drug Deliv Rev. 1999;36:3–16. doi: 10.1016/s0169-409x(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 88.Freeman D.J., Niven R.W. The influence of sodium glycocholate and other additives on the in vivo transfection of plasmid DNA in the lungs. Pharm Res. 1996;13:202–209. doi: 10.1023/a:1016078728202. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez H., Hwang S.J., Davis M.E. New class of polymers for the delivery of macromolecular therapeutics. Bioconjugate Chem. 1999;10:1068–1074. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- 90.Suzie H.P., Davis M.E. Development of a nonviral gene delivery vehicle for systemic application. Bioconjugate Chem. 2002;13:630–639. doi: 10.1021/bc0155768. [DOI] [PubMed] [Google Scholar]
- 91.Hu-Lieskovan S., Heidel J.D., Bartlett D.W., Davis M.E., Triche T.J. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing's sarcoma. Canc Res. 2005;65:8984–8992. doi: 10.1158/0008-5472.CAN-05-0565. [DOI] [PubMed] [Google Scholar]
- 92.Bellocq N.C., Pun S.H., Jensen G.S., Davis M.E. Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjugate Chem. 2003;14:1122–1132. doi: 10.1021/bc034125f. [DOI] [PubMed] [Google Scholar]
- 93.Mellet C.O., Fernández J.M.G., Benito J.M. Cyclodextrin-based gene delivery systems. Chem Soc Rev. 2011;40:1586–1608. doi: 10.1039/c0cs00019a. [DOI] [PubMed] [Google Scholar]
- 94.Heidel J.D., Yu Z., Liu J.Y.C., Rele S.M., Liang Y., Zeidan R.K. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Natl Acad Sci U S A. 2007;104:5715–5721. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nabel G.J., Nabel E.G., Yang Z.Y., Fox B.A., Plautz G.E., Gao X. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc Natl Acad Sci U S A. 1993;90:11307–11311. doi: 10.1073/pnas.90.23.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caplen N.J., Alton E.W.F.W., Mddleton P.G., Dorin J.R., Stevenson B.J., Gao X. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1:39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- 97.Farhood H., Serbina N., Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim Biophys Acta. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 98.Maitani Y., Igarashi S., Sato M., Hattori Y. Cationic liposome (DC-Chol/DOPE = 1:2) and a modified ethanol injection method to prepare liposomes, increased gene expression. Int J Pharm. 2007;342:33–39. doi: 10.1016/j.ijpharm.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 99.Dass C.R., Choong P.F.M. Selective gene delivery for cancer therapy using cationic liposomes: in vivo proof of applicability. J Control Release. 2006;113:155–163. doi: 10.1016/j.jconrel.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 100.Oupicky D., Ogris M., Howard K.A., Dash P.R., Ulbrich K., Seymour L.W. Importance of lateral and steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol Ther. 2002;5:463–472. doi: 10.1006/mthe.2002.0568. [DOI] [PubMed] [Google Scholar]
- 101.Lee J., Ahn H.J. PEGylated DC-Chol/DOPE cationic liposomes containing KSP siRNA as a systemic siRNA delivery carrier for ovarian cancer therapy. Biochem Biophys Res Commun. 2018;503:1716–1722. doi: 10.1016/j.bbrc.2018.07.104. [DOI] [PubMed] [Google Scholar]
- 102.Liu X., Madhankumar A.B., Slagle-Webb B., Sheehan J.M., Surguladze N., Connor J.R. Heavy chain ferritin siRNA delivered by cationic liposomes increases sensitivity of cancer cells to chemotherapeutic agents. Canc Res. 2011;71:2240–2249. doi: 10.1158/0008-5472.CAN-10-1375. [DOI] [PubMed] [Google Scholar]
- 103.Seraj S., Lee J., Ahn H.J. Systemic delivery of Eg5 shRNA-expressing plasmids using PEGylated DC-Chol/DOPE cationic liposome: long-term silencing and anticancer effects in vivo. Biochem Pharmacol. 2019;166:192–202. doi: 10.1016/j.bcp.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 104.Tseng W.C., Tang C.H., Fang T.Y., Su L.Y. Using disaccharides to enhance in vitro and in vivo transgene expression mediated by a lipid-based gene delivery system. J Gene Med. 2007;9:659–667. doi: 10.1002/jgm.1063. [DOI] [PubMed] [Google Scholar]
- 105.Yang S ye., Zheng Y., Chen J. yin., Zhang Q. yang., Zhao D., Han D. en. Comprehensive study of cationic liposomes composed of DC-Chol and cholesterol with different mole ratios for gene transfection. Colloids Surf B Biointerfaces. 2013;101:6–13. doi: 10.1016/j.colsurfb.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 106.Bennett M.J., Nantz M.H., Balasubramaniam R.P., Gruenert D.C., Malone R.W. Cholesterol enhances cationic liposome-mediated DNA transfection of human respiratory epithelial cells. Biosci Rep. 1995;15:47–53. doi: 10.1007/BF01200214. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y., Sun J., Lu Y., Tao C., Huang J., Zhang H. Complexes containing cationic and anionic pH-sensitive liposomes: comparative study of factors influencing plasmid DNA gene delivery to tumors. Int J Nanomed. 2013;8:1573–1593. doi: 10.2147/IJN.S42800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nchinda G., Überla K., Zschörnig O. Characterization of cationic lipid DNA transfection complexes differing in susceptibility to serum inhibition. BMC Biotechnol. 2002;2:1–10. doi: 10.1186/1472-6750-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kisoon N., Ariatti M., Moodley T. A novel cationic cholesterol derivative, its formulation into liposomes, and the efficient transfection of the transformed human cell lines HepG2 and HeLa. Drug Deliv J Deliv Target Ther Agents. 2002;9:161–167. doi: 10.1080/15227950290097598. [DOI] [PubMed] [Google Scholar]
- 110.Hattori Y., Nakamura M., Takeuchi N., Tamaki K., Shimizu S., Yoshiike Y. Effect of cationic lipid in cationic liposomes on siRNA delivery into the lung by intravenous injection of cationic lipoplex. J Drug Target. 2019;27:217–227. doi: 10.1080/1061186X.2018.1502775. [DOI] [PubMed] [Google Scholar]
- 111.Lechanteur A., Sanna V., Duchemin A., Evrard B., Mottet D., Piel G. Cationic liposomes carrying siRNA: impact of lipid composition on physicochemical properties, cytotoxicity and endosomal escape. Nanomaterials. 2018;8:1–12. doi: 10.3390/nano8050270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lechanteur A., Furst T., Evrard B., Delvenne P., Hubert P., Piel G. PEGylation of lipoplexes: the right balance between cytotoxicity and siRNA effectiveness. Eur J Pharmaceut Sci. 2016;93:493–503. doi: 10.1016/j.ejps.2016.08.058. [DOI] [PubMed] [Google Scholar]
- 113.Behr J.P., Demeneix B., Loeffler J.P., Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci U S A. 1989;86:6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.San H., Yang Z.Y., Pompili V.J., Jaffe M.L., Plautz G.E., Xu L. Safety and short-term toxicity of a novel cationic lipid formulation for human gene therapy. Hum Gene Ther. 1993;4:781–788. doi: 10.1089/hum.1993.4.6-781. [DOI] [PubMed] [Google Scholar]
- 115.Wagner E., Cotten M., Foisner R., Birnstiel M.L. Transferrin‒polycation‒DNA complexes: the effect of polycations on the structure of the complex and DNA delivery to cells. Proc Natl Acad Sci U S A. 1991;88:4255–4259. doi: 10.1073/pnas.88.10.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gao X., Huang L. Potentiation of cationic liposome-mediated gene delivery by polycations. Biochemistry. 1996;35:1027–1036. doi: 10.1021/bi952436a. [DOI] [PubMed] [Google Scholar]
- 117.Gao J., Chen H., Yu Y., Song J., Song H., Su X. Inhibition of hepatocellular carcinoma growth using immunoliposomes for co-delivery of adriamycin and ribonucleotide reductase M2 siRNA. Biomaterials. 2013;34:10084–10098. doi: 10.1016/j.biomaterials.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 118.Li S.D., Chono S., Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol Ther. 2008;16:942–946. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li S.D., Huang L. Surface-modified LPD nanoparticles for tumor targeting. Ann N Y Acad Sci. 2006;1082:1–8. doi: 10.1196/annals.1348.001. [DOI] [PubMed] [Google Scholar]
- 120.Chen Y., Huang L. Tumor-targeted delivery of siRNA by non-viral vector: safe and effective cancer therapy. Expet Opin Drug Deliv. 2008;5:1301–1311. doi: 10.1517/17425240802568505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen Y., Wu J.J., Huang L. Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy. Mol Ther. 2010;18:828–834. doi: 10.1038/mt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen Y., Bathula S.R., Yang Q., Huang L. Targeted nanoparticles deliver siRNA to melanoma. J Invest Dermatol. 2010;130:2790–2798. doi: 10.1038/jid.2010.222. [DOI] [PubMed] [Google Scholar]
- 123.Deng L., Zhang Y., Ma L., Jing X., Ke X., Lian J. Comparison of anti-EGFR-Fab’ conjugated immunoliposomes modified with two different conjugation linkers for siRNA delivery in SMMC-7721 cells. Int J Nanomed. 2013;8:3271–3283. doi: 10.2147/IJN.S47597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen Y., Sen J., Bathula S.R., Yang Q., Fittipaldi R., Huang L. Novel cationic lipid that delivers siRNA and enhances therapeutic effect in lung cancer cells. Mol Pharm. 2009;6:696–705. doi: 10.1021/mp800136v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li F., Zhu Z., Xue M., He W., Zhang T., Feng L. SiRNA-based breast cancer therapy by suppressing 17β-hydroxysteroid dehydrogenase type 1 in an optimized xenograft cell and molecular biology model in vivo. Drug Des Dev Ther. 2019;13:757–766. doi: 10.2147/DDDT.S180836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu Y., Singh R., Deng Z., Mintz A., Hsu W. Liposome-protamine-DNA nanoparticle-mediated delivery of short hairpin RNA targeting brachyury inhibits chordoma cell growth. J Biomed Nanotechnol. 2016;12:1952–1961. doi: 10.1166/jbn.2016.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jalali S.A., Sankian M., Tavakkol-Afshari J., Jaafari M.R. Induction of tumor-specific immunity by multi-epitope rat HER2/neu-derived peptides encapsulated in LPD Nanoparticles. Nanomed Nanotechnol Biol Med. 2012;8:692–701. doi: 10.1016/j.nano.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 128.Zhao Y., Wang W., Guo S., Wang Y., Miao L., Xiong Y. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat Commun. 2016;7:1–9. doi: 10.1038/ncomms11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao J., Liu W., Xia Y., Li W., Sun J., Chen H. The promotion of siRNA delivery to breast cancer overexpressing epidermal growth factor receptor through anti-EGFR antibody conjugation by immunoliposomes. Biomaterials. 2011;32:3459–3470. doi: 10.1016/j.biomaterials.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 130.Li J., Chen Y.C., Tseng Y.C., Mozumdar S., Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J Control Release. 2010;142:416–421. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang J.L., Chen H.Z., Gao X.L. Lipid-coated calcium phosphate nanoparticle and beyond: a versatile platform for drug delivery. J Drug Target. 2018;26:398–406. doi: 10.1080/1061186X.2017.1419360. [DOI] [PubMed] [Google Scholar]
- 132.Maitra A. Calcium phosphate nanoparticles: second-generation nonviral vectors in gene therapy. Expert Rev Mol Diagn. 2005;5:893–905. doi: 10.1586/14737159.5.6.893. [DOI] [PubMed] [Google Scholar]
- 133.Sokolova V.V., Radtke I., Heumann R., Epple M. Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomaterials. 2006;27:3147–3153. doi: 10.1016/j.biomaterials.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 134.Liu T., Tang A., Zhang G., Chen Y., Zhang J., Peng S. Calcium phosphate nanoparticles as a novel nonviral vector for efficient transfection of DNA in cancer gene therapy. Cancer Biother Radiopharm. 2005;20:141–149. doi: 10.1089/cbr.2005.20.141. [DOI] [PubMed] [Google Scholar]
- 135.Zhou C., Yu B., Yang X., Huo T., Lee L.J., Barth R.F. Lipid-coated nano-calcium-phosphate (LNCP) for gene delivery. Int J Pharm. 2010;392:201–208. doi: 10.1016/j.ijpharm.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X., Zhang M., Zhang L., Li L., Li S., Wang C. Designed Synthesis of lipid-coated polyacrylic acid/calcium phosphate nanoparticles as dual pH-responsive drug-delivery vehicles for cancer chemotherapy. Chem Eur J. 2017;23:6586–6595. doi: 10.1002/chem.201700060. [DOI] [PubMed] [Google Scholar]
- 137.Tseng Y.C., Xu Z., Guley K., Yuan H., Huang L. Lipid-calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials. 2014;35:4688–4698. doi: 10.1016/j.biomaterials.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang M., Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 139.Lee N., Choi S.H., Hyeon T. Nano-sized CT contrast agents. Adv Mater. 2013;25:2641–2660. doi: 10.1002/adma.201300081. [DOI] [PubMed] [Google Scholar]
- 140.Curry T., Kopelman R., Shilo M., Popovtzer R. Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy. Contrast Media Mol Imaging. 2014;9:53–61. doi: 10.1002/cmmi.1563. [DOI] [PubMed] [Google Scholar]
- 141.Goldman L.W. Principles of CT: radiation dose and image quality. J Nucl Med Technol. 2007;35:213–225. doi: 10.2967/jnmt.106.037846. [DOI] [PubMed] [Google Scholar]
- 142.Zhu L., Torchilin V.P. Stimulus-responsive nanopreparations for tumor targeting. Integr Biol. 2013;5:96–107. doi: 10.1039/c2ib20135f. (Camb) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kreuter J. Nanoparticles and microparticles for drug and vaccine delivery. J Anat. 1996;189:503–505. [PMC free article] [PubMed] [Google Scholar]
- 144.Amendola V., Pilot R., Frasconi M., Maragò O.M., Iatì M.A. Surface plasmon resonance in gold nanoparticles: a review. J Phys Condens Matter. 2017;29:1–48. doi: 10.1088/1361-648X/aa60f3. [DOI] [PubMed] [Google Scholar]
- 145.Kong F.Y., Zhang J.W., Li R.F., Wang Z.X., Wang W.J., Wang W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017;22:1–13. doi: 10.3390/molecules22091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Prusty K., Swain S.K. Nano silver decorated polyacrylamide/dextran nanohydrogels hybrid composites for drug delivery applications. Mater Sci Eng C. 2018;85:130–141. doi: 10.1016/j.msec.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 147.Jain T.K., Morales M.A., Sahoo S.K., Leslie-Pelecky D.L., Labhasetwar V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharm. 2005;2:194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- 148.Ngamcherdtrakul W., Morry J., Gu S., Castro D.J., Goodyear S.M., Sangvanich T. Cationic polymer modified mesoporous silica nanoparticles for targeted siRNA delivery to HER2+ breast cancer. Adv Funct Mater. 2015;25:2646–2659. doi: 10.1002/adfm.201404629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee J.S., Green J.J., Love K.T., Sunshine J., Langer R., Anderson D.G. Gold, poly(β-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009;9:2402–2406. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kango S., Kalia S., Celli A., Njuguna J., Habibi Y., Kumar R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—a review. Prog Polym Sci. 2013;38:1232–1261. [Google Scholar]
- 151.Sperling R.A., Parak W.J. Surface modification, functionalization and bioconjugation of colloidal Inorganic nanoparticles. Philos Trans R Soc A Math Phys Eng Sci. 2010;368:1333–1383. doi: 10.1098/rsta.2009.0273. Available from: [DOI] [PubMed] [Google Scholar]
- 152.Roca M., Haes A.J. Probing cells with noble metal nanoparticle aggregates. Nanomedicine. 2008;3:555–565. doi: 10.2217/17435889.3.4.555. [DOI] [PubMed] [Google Scholar]
- 153.Vilela D., González M.C., Escarpa A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: chemical creativity behind the assay. A review. Anal Chim Acta. 2012;751:24–43. doi: 10.1016/j.aca.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 154.Ding Y., Jiang Z., Saha K., Kim C.S., Kim S.T., Landis R.F. Gold nanoparticles for nucleic acid delivery. Mol Ther. 2014;22:1075–1083. doi: 10.1038/mt.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rosi N.L., Giljohann D.A., Thaxton C.S., Lytton-Jean A.K.R., Han M.S., Mirkin C.A. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 156.Seferos D.S., Prigodich A.E., Giljohann D.A., Patel P.C., Mirkin C.A. Polyvalent DNA nanoparticle conjugates stabilize nucleic acids. Nano Lett. 2009;9:308–311. doi: 10.1021/nl802958f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jin R., Wu G., Li Z., Mirkin C.A., Schatz G.C. What controls the melting properties of DNA-linked gold nanoparticle assemblies? J Am Chem Soc. 2003;125:1643–1654. doi: 10.1021/ja021096v. [DOI] [PubMed] [Google Scholar]
- 158.Lytton-Jean A.K.R., Mirkin C.A. A thermodynamic investigation into the binding properties of DNA functionalized gold nanoparticle probes and molecular fluorophore probes. J Am Chem Soc. 2005;127:12754–12755. doi: 10.1021/ja052255o. [DOI] [PubMed] [Google Scholar]
- 159.Thaxton C.S., Hill H.D., Georganopoulou D.G., Stoeva S.I., Mirkin C.A. A bio-bar-code assay based upon dithiothreitol-induced oligonucleotide release. Anal Chem. 2005;77:8174–8178. doi: 10.1021/ac0514265. [DOI] [PubMed] [Google Scholar]
- 160.Giljohann D.A., Seferos D.S., Patel P.C., Millstone J.E., Rosi N.L., Mirkin C.A. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007;7:3818–3821. doi: 10.1021/nl072471q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hurst S.J., Lytton-Jean A.K.R., Mirkin C.A. Maximizing DNA loading on a range of gold nanoparticle sizes. Anal Chem. 2006;78:8313–8318. doi: 10.1021/ac0613582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee J.S., Seferos D.S., Giljohann D.A., Mirkin C.A. Thermodynamically controlled separation of polyvalent 2-nm gold nanoparticle-oligonucleotide conjugates. J Am Chem Soc. 2008;130:5430–5431. doi: 10.1021/ja800797h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Giljohann D.A., Seferos D.S., Daniel W.L., Massich M.D., Patel P.C., Mirkin C.A. Gold nanoparticles for biology and medicine. Angew Chem Int Ed. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Patel P.C., Giljohann D.A., Daniel W.L., Zheng D., Prigodich A.E., Mirkin C.A. Scavenger receptors mediate cellular uptake of polyvalent oligonucleotide-functionalized gold nanoparticles. Bioconjugate Chem. 2010;21:2250–2256. doi: 10.1021/bc1002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zani I., Stephen S., Mughal N., Russell D., Homer-Vanniasinkam S., Wheatcroft S. Scavenger receptor structure and function in health and disease. Cells. 2015;4:178–201. doi: 10.3390/cells4020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang K., Hao L., Hurst S.J., Mirkin C.A. Antibody-linked spherical nucleic acids for cellular targeting. J Am Chem Soc. 2012;134:16488–16491. doi: 10.1021/ja306854d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hao L., Patel P.C., Alhasan A.H., Giljohann D.A., Mirkin C.A. Nucleic acid-gold nanoparticle conjugates as mimics of microRNA. Small. 2011;7:3158–3162. doi: 10.1002/smll.201101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oishi M., Nakaogami J., Ishii T., Nagasaki Y. Smart PEGylated gold nanoparticles for the cytoplasmic delivery of siRNA to induce enhanced gene silencing. Chem Lett. 2006;35:1046–1047. [Google Scholar]
- 169.Giljohann D.A., Seferos D.S., Prigodich A.E., Patel P.C., Mirkin C.A. Gene regulation with polyvalent siRNA-nanoparticle conjugates. J Am Chem Soc. 2009;131:2072–2073. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zheng D., Giljohann D.A., Chen D.L., Massich M.D., Wang X.Q., Iordanov H. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc Natl Acad Sci U S A. 2012;109:11975–11980. doi: 10.1073/pnas.1118425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Massich M.D., Giljohann D.A., Seferos D.S., Ludlow L.E., Horvath C.M., Mirkin C.A. Regulating immune response using polyvalent nucleic acid-gold nanoparticle conjugates. Mol Pharm. 2009;6:1934–1940. doi: 10.1021/mp900172m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cheung W., Pontoriero F., Taratula O., Chen A.M., He H. DNA and carbon nanotubes as medicine. Adv Drug Deliv Rev. 2010;62:633–649. doi: 10.1016/j.addr.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 173.Qi L., Gao X. Quantum dot—amphipol nanocomplex for intracellular delivery and real-time imaging of siRNA. ACS Nano. 2008;2:1403–1410. doi: 10.1021/nn800280r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Boisselier E., Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 175.Kim S.T., Chompoosor A., Yeh Y.C., Agasti S.S., Solfiell D.J., Rotello V.M. Dendronized gold nanoparticles for siRNA delivery. Small. 2012;8:3253–3256. doi: 10.1002/smll.201201141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fitzgerald K.A., Rahme K., Guo J., Holmes J.D., O'Driscoll C.M. Anisamide-targeted gold nanoparticles for siRNA delivery in prostate cancer—synthesis, physicochemical characterisation and in vitro evaluation. J Mater Chem B. 2016;4:2242–2252. doi: 10.1039/c6tb00082g. [DOI] [PubMed] [Google Scholar]
- 177.Ahwazi R.P., Kiani M., Dinarvand M., Assali A., Tekie F.S.M., Dinarvand R. Immobilization of HIV-1 TAT peptide on gold nanoparticles: a feasible approach for siRNA delivery. J Cell Physiol. 2020;235:2049–2059. doi: 10.1002/jcp.29105. [DOI] [PubMed] [Google Scholar]
- 178.Shirazi A.N., Paquin K.L., Howlett N.G., Mandal D., Parang K. Cyclic peptide-capped gold nanoparticles for enhanced siRNA delivery. Molecules. 2014;19:13319–13331. doi: 10.3390/molecules190913319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Paul A.M., Shi Y., Acharya D., Douglas J.R., Cooley A., Anderson J.F. Delivery of antiviral small interfering RNA with gold nanoparticles inhibits dengue virus infection in vitro. J Gen Virol. 2014;95:1712–1722. doi: 10.1099/vir.0.066084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 181.Jensen S.A., Day E.S., Ko C.H., Hurley L.A., Luciano J.P., Kouri F.M. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5:1–22. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huang X., Hu Q., Braun G.B., Pallaoro A., Morales D.P., Zasadzinski J. Light-activated RNA interference in human embryonic stem cells. Biomaterials. 2015;63:70–79. doi: 10.1016/j.biomaterials.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 183.Jiang Y., Huo S., Hardie J., Liang X.J., Rotello V.M. Progress and perspective of inorganic nanoparticle-based siRNA delivery systems. Expet Opin Drug Deliv. 2016;13:547–559. doi: 10.1517/17425247.2016.1134486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Elbakry A., Zaky A., Liebl R., Rachel R., Goepferich A., Breunig M. Layer-by-layer assembled gold nanoparticles for siRNA delivery. Nano Lett. 2009;9:2059–2064. doi: 10.1021/nl9003865. [DOI] [PubMed] [Google Scholar]
- 185.Lee S.K., Han M.S., Asokan S., Tung C.H. Effective gene silencing by multilayered siRNA-coated gold nanoparticles. Small. 2011;7:364–370. doi: 10.1002/smll.201001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee M.Y., Park S.J., Park K., Kim K.S., Lee H., Hahn S.K. Target-specific gene silencing of layer-by-layer assembled gold-cysteamine/siRNA/PEI/HA nanocomplex. ACS Nano. 2011;5:6138–6147. doi: 10.1021/nn2017793. [DOI] [PubMed] [Google Scholar]
- 187.Jhaveri A.M., Torchilin V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol. 2014;5:1–26. doi: 10.3389/fphar.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Falamarzian A., Xiong X.B., Uludag H., Lavasanifar A. Polymeric micelles for siRNA delivery. J Drug Deliv Sci Technol. 2012;43–54 [Google Scholar]
- 189.Fernandez A.M., Van derpoorten K., Dasnois L., Lebtahi K., Dubois V., Lobl T.J. N-Succinyl-(β-alanyl-l-leucyl-l-alanyl-l-leucyl)doxorubicin: an extracellularly tumor-activated prodrug devoid of intravenous acute toxicity. J Med Chem. 2001;44:3750–3753. doi: 10.1021/jm0108754. [DOI] [PubMed] [Google Scholar]
- 190.Lu Y., Park K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm. 2013;453:198–214. doi: 10.1016/j.ijpharm.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 192.Kedar U., Phutane P., Shidhaye S., Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed Nanotechnol Biol Med. 2010;6:714–729. doi: 10.1016/j.nano.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 193.Iyer A.K., Khaled G., Fang J., Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 194.Hobbs S.K., Monsky W.L., Yuan F., Roberts W.G., Griffith L., Torchilin V.P. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xu X., Ho W., Zhang X., Bertrand N., Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends Mol Med. 2015;21:223–232. doi: 10.1016/j.molmed.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Danhier F., Feron O., Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 197.Kirpotin D.B., Drummond D.C., Shao Y., Shalaby M.R., Hong K., Nielsen U.B. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Canc Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 198.Jin C., Qian N., Zhao W., Yang W., Bai L., Wu H. Improved therapeutic effect of DOX-PLGA-PEG micelles decorated with bivalent fragment HAb18 F(ab')2 for hepatocellular carcinoma. Biomacromolecules. 2010;11:2422–2431. doi: 10.1021/bm1005992. [DOI] [PubMed] [Google Scholar]
- 199.Cheng R., Meng F., Deng C., Klok H.A., Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34:3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. Available from: [DOI] [PubMed] [Google Scholar]
- 200.Liberti M.V., Locasale J.W. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gerweck L.E., Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Canc Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 202.Yoshida T., Lai T.C., Kwon G.S., Sako K. PH-and ion-sensitive polymers for drug delivery. Expet Opin Drug Deliv. 2013;10:1497–1513. doi: 10.1517/17425247.2013.821978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jiang B., Hom W.L., Chen X., Yu P., Pavelka L.C., Kisslinger K. Magnetic Hydrogels from alkyne/cobalt carbonyl-functionalized ABA triblock copolymers. J Am Chem Soc. 2016;138:4616–4625. doi: 10.1021/jacs.6b01271. [DOI] [PubMed] [Google Scholar]
- 204.Panja S., Dey G., Bharti R., Kumari K., Maiti T.K., Mandal M. Tailor-made temperature-sensitive micelle for targeted and on-demand release of anticancer drugs. ACS Appl Mater Interfaces. 2016;8:12063–12074. doi: 10.1021/acsami.6b03820. [DOI] [PubMed] [Google Scholar]
- 205.Blanco E., Kessinger C.W., Sumer B.D., Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med. 2009;234:123–131. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Duong H.H.P., Yung L.Y.L. Synergistic co-delivery of doxorubicin and paclitaxel using multi-functional micelles for cancer treatment. Int J Pharm. 2013;454:486–495. doi: 10.1016/j.ijpharm.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 207.Christie R.J., Matsumoto Y., Miyata K., Nomoto T., Fukushima S., Osada K. Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano. 2012;6:5174–5189. doi: 10.1021/nn300942b. [DOI] [PubMed] [Google Scholar]
- 208.Lu H.H., Huang C.H., Shiue T.Y., Wang F.S., Chang K.K., Chen Y. Erratum: highly efficient gene release in spatiotemporal precision approached by light and pH dual responsive copolymers. Chem Sci. 2019;10:284–292. doi: 10.1039/c8sc01494a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hao F., Lee R.J., Yang C., Zhong L., Sun Y., Dong S. Targeted co-delivery of siRNA and methotrexate for tumor therapy via mixed micelles. Pharmaceutics. 2019;11:1–19. doi: 10.3390/pharmaceutics11020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hazekawa M., Nishinakagawa T., Kawakubo-Yasukochi T., Nakashima M. Glypican-3 gene silencing for ovarian cancer using siRNA-PLGA hybrid micelles in a murine peritoneal dissemination model. J Pharmacol Sci. 2019;139:231–239. doi: 10.1016/j.jphs.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 211.Hao F., Dong S., Yang C., Li Z., Cheng Z., Zhong L. Targeted and efficient delivery of siRNA using tunable polymeric hybrid micelles for tumor therapy. Anticancer Res. 2019;39:1169–1178. doi: 10.21873/anticanres.13226. [DOI] [PubMed] [Google Scholar]
- 212.Xu C., Li D., Cao Z., Xiong M., Yang X., Wang J. Facile hydrophobization of siRNA with anticancer drug for non-cationic nanocarrier-mediated systemic delivery. Nano Lett. 2019;19:2688–2693. doi: 10.1021/acs.nanolett.9b00657. [DOI] [PubMed] [Google Scholar]
- 213.Wang X., Hhua Y., Xu G., Deng S., Yang D., Gao X. Targeting EZH2 for glioma therapy with a novel nanoparticle‒siRNA complex. Int J Nanomed. 2019;14:2637–26353. doi: 10.2147/IJN.S189871. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li G., Gao Y., Gong C., Han Z., Qiang L., Tai Z. Dual-blockade immune checkpoint for breast cancer treatment based on a tumor-penetrating peptide assembling nanoparticle. ACS Appl Mater Interfaces. 2019;11:39513–39524. doi: 10.1021/acsami.9b13354. [DOI] [PubMed] [Google Scholar]
- 215.Yang Y., Meng Y., Ye J., Xia X., Wang H., Li L. Sequential delivery of VEGF siRNA and paclitaxel for PVN destruction, anti-angiogenesis, and tumor cell apoptosis procedurally via a multi-functional polymer micelle. J Control Release. 2018;287:103–120. doi: 10.1016/j.jconrel.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 216.Suo A., Qian J., Xu M., Xu W., Zhang Y., Yao Y. Folate-decorated PEGylated triblock copolymer as a pH/reduction dual-responsive nanovehicle for targeted intracellular co-delivery of doxorubicin and Bcl-2 siRNA. Mater Sci Eng C. 2017;76:659–672. doi: 10.1016/j.msec.2017.03.124. [DOI] [PubMed] [Google Scholar]
- 217.Lee S.Y., Yang C.Y., Peng C.L., Wei M.F., Chen K.C., Yao C.J. A theranostic micelleplex co-delivering Sn-38 and VEGF siRNA for colorectal cancer therapy. Biomaterials. 2016;86:92–105. doi: 10.1016/j.biomaterials.2016.01.068. [DOI] [PubMed] [Google Scholar]
- 218.Kanazawa T., Sugawara K., Tanaka K., Horiuchi S., Takashima Y., Okada H. Suppression of tumor growth by systemic delivery of anti-VEGF siRNA with cell-penetrating peptide-modified MPEG-PCL nanomicelles. Eur J Pharm Biopharm. 2012;81:470–477. doi: 10.1016/j.ejpb.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 219.Wu Y., Zhang Y., Zhang W., Sun C., Wu J., Tang J. Reversing of multidrug resistance breast cancer by co-delivery of P-gp siRNA and doxorubicin via folic acid-modified core-shell nanomicelles. Colloids Surf B Biointerfaces. 2016;138:60–69. doi: 10.1016/j.colsurfb.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 220.Fang S., Wu L., Li M., Yi H., Gao G., Sheng Z. ZEB1 knockdown mediated using polypeptide cationic micelles inhibits metastasis and effects sensitization to a chemotherapeutic drug for cancer therapy. Nanoscale. 2014;6:10084–10094. doi: 10.1039/c4nr01518e. [DOI] [PubMed] [Google Scholar]
- 221.Xiong X.B., Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS Nano. 2011;5:5202–5213. doi: 10.1021/nn2013707. [DOI] [PubMed] [Google Scholar]
- 222.Yu S.S., Lau C.M., Barham W.J., Onishko H.M., Nelson C.E., Li H. Macrophage-specific RNA interference targeting via “click”, mannosylated polymeric micelles. Mol Pharm. 2013;10:975–987. doi: 10.1021/mp300434e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li Y., Ma J., Zhu H., Gao X., Dong H., Shi D. Green synthetic, multifunctional hybrid micelles with shell embedded magnetic nanoparticles for theranostic applications. ACS Appl Mater Interfaces. 2013;5:7227–7235. doi: 10.1021/am401573b. [DOI] [PubMed] [Google Scholar]
- 224.Song N., Ding M., Pan Z., Li J., Zhou L., Tan H. Construction of targeting-clickable and tumor-cleavable polyurethane nanomicelles for multifunctional intracellular drug delivery. Biomacromolecules. 2013;14:4407–4419. doi: 10.1021/bm401342t. [DOI] [PubMed] [Google Scholar]
- 225.Ding M., Song N., He X., Li J., Zhou L., Tan H. Toward the next-generation nanomedicines: design of multifunctional multiblock polyurethanes for effective cancer treatment. ACS Nano. 2013;7:1918–1928. doi: 10.1021/nn4002769. [DOI] [PubMed] [Google Scholar]
- 226.Zhu L., Wang T., Perche F., Taigind A., Torchilin V.P. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc Natl Acad Sci U S A. 2013;110:17047–17052. doi: 10.1073/pnas.1304987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tang X., Rao J., Yin S., Wei J., Xia C., Li M. PD-L1 knockdown via hybrid micelle promotes paclitaxel induced cancer-immunity cycle for melanoma treatment. Eur J Pharmaceut Sci. 2019;127:161–174. doi: 10.1016/j.ejps.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 228.Pan J., Mendes L.P., Yao M., Filipczak N., Garai S., Thakur G.A. Polyamidoamine dendrimers-based nanomedicine for combination therapy with siRNA and chemotherapeutics to overcome multidrug resistance. Eur J Pharm Biopharm. 2019;136:18–28. doi: 10.1016/j.ejpb.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kim S.H., Jeong J.H., Lee S.H., Kim S.W., Park T.G. Local and systemic delivery of VEGF siRNA using polyelectrolyte complex micelles for effective treatment of cancer. J Control Release. 2008;129:107–116. doi: 10.1016/j.jconrel.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 230.Decuzzi P., Ferrari M. The receptor-mediated endocytosis of nonspherical particles. Biophys J. 2008;94:3790–3797. doi: 10.1529/biophysj.107.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xiong X.B., Uludaĝ H., Lavasanifar A. Virus-mimetic polymeric micelles for targeted siRNA delivery. Biomaterials. 2010;31:5886–5893. doi: 10.1016/j.biomaterials.2010.03.075. [DOI] [PubMed] [Google Scholar]
- 232.Rabideau A.E., Pentelute B.L. Delivery of non-native cargo into mammalian cells using anthrax lethal toxin. ACS Chem Biol. 2016;11:1490–1501. doi: 10.1021/acschembio.6b00169. [DOI] [PubMed] [Google Scholar]
- 233.Cheng L., Yang L., Meng F., Zhong Z. Protein nanotherapeutics as an emerging modality for cancer therapy. Adv Healthc Mater. 2018;7:1–9. doi: 10.1002/adhm.201800685. [DOI] [PubMed] [Google Scholar]
- 234.Liu X., Wu F., Ji Y., Yin L. Recent advances in anti-cancer protein/peptide delivery. Bioconjugate Chem. 2019;30:305–324. doi: 10.1021/acs.bioconjchem.8b00750. [DOI] [PubMed] [Google Scholar]
- 235.Stewart M.P., Langer R., Jensen K.F. Intracellular delivery by membrane disruption: mechanisms, strategies, and concepts. Chem Rev. 2018;118:7409–7531. doi: 10.1021/acs.chemrev.7b00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yang J., Zhang Q., Chang H., Cheng Y. Surface-engineered dendrimers in gene delivery. Chem Rev. 2015;115:5274–5300. doi: 10.1021/cr500542t. [DOI] [PubMed] [Google Scholar]
- 237.Wang H., Miao W., Wang F., Cheng Y. A Self-assembled coumarin-anchored dendrimer for efficient gene delivery and light-responsive drug delivery. Biomacromolecules. 2018;19:2194–2201. doi: 10.1021/acs.biomac.8b00246. [DOI] [PubMed] [Google Scholar]
- 238.Liu H., Chang H., Lv J., Jiang C., Li Z., Wang F. Screening of efficient siRNA carriers in a library of surface-engineered dendrimers. Sci Rep. 2016;6:1–11. doi: 10.1038/srep25069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yang J., Hendricks W., Liu G., McCaffery J.M., Kinzler K.W., Huso D.L. A nanoparticle formulation that selectively transfects metastatic tumors in mice. Proc Natl Acad Sci U S A. 2013;110:14717–14722. doi: 10.1073/pnas.1313330110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hashim P.K., Okuro K., Sasaki S., Hoashi Y., Aida T. Reductively cleavable nanocaplets for siRNA delivery by template-assisted oxidative polymerization. J Am Chem Soc. 2015;137:15608–15611. doi: 10.1021/jacs.5b08948. [DOI] [PubMed] [Google Scholar]
- 241.Yang X.Z., Du J.Z., Dou S., Mao C.Q., Long H.Y., Wang J. Sheddable ternary nanoparticles for tumor acidity-targeted siRNA delivery. ACS Nano. 2012;6:771–781. doi: 10.1021/nn204240b. [DOI] [PubMed] [Google Scholar]
- 242.Bakker M.H., Lee C.C., Meijer E.W., Dankers P.Y.W., Albertazzi L. Multicomponent supramolecular polymers as a modular platform for intracellular delivery. ACS Nano. 2016;10:1845–1852. doi: 10.1021/acsnano.5b05383. [DOI] [PubMed] [Google Scholar]
- 243.Juanes M., Creese O., Fernández-Trillo P., Montenegro J. Messenger RNA delivery by hydrazone-activated polymers. MedChemComm. 2019;10:1138–1144. doi: 10.1039/c9md00231f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Spagnou S., Miller A.D., Keller M. Lipidic carriers of siRNA: differences in the formulation, cellular uptake, and delivery with plasmid DNA. Biochemistry. 2004;43:13348–13356. doi: 10.1021/bi048950a. [DOI] [PubMed] [Google Scholar]
- 245.Moghimi S.M., Symonds P., Murray J.C., Hunter A.C., Debska G., Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 246.Namgung R., Kim J., Singha K., Kim C.H., Kim W.J. Synergistic effect of low cytotoxic linear polyethylenimine and multiarm polyethylene glycol: study of physicochemical properties and in vitro gene transfection. Mol Pharm. 2009;6:1826–1835. doi: 10.1021/mp900096u. [DOI] [PubMed] [Google Scholar]
- 247.Mao S., Neu M., Germershaus O., Merkel O., Sitterberg J., Bakowsky U. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjugate Chem. 2006;17:1209–1218. doi: 10.1021/bc060129j. [DOI] [PubMed] [Google Scholar]
- 248.Park I.K., Singha K., Arote R.B., Choi Y.J., Kim W.J., Cho C.S. pH-responsive polymers as gene carriers. Macromol Rapid Commun. 2010;31:1122–1133. doi: 10.1002/marc.200900867. [DOI] [PubMed] [Google Scholar]
- 249.De las Heras Alarcón C., Pennadam S., Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34:276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 250.Oskuee R.K., Philipp A., Dehshahri A., Wagner E., Ramezani M. The impact of carboxyalkylation of branched polyethylenimine on effectiveness in small interfering RNA delivery. J Gene Med. 2010;12:729–738. doi: 10.1002/jgm.1490. [DOI] [PubMed] [Google Scholar]
- 251.Hunter A.C., Moghimi S.M. Therapeutic synthetic polymers: a game of Russian roulette? Drug Discov Today. 2002;7:998–1001. doi: 10.1016/s1359-6446(02)02444-3. [DOI] [PubMed] [Google Scholar]
- 252.Philipp A., Zhao X., Tarcha P., Wagner E., Zintchenko A. Hydrophobically modified oligoethylenimines as highly efficient transfection agents for siRNA delivery. Bioconjugate Chem. 2009;20:2055–2061. doi: 10.1021/bc9001536. [DOI] [PubMed] [Google Scholar]
- 253.Kim T.-il., Lee M., Kim S.W. A guanidinylated bioreducible polymer with high nuclear localization ability for gene delivery systems. Biomaterials. 2010;31:1798–1804. doi: 10.1016/j.biomaterials.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Son S., Singha K., Kim W.J. Bioreducible BPEI-SS-PEG-cNGR polymer as a tumor targeted nonviral gene carrier. Biomaterials. 2010;31:6344–6354. doi: 10.1016/j.biomaterials.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 255.Son S., Hwang D.W., Singha K., Jeong J.H., Park T.G., Lee D.S. RVG peptide tethered bioreducible polyethylenimine for gene delivery to brain. J Control Release. 2011;155:18–25. doi: 10.1016/j.jconrel.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 256.Singha K., Namgung R., Kim W.J. Polymers in small-interfering RNA delivery. Nucleic Acid Therapeut. 2011;21:133–147. doi: 10.1089/nat.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kim W.J., Chang C.W., Lee M., Kim S.W. Efficient siRNA delivery using water soluble lipopolymer for anti-angiogenic gene therapy. J Control Release. 2007;118:357–363. doi: 10.1016/j.jconrel.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 258.Biswal B.K., Debata N.B., Verma R.S. Development of a targeted siRNA delivery system using FOL‒PEG‒PEI conjugate. Mol Biol Rep. 2010;37:2919–2926. doi: 10.1007/s11033-009-9853-3. [DOI] [PubMed] [Google Scholar]
- 259.Yamaoka T., Tabata Y., Ikada Y. Body distribution profile of polysaccharides after intravenous administration. Drug Deliv. 1993;1:75–82. [Google Scholar]
- 260.Park I.K., Ihm J.E., Park Y.H., Choi Y.J., Kim S.I., Kim W.J. Galactosylated chitosan (GC)-graft-poly(vinyl pyrrolidone) (PVP) as hepatocyte-targeting DNA carrier: preparation and physicochemical characterization of GC‒graft‒PVP/DNA complex (1) J Control Release. 2003;86:349–359. doi: 10.1016/s0168-3659(02)00365-6. [DOI] [PubMed] [Google Scholar]
- 261.Kim S.K., Park K.M., Singha K., Kim J., Ahn Y., Kim K. Galactosylated cucurbituril-inclusion polyplex for hepatocyte-targeted gene delivery. Chem Commun. 2010;46:692–694. doi: 10.1039/b920753h. [DOI] [PubMed] [Google Scholar]
- 262.Lee K., Bae K.H., Lee Y., Lee S.H., Ahn C.H., Park T.G. Pluronic/polyethylenimine shell crosslinked nanocapsules with embedded magnetite nanocrystals for magnetically triggered delivery of siRNA. Macromol Biosci. 2010;10:239–245. doi: 10.1002/mabi.200900291. [DOI] [PubMed] [Google Scholar]
- 263.Yuan X., Li N., Rathinavelu A., Hao J., Narasimhan M., He M. siRNA drug delivery by biodegradable polymeric nanoparticles. J Nanosci Nanotechnol. 2006;6:2821–2828. doi: 10.1166/jnn.2006.436. [DOI] [PubMed] [Google Scholar]
- 264.Son S., Kim W.J. Biodegradable nanoparticles modified by branched polyethylenimine for plasmid DNA delivery. Biomaterials. 2010;31:133–143. doi: 10.1016/j.biomaterials.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 265.Tahara K., Sakai T., Yamamoto H., Takeuchi H., Kawashima Y. Establishing chitosan coated PLGA nanosphere platform loaded with wide variety of nucleic acid by complexation with cationic compound for gene delivery. Int J Pharm. 2008;354:210–216. doi: 10.1016/j.ijpharm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 266.Cun D., Foged C., Yang M., Frøkjær S., Nielsen H.M. Preparation and characterization of poly(d,l-lactide-co-glycolide) nanoparticles for siRNA delivery. Int J Pharm. 2010;390:70–75. doi: 10.1016/j.ijpharm.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 267.Cun D., Jensen D.K., Maltesen M.J., Bunker M., Whiteside P., Scurr D. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: quality by design optimization and characterization. Eur J Pharm Biopharm. 2011;77:26–35. doi: 10.1016/j.ejpb.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 268.Wang J., Feng S.S., Wang S., Chen Z-ying. Evaluation of cationic nanoparticles of biodegradable copolymers as siRNA delivery system for hepatitis B treatment. Int J Pharm. 2010;400:194–200. doi: 10.1016/j.ijpharm.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 269.Hombach J., Bernkop-Schnürch A. Chitosan solutions and particles: evaluation of their permeation enhancing potential on MDCK cells used as blood brain barrier model. Int J Pharm. 2009;376:104–109. doi: 10.1016/j.ijpharm.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 270.Andersen M., Lichawska A., Arpanaei A., Rask Jensen S.M., Kaur H., Oupicky D. Surface functionalisation of PLGA nanoparticles for gene silencing. Biomaterials. 2010;31:5671–5677. doi: 10.1016/j.biomaterials.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 271.Alshamsan A., Haddadi A., Hamdy S., Samuel J., El-Kadi A.O.S., Uludaǧ H. STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response. Mol Pharm. 2010;7:1643–1654. doi: 10.1021/mp100067u. [DOI] [PubMed] [Google Scholar]
- 272.Oster C.G., Kissel T. Comparative study of DNA encapsulation into PLGA microparticles using modified double emulsion methods and spray drying techniques. J Microencapsul. 2005;22:235–244. doi: 10.1080/02652040500100295. [DOI] [PubMed] [Google Scholar]
- 273.Katas H., Cevher E., Alpar H.O. Preparation of polyethyleneimine incorporated poly(d,l-lactide-co-glycolide) nanoparticles by spontaneous emulsion diffusion method for small interfering RNA delivery. Int J Pharm. 2009;369:144–154. doi: 10.1016/j.ijpharm.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 274.Risnayanti C., Jang Y.S., Lee J., Ahn H.J. PLGA nanoparticles co-delivering MDR1 and BCL2 siRNA for overcoming resistance of paclitaxel and cisplatin in recurrent or advanced ovarian cancer. Sci Rep. 2018;8:1–12. doi: 10.1038/s41598-018-25930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Patil Y., Panyam J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int J Pharm. 2009;367:195–203. doi: 10.1016/j.ijpharm.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hasan W., Chu K., Gullapalli A., Dunn S.S., Enlow E.M., Luft J.C. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 2012;12:287–292. doi: 10.1021/nl2035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wang L., Griffel B., Xu X. Synthesis of PLGA–lipid hybrid nanoparticles for siRNA delivery using the emulsion method PLGA‒PEG–lipid nanoparticles for siRNA delivery. Methods Mol Biol. 2017;1632:231–240. doi: 10.1007/978-1-4939-7138-1_15. [DOI] [PubMed] [Google Scholar]
- 278.Mao S., Sun W., Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62:12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 279.Opanasopit P., Techaarpornkul S., Rojanarata T., Ngawhirunpat T., Ruktanonchai U. Nucleic acid delivery with chitosan hydroxybenzotriazole. Oligonucleotides. 2010;20:127–136. doi: 10.1089/oli.2009.0227. [DOI] [PubMed] [Google Scholar]
- 280.Rojanarata T., Opanasopit P., Techaarpornkul S., Ngawhirunpat T., Ruktanonchai U. Chitosan-thiamine pyrophosphate as a novel carrier for siRNA delivery. Pharm Res. 2008;25:2807–2814. doi: 10.1007/s11095-008-9648-6. [DOI] [PubMed] [Google Scholar]
- 281.Roy K., Ghosn B., Kasturi S. Enhancing polysaccharide-mediated delivery of nucleic acids through functionalization with secondary and tertiary amines. Curr Top Med Chem. 2008;8:331–340. doi: 10.2174/156802608783790947. [DOI] [PubMed] [Google Scholar]
- 282.Howard K.A., Rahbek U.L., Liu X., Damgaard C.K., Glud S.Z., Andersen M. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 283.Choi B., Cui Z.K., Kim S., Fan J., Wu B.M., Lee M. Glutamine-chitosan modified calcium phosphate nanoparticles for efficient siRNA delivery and osteogenic differentiation. J Mater Chem B. 2015;3:6448–6455. doi: 10.1039/C5TB00843C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ni S., Xie Y., Tang Y., Liu Y., Chen J., Zhu S. Nebulized anionic guanidinylated O-carboxymethyl chitosan/N-2-hydroxypropyltimehyl ammonium chloride chitosan nanoparticles for siRNA pulmonary delivery: preparation, characterization and in vitro evaluation. J Drug Target. 2017;25:451–462. doi: 10.1080/1061186X.2016.1278219. [DOI] [PubMed] [Google Scholar]
- 285.Sun P., Huang W., Jin M., Wang Q., Fan B., Kang L. Chitosan-based nanoparticles for survivin targeted siRNA delivery in breast tumor therapy and preventing its metastasis. Int J Nanomed. 2016;11:4931–4945. doi: 10.2147/IJN.S105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Shahana S., Kampf C., Roomans G.M. Effects of the cationic protein poly-l-arginine on airway epithelial cells in vitro. Mediat Inflamm. 2002;11:141–148. doi: 10.1080/09622935020138172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Noh S.M., Park M.O., Shim G., Han S.E., Lee H.Y., Huh J.H. Pegylated poly-l-arginine derivatives of chitosan for effective delivery of siRNA. J Control Release. 2010;145:159–164. doi: 10.1016/j.jconrel.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 288.Kim E.J., Shim G., Kim K., Kwon I.C., Oh Y.K., Shim C.K. Hyaluronic acid complexed to biodegradable poly l-arginine for targeted delivery of siRNAs. J Gene Med. 2009;11:791–803. doi: 10.1002/jgm.1352. [DOI] [PubMed] [Google Scholar]
- 289.Plianwong S., Opanasopit P., Ngawhirunpat T., Rojanarata T. Chitosan combined with poly-l-arginine as efficient, safe, and serum-insensitive vehicle with RNase protection ability for siRNA delivery. BioMed Res Int. 2013;2013:1–9. doi: 10.1155/2013/574136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wan K., Li J., Li D., Ge J., Wang Y., Li X. Novel hydroxybutyl chitosan nanoparticles for siRNA delivery targeting tissue factor inhibits proliferation and induces apoptosis in human vascular smooth muscle cells. Mol Med Rep. 2015;12:7957–7962. doi: 10.3892/mmr.2015.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Arami S., Rashidi M.R., Mahdavi M., Fathi M., Entezami A.A. Synthesis and characterization of Fe3O4-PEG-LAC-chitosan-PEI nanoparticle as a survivin siRNA delivery system. Hum Exp Toxicol. 2017;36:227–237. doi: 10.1177/0960327116646618. [DOI] [PubMed] [Google Scholar]
- 292.Shen W., Wang R., Fan Q., Gao X., Wang H., Shen Y. Natural polyphenol inspired polycatechols for efficient siRNA delivery. CCS Chem. 2020;2:146–157. [Google Scholar]
- 293.Chung J.E., Tan S., Gao S.J., Yongvongsoontorn N., Kim S.H., Lee J.H. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat Nanotechnol. 2014;9:907–912. doi: 10.1038/nnano.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Shen W., Wang Q., Shen Y., Gao X., Li L., Yan Y. Green tea catechin dramatically promotes RNAi mediated by low-molecular-weight polymers. ACS Cent Sci. 2018;4:1326–1333. doi: 10.1021/acscentsci.8b00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hassler M.R., Turanov A.A., Alterman J.F., Haraszti R.A., Coles A.H., Osborn M.F. Comparison of partially and fully chemically-modified siRNA in conjugate-mediated delivery in vivo. Nucleic Acids Res. 2018;46:2185–2196. doi: 10.1093/nar/gky037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Mansoori B., Mohammadi A., Shir Jang S., Baradaran B. Mechanisms of immune system activation in mammalians by small interfering RNA (siRNA). Artif Cells. Nanomedicine Biotechnol. 2016;44:1589–1596. doi: 10.3109/21691401.2015.1102738. [DOI] [PubMed] [Google Scholar]
- 297.Huntzinger E., Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 298.Deleavey G.F., Watts J.K., Damha M.J. Chemical modification of siRNA. Current Protocols in Nucleic Acid Chemistry. 2009;39:1. doi: 10.1002/0471142700.nc1603s39. [DOI] [PubMed] [Google Scholar]
- 299.Allerson C.R., Sioufi N., Jarres R., Prakash T.P., Naik N., Berdeja A. Fully 2′-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J Med Chem. 2005;48:901–904. doi: 10.1021/jm049167j. [DOI] [PubMed] [Google Scholar]
- 300.Shan G., Li Y., Zhang J., Li W., Szulwach K.E., Duan R. A small molecule enhances RNA interference and promotes microRNA processing. Nat Biotechnol. 2008;26:933–940. doi: 10.1038/nbt.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Diebold S.S., Massacrier C., Akira S., Paturel C., Morel Y., Reis e Sousa C. Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur J Immunol. 2006;36:3256–3267. doi: 10.1002/eji.200636617. [DOI] [PubMed] [Google Scholar]
- 302.Wei P., Sun M., Yang B., Xiao J., Du J. Ultrasound-responsive polymersomes capable of endosomal escape for efficient cancer therapy. J Control Release. 2020;322:81–94. doi: 10.1016/j.jconrel.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 303.Puri A., Viard M., Zakrevsky P., Zampino S., Chen A., Isemann C. Photoactivation of sulfonated polyplexes enables localized gene silencing by DsiRNA in breast cancer cells. Nanomed Nanotechnol Biol Med. 2020;26:102176. doi: 10.1016/j.nano.2020.102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Song E., Zhu P., Lee S.K., Chowdhury D., Kussman S., Dykxhoorn D.M. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 305.Sarett S.M., Werfel T.A., Lee L., Jackson M.A., Kilchrist K.V., Brantley-Sieders D. Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proc Natl Acad Sci U S A. 2017;114:E6490–E6497. doi: 10.1073/pnas.1621240114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sato Y., Nakamura T., Yamada Y., Harashima H. Development of a multifunctional envelope-type nano device and its application to nanomedicine. J Control Release. 2016;244:194–204. doi: 10.1016/j.jconrel.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 307.Khalil I.A., Kogure K., Futaki S., Hama S., Akita H., Ueno M. Octaarginine-modified multifunctional envelope-type nanoparticles for gene delivery. Gene Ther. 2007;14:682–689. doi: 10.1038/sj.gt.3302910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Moriguchi R., Kogure K., Akita H., Futaki S., Miyagishi M., Taira K. A multifunctional envelope-type nano device for novel gene delivery of siRNA plasmids. Int J Pharm. 2005;301:277–285. doi: 10.1016/j.ijpharm.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 309.Kortylewski M., Swiderski P., Herrmann A., Wang L., Kowolik C., Kujawski M. in vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Khalil I.A., Kimura S., Sato Y., Harashima H. Synergism between a cell penetrating peptide and a pH-sensitive cationic lipid in efficient gene delivery based on double-coated nanoparticles. J Control Release. 2018;275:107–116. doi: 10.1016/j.jconrel.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 311.Rungta R.L., Choi H.B., Lin P.J.C., Ko R.W.Y., Ashby D., Nair J. Lipid nanoparticle delivery of siRNA to silence neuronal gene expression in the brain. Mol Ther Nucleic Acids. 2013;2 doi: 10.1038/mtna.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chaudhary A., Garg S. siRNA delivery using polyelectrolyte-gold nanoassemblies in neuronal cells for BACE1 gene silencing. Mater Sci Eng C. 2017;80:18–28. doi: 10.1016/j.msec.2017.05.101. [DOI] [PubMed] [Google Scholar]
- 313.Solanki A., Shah S., Yin P.T., Lee K.B. Nanotopography-mediated reverse uptake for siRNA delivery into neural stem cells to enhance neuronal differentiation. Sci Rep. 2013;3:1–7. doi: 10.1038/srep01553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kumar P., Wu H., McBride J.L., Jung K.E., Hee Kim M., Davidson B.L. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 315.Huwyler J., Drewe J., Krähenbühl S. Tumor targeting using liposomal antineoplastic drugs. Int J Nanomed. 2008;3:21–29. [PMC free article] [PubMed] [Google Scholar]
- 316.Dokka S., Toledo D., Shi X., Castranova V., Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res. 2000;17:521–525. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- 317.Akhtar S., Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev. 2007;59:164–182. doi: 10.1016/j.addr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 318.Filion M.C., Phillips N.C. Major limitations in the use of cationic liposomes for DNA delivery. Int J Pharm. 1998;162:159–170. [Google Scholar]
- 319.Sharma A., Madhunapantula S.V., Robertson G.P. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expet Opin Drug Metabol Toxicol. 2012;8:47–69. doi: 10.1517/17425255.2012.637916. [DOI] [PMC free article] [PubMed] [Google Scholar]